Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells
Abstract
1. Introduction
2. Results
2.1. B Cells and CR3-Expressing Immune Cell Types in Spleen Bind Complement-Opsonized NC at High Extent
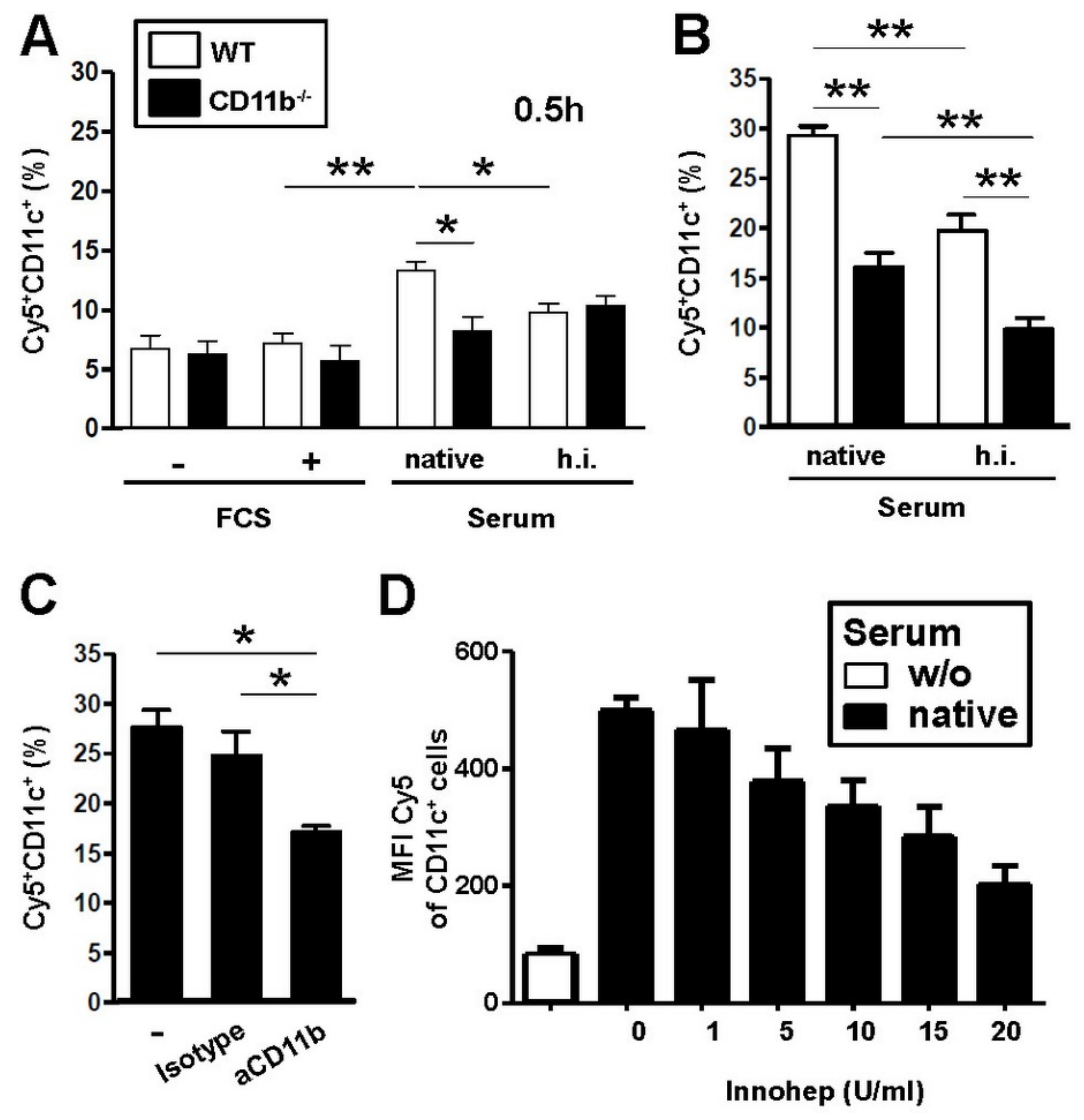
2.2. Concomitant Expression of CR4 Does Not Increase Binding of Serum-Pretreated NC to DC
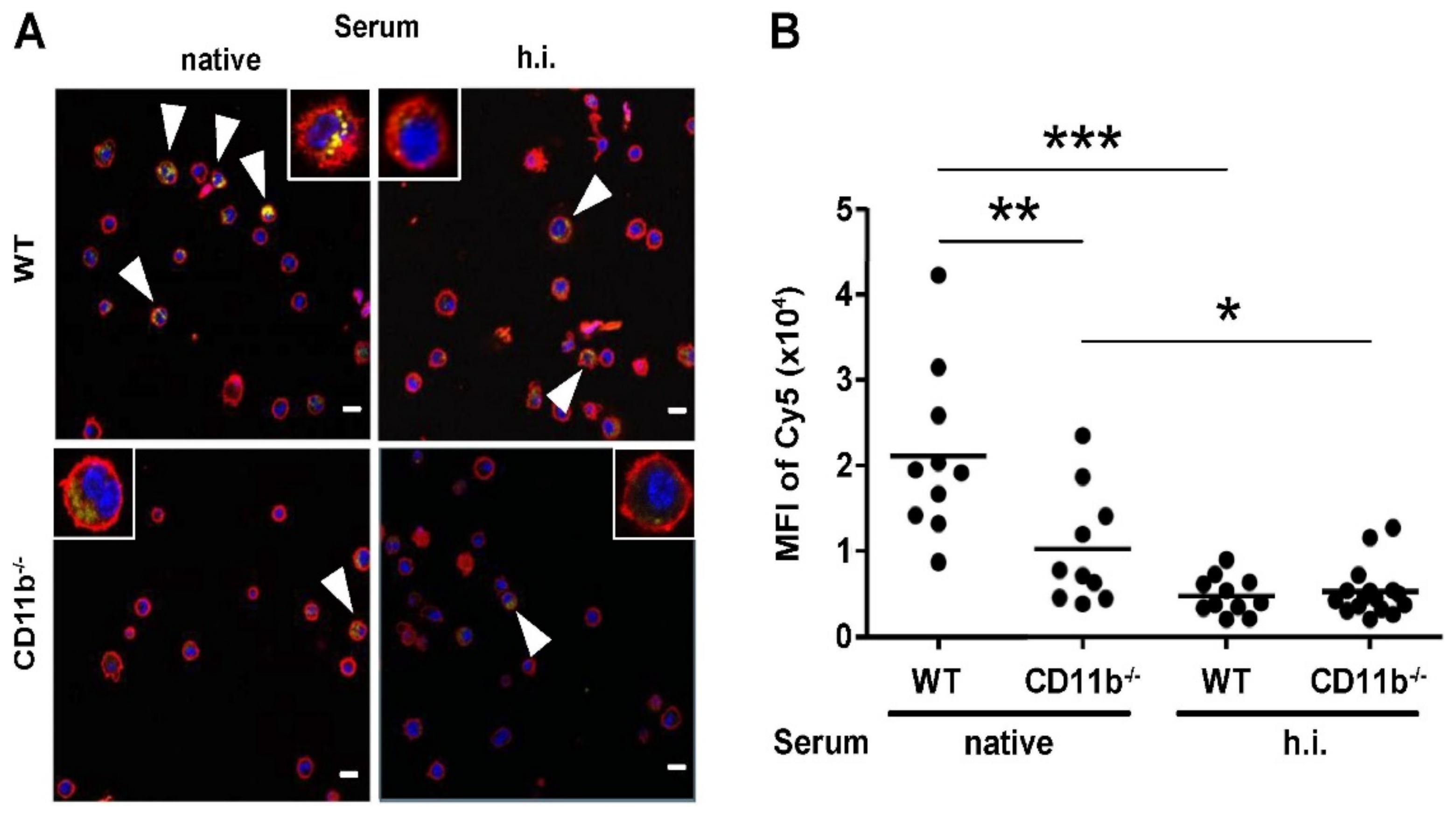
2.3. CR3, but Not CR4 Is Required for Binding and Uptake of Complement-Opsonized NC by DC
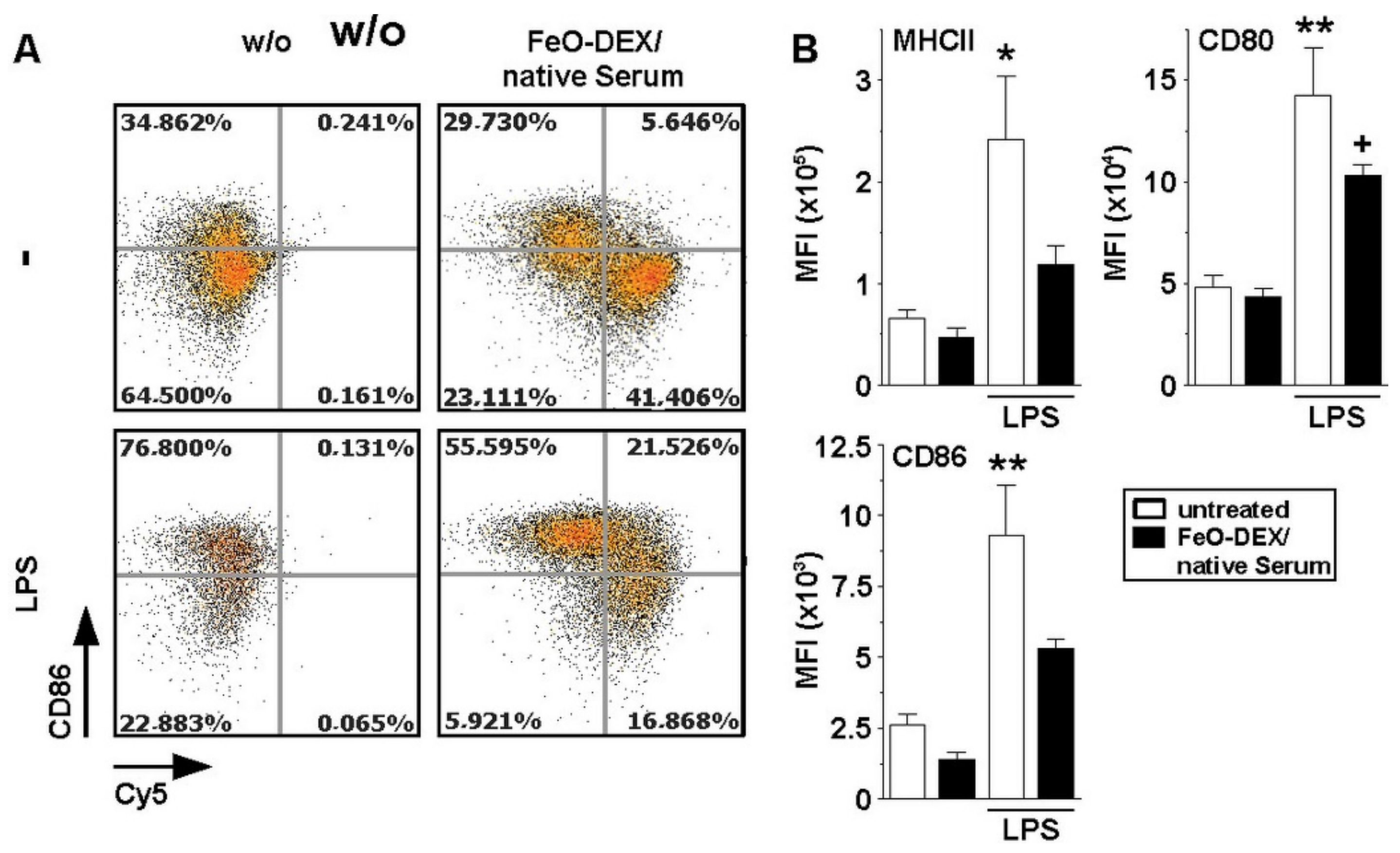
2.4. Binding of Complement-Coated NC Interferes with DC Activation
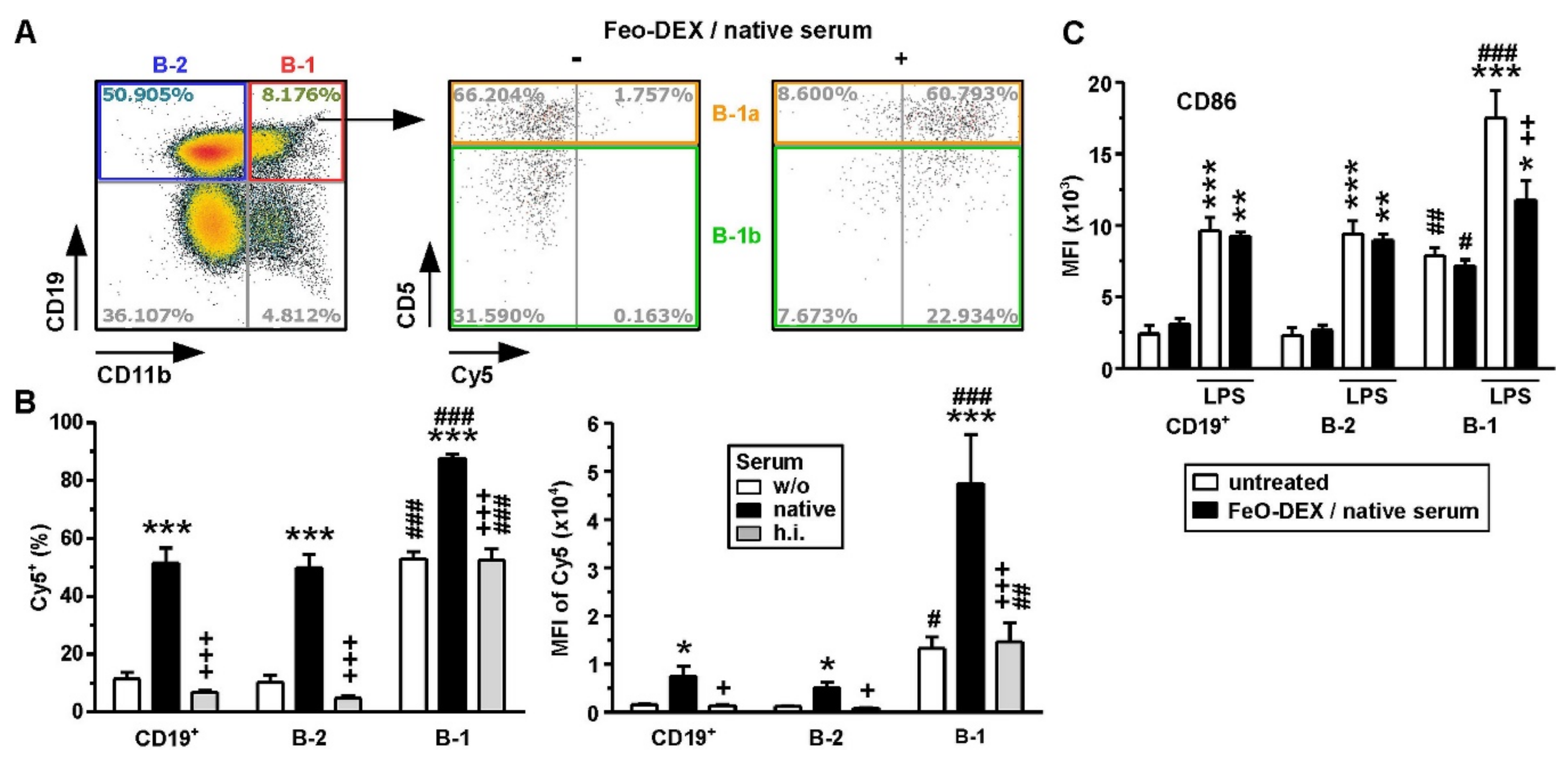
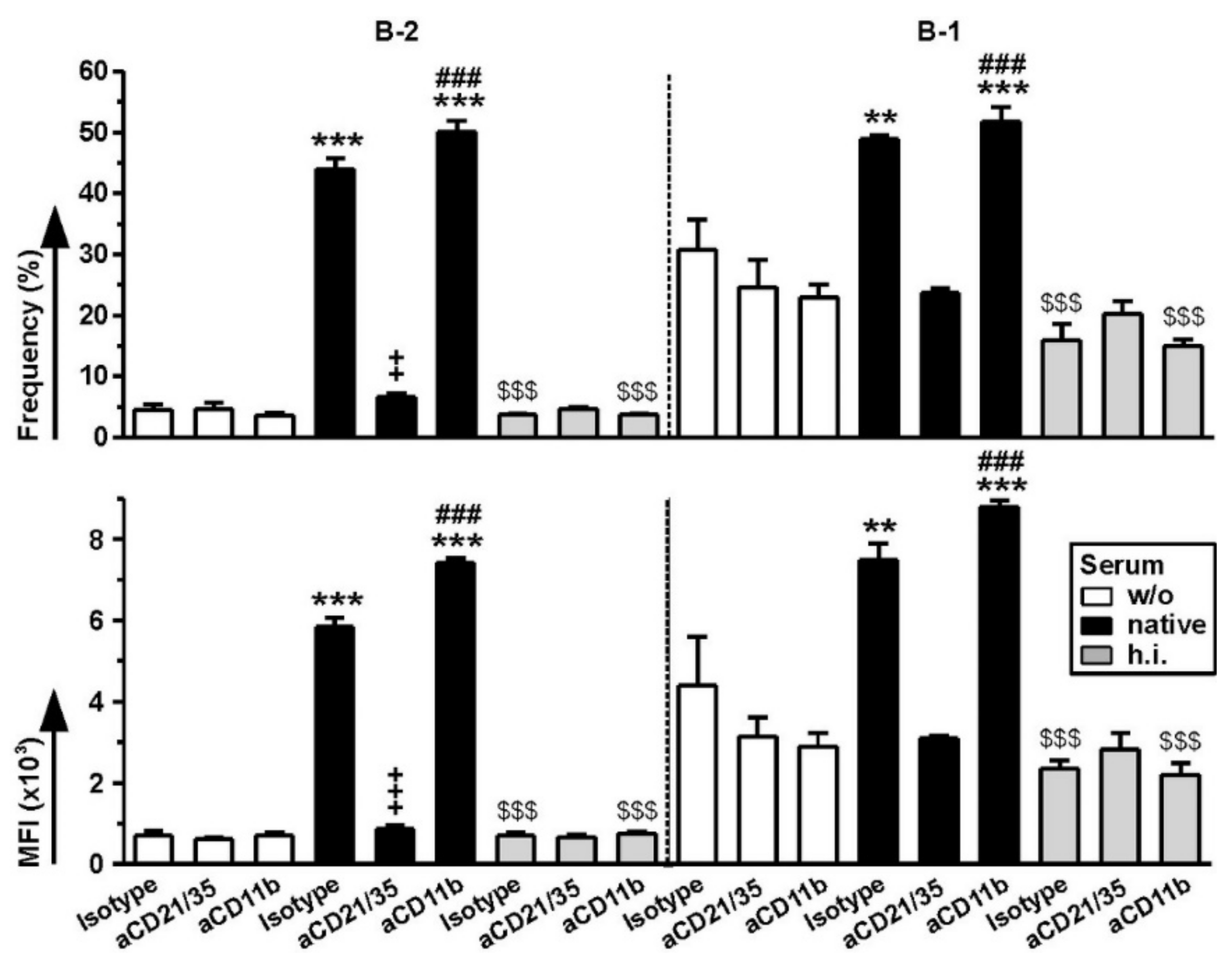
2.5. Among B Cells, FeO-DEX Are Highly Engaged by B-1 Cells
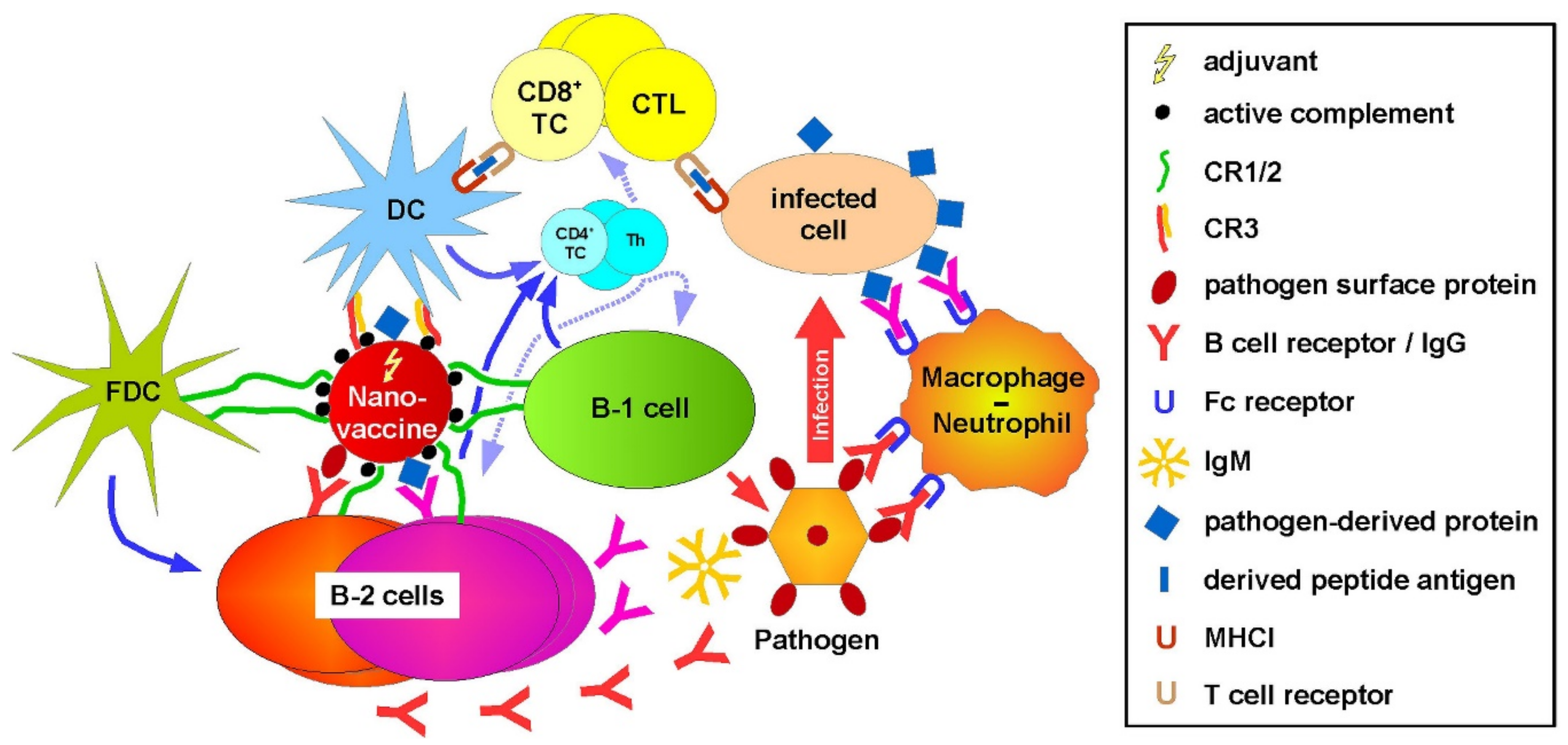
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mice
4.3. Immune Cells
4.4. NP Binding Studies
4.5. Flow Cytometry
4.6. CLSM
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| BM | Bone marrow |
| CR | Complement receptor |
| cDC | Conventional dendritic cell |
| CLSM | Confocal laser scanning microscopy |
| DC | Dendritic cell |
| FCS | Fetal calf serum |
| FDC | Follicular dendritic cells |
| LSEC | Liver sinusoidal endothelial cell |
| MAC | Macrophage |
| NC | Nanocarrier |
| NPC | Nonparenchymal cell |
| NK | Natural killer cell |
| ODN | Oligodeoxynucleotides |
| OVA | Ovalbumin |
| pDC | Plasmacytoid DC |
| PMN | Polymorphonuclear cell |
| SPION | Superparamagnetic iron oxide nanoparticles |
| SR-A | Scavenger receptor class A |
| Th | T helper cell |
| TLR | Toll-like receptor |
| WT | Wild type |
References
- Du, G.; Sun, X. Engineering nanoparticulate vaccines for enhancing antigen cross-presentation. Curr. Opin. Biotechnol. 2020, 66, 113–122. [Google Scholar] [CrossRef]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailänder, V.; Landfester, K.; Grabbe, S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.L.Y.; Lazarovits, J.; Chan, W.C.W. An Analysis of the Binding Function and Structural Organization of the Protein Corona. J. Am. Chem. Soc. 2020, 142, 8827–8836. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitayama, Y.; Sasao, R.; Yamada, T.; Toh, K.; Matsumoto, Y.; Kataoka, K. Molecularly Imprinted Nanogels Acquire Stealth In Situ by Cloaking Themselves with Native Dysopsonic Proteins. Angew. Chem. Int. Ed. Engl. 2017, 56, 7088–7092. [Google Scholar] [CrossRef]
- Mortimer, G.M.; Butcher, N.J.; Musumeci, A.W.; Deng, Z.J.; Martin, D.J.; Minchin, R.F. Cryptic epitopes of albumin determine mononuclear phagocyte system clearance of nanomaterials. ACS Nano 2014, 8, 3357–3366. [Google Scholar] [CrossRef]
- Vincent, M.P.; Bobbala, S.; Karabin, N.B.; Frey, M.; Liu, Y.; Navidzadeh, J.O.; Stack, T.; Scott, E.A. Surface chemistry-mediated modulation of adsorbed albumin folding state specifies nanocarrier clearance by distinct macrophage subsets. Nat. Commun. 2021, 12, 648. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Tenzer, S.; Storck, W.; Hobernik, D.; Raker, V.K.; Fischer, K.; Decker, S.; Dzionek, A.; Krauthäuser, S.; Diken, M.; et al. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. J. Allergy Clin. Immunol. 2018, 142, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Griffin, J.I.; Inturi, S.; Brenneman, B.; Banda, N.K.; Holers, V.M.; Moghimi, S.M.; Simberg, D. In Vitro and In Vivo Differences in Murine Third Complement Component (C3) Opsonization and Macrophage/Leukocyte Responses to Antibody-Functionalized Iron Oxide Nanoworms. Front. Immunol. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, M.; Stege, H.; Grabbe, S.; Bros, M. β2 Integrins-Multi-Functional Leukocyte Receptors in Health and Disease. Int. J. Mol. Sci. 2020, 21, 1402. [Google Scholar] [CrossRef]
- Chao, Y.; Karmali, P.P.; Simberg, D. Role of carbohydrate receptors in the macrophage uptake of dextran-coated iron oxide nanoparticles. Adv. Exp. Med. Biol. 2012, 733, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Makale, M.; Karmali, P.P.; Sharikov, Y.; Tsigelny, I.; Merkulov, S.; Kesari, S.; Wrasidlo, W.; Ruoslahti, E.; Simberg, D. Recognition of dextran-superparamagnetic iron oxide nanoparticle conjugates (Feridex) via macrophage scavenger receptor charged domains. Bioconjug. Chem. 2012, 23, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Karmali, P.P.; Mukthavaram, R.; Kesari, S.; Kouznetsova, V.L.; Tsigelny, I.F.; Simberg, D. Direct recognition of superparamagnetic nanocrystals by macrophage scavenger receptor SR-AI. ACS Nano 2013, 7, 4289–4298. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Serkova, N.J.; Groman, E.V.; Scheinman, R.I.; Simberg, D. Feraheme (Ferumoxytol) Is Recognized by Proinflammatory and Anti-inflammatory Macrophages via Scavenger Receptor Type AI/II. Mol. Pharm. 2019, 16, 4274–4281. [Google Scholar] [CrossRef]
- Erdei, A.; Isaák, A.; Török, K.; Sándor, N.; Kremlitzka, M.; Prechl, J.; Bajtay, Z. Expression and role of CR1 and CR2 on B and T lymphocytes under physiological and autoimmune conditions. Mol. Immunol. 2009, 46, 2767–2773. [Google Scholar] [CrossRef]
- Ross, G.D.; Vĕtvicka, V. CR3 (CD11b, CD18): A phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin. Exp. Immunol. 1993, 92, 181–184. [Google Scholar] [CrossRef]
- Yoshimoto, M. The ontogeny of murine B-1a cells. Int. J. Hematol. 2020, 111, 622–627. [Google Scholar] [CrossRef]
- Novaes, E.B.R.R.; Dos Santos Toledo, M.; Labussiere, G.M.; Dupin, T.V.; de Campos Reis, N.F.; Perez, E.C.; Xander, P. B-1 cell response in immunity against parasites. Parasitol. Res. 2019, 118, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.H.; Jain, M.; Lee, K.; Kandimalla, E.R.; Faridi, M.H.; Buyon, J.P.; Gupta, V.; Clancy, R.M. Complement receptor 3 influences toll-like receptor 7/8-dependent inflammation: Implications for autoimmune diseases characterized by antibody reactivity to ribonucleoproteins. J. Biol. Chem. 2013, 288, 9077–9083. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.K.; Hamerman, J.A. β(2) integrins inhibit TLR responses by regulating NF-κB pathway and p38 MAPK activation. Eur. J. Immunol. 2013, 43, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Vorup-Jensen, T.; Jensen, R.K. Structural Immunology of Complement Receptors 3 and 4. Front. Immunol. 2018, 9, 2716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.J.; Yang, Z.F.; Chen, Y.; Wang, J.; Rosmarin, A.G. PU.1 is essential for CD11c expression in CD8(+)/CD8(-) lymphoid and monocyte-derived dendritic cells during GM-CSF or FLT3L-induced differentiation. PLoS ONE 2012, 7, e52141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Xiang, Y.; Xin, V.W.; Wang, X.W.; Peng, X.C.; Liu, X.Q.; Wang, D.; Li, N.; Cheng, J.T.; Lyv, Y.N.; et al. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020, 13, 107. [Google Scholar] [CrossRef]
- Shen, L.; Krauthauser, S.; Fischer, K.; Hobernik, D.; Abassi, Y.; Dzionek, A.; Nikolaev, A.; Voltz, N.; Diken, M.; Krummen, M.; et al. Vaccination with trifunctional nanoparticles that address CD8(+) dendritic cells inhibits growth of established melanoma. Nanomedicine 2016, 11, 2647–2662. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Medina-Montano, C.; Kaps, L.; Kappel, C.; Gehring, S.; Bros, M. Role of Liver-Mediated Tolerance in Nanoparticle-Based Tumor Therapy. Cells 2020, 9, 1985. [Google Scholar] [CrossRef]
- Na, Y.R.; Jung, D.; Gu, G.J.; Seok, S.H. GM-CSF Grown Bone Marrow Derived Cells Are Composed of Phenotypically Different Dendritic Cells and Macrophages. Mol. Cells 2016, 39, 734–741. [Google Scholar] [CrossRef]
- Ludwig, R.J. Therapeutic use of heparin beyond anticoagulation. Curr. Drug Discov. Technol. 2009, 6, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Klempp, C.; Büchler, M.W.; Märten, A. Release of iC3b from apoptotic tumor cells induces tolerance by binding to immature dendritic cells in vitro and in vivo. Cancer Immunol. Immunother. 2006, 55, 31–38. [Google Scholar] [CrossRef]
- Margry, B.; Wieland, W.H.; van Kooten, P.J.; van Eden, W.; Broere, F. Peritoneal cavity B-1a cells promote peripheral CD4+ T-cell activation. Eur. J. Immunol. 2013, 43, 2317–2326. [Google Scholar] [CrossRef]
- Metlay, J.P.; Witmer-Pack, M.D.; Agger, R.; Crowley, M.T.; Lawless, D.; Steinman, R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990, 171, 1753–1771. [Google Scholar] [CrossRef]
- Bertholon, I.; Vauthier, C.; Labarre, D. Complement activation by core-shell poly(isobutylcyanoacrylate)-polysaccharide nanoparticles: Influences of surface morphology, length, and type of polysaccharide. Pharm. Res. 2006, 23, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Calderó, G.; Mitjans, M.; Vinardell, M.P.; Solans, C.; Vauthier, C. Interactions of PLGA nanoparticles with blood components: Protein adsorption, coagulation, activation of the complement system and hemolysis studies. Nanoscale 2015, 7, 6045–6058. [Google Scholar] [CrossRef] [PubMed]
- Kokate, R.A.; Chaudhary, P.; Sun, X.; Thamake, S.I.; Maji, S.; Chib, R.; Vishwanatha, J.K.; Jones, H.P. Rationalizing the use of functionalized poly-lactic-co-glycolic acid nanoparticles for dendritic cell-based targeted anticancer therapy. Nanomedicine 2016, 11, 479–494. [Google Scholar] [CrossRef] [PubMed]
- El-Maghawry, E.; Tadros, M.I.; Elkheshen, S.A.; Abd-Elbary, A. Eudragit(®)-S100 Coated PLGA Nanoparticles for Colon Targeting of Etoricoxib: Optimization and Pharmacokinetic Assessments in Healthy Human Volunteers. Int. J. Nanomed. 2020, 15, 3965–3980. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, T.D.; Dolgova, N.V.; Lewis, J.S.; Hamaker, K.; Clare-Salzler, M.J.; Keselowsky, B.G. Macrophage integrins modulate response to ultra-high molecular weight polyethylene particles and direct particle-induced osteolysis. Biomaterials 2017, 115, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.; Mlady, G.; Eberhardt, S.C.; Sillerud, L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. AJR Am. J. Roentgenol. 2015, 204, W302–W313. [Google Scholar] [CrossRef]
- Von Zur Muhlen, C.; von Elverfeldt, D.; Bassler, N.; Neudorfer, I.; Steitz, B.; Petri-Fink, A.; Hofmann, H.; Bode, C.; Peter, K. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): Implications on imaging of atherosclerotic plaques. Atherosclerosis 2007, 193, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Sun, Q.; Zhao, L.; Tang, P.; Suo, Z.; Zhang, S.; Zhang, Y.; Zhang, M.; Wang, W.; Li, H. Protein corona of metal-organic framework nanoparticals: Study on the adsorption behavior of protein and cell interaction. Int. J. Biol. Macromol. 2019, 140, 709–718. [Google Scholar] [CrossRef]
- O’Brien, X.M.; Heflin, K.E.; Lavigne, L.M.; Yu, K.; Kim, M.; Salomon, A.R.; Reichner, J.S. Lectin site ligation of CR3 induces conformational changes and signaling. J. Biol. Chem. 2012, 287, 3337–3348. [Google Scholar] [CrossRef]
- Zhang, Y.; Hayenga, H.N.; Sarantos, M.R.; Simon, S.I.; Neelamegham, S. Differential regulation of neutrophil CD18 integrin function by di- and tri-valent cations: Manganese vs. gadolinium. Ann. Biomed. Eng. 2008, 36, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Balkow, S.; Wild, M.K.; Stadtbaeumer, A.; Krummen, M.; Rothoeft, T.; Higuchi, T.; Beissert, S.; Wethmar, K.; Scharffetter-Kochanek, K.; et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 2007, 109, 661–669. [Google Scholar] [CrossRef]
- Stevanin, M.; Busso, N.; Chobaz, V.; Pigni, M.; Ghassem-Zadeh, S.; Zhang, L.; Acha-Orbea, H.; Ehirchiou, D. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur. J. Immunol. 2017, 47, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jin, J.; Xu, S.; Liu, H.; Li, N.; Cao, X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 2010, 11, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Behrens, E.M.; Sriram, U.; Shivers, D.K.; Gallucci, M.; Ma, Z.; Finkel, T.H.; Gallucci, S. Complement receptor 3 ligation of dendritic cells suppresses their stimulatory capacity. J. Immunol. 2007, 178, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Zahorchak, A.F.; Logar, A.J.; Papworth, G.D.; Wang, Z.; Watkins, S.C.; Falo, L.D., Jr.; Thomson, A.W. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: Dependence on complement receptors and effect on cytokine production. Blood 2003, 101, 611–620. [Google Scholar] [CrossRef]
- Macri, C.; Fancke, B.; Radford, K.J.; O’Keeffe, M. Monitoring Dendritic Cell Activation and Maturation. Methods Mol. Biol. 2019, 1988, 403–418. [Google Scholar] [CrossRef]
- Lynn, W.A.; Raetz, C.R.; Qureshi, N.; Golenbock, D.T. Lipopolysaccharide-induced stimulation of CD11b/CD18 expression on neutrophils. Evidence of specific receptor-based response and inhibition by lipid A-based antagonists. J. Immunol. 1991, 147, 3072–3079. [Google Scholar]
- Ammon, C.; Meyer, S.P.; Schwarzfischer, L.; Krause, S.W.; Andreesen, R.; Kreutz, M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology 2000, 100, 364–369. [Google Scholar] [CrossRef]
- Barillet, S.; Fattal, E.; Mura, S.; Tsapis, N.; Pallardy, M.; Hillaireau, H.; Kerdine-Römer, S. Immunotoxicity of poly (lactic-co-glycolic acid) nanoparticles: Influence of surface properties on dendritic cell activation. Nanotoxicology 2019, 13, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017, 12, 515–531. [Google Scholar] [CrossRef] [PubMed]
- De Winde, C.M.; Munday, C.; Acton, S.E. Molecular mechanisms of dendritic cell migration in immunity and cancer. Med. Microbiol. Immunol. 2020, 209, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Puri, B.K.; Olive, L.; Carvalho, A.F.; Berk, M.; Maes, M. Emerging role of innate B1 cells in the pathophysiology of autoimmune and neuroimmune diseases: Association with inflammation, oxidative and nitrosative stress and autoimmune responses. Pharmacol. Res. 2019, 148, 104408. [Google Scholar] [CrossRef]
- Waffarn, E.E.; Hastey, C.J.; Dixit, N.; Soo Choi, Y.; Cherry, S.; Kalinke, U.; Simon, S.I.; Baumgarth, N. Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat. Commun. 2015, 6, 8991. [Google Scholar] [CrossRef]
- Ding, C.; Ma, Y.; Chen, X.; Liu, M.; Cai, Y.; Hu, X.; Xiang, D.; Nath, S.; Zhang, H.G.; Ye, H.; et al. Integrin CD11b negatively regulates BCR signalling to maintain autoreactive B cell tolerance. Nat. Commun. 2013, 4, 2813. [Google Scholar] [CrossRef]
- Aziz, M.; Holodick, N.E.; Rothstein, T.L.; Wang, P. The role of B-1 cells in inflammation. Immunol. Res. 2015, 63, 153–166. [Google Scholar] [CrossRef]
- Griffin, D.O.; Rothstein, T.L. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med. 2011, 208, 2591–2598. [Google Scholar] [CrossRef]
- Aziz, M.; Brenner, M.; Wang, P. Therapeutic Potential of B-1a Cells in COVID-19. Shock 2020, 54, 586–594. [Google Scholar] [CrossRef]
- Schoenlaub, L.; Elliott, A.; Freches, D.; Mitchell, W.J.; Zhang, G. Role of B cells in host defense against primary Coxiella burnetii infection. Infect. Immun. 2015, 83, 4826–4836. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Baumgarth, N. The Multifaceted B Cell Response to Influenza Virus. J. Immunol. 2019, 202, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.K.; Pazgier, M.; Evans, D.T.; Ferrari, G.; Bournazos, S.; Parsons, M.S.; Bernard, N.F.; Finzi, A. Beyond Viral Neutralization. AIDS Res. Hum. Retrovir. 2017, 33, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, Y.; Jiang, X. Antibody and antibody fragments for cancer immunotherapy. J. Control. Release 2020, 328, 395–406. [Google Scholar] [CrossRef]
- Adamus, T.; Kortylewski, M. The revival of CpG oligonucleotide-based cancer immunotherapies. Contemp. Oncol. 2018, 22, 56–60. [Google Scholar] [CrossRef]
- Macri, C.; Dumont, C.; Johnston, A.P.; Mintern, J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016, 5, e66. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.J.; Yuen, D.; Rae, J.; Johnston, A.P.; Parton, R.G.; Kent, S.J.; De Rose, R. Human immune cell targeting of protein nanoparticles-caveospheres. Nanoscale 2016, 8, 8255–8265. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jackman, J.A.; Ferhan, A.R.; Belling, J.N.; Mokrzecka, N.; Weiss, P.S.; Cho, N.J. Cloaking Silica Nanoparticles with Functional Protein Coatings for Reduced Complement Activation and Cellular Uptake. ACS Nano 2020, 14, 11950–11961. [Google Scholar] [CrossRef]
- Janco, E.W.; Hulander, M.; Andersson, M. Curvature-dependent effects of nanotopography on classical immune complement activation. Acta Biomater. 2018, 74, 112–120. [Google Scholar] [CrossRef]
- Tavano, R.; Gabrielli, L.; Lubian, E.; Fedeli, C.; Visentin, S.; Polverino De Laureto, P.; Arrigoni, G.; Geffner-Smith, A.; Chen, F.; Simberg, D.; et al. C1q-Mediated Complement Activation and C3 Opsonization Trigger Recognition of Stealth Poly(2-methyl-2-oxazoline)-Coated Silica Nanoparticles by Human Phagocytes. ACS Nano 2018, 12, 5834–5847. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Perez-Potti, A.; Herda, L.M.; Adumeau, L.; Dawson, K.A.; Yan, Y. Differential Recognition of Nanoparticle Protein Corona and Modified Low-Density Lipoprotein by Macrophage Receptor with Collagenous Structure. ACS Nano 2018, 12, 4930–4937. [Google Scholar] [CrossRef]
- Zou, Y.; Ito, S.; Yoshino, F.; Suzuki, Y.; Zhao, L.; Komatsu, N. Polyglycerol Grafting Shields Nanoparticles from Protein Corona Formation to Avoid Macrophage Uptake. ACS Nano 2020, 14, 7216–7226. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.M.G.; Jaafari, M.R.; Hatamipour, M.; Penson, P.E.; Sahebkar, A. Liposome Circulation Time is Prolonged by CD47 Coating. Protein Pept. Lett. 2020, 27, 1029–1037. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.U.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Lukácsi, S.; Mácsik-Valent, B.; Nagy-Baló, Z.; Kovács, K.G.; Kliment, K.; Bajtay, Z.; Erdei, A. Utilization of complement receptors in immune cell-microbe interaction. FEBS Lett. 2020, 594, 2695–2713. [Google Scholar] [CrossRef] [PubMed]
- Noubade, R.; Majri-Morrison, S.; Tarbell, K.V. Beyond cDC1: Emerging Roles of DC Crosstalk in Cancer Immunity. Front. Immunol. 2019, 10, 1014. [Google Scholar] [CrossRef]
- Bosteels, C.; Fierens, K.; De Prijck, S.; Van Moorleghem, J.; Vanheerswynghels, M.; De Wolf, C.; Chalon, A.; Collignon, C.; Hammad, H.; Didierlaurent, A.M.; et al. CCR2- and Flt3-Dependent Inflammatory Conventional Type 2 Dendritic Cells Are Necessary for the Induction of Adaptive Immunity by the Human Vaccine Adjuvant System AS01. Front. Immunol. 2020, 11, 606805. [Google Scholar] [CrossRef]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Croix, D.A.; Ahearn, J.M.; Rosengard, A.M.; Han, S.; Kelsoe, G.; Ma, M.; Carroll, M.C. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J. Exp. Med. 1996, 183, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Luxembourg, A.T.; Cooper, N.R. Modulation of signaling via the B cell antigen receptor by CD21, the receptor for C3dg and EBV. J. Immunol. 1994, 153, 4448–4457. [Google Scholar] [PubMed]
- Ali, M.G.; Zhang, Z.; Gao, Q.; Pan, M.; Rowan, E.G.; Zhang, J. Recent advances in therapeutic applications of neutralizing antibodies for virus infections: An overview. Immunol. Res. 2020, 68, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Webb, N.E.; Bernshtein, B.; Alter, G. Tissues: The unexplored frontier of antibody mediated immunity. Curr. Opin. Virol. 2021, 47, 52–67. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Chenoweth, A.M.; Wines, B.D.; Anania, J.C.; Mark Hogarth, P. Harnessing the immune system via FcγR function in immune therapy: A pathway to next-gen mAbs. Immunol. Cell Biol. 2020, 98, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Taylor, R.P. The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer mAbs. Antibodies 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, H.J.; Nijhof, I.S.; Mutis, T.; Chamuleau, M.E.D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers 2020, 12, 3041. [Google Scholar] [CrossRef]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Amon, L.; Hatscher, L.; Heger, L.; Dudziak, D.; Lehmann, C.H.K. Harnessing the Complete Repertoire of Conventional Dendritic Cell Functions for Cancer Immunotherapy. Pharmaceutics 2020, 12, 663. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol. Rev. 2020, 296, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Heath, W.R.; Mueller, S.N. CD4(+) T-cell help amplifies innate signals for primary CD8(+) T-cell immunity. Immunol. Rev. 2016, 272, 52–64. [Google Scholar] [CrossRef]
- Aloulou, M.; Fazilleau, N. Regulation of B cell responses by distinct populations of CD4 T cells. Biomed. J. 2019, 42, 243–251. [Google Scholar] [CrossRef]
- Heesters, B.A.; Myers, R.C.; Carroll, M.C. Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol. 2014, 14, 495–504. [Google Scholar] [CrossRef]
- Roozendaal, R.; Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 2007, 219, 157–166. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, M.; Medina-Montano, C.; Fittler, F.J.; Stege, H.; Roskamp, M.; Kuske, M.; Langer, C.; Vahldieck, M.; Montermann, E.; Tubbe, I.; et al. Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells. Int. J. Mol. Sci. 2021, 22, 2869. https://doi.org/10.3390/ijms22062869
Bednarczyk M, Medina-Montano C, Fittler FJ, Stege H, Roskamp M, Kuske M, Langer C, Vahldieck M, Montermann E, Tubbe I, et al. Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells. International Journal of Molecular Sciences. 2021; 22(6):2869. https://doi.org/10.3390/ijms22062869
Chicago/Turabian StyleBednarczyk, Monika, Carolina Medina-Montano, Frederic Julien Fittler, Henner Stege, Meike Roskamp, Michael Kuske, Christian Langer, Marco Vahldieck, Evelyn Montermann, Ingrid Tubbe, and et al. 2021. "Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells" International Journal of Molecular Sciences 22, no. 6: 2869. https://doi.org/10.3390/ijms22062869
APA StyleBednarczyk, M., Medina-Montano, C., Fittler, F. J., Stege, H., Roskamp, M., Kuske, M., Langer, C., Vahldieck, M., Montermann, E., Tubbe, I., Röhrig, N., Dzionek, A., Grabbe, S., & Bros, M. (2021). Complement-Opsonized Nano-Carriers Are Bound by Dendritic Cells (DC) via Complement Receptor (CR)3, and by B Cell Subpopulations via CR-1/2, and Affect the Activation of DC and B-1 Cells. International Journal of Molecular Sciences, 22(6), 2869. https://doi.org/10.3390/ijms22062869







