Abstract
Recent studies on cyclin-dependent kinase (CDK) inhibitors have revealed that small molecule drugs have become very attractive for the treatment of cancer and neurodegenerative disorders. Most CDK inhibitors have been developed to target the ATP binding pocket. However, CDK kinases possess a very similar catalytic domain and three-dimensional structure. These features make it difficult to achieve required selectivity. Therefore, inhibitors which bind outside the ATP binding site present a great interest in the biomedical field, both from the fundamental point of view and for the wide range of their potential applications. This review tries to explain whether the ATP competitive inhibitors are still an option for future research, and highlights alternative approaches to discover more selective and potent small molecule inhibitors.
1. Cyclin-Dependent Kinases (CDKs)
Protein phosphorylation is a necessary mechanism to drive numerous cellular processes such as cell division, migration, differentiation and programmed cell death. This process is regulated by many enzymes, including cyclin-dependent kinases (CDKs) which phosphorylate proteins on their serine and threonine amino acid residues. The 20 members of CDK family known to this day regulate the cell cycle, transcription and splicing [1]. A number of kinase inhibitors are emerging every day as potential small molecule drugs, with some of them already being approved by the United States Food and Drug Administration (FDA). Moreover, these already approved kinase targeting drugs now account for more than a quarter of all available drugs [2]. In relation to CDK inhibitors, drugs such as Palbociclib 1, Ribociclib 2 and Abemaciclib 3, have been approved for ER+/HER2- advanced breast cancer treatment [3]. Until recently, the focus of the research was aimed at the highly conserved ATP binding sites of each CDK kinase. Hence, the development of CDK inhibitors has been extremely challenging due to the difficulty of obtaining sufficient selectivity with typical ATP-mimetic compounds. The greatest number of reported compounds has been identified to target the ATP binding pocket. Most recent studies suggest that inhibitors targeting hydrophobic pockets outside the ATP binding site may provide an opportunity for rational target selectivity [4]. Figure 1 illustrates the typical protein structure of the CDK enzyme. The diagram depicts the structural features of a typical kinase domain. Specifically highlighted are the binding pockets of different types of inhibitors, as well as the activation loop.
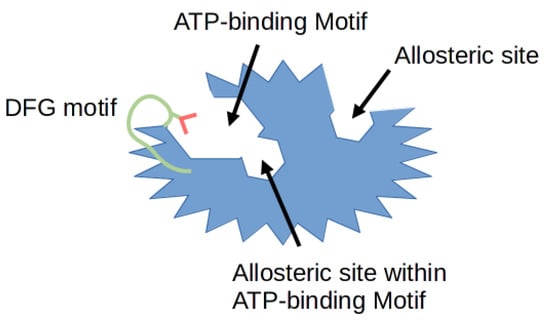
Figure 1.
Schematic representation of different types of binding pockets. The protein kinase is shown in blue, with the Asp-Phe-Gly (DFG) motif in green. Red color denotes the aspartate amino acid residue of the DFG motif. The particular regions where different types of inhibitors bind are described below, the allosteric pocket is only a visualization and its place can be anywhere outside the ATP binding site.
2. Cyclin-Dependent Kinase (CDK) Inhibitors in Drug Development
CDK family is known to regulating the cell cycle, transcription and splicing. Deregulation of any of the stages of the cell cycle or transcription leads to apoptosis but, if uncorrected, it can result in a series of diseases such as cancer or neurodegenerative diseases [1,5,6,7].
Within the last 20 years important advances have been achieved in the development of effective strategies to inhibit CDK kinases. Access of the substrate to the active site of CDK kinase is regulated by the activation loop (A-loop) which is very flexible. The A-loop contains between 20–30 amino acids marked by the conserved Asp-Phe-Gly (DFG) tripeptide motif at the proximal end. Phosphorylation of the activation loop activates the kinase. In this state, the DFG sequence fits snugly into a hydrophobic back pocket adjacent to the ATP binding site. Conversely, in the inactive state the DFG motif swings outwards by partially blocking both the ATP and substrate binding pockets [8].
To date, six types of small molecule kinase inhibitors have been defined by the pharmaceutical industry based on their biochemical mechanisms of action (Figure 2). Type I inhibitors interact directly with the ATP binding site and react with the active form of the kinase which is in the DFG-in state and with a phosphorylated activation loop (activation segment). These inhibitors mimic the hydrogen bonds created between the adenine ring of the ATP and the hinge region of the enzyme. Type II inhibitors interact with a DFG-out catalytically inactive conformation of the enzyme and, like type I inhibitors, explore the hinge region and the adenine binding pocket. Type III inhibitors are non-competitive with ATP as they bind to the hydrophobic pocket next to the ATP-binding site, while type IV inhibitors bind away from the ATP binding pocket. Both, type III and IV inhibitors are allosteric in nature [8]. Type V inhibitors interact with two separate regions of the protein kinase domain. This group of inhibitors has been classified as bi-substrate inhibitors. These five classes of inhibitors interact reversibly, while type VI inhibitors form a covalent bond with their target kinase (Figure 2) [9].
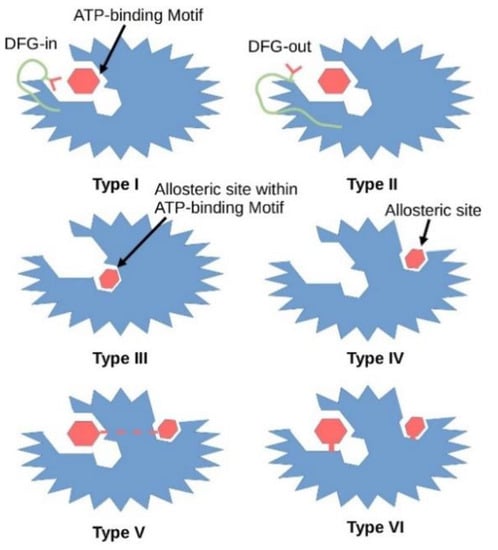
Figure 2.
Graphical illustration of different types of kinase inhibitors and their mode of action. Dark red hexagon represents an inhibitor. The protein kinase is shown in blue, the DFG motif in green, the aspartate amino acid residue of the DFG motif in red. In 2015 Wu demonstrated that co-crystal structure of 3-phosphoinositide-dependent protein kinase 1 (PDPK1, PDK1) with ATP showed that type I inhibitors interact with the active conformation of the enzyme where the aspartate residue of the DFG motif points into the ATP binding pocket, while type II inhibitors stabilize the inactive conformation of the enzyme where the aspartate residue faces outward of the binding site (PDB entry: 4RRV). Type III inhibitors interact with the allosteric site within the ATP binding pocket. Type IV inhibitors interact with the allosteric site. However, the allosteric pocket is only a visualization and its place can be anywhere outside the ATP binding site. Type V inhibitors interact with both the allosteric and ATP binding pockets. Type VI inhibitors form covalent bonds with either the ATP binding pocket or the allosteric pocket.
3. Type I Inhibitors
Many heterocyclic compounds can mimic the hydrogen binding motif of adenine, therefore many type I inhibitors have been discovered. As mentioned above, these compounds interact with the ATP-site of the kinase in its active (DFG-in) conformation and with phosphorylated polypeptide region (activation segment) which lies outside the active site pocket. First generation of structurally diverse ATP competitive small molecule type I CDK inhibitors, produced in the late 1990s and early 2000s, have entered clinical trials to treat numerous solid tumors and hematopoietic malignances. Among the list of compounds that have been synthesized as CDK inhibitors, Flavopiridol (Alvocidib) 4 (Figure 3), a flavonoid derived from an indigenous plant from India, is active against CDK1, CDK2, CDK4, CDK6, and CDK9 with IC50 values in the 20–100 nM range (Table 1) [10,11,12,13]. Flavopiridol can inhibit cell cycle progression in G1 as well as G2 phase due to inhibition of CDK2/4 and CDK1 activity, respectively. Early clinical trials proved ineffective because of unsatisfactory efficacy and high toxicity [14,15]. However, later studies confirmed its clinical efficacy in hematological malignancies, and it was granted orphan drug designation for the treatment of patients with acute myeloid leukemia (Table 2) [16].
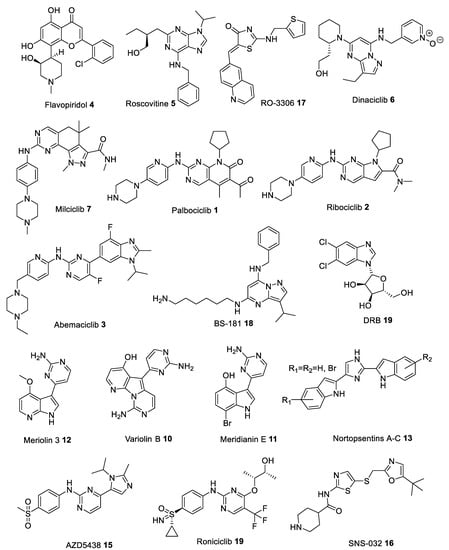
Figure 3.
Chemical structures of some of the most studied type I cyclin-dependent kinase (CDK) inhibitors.
Roscovitine (Seliciclib) 5 (Figure 3), one of the best known CDK inhibitors, is active against CDK2, CDK5, CDK7 and CDK9 (Table 1). This compound is, by far, the most effective inhibitor of CDK5/p25 (IC50 = 160 nM [17]), as shown by numerous studies using this compound as a potential drug against cancer, neurodegenerative or viral diseases, inflammation, polycystic kidney disease (PKD) and glomerulonephritis (Table 2) [18,19,20,21,22]. However, despite many successful preclinical studies, results from several clinical trials are not that promising [23].
Another compound is Dinaciclib 6 (Figure 3), which proved to be a very effective small molecule inhibitor against CDK5 (IC50 = 1 nM [24]) (Table 1). Preclinical studies have shown that Dinaciclib is effective against solid tumors and chronic lymphocytic leukemia (CLL), without adversely affecting T-lymphocytes and their numbers (Table 2) [25].
Moreover, Milciclib 7 (Figure 3), an orally bioavailable inhibitor of cyclin-dependent kinases (CDKs) and several other protein kinases responsible for controlling cell growth and replication, has recently obtained the orphan drug designation for thymic carcinoma. It is currently under investigation as a potential drug target for treatment of glioma and hepatocellular carcinoma (HCC) (Table 2) [26,27]. It inhibits CDK2 with IC50 of 45 nM and exhibits submicromolar activity against other CDKs including CDK1, CDK4 and CDK5 resulting in a block in the G1 (gap) phase of the cell cycle (Table 1) [28]. Furthermore, Milciclib was found to reduce levels of microRNAs, miR-221 and miR-222, which promote the formation of blood supply (angiogenesis) in cancer tumors [29].
And finally, Palbociclib 1 and Ribociclib 2 (Figure 3), novel CDK4/6 inhibitors, were approved as effective drugs against HR+/HER2- metastatic breast cancer (Table 2) [30,31]. They selectively inhibit CDK4/6 (Table 1), thereby inhibiting retinoblastoma (Rb) protein phosphorylation early in the G1 phase leading to cell cycle arrest, causing defects in DNA replication and efficiently suppress cancer cell proliferation. Most recent data show that both drugs demonstrate a synergistic effect when combined with other drugs, for example Palbociclib and aromatase inhibitor Letrozole [32], Ribociclib and either anaplastic lymphoma kinase (ALK) inhibitor or the mitogen-activated protein kinase kinase (MAP2K, MEK) inhibitor Trametinib [33]. Moreover, utilizing this approach leads to a significant reduction in the development of resistance during prolonged treatment courses [31].
In addition, Tamoxifen 8 has been found to be effective against breast cancer. It reduces CDK5 activity by interacting with p25 and p35, thus preventing CDK5 activation. Tamoxifen can also lower Tau protein phosphorylation, which may suggest that tamoxifen could be used against Alzheimer’s disease [34].
Yet another inhibitor, 5,6-dichlorobenzimidazone-1-β-D-ribofuranoside (DRB) 9 (Figure 3) possesses high selectivity against CDK9, with nearly 25-fold difference in potency over CDK2 and CDK7 (Table 1) [35]. In HeLa cells, DRB (75 μM) inhibited 60-75% of nuclear heterogeneous RNA (hnRNA) synthesis. DRB inhibited a HeLa protein kinase which phosphorylated an RNA polymerase II-derived peptide [36]. DRB can also inhibit HIV transcription (IC50 = ~4 μM) by targeting elongation enhanced by the HIV-encoded transactivator Tat (Table 2) [37].

Table 1.
Kinase inhibitory activities of type I CDK inhibitors.
Table 1.
Kinase inhibitory activities of type I CDK inhibitors.
| Inhibitor | Kinase IC50 [nM] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CDK1/B | CDK2/A | CDK2/E | CDK4/D | CDK5/p25 | CDK6/D | CDK7/H | CDK8/C | CDK9/T1 | |
| Flavopiridol 4 [38,39] | 30 | 100 | 100 | 20–40 | - | 60 | 110 | - | 20 |
| Roscovitine 5 [40] | 650 | 700 | 700 | >100,000 | 160 | >100,000 | 460 | >100,000 | 600 |
| RO-3306 17 [41] | 35 | - | 340 | >2000 | - | - | - | - | - |
| Dinaciclib 6 [42] | 3 | 1 | 1 | 100 | 1 | - | - | - | 4 |
| Milciclib 7 [28] | 398 | 45 | 363 | 160 | 265 | - | 150 | - | - |
| Palbociclib 1 [43] | >10,000 | >10,000 | >10,000 | 11 | >10,000 | 15 | - | - | - |
| Ribociclib 2 [44] | 113,000 | 76,000 | 76,000 | 10 | 43,900 | 39 | - | - | - |
| Abemaciclib 3 [45] | 1627 | - | 504 | 2 | 355 | 10 | 3910 | - | 57 |
| BS-181 18 [46] | 8100 | 730 | 880 | 33,000 | 3000 | 47,000 | 21 | - | 4200 |
| DRB 9 [47] | 17,000 | - | >10,000 | >10,000 | - | - | >10,000 | >10,000 | 340 |
| Meriolin 3 12 [48] | 170 | 11 | - | >100,000 | 170 | >100,000 | >100,000 | - | 6 |
| Variolin B 10 [49] | 60 | 80 | - | >10,000 | 90 | >10,000 | >1000 | - | 26 |
| Meridianin E 11 [50] | 180 | 800 | 1800 | 3000 | 150 | - | - | - | 18 |
| Nortopsentins 13 [51] | 310–900 | - | - | - | - | - | - | - | - |
| AZD5438 15 [52] | 16 | 45 | 6 | 449 | 14 | 21 | 821 | - | 20 |
| Roniciclib 19 [53] | 7 | - | 9 | 11 | - | - | 25 | - | 5 |
| SNS-032 16 [54] | 480 | 38 | 48 | 925 | 340 (CDK5/p35) | - | 62 | - | 4 |

Table 2.
Type I CDK inhibitors at different phases of clinical and pre-clinical studies. Trial information obtained from ClinicalTrials.gov as of January 2021.
Table 2.
Type I CDK inhibitors at different phases of clinical and pre-clinical studies. Trial information obtained from ClinicalTrials.gov as of January 2021.
| Inhibitor | Main Targets | Condition or Disease | Phase | Status | Identifier |
|---|---|---|---|---|---|
| Flavopiridol 4 | CDK1, CDK2, CDK4, CDK6, CDK9 | Acute Myeloid Leukemia (AML) | on the market | “orphan drug” | - |
| Roscovitine 5 | CDK2, CDK7, CDK9 | Pituitary Cushing Disease | II | active | NCT02160730 NCT03774446 |
| Cystic Fibrosis | II | terminated | NCT02649751 | ||
| Advanced Solid Tumors | I | terminated | NCT00999401 | ||
| Lung Cancer | II | terminated | NCT00372073 | ||
| RO-3306 17 [41] | CDK1 | Acute Myeloid Leukemia (AML) | pre-clinical | - | - |
| Dinaciclib 6 | CDK1, CDK2, CDK5, CDK9 | Chronic Lymphocytic Leukemia (CLL) | on the market | “orphan drug” | - |
| Breast and Lung Cancers | II | terminated | NCT00732810 | ||
| Milciclib 7 | CDK1, CDK2, CDK4, CDK7 | Hepatocellular Carcinoma (HCC) | II | active | NCT03109886 |
| Thymic Carcinoma | II | terminated | NCT01301391 NCT01011439 | ||
| Palbociclib 1 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Letrozole | - |
| III | active, to be used with other drugs like Fulvestrant | NCT02692755 | |||
| Head and Neck, Brain, Colon, and other Solid Cancers | II | active, to be used alone and in combination with different drugs | NCT02255461 NCT03446157 NCT02896335 NCT03965845 | ||
| Ribociclib 2 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Letrozole | - |
| III | active, to be used with other drugs like Fulvestrant | NCT02422615 NCT03439046 NCT03294694 | |||
| Prostate, and other Solid Cancers | II | active, to be used alone and in combination with different drugs | NCT02555189 NCT01543698 NCT02934568 | ||
| Abemaciclib 3 | CDK4, CDK6 | HR+/HER2- Breast Cancer | on the market | used in combination with Fulvestrant | - |
| III | active, to be used with other drugs like Letrozole | NCT02763566 | |||
| Lung, Brain, Colon, and other Solid Cancers | II or III | active, to be used alone and in combination with different drugs | NCT04545710 NCT02152631 NCT03220646 NCT04616183 NCT03310879 | ||
| BS-181 18 [46] | CDK7 | Breast, Lung, Prostate and Colorectal Cancers | pre-clinical | - | - |
| DRB 9 [55] | CDK7, CDK8, CDK9 | HIV Transcription | pre-clinical | - | - |
| Meriolin 3 12 [48] | CDK1, CDK2, CDK5, CDK9 | Neuroblastoma, Glioma, Myeloma, Colon Cancer | pre-clinical | - | - |
| Variolin B 10 [56] | CDK1, CDK2, CDK5, CDK9 | Murine Leukemia | pre-clinical | - | - |
| Meridianin E 11 [57] | CDK1, CDK5, CDK9 | Larynx Carcinoma, Myeloid Leukemia | pre-clinical | - | - |
| Nortopsentins 13 [58] | CDK1 | Malignant Pleural Mesothelioma (MPM) | pre-clinical | - | - |
| AZD5438 15 | CDK1, CDK2, CDK5, CDK6, CDK9 | Advanced Solid Malignancies | I | terminated | NCT00088790 |
| Roniciclib 19 | CDK1, CDK2, CDK4, CDK7, CDK9 | Lung and Advanced Solid Cancers | II | terminated | NCT02161419 NCT01573338 NCT02656849 |
| SNS-032 16 | CDK2, CDK7, CDK9 | Chronic Lymphocytic Leukemia and other Solid Cancers | I | terminated | NCT00446342 NCT00292864 |
Novel alkaloids, acting as CDK inhibitors, were also found in some marine organisms. Variolins, 7-azaindole based alkaloids isolated from the antarctic sponge Kirkpatrickia variolosa [59,60], showed in vitro activity against a murine (P388) leukemia cell line with submicromolar potencies by preventing cell proliferation, and inducing apoptosis (Table 2) [56,59]. Variolin B 10 (Figure 3), in particular, was found to inhibit CDK1 and CDK2 kinases, in the micromolar concentration range (Table 1) [61]. Meridianins A-G, a family of 3-(2-aminopyrimidine)indoles, which originate from the ascidian Aplidium meridianum, were demonstrated to inhibit several protein kinases, especially Meridianin E 11 (Figure 3), which can selectively inhibit CDK1 and CDK5 in the low micromolar range (Table 1) [62]. Based on the latter two compounds, Meriolins 12, a new class of inhibitors, have been designed. These new derivatives have been reported to strongly inhibit various protein kinases, especially CDK1, CDK2, CDK4 and CDK9 (Table 1) [48]. Most recent analysis provides a high potential of Meriolins in the treatment of cancer and noncancer pathologies such as polycystic kidney disease, neurodegenerative diseases, stroke, chronic inflammation, and bipolar disorders (Table 2) [48]. Nortopsentins A-C 13 (Figure 3), antifungal 1,4-bisindolylimidazole marine alkaloids, having an imidazole as a spacer between the two indole units, isolated from the Caribbean deep sea Spongosorites ruetzleri, displayed in vitro cytotoxicity against P388 leukemia cells (IC50 4.5–20.7 µM). Analogues in which the imidazole ring of the alkaloid was replaced by other five or six membered heterocycles were able to inhibit the activity of the cyclin-dependent kinase 1 (CDK1) with submicromolar IC50 values (in particular 3-[(2-indolyl)-5-phenyl]-pyridines, phenyl-thiazolyl-7-azaindoles, indolyl-thiazolyl-4-azaindole and indolyl-thiazolyl-7-azaindole derivatives) (Table 1) [51]. Preliminary results indicate, that Nortopsentins, and their analogues, were active against malignant pleural mesothelioma (MPM), a very aggressive human malignancy poorly responsive to currently available therapies (Table 2) [58].
Recent development has enabled combinatorial treatment regimens which can demonstrate synergistic anticancer mechanisms. For instance, THZ1 14 (Figure 7) a covalent CDK7 inhibitor, was found to selectively downregulate CDK7-mediated phosphorylation of RNA polymerase II, indicative of transcriptional inhibition. Further investigations revealed that the survival of triple negative breast cancer (TNBC) cells relied heavily on the B-cell lymphoma 2 (BCL-2)/B-cell lymphoma-extra large (BCL-XL) signaling axes in cells. Thus, combining the CDK7 inhibitor THZ1 with the BCL-2/BCL-XL inhibitors (ABT-263/ABT199) offer a preclinical proof to significantly improve the poor prognosis in TNBC [63].
However, the complexity of CDK biology and the undesired toxicity related to the off-target effects of the existing pan-CDK inhibitors, led to decisions by several pharmaceutical companies to discontinue the development of many potential anti-cancer agents, exampled with AZD5438 15, Roniciclib, SNS-032 16, RO-3306 17, BS-181 18 and Roniciclib 19 (Figure 3) (Table 1 and Table 2) [64,65,66]. Therefore, new classes of more selective CDK inhibitors, with strong potential to deliver a meaningful therapeutic impact, were needed.
One of those compounds is CDK5 inhibitory protein (CIP), a small protein which contribute to nerve cells’ degeneration. CIP specifically blocks the hyperactivated state of CDK5 only when it is linked to p25/p29, while allowing normal activation of CDK5 by p35/p39. The selective inhibition of p25/CDK5 hyperactivation in vivo, through overexpression of CIP, reduced neurodegeneration and improved cognitive function of transgenic mice, without affecting normal neurodevelopment [67]. These findings suggest that CIP could possibly be used to selectively inhibit the p25/CDK5 hyperactivation as a potential therapeutic target to treat certain cancers caused by aberrant CDK5 activation.
4. Type 1.5 Inhibitors
Another strategy to generate novel class of inhibitors has been devised by targeting the inactive unphosphorylated monomeric kinase, not the heterodimer complex. In case of CDK2, a series of compounds based on a quinoline scaffold which bind tightly to the ATP binding site and adjacent back pocket behind the gatekeeper have been synthesized. The binding mode with quinoline-based derivative 20 (Figure 4) in CDK2 demonstrates that the DGF motif is in the “in” state, and 20 interacts not only with the ATP binding site but also disrupts the binding of cyclin A by inducing extensive conformational changes in the C helix (PDB entry 4NJ3). This type of binding to the DFG-in inactive conformation is also referred to as type 1.5 inhibition [68].
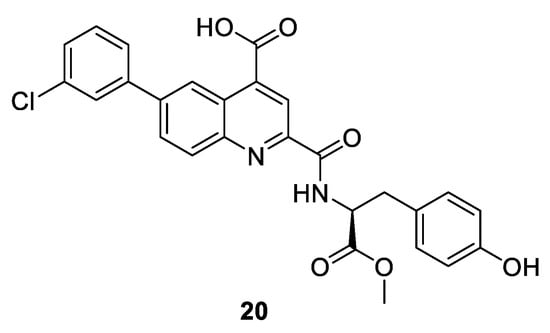
Figure 4.
Chemical structure of quinoline-based type 1.5 inhibitor of monomeric CDK2.
Despite showing significant binding affinities none of the compounds exhibited high cellular activity. Quinoline-based 20 bound CDK2 with a Ki value of 0.14 µM determined using the CDK2 fluorescence polarization (FP) binding assay and Kd = 0.3 µM using the temperature-dependent circular dichroism assay (TdCD). Moreover, the IC50 is greater than 10 µM in the CDK2/cyclin A enzyme assay. The explanation might stem from the poor permeability resulting from the carboxyl group, as well as the competition of these inhibitors with cyclin A binding to the monomeric CDK2 (Table 3) [68]. These findings clearly demonstrate the potential of these CDK2 inhibitors. Hopefully, by blocking the interaction with cyclin A, these agents will exhibit different cellular effects which can translate into novel therapeutic possibilities.

Table 3.
Selected biological data obtained from different assays with quinoline-based compound demonstrating that it targets monomeric CDK2.
5. Type II Inhibitors
It has been observed that the CDK active site cleft is very spacious and this fact has been widely exploited in drug discovery. It consists of two regions: the front and back clefts, which are separated by the hydrophobic gatekeeper residue (phenylalanine in nearly all CDK members, methionine in CDK10 and CDK11) [69,70]. The residues necessary to adopt DFG-out conformation are the amino acid at the gatekeeper position and the residue immediately prior to the DFG motif (DFG-1) [71].
The conformational plasticity of the DFG-out binding pocket present a huge opportunity to develop many binding site structural variants which hopefully will be trapped and stabilized by inhibitors [72]. This binding pocket has attracted considerable attention, paving the way for the development of type II inhibitors. Type II inhibitors are anticipated not only to address the problem of kinase inhibitor selectivity but also obtain additional therapeutic benefits such as extended drug target residence times, possess better safety profiles and have fewer side effects [73].
Initially, the development of type II inhibitors had been hampered a little because of the notion that only the simplest amino acids, such as threonine or alanine, as a gatekeeper residue allow the back cleft to be accessible, bulky residues (leucine, methionine or phenylalanine) on the other hand stop a potential small molecule inhibitor from entering the back pocket through this internal gate [74]. Recent studies, however, have shown that kinases with bulkier gatekeeper residues are also able to bind type II inhibitors in the DFG-out state [75]. Moreover, cancerous mutations into larger gatekeeper amino acids generally result in kinase activation, thereby stabilizing the active state of the kinase [76,77,78]. Whether kinases with smaller gatekeeper residues still favor the DFG-out motif has yet to be exemplified.
Although, the factors modulating the DFG-out conformation still remain to be elucidated the initial conclusions can be easily drawn. The results reveal that certain protein kinases such as CDK6, receptor-type tyrosine-protein kinase (RTK, FLT3, CD135), coagulation factor II (thrombin) receptor (PAR1), RAC-b serine/threonine-protein kinase (AKT2), mitogen-activated protein kinase 14 (MAPK14, p38a) and bacterial cell membrane non-specific serine/threonine protein kinase (STK1) favor a classical DFG-out conformation even without the presence of type II inhibitor [79,80], whereas the other inactive, unphosphorylated kinases can be shown to assume the DFG-in conformation [81]. Moreover, Molecular Dynamics (MD) simulations carried out for the Abl tyrosine kinase indicate that DFG binding mode selection might be pH-dependent [82]. Additionally, site-directed mutagenesis (SDM) was used to identify that not only the gatekeeper residue but also the residue located at the N-terminal to the DFG motif play a key role in stabilizing the DFG-out inactive state [71]. Moreover, a comparative analysis of a small library of type II inhibitors showed that over 200 kinases can be targeted, which does not make them intrinsically more selective than type I inhibitors [8]. Moreover, a number of kinases, bound to a type II inhibitor, can exhibit many intermediate states of the DFG-in and DFG-out conformations [83]. The advantages of knowing which and how many enzymes may be targeted by type II inhibitors will be of great value.
Alanine is the most frequently observed amino acid residue at the DFG-1 position (Ala144 in case of CDK2) [84]. However, more data is needed to demonstrate its role in the stabilization of the DFG-out state as one group stated that mutating leucine to cysteine at the DFG-1 position in Mitogen-activated protein kinase 1 (MAPK1) makes it impossible to bind a type II inhibitor by disrupting the DFG-out state [71], while the other one showed that by changing alanine to either cysteine or glycine seem to participate in the stabilization of the DFG-out conformation in CDK2 [85]. Moreover, there are protein kinases which have a cysteine at this position, such as Mitogen-activated protein kinase kinase kinase 7 (MAP3K7, TAK1) which hopefully could bind type II inhibitors [86]. Until more recent information becomes available, it is worth noting that each type of protein kinase should be considered individually, and be limited to the specific case of particular type II inhibitor structure [85].
First attempts to synthesize type II inhibitors of CDK2, the most studied CDK kinase, were based upon their endogenous inhibitors such as the INhibitors of CDK4 (INK4) (p16 and p18) and the CDK interacting proteins/Kinase inhibitory proteins (Cip/Kip) (p21, p27 and p57). The structural studies focused on the interaction of p27 with the CDK2 N-terminal lobe and the cyclin A box revealed that p27 inserts itself into the ATP binding site, thus preventing its conformational activation (PDB entry 1JSU) [87]. The INK4 family inhibitor p18 in the p18–CDK6/cyclin K ternary complex was also found to inactivate the CDK/cyclin dimer structure by distorting the ATP binding site and misaligning catalytic residues (PDB entry 1G3N) [88]. These observations support the model that the other CDKs may undergo similar inhibitory conformational changes by binding to their respective CDK inhibitors. Numerous peptides and peptidomimetics, based on the sequence alignment of the cyclin-binding motif found in many CDK inhibitory proteins (especially p21 and p27), have been synthesized. Two 8-amino-acid oligopeptidic units H-His-Ala-Lys-Arg-Arg-Leu-Ile-Phe-NH2 21 and H-Ala-Ala-Abu-Arg-Arg-Leu-Ile-pFPhe-NH2 22 showed the highest growth-inhibitory activities in both the cyclin A competitive binding assay and the CDK2/cyclin A kinase functional assay with IC50 values in the low nanomolar region (Table 4) [89,90]. Another example of the peptidomimetic molecule is MM-D37K 23, derived from p16, which was found to be the first cyclin D-CDK4/6 alternative class inhibitor in the clinic for colorectal cancer. Collected data will certainly allow interesting comparisons with existing type I inhibitors [91].

Table 4.
Type II CDK inhibitors under clinical evaluation.
The first small molecule inhibitor which induced the DFG-out motif was Sorafenib 24 (Figure 5), a well-known multikinase type II inhibitor, bound to CDK8/Cyclin C heterodimer complex (PDB entry 3RGF). In case of CDK8 the DFG motif is replaced by a unique Asp-Met-Gly tripeptide motif (DMG) [93]. Sorafenib has been found to disrupt mitogen-activated protein kinase (Ras-MAPK) signaling in many cell-based assays, such as colon, liver, kidney, lung, and breast cancer cell lines [95,96]. The function of Ras-MAPK pathway is to transduce signals from the extracellular receptor to the DNA in the cell nucleus where specific genes are activated for cell growth, division and differentiation [97]. Structure-guided modification of Sorafenib resulted in a series of potent CDK8 inhibitors stabilizing the DMG-out conformation such as compound 25 (Figure 5). However, these inhibitors (including Sorafenib) demonstrated weak activity in cellular assays (they neither suppressed the Wnt/β-catenin pathway nor phosphorylated Signal transducer and activator of transcription 1 (STAT1) at Ser727). These findings also suggest that type II inhibitors target the inactive form of CDK8 which is poorly accessible in cells due to the fact that it either forms the Mediator or the kinase-module [94,98]. Most recent data show that the antitumor efficacy of Sorafenib can be enhanced by the addition of Flavopiridol in both Sorafenib-sensitive and Sorafenib-resistant hepatocellular carcinoma (HCC) cell lines. The enhancing effects result from the synergistic effect of co-targeting two different biological mechanisms: CDKs (Flavopiridol) and the Ras-MAPK pathway (Sorafenib), both being linked to the suppression of Mcl-1 expression [99].
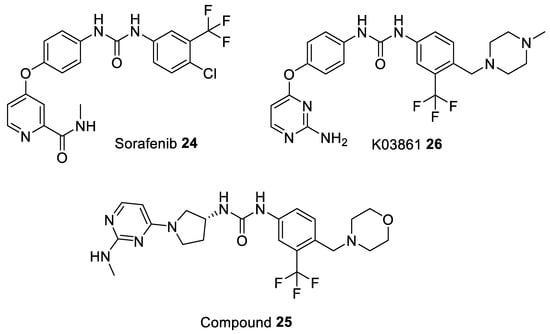
Figure 5.
Chemical structures of some of the most studied type II CDK inhibitors.
Another example of inhibitor able to adopt the DFG-out conformation is K03861 (AUZ454) 26 (Figure 5) an aminopyrimidine-phenyl urea inhibitor. This is a type II CDK2 inhibitor with Kd values of 50 nM, 18.6 nM, 15.4 nM, and 9.7 nM for CDK2 (wild type), CDK2(C118L), CDK2(A144C), and CDK2(C118L/A144C), respectively (Table 4). The co-crystal structure of K03861 bound to cyclin-free CDK2 exhibit a type II binding mode with the DFG-out state (PDB entry 1b38). Further analysis of this compound, obtained from kinetic binding experiments, revealed slow off-rates, meaning that compounds exhibiting slow dissociation rates could be considered as a clinically important and statistically significant benefit to patients since they interact with a kinase for much longer [85].
6. Type III Inhibitors
This type of inhibitors are compounds which make specific interactions with an exclusive pocket, known as the back pocket of the kinase, which is adjacent to the ATP binding site. Type I/II kinase inhibitors are very sensitive to the gatekeeper mutations affecting the residues within the ATP pocket, the region that has been recognized as responsible for acquired resistance to type I and II kinase inhibitors [100,101].
PD184352 (CI-1040), a selective oral mitogen-activated protein kinase kinase 1/2 (MAP2K1/2, MEK1/2) inhibitor, was the first type III inhibitor to enter clinical trials, that laid the groundwork for the discovery of additional non-ATP-competitive inhibitors [102]. However, no such molecule has been reported in case of type III CDK inhibitors.
7. Type IV Inhibitors
Type IV inhibitors have been defined as compounds which bind to unique structural features remote from the ATP binding pocket and are able to interact with these allosteric regions by stabilizing inactive conformations. The allosteric pocket of type IV inhibitors can be located anywhere within the kinase, with one exception for the hydrophobic pocket close to the ATP-binding site which is targeted by type III inhibitors [103]. Potential compounds able to allosterically regulate kinase enzymatic activity will offer the possibility of achieving distinctive advantages which could make them very valuable. Type IV inhibitors do not need to interfere with the phosphorylation of all native substrates but only some, allowing them to block the kinase functions responsible for a particular disease but at the same time preserving their positive functions. However, it is still very difficult to determine what sites are necessary for a certain biological function. The arduous investigation to predict potential allosteric kinase hot spots identified ten different sites outside the ATP site that can be utilized in future development of type IV kinase inhibitors, and their applications in regulating kinase activity in a variety of disease states [4,104,105].
Based on structural features of CDK2, a novel allosteric inhibitor, 8-anilino-1-naphthalene sulfonate (ANS) 27, was discovered (Figure 6).
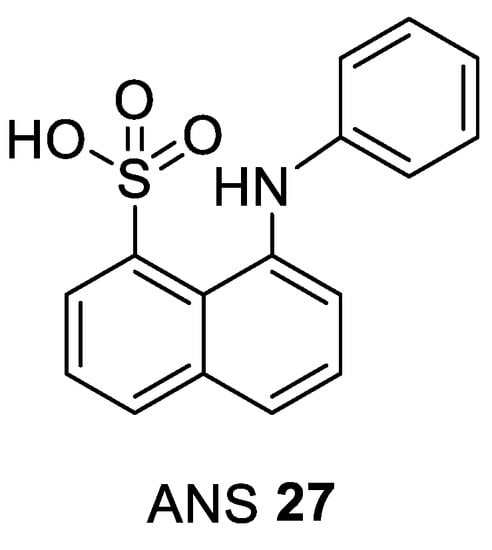
Figure 6.
Chemical structure of 8-anilino-1-naphthalene sulfonate (ANS) the most studied type IV CDK inhibitor.
It was found to bind to monomeric CDK2 by exploring a cavity very close to the DFG region which results in a structural transformation able to disrupt interactions with the CDK2 activator cyclin A. The activation loop adopts the active DFG-in conformation (PDB entry 3PXF). Consistent with its weak binding affinity to CDK2 (Kd = 37 µM) ANS can be easily displaced from the enzyme by cyclin A with an EC50 value of 0.6 μM. In addition, ANS was found to inhibit the active, phosphorylated CDK2/cyclin A dimer complex with a poor IC50 value of 91 μM (Table 5). It has been concluded that inhibitors with an ANS-like binding mode must interact more efficiently with monomeric CDK2 to noticeably improve their binding affinity, in order to inhibit complex formation with cyclins [106].

Table 5.
Selected biological data obtained from different experiments with ANS which demonstrate that it targets monomeric CDK2.
8. Type V Inhibitors
The preparation of type V inhibitors is considered as a new method to discover compounds which target both the ATP-binding site as well as distinct structural elements unique to each protein kinase in order increase their potency and selectivity. This group of compounds refers to bi-substrate inhibitors. However, the key problem relating to this class of agents is maintaining a balance between potency and selectivity in order to modulate their cellular activity or physicochemical properties [107,108]. A series of highly selective and potent type V inhibitors targeting tyrosine and serine/threonine kinases have been synthesized [109,110,111,112], but as of yet, no such molecule has been reported to be active against CDK family members.
9. Type VI Inhibitors
In recent years, there has been rapid progress made in the development of kinase inhibitors which can make covalent, very often irreversible, bond with the kinase active pocket (Figure 7).
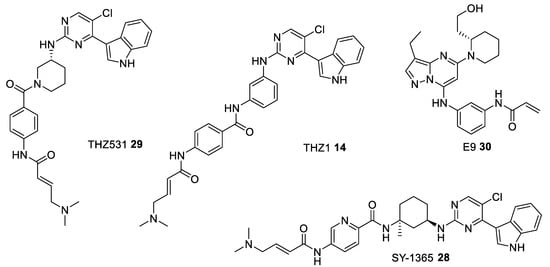
Figure 7.
Chemical structures of some of the most studied type VI CDK inhibitors.
The initial research findings, relating to the covalent-binding drugs, indicated that some of these agents can be beneficial to our health and some not necessarily. For instance, aspirin binds with a serine residue of the cyclooxygenase-1 (COX-1) enzyme, by forming covalent adducts, hence preventing the production of proinflammatory cytokines [113]. In contrast, paracetamol metabolizes into highly reactive radicals, although only in about 3%, when overdosed can cause oxidative stress, by forming toxic covalent adducts with liver proteins [114]. However, the most recent advances in Computational Biosciences have made it possible to design compounds with augmented selectivity and efficacy, and limited adverse effects.
Type VI inhibitors utilize chemical properties of type I-IV inhibitors, but they possess additional electrophilic groups (known as warheads) which mainly react with a nucleophilic cysteine residue in the active site (occasionally they also target lysine and tyrosine residues). Irreversible kinase inhibitors are meant to limit drug resistance given by protein kinase mutations, as well as overcome the competition from endogenous ATP. The adduct is generated in the Michael reaction through an acrylamide group (electrophilic warhead) which favors the formation of bonds with cysteine residues. It is thought that by lowering the reactivity of the warhead of type VI inhibitor to generate new shorter-acting reversible type IV agents could reduce their toxicity and off-target reactivity [115].
The first irreversible kinase inhibitor is THZ1 14 (Figure 7) which covalently binds to a cysteine residue (Cys312) in the ATP binding pocket (PDB entry 1UA2). This compound inhibits CDK7. At higher concentration it also demonstrates some activity against closely related kinases CDK12 and CDK13 (Table 6) [116]. Based on the THZ1 scaffold a new more selective covalent inhibitors, SY-1365 28 and THZ531 29 (Figure 7), have been identified. SY-1365 is currently being investigated for the treatment of ovarian and breast cancers (NCT03134638) [117], whereas THZ531 turned out to be a selective covalent inhibitor of CDK12/13 [118] (Table 6). To further optimize the structure of THZ1-like inhibitors another generation of irreversible CDK inhibitors, E9 30 (Figure 7), has been proposed. The findings show that E9 can overcome a common problem of resistance to the THZ1-like agents by ABC transporter-mediated drug efflux, and it covalently targets CDK12 (Table 6) [119].

Table 6.
Type VI CDK inhibitors under clinical evaluation.
Comparative studies of type VI inhibitors targeting other kinases, such as Ibrutinib or Afatinib, with type I and type II inhibitors demonstrated long-term clinical benefits of early treatment of patients with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL) [120] and lung cancer [121]. However, the research aimed at targeting only one amino acid (cysteine) can lead to a single point mutation resulting in acquired resistance to this particular agent. Therefore, the latest studies are focused on the development of type VI CDK inhibitors able to utilize the reactivity of other nucleophilic amino acids, such as lysine, tyrosine or even aspartic acid residues [115,122]. Hopefully, this theoretical data will soon generate new type VI CDK inhibitors.
10. Targeted Protein Degradation (TPD)
Recent advances in medical modalities gave rise to an appealing and promising technology known as Targeted Protein Degradation (TPD). TPD is a highly efficient method for selectively targeting proteins for removal from the cell, rather than inhibiting their activity. It is anticipated that, by using this method, toxic and disease-causing proteins could be depleted from cells under the potentially effective low-dose treatment. Small molecules able to induce degradation of target proteins can be divided into three major classes based on the structure of the compounds and their mechanism of action [123]. Single-ligand molecules able to create a direct interaction with the target protein to induce degradation belong to the first class of compounds. This group of compounds is represented by the aforementioned Fulvestrant, a selective estrogen receptor downregulator (SERD) which reduces the estrogen receptor-α (ERα) protein level [124]. However, this approach is limited to the finite number of target proteins.
Compounds that interact with E3 ubiquitin ligase to modulate substrate selectivity to modulate substrate selectivity are known as E3 modulators or molecular glues. The processes by which degradation of proteins is induced include: ubiquitination, targeting to the proteasome, proteolysis and functional silencing. Molecular glues act sub-stoichiometrically to facilitate rapid depletion of previously inaccessible proteins, but have mostly been identified somewhat serendipitously [125]. The first molecular glue was thalidomide which was identified to interact with CRBN gene (Cereblon) [126], a substrate recognition subunit of the Cullin-RING E3 ubiquitin ligase (CRL4) [127].
In relation to CDK molecular glues, the first compound acting as molecular glue degrader is R‑CR8 31, a pan-selective cyclin-dependent kinase (CDK) inhibitor (very similar to R-Roscovitine) (Figure 8) [128]. R-CR8 binds to the CDK12/cyclin K dimer, the resultant surface-exposed 2-pyridyl moiety facilitates CDK12/cyclin K complex formation with DDB1, the CUL4 adaptor protein, by circumventing the necessity for a substrate receptor and triggers rapid proteasomal degradation of cyclin K [129].
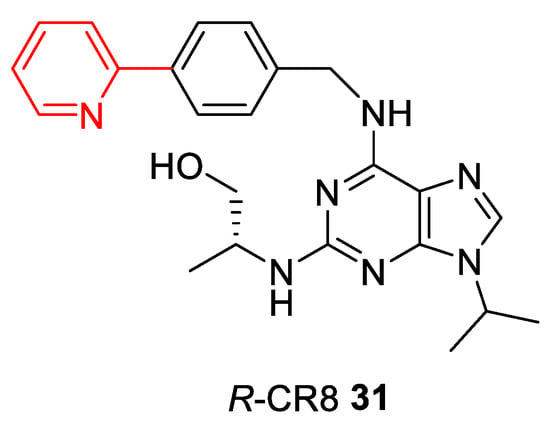
Figure 8.
Chemical structure of CR8. A surface-exposed 2-pyridyl moiety of CR8 is responsible for glue degrader properties.
The third class encompasses the chimeric small molecules, where an E3 ligase component and a protein of interest are linked to form a new and unique molecule. This group of compounds was developed under different names such as PROteolysis TArgeting Chimeras (PROTACs) and Specific and Non-genetic IAP-dependent Protein Erasers (SNIPERs). They target different proteins, but their mechanism of action is almost identical. Both, PROTACs and SNIPERs initiate the degradation of targeted protein by linking the protein of interest to an E3 ubiquitin ligase using the cell’s natural ubiquitin proteasome pathway (UPS) [130].
In relation to CDK kinases a series of PROTECs molecules have been reported. First dual CDK4/6 degraders 32, synthesized by linking Pomalidomide and Palbociclib, were reported by Burgess which efficiently degraded CDK4/6 with DC50 values ranging from 20–50 nM (Figure 9).
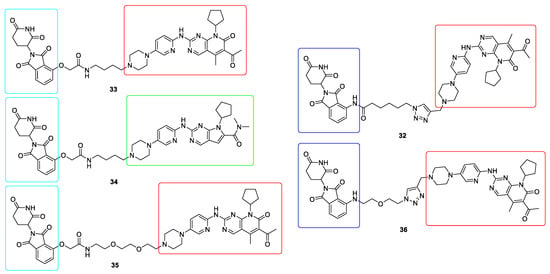
Figure 9.
Chemical structures of CDK4/6 PROteolysis TArgeting Chimeras (PROTACs). Red rectangle denotes the palbociclib moiety, green rectangle denotes the ribociclib moiety, light blue rectangle denotes the thalidomide moiety and dark blue rectangle denotes the pomalidomide moiety.
However, these compounds were not active in cells with overexpressed CDK4/6 [131]. Another group identified both dual CDK4/6 degraders 33 (based on Thalidomide and Palbociclib), as well as selective CDK4 34 (based on Thalidomide and Ribociclib) and CDK6 35 (based on Thalidomide and Palbociclib) degraders (Figure 9). These compounds exhibited good target degradation at 100 nM and showed more profound antiproliferative activities [132,133]. Very promising CDK6 degrader 36 was synthesized by linking Pomalidomide and Palbociclib (Figure 9). It possessed high CDK6 degradation capacity with a DC50 value of 2.1 nM. Moreover, it inhibited the proliferation of hematopoietic cancer cells, even with copy-amplified/mutated forms of CDK6 [134]. However, it is worth noting that their impact is still limited due to resistance development, which is the biggest challenge for PROTAC-based therapies at the moment [135].
As the effectiveness of traditional CDK8 inhibitors in the treatment of numerous cancers has yet to be confirmed, hence the need to elaborate new PROTACs for degrading the protein CDK8 became a driving force to overcome these shortcomings [136]. Cortistatin A was used to develop new derivatives. One of these compounds JH-XI-10-02 (37) is a potent CDK8 degrader (Figure 10). Its efficacy was verified by carrying out the degradation experiments in Jurkat and CRBN knockout Molt14 cells [137]. The synthesis of CDK8 degraders will definitely help to clarify whether targeting CDK8 is an effective strategy for treating cancer.
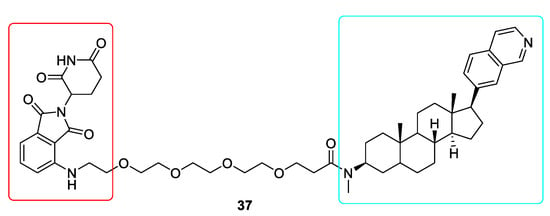
Figure 10.
Chemical structure of CDK8 PROTAC. Red rectangle denotes the pomalidomide moiety, light blue rectangle denotes the Cortistatin A derivative JH-VIII-49 moiety.
CDK9 forms a part of the positive transcription elongation factor b (P-TEFb) complex which together with cyclin T is responsible for the transcription elongation. CDK9 was found to be present in all tissues and numerous malignancies [138]. Due to the fact that CDK9 shares a high level of conservation sequence with other CDK members, it is difficult to obtain satisfactory selectivity [139]. In order to develop effective CDK9-targeting PROTAC it is necessary to identify lysine residues which can be targeted for ubiquitination and degradation [140]. The first selective CDK9 degrader 38 was developed on the basis of aminopyrazole derivative and Thalidomide (Figure 11). This CDK9 degrader reduced CDK9 protein activity in HCT116 cells by 56 and 65% at 10 and 20 µM, respectively, without affecting other CDKs [141]. Another CDK9 degrader, THAL-SNS-032 39 was developed by conjugating pan-selective CDK inhibitor SNS-032 and Pomalidomide (Figure 11). It selectively degraded CDK9 with a 99% Dmax at 250 nM in MOLT 4 cells after 6h treatment. Moreover, THAL-SNS-032 exhibited slower dissociation rates [142]. Yet another CDK9 degrader 40 was generated by conjugation of the natural compound Wogonin to Pomalidomide (Figure 11). This PROTAC induced the rapid degradation and showed more potency (IC50 = 17 ± 1.9 μM) than Wogonin (IC50 = 30 ± 3.5 μM) in MCF7 cells [143].
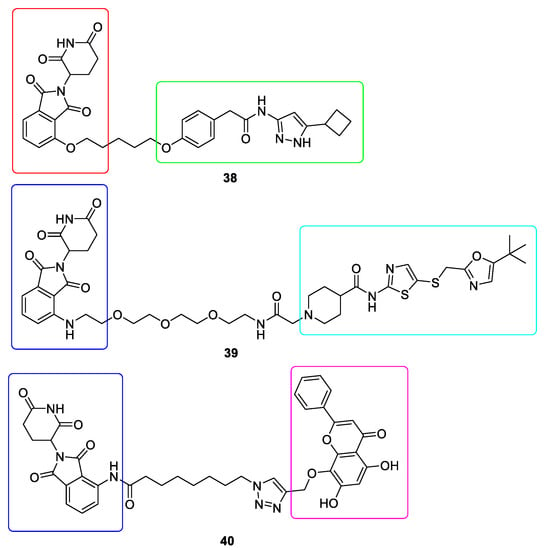
Figure 11.
Chemical structures of CDK9 PROTACs. Red rectangle denotes the Thalidomide moiety, dark blue rectangle denotes the Pomalidomide moiety, green rectangle denotes the aminopyrazole derivative moiety, light blue rectangle denotes the SNS-032 moiety and violet rectangle denotes the Wogonin moiety.
11. Conclusions
Cyclin-dependent kinases (CDKs) have unique tissue specific functions and dysregulation of CDKs and their cyclin partners is observed in a range of tumor types, and some of them have emerged as promising therapeutic targets in cancer. The major challenges in the CDK-targeted drug discovery are selectivity and bad responses, or resistance to treatments. However, the latest advances in the field provide encouragement that highly selective and potent inhibitors of human cyclin-dependent kinases with favorable pharmacokinetic properties will be identified.
Author Contributions
Conceptualization, P.Ł.; Funding acquisition, I.G.; Visualization, I.B.-B.; Writing—original draft, P.Ł.; Writing—review & editing, K.K., I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by statutory budget of Department of Medical Chemistry Pomeranian Medical University in Szczecin.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare they have no actual or potential competing financial interests.
Ethical Approval
The study was performed in accordance with the Declaration of Helsinki.
References
- Pavletich, N.P. Mechanisms of cyclin-dependent kinase regulation: Structures of cdks, their cyclin activators, and cip and INK4 inhibitors. J. Mol. Biol. 1999, 287, 821–828. [Google Scholar] [CrossRef]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef]
- Marra, A.; Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Clausen, M.H.; Nielsen, T.E. Allosteric small-molecule kinase inhibitors. Pharmacol. Ther. 2015, 156, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.O. The Cell Cycle: Principles of Control, 1st ed.; New Science Press: London, UK, 2007. [Google Scholar]
- Patrick, G.N.; Zukerberg, L.R.; Nikolic, M.; De La Monte, S.; Dikkes, P.; Tsai, L.-H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nat. Cell Biol. 1999, 402, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.-E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, H.; Wang, L.; Liu, Y.; Knapp, S.; Liu, Q.; Gray, N.S. Exploration of Type II Binding Mode: A Privileged Approach for Kinase Inhibitor Focused Drug Discovery? ACS Chem. Biol. 2014, 9, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Rabiller, M.; Getlik, M.; Klüter, S.; Richters, A.; Tückmantel, S.; Simard, J.R.; Rauh, D. Proteus in the World of Proteins: Conformational Changes in Protein Kinases. Arch. Pharm. 2010, 343, 193–206. [Google Scholar] [CrossRef]
- Baumli, S.; Lolli, G.; Lowe, E.D.; Troiani, S.; Rusconi, L.; Bullock, A.N.; Debreczeni, J.É.; Knapp, S.; Johnson, L.N. The structure of P-TEFb (CDK9/cyclin T1), its complex with flavopiridol and regulation by phosphorylation. EMBO J. 2008, 27, 1907–1918. [Google Scholar] [CrossRef]
- Chao, S.-H.; Fujinaga, K.; Marion, J.E.; Taube, R.; Sausville, E.A.; Senderowicz, A.M.; Peterlin, B.M.; Price, D.H. Flavopiridol Inhibits P-TEFb and Blocks HIV-1 Replication. J. Biol. Chem. 2000, 275, 28345–28348. [Google Scholar] [CrossRef] [PubMed]
- A Carlson, B.; Dubay, M.M.; A Sausville, E.; Brizuela, L.; Worland, P.J. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996, 56, 2973–2978. [Google Scholar] [PubMed]
- De Azevedo, W.F.; Mueller-Dieckmann, H.J.; Schulzegahmen, U.; Worland, P.J.; A Sausville, E.; Kim, S.H. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 2735–2740. [Google Scholar] [CrossRef]
- E Kahn, M.; Senderowicz, A.; A Sausville, E.; E Barrett, K. Possible mechanisms of diarrheal side effects associated with the use of a novel chemotherapeutic agent, flavopiridol. Clin. Cancer Res. 2001, 7, 343. [Google Scholar]
- Stadler, W.M.; Vogelzang, N.J.; Amato, R.; Sosman, J.; Taber, D.; Liebowitz, D.; Vokes, E.E. Flavopiridol, A Novel Cyclin-Dependent Kinase Inhibitor, in Metastatic Renal Cancer: A University of Chicago Phase II Consortium Study. J. Clin. Oncol. 2000, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R. Chronic Lymphocytic Leukemia: A Niche for Flavopiridol? Clin. Cancer Res. 2005, 11, 3971–3973. [Google Scholar] [CrossRef]
- De Azevedo, W.F., Jr.; Gaspar, R.T.; Canduri, F.; Camera, J.C., Jr.; da Silveira, N.J.F.J.B. Molecular model of cyclin-dependent kinase 5 complexed with roscovitine. Biochem. Biophy. Res. Commun. 2002, 297, 1154–1158. [Google Scholar] [CrossRef]
- Blachly, J.S.; Byrd, J.C. Emerging drug profile: Cyclin-dependent kinase inhibitors. Leuk. Lymphoma 2013, 54, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Pippin, J.W.; Qu, Q.; Meijer, L.; Shankland, S.J. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J. Clin. Investig. 1997, 100, 2512–2520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoogendijk, A.J.; Roelofs, J.J.T.H.; Duitman, J.; Van Lieshout, M.H.P.; Blok, D.C.; Van Der Poll, T.; Wieland, C.W. R-roscovitine Reduces Lung Inflammation Induced by Lipoteichoic Acid and Streptococcus pneumoniae. Mol. Med. 2012, 18, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Schang, L.M.; Bantly, A.; Knockaert, M.; Shaheen, F.; Meijer, L.; Malim, M.H.; Gray, N.S.; Schaffer, P.A. Pharmacological Cyclin-Dependent Kinase Inhibitors Inhibit Replication of Wild-Type and Drug-Resistant Strains of Herpes Simplex Virus and Human Immunodeficiency Virus Type 1 by Targeting Cellular, Not Viral, Proteins. J. Virol. 2002, 76, 7874–7882. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.; Crews, L.; Desplats, P.; Dumaop, W.; Rockenstein, E.; Achim, C.L.; Everall, I.P.; Masliah, E. Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am. J. Pathol. 2011, 178, 1646–1661. [Google Scholar] [CrossRef]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar]
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.; Jones, J.; Johnson, A.J.; Andritsos, L.; Maddocks, K.; Jaglowski, S.; Hessler, J.; Grever, M.R.; Im, E.; Zhou, H.; et al. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 2015, 29, 1524–1529. [Google Scholar] [CrossRef]
- Yakisich, J.S.; Vita, M.F.; Siden, Å.; Tasat, D.R.; Cruz, M. Strong inhibition of replicative DNA synthesis in the developing rat cerebral cortex and glioma cells by roscovitine. Investig. New Drugs 2009, 28, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Faivre, S.; Laurence, V.; Delbaldo, C.; Vera, K.; Girre, V.; Chiao, J.; Armour, S.; Frame, S.; Green, S.R.; et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur. J. Cancer 2010, 46, 3243–3250. [Google Scholar] [CrossRef]
- Brasca, M.G.; Amboldi, N.; Ballinari, D.; Cameron, A.; Casale, E.; Cervi, G.; Colombo, M.; Colotta, F.; Croci, V.; D’Alessio, R.; et al. Identification of N,1,4,4-Tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-pyrazolo [4,3-h]quinazoline-3-carboxamide (PHA-848125), a Potent, Orally Available Cyclin Dependent Kinase Inhibitor. J. Med. Chem. 2009, 52, 5152–5163. [Google Scholar] [CrossRef] [PubMed]
- Milciclib. Available online: http://www.tizianalifesciences.com/drug-pipeline/milciclib/ (accessed on 20 January 2021).
- Rocca, A.; Farolfi, A.; Bravaccini, S.; Schirone, A.; Amadori, D. Palbociclib (PD 0332991): Targeting the cell cycle machinery in breast cancer. Expert Opin. Pharmacother. 2014, 15, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Samson, K. LEE011 CDK Inhibitor Showing Early Promise in Drug-Resistant Cancers. Oncol. Times 2014, 36, 39–40. [Google Scholar] [CrossRef]
- Diéras, V.; Harbeck, N.; Joy, A.A.; Gelmon, K.; Ettl, J.; Verma, S.; Lu, D.R.; Gauthier, E.; Schnell, P.; Mori, A.; et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2− Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019, 24, 1514. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kittaneh, M.; Lolkema, M.P.J.K.; Postow, M.A.; Schwartz, G.; Franklin, C.; Matano, A.; Bhansali, S.; Parasuraman, S.; Kim, K. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J. Clin. Oncol. 2014, 32, 9009. [Google Scholar] [CrossRef]
- Corbel, C.; Zhang, B.; Le Parc, A.; Baratte, B.; Colas, P.; Couturier, C.; Kosik, K.S.; Landrieu, I.; Le Tilly, V.; Bach, S. Tamoxifen Inhibits CDK5 Kinase Activity by Interacting with p35/p25 and Modulates the Pattern of Tau Phosphorylation. Chem. Biol. 2015, 22, 472–482. [Google Scholar] [CrossRef]
- Maccallum, D.E. Seliciclib (CYC202, R-Roscovitine) Induces Cell Death in Multiple Myeloma Cells by Inhibition of RNA Polymerase II-Dependent Transcription and Down-regulation of Mcl-1. Cancer Res. 2005, 65, 5399–5407. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.; Maupin, M.K. 5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole inhibits a HeLa protein kinase that phosphorylates an RNA polymerase II-derived peptide. Biochem. Biophys. Res. Commun. 1989, 159, 508–515. [Google Scholar] [CrossRef]
- Zhu, Y.; Pe’Ery, T.; Peng, J.; Ramanathan, Y.; Marshall, N.; Marshall, T.; Amendt, B.; Mathews, M.B.; Price, D.H. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997, 11, 2622–2632. [Google Scholar] [CrossRef]
- Sedlacek, H. Mechanisms of action of flavopiridol. Crit. Rev. Oncol. 2001, 38, 139–170. [Google Scholar] [CrossRef]
- Montagnoli, A.; Valsasina, B.; Croci, V.; Menichincheri, M.; Rainoldi, S.; Marchesi, V.; Tibolla, M.; Tenca, P.; Brotherton, D.; Albanese, C.; et al. A Cdc7 kinase inhibitor restricts initiation of DNA replication and has antitumor activity. Nat. Chem. Biol. 2008, 4, 357–365. [Google Scholar] [CrossRef]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine Targets, Protein Kinases and Pyridoxal Kinase. J. Biol. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Tovar, C.; Chen, S.; Knezevic, D.; Zhao, X.; Sun, H.; Heimbrook, D.C.; Chen, L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. USA 2006, 103, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mita, M.; Shapiro, G.I.; Poon, J.; Small, K.; Tzontcheva, A.; Kantesaria, B.; Zhu, Y.; Bannerji, R.; Statkevich, P. Effect of aprepitant on the pharmacokinetics of the cyclin-dependent kinase inhibitor dinaciclib in patients with advanced malignancies. Cancer Chemother. Pharmacol. 2012, 70, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer 2004, 3, 1427. [Google Scholar]
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262. [Google Scholar] [CrossRef]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef]
- Ali, S.; Heathcote, D.A.; Kroll, S.H.B.; Jogalekar, A.S.; Scheiper, B.; Patel, H.; Brackow, J.; Siwicka, A.; Fuchter, M.J.; Periyasamy, M.; et al. The Development of a Selective Cyclin-Dependent Kinase Inhibitor That Shows Antitumor Activity. Cancer Res. 2009, 69, 6208–6215. [Google Scholar] [CrossRef]
- Wang, S.; Fischer, P. Cyclin-dependent kinase 9: A key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 2008, 29, 302–313. [Google Scholar] [CrossRef]
- Echalier, A.; Bettayeb, K.; Ferandin, Y.; Lozach, O.; Clément, M.; Valette, A.; Liger, F.; Marquet, B.; Morris, J.C.; Endicott, J.A.; et al. Meriolins (3-(Pyrimidin-4-yl)-7-azaindoles): Synthesis, Kinase Inhibitory Activity, Cellular Effects, and Structure of a CDK2/Cyclin A/Meriolin Complex†. J. Med. Chem. 2008, 51, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Bettayeb, K.; Tirado, O.M.; Marionneau-Lambot, S.; Ferandin, Y.; Lozach, O.; Morris, J.C.; Mateo-Lozano, S.; Drueckes, P.; Schächtele, C.; Kubbutat, M.H.; et al. Meriolins, a New Class of Cell Death–Inducing Kinase Inhibitors with Enhanced Selectivity for Cyclin-Dependent Kinases. Cancer Res. 2007, 67, 8325–8334. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, Y.A.; Taylor, M.A.; Napoleon, J.V.; Rana, S.; Contreras, J.I.; Natarajan, A. Cyclin Dependent Kinase 9 Inhibitors for Cancer Therapy. J. Med. Chem. 2016, 59, 8667–8684. [Google Scholar] [CrossRef]
- Parrino, B.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Diana, P.; Cirrincione, G.; Carbone, A. Synthesis, antitumor activity and CDK1 inhibiton of new thiazole nortopsentin analogues. Eur. J. Med. Chem. 2017, 138, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Byth, K.F.; Thomas, A.; Hughes, G.; Forder, C.; McGregor, A.; Geh, C.; Oakes, S.; Green, C.; Walker, M.; Newcombe, N.; et al. AZD5438, a potent oral inhibitor of cyclin-dependent kinases 1, 2, and 9, leads to pharmacodynamic changes and potent antitumor effects in human tumor xenografts. Mol. Cancer Ther. 2009, 8, 1856–1866. [Google Scholar] [CrossRef]
- Siemeister, G.; Lücking, U.; Wengner, A.M.; Lienau, P.; Steinke, W.; Schatz, C.; Mumberg, D.; Ziegelbauer, K. BAY 1000394, a Novel Cyclin-Dependent Kinase Inhibitor, with Potent Antitumor Activity in Mono- and in Combination Treatment upon Oral Application. Mol. Cancer Ther. 2012, 11, 2265–2273. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wierda, W.G.; Chubb, S.; Hawtin, R.E.; Fox, J.A.; Keating, M.J.; Gandhi, V.; Plunkett, W. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood 2009, 113, 4637–4645. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, G.; Mediouni, S.; Valente, S.T. Targeting HIV transcription: The quest for a functional cure. Curr. Top. Microbiol. Immunol. 2015, 389, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and Related Alkaloids. Chem. Rev. 2009, 109, 3080–3098. [Google Scholar] [CrossRef]
- Gompel, M.; Leost, M.; Joffe, E.B.D.K.; Puricelli, L.; Franco, L.H.; Palermo, J.; Meijer, L. Meridianins, a new family of protein kinase inhibitors isolated from the Ascidian Aplidium meridianum. Bioorganic Med. Chem. Lett. 2004, 14, 1703–1707. [Google Scholar] [CrossRef]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M.; et al. Novel 1H-Pyrrolo[2,3-b]pyridine Derivative Nortopsentin Analogues: Synthesis and Antitumor Activity in Peritoneal Mesothelioma Experimental Models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef]
- Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Parkin, S.; Hope, H. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Tetrahedron 1994, 50, 3987–3992. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Faulkner, D.; Perry, N.B.; Ettouati, L.; Litaudon, M.; Blunt, J.W.; Munro, M.H.; Jameson, G.B. Alkaloids from the antarctic sponge Kirkpatrickia varialosa. Part 2: Variolin A and N(3′)-methyl tetrahydrovariolin B. Tetrahedron 1994, 50, 3993–4000. [Google Scholar] [CrossRef]
- Simone, M.; Erba, E.; Damia, G.; Vikhanskaya, F.; Di Francesco, A.M.; Riccardi, R.; Bailly, C.; Cuevas, C.; Sousa-Faro, J.M.F.; D’Incalci, M. Variolin B and its derivate deoxy-variolin B: New marine natural compounds with cyclin-dependent kinase inhibitor activity. Eur. J. Cancer 2005, 41, 2366–2377. [Google Scholar] [CrossRef]
- Bharate, S.B.; Yadav, R.R.; Battula, S.; Vishwakarma, R.A. Meridianins: Marine-derived potent kinase inhibitors. Mini-Reviews Med. Chem. 2012, 12, 618–631. [Google Scholar] [CrossRef]
- Li, B.; Chonghaile, T.N.; Fan, Y.; Madden, S.F.; Klinger, R.; O’Connor, A.E.; Walsh, L.; O’Hurley, G.; Udupi, G.M.; Joseph, J.; et al. Therapeutic Rationale to Target Highly Expressed CDK7 Conferring Poor Outcomes in Triple-Negative Breast Cancer. Cancer Res. 2017, 77, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Walsby, E.; Lazenby, M.; Pepper, C.; Burnett, A.K. The cyclin-dependent kinase inhibitor SNS-032 has single agent activity in AML cells and is highly synergistic with cytarabine. Leukemia 2011, 25, 411–419. [Google Scholar] [CrossRef]
- Reck, M.; Horn, L.; Novello, S.; Barlesi, F.; Albert, I.; Juhász, E.; Kowalski, D.; Robinet, G.; Cadranel, J.; Bidoli, P.; et al. Phase II Study of Roniciclib in Combination with Cisplatin/Etoposide or Carboplatin/Etoposide as First-Line Therapy in Patients with Extensive-Disease Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Boss, D.; Schwartz, G.; Middleton, M.; Amakye, D.; Swaisland, H.; Midgley, R.; Ranson, M.; Danson, S.; Calvert, H.; Plummer, R.; et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours. Ann. Oncol. 2010, 21, 884–894. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Bin Sulaimee, N.H.; Pareek, T.; Asad, A.B.M.A.; Rajkumar, R.; Cheong, W.F.; Wenk, M.R.; Dawe, G.S.; Chuang, K.-H.; et al. Specific Inhibition of p25/Cdk5 Activity by the Cdk5 Inhibitory Peptide Reduces Neurodegeneration In Vivo. J. Neurosci. 2013, 33, 334–343. [Google Scholar] [CrossRef]
- Deng, Y.; Shipps, G.W.; Zhao, L.; Siddiqui, M.A.; Popovici-Muller, J.; Curran, P.J.; Duca, J.S.; Hruza, A.W.; Fischmann, T.O.; Madison, V.S.; et al. Modulating the interaction between CDK2 and cyclin A with a quinoline-based inhibitor. Bioorganic Med. Chem. Lett. 2014, 24, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; McNae, I.; Kontopidis, G.; McClue, S.J.; McInnes, C.; Stewart, K.J.; Wang, S.; Zheleva, D.I.; Marriage, H.; Lane, D.P.; et al. Discovery of a Novel Family of CDK Inhibitors with the Program LIDAEUS: Structural Basis for Ligand-Induced Disordering of the Activation Loop. Structure 2003, 11, 399–410. [Google Scholar] [CrossRef]
- Martin, M.P.; Endicott, J.A.; Noble, M.E. Structure-based discovery of cyclin-dependent protein kinase inhibitors. Essays Biochem. 2017, 61, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Hari, S.B.; Merritt, E.A.; Maly, D.J. Sequence Determinants of a Specific Inactive Protein Kinase Conformation. Chem. Biol. 2013, 20, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Jura, N.; Zhang, X.; Endres, N.F.; Seeliger, M.A.; Schindler, T.; Kuriyan, J. Catalytic Control in the EGF Receptor and Its Connection to General Kinase Regulatory Mechanisms. Mol. Cell 2011, 42, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.; Robert, L.J.-L.J.A.A.C. Targeting Protein Multiple Conformations: A Structure-Based Strategy for Kinase Drug Design. Curr. Top. Med. Chem. 2007, 7, 1394–1407. [Google Scholar] [CrossRef]
- Wohlbold, L.; Merrick, K.A.; De, S.; Amat, R.; Kim, J.H.; LaRochelle, S.; Allen, J.J.; Zhang, C.; Shokat, K.M.; Petrini, J.H.J.; et al. Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells. PLoS Genet. 2012, 8, e1002935. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nishigaki, N.; Washio, Y.; Kano, K.; Harris, P.A.; Sato, H.; Mori, I.; West, R.I.; Shibahara, M.; Toyoda, H.; et al. Discovery of Novel Benzimidazoles as Potent Inhibitors of TIE-2 and VEGFR-2 Tyrosine Kinase Receptors. J. Med. Chem. 2007, 50, 4453–4470. [Google Scholar] [CrossRef]
- Emrick, M.A.; Lee, T.; Starkey, P.J.; Mumby, M.C.; Resing, K.A.; Ahn, N.G. The gatekeeper residue controls autoactivation of ERK2 via a pathway of intramolecular connectivity. Proc. Natl. Acad. Sci. USA 2006, 103, 18101–18106. [Google Scholar] [CrossRef]
- Azam, M.; A Seeliger, M.; Gray, N.S.; Kuriyan, J.; Daley, G.Q. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat. Struct. Mol. Biol. 2008, 15, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Cardama, A.; Tong, W.; Manshouri, T.; Vega, F.; A Lennon, P.; Cools, J.; Gilliland, D.G.; Lee, F.; Cortés, J.; Kantarjian, H.; et al. Activity of tyrosine kinase inhibitors against human NUP214-ABL1-positive T cell malignancies. Leukemia 2008, 22, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.S.K.; He, P.; Modi, V.; Duong-Ly, K.C.; Ma, H.; Peterson, J.R.; Dunbrack, R.L.; Levy, R.M. Conformational Analysis of the DFG-Out Kinase Motif and Biochemical Profiling of Structurally Validated Type II Inhibitors. J. Med. Chem. 2015, 58, 466–479. [Google Scholar] [CrossRef]
- Griffith, J.; Black, J.; Faerman, C.; Swenson, L.; Wynn, M.; Lu, F.; Lippke, J.; Saxena, K. The Structural Basis for Autoinhibition of FLT3 by the Juxtamembrane Domain. Mol. Cell 2004, 13, 169–178. [Google Scholar] [CrossRef]
- Wang, Z.; Harkins, P.C.; Ulevitch, R.J.; Han, J.; Cobb, M.H.; Goldsmith, E.J. The structure of mitogen-activated protein kinase p38 at 2.1-Å resolution. Proc. Natl. Acad. Sci. USA 1997, 94, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Seeliger, M.A.; Eastwood, M.P.; Frank, F.; Xu, H.; Jensen, M.Ø.; Dror, R.O.; Kuriyan, J.; Shaw, D.E. A conserved protonation-dependent switch controls drug binding in the Abl kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Atwell, S.; Adams, J.M.; Badger, J.; Buchanan, M.D.; Feil, I.K.; Froning, K.J.; Gao, X.; Hendle, J.; Keegan, K.; Leon, B.C.; et al. A Novel Mode of Gleevec Binding Is Revealed by the Structure of Spleen Tyrosine Kinase. J. Biol. Chem. 2004, 279, 55827–55832. [Google Scholar] [CrossRef]
- Liao, J.J.-L. Molecular Recognition of Protein Kinase Binding Pockets for Design of Potent and Selective Kinase Inhibitors. J. Med. Chem. 2007, 50, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.T.; Möbitz, H.; Drueckes, P.; Savitsky, P.; Fedorov, O.; Elkins, J.M.; Deane, C.M.; Cowan-Jacob, S.W.; Knapp, S. Type II Inhibitors Targeting CDK2. ACS Chem. Biol. 2015, 10, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Gurbani, D.; Weisberg, E.L.; Hunter, J.C.; Li, L.; Jones, D.S.; Ficarro, S.B.; Mowafy, S.; Tam, C.-P.; Rao, S.; et al. Structure-guided development of covalent TAK1 inhibitors. Bioorganic Med. Chem. 2017, 25, 838–846. [Google Scholar] [CrossRef]
- Russo, A.A.; Jeffrey, P.D.; Patten, A.K.; Massagué, J.; Pavletich, N.P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inibitor bound to the cyclin A–Cdk2 complex. Nature 1996, 382, 325–331. [Google Scholar] [CrossRef]
- Jeffrey, P.D.; Tong, L.; Pavletich, N.P. Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 2000, 14, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- McInnes, C.; Andrews, M.J.I.Z.; Daniella, I.; Lane, D.P.; Fischer, P.M. Peptidomimetic Design of CDK Inhibitors Targeting theRecruitment Site of the Cyclin Subunit. Curr. Med. Chem. Anti-Cancer Agents 2003, 3, 57–69. [Google Scholar]
- Kontopidis, G.; Andrews, M.J.; McInnes, C.; Plater, A.; Innes, L.; Renachowski, S.; Cowan, A.; Fischer, P.M. Truncation and Optimisation of Peptide Inhibitors of Cyclin-Dependent Kinase 2-Cyclin A Through Structure-Guided Design. ChemMedChem 2009, 4, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, C.; Gelbert, L.M.; Lallena, M.J.; de Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorganic Med. Chem. Lett. 2015, 25, 3420–3435. [Google Scholar] [CrossRef]
- MM D37K. Available online: https://adisinsight.springer.com/drugs/800040966 (accessed on 20 January 2021).
- Schneider, E.V.; Böttcher, J.; Blaesse, M.; Neumann, L.; Huber, R.; Maskos, K. The Structure of CDK8/CycC Implicates Specificity in the CDK/Cyclin Family and Reveals Interaction with a Deep Pocket Binder. J. Mol. Biol. 2011, 412, 251–266. [Google Scholar] [CrossRef]
- Bergeron, P.; Koehler, M.F.T.; Blackwood, E.M.; Bowman, K.; Clark, K.; Firestein, R.; Kiefer, J.R.; Maskos, K.; McCleland, M.L.; Orren, L.; et al. Design and Development of a Series of Potent and Selective Type II Inhibitors of CDK8. ACS Med. Chem. Lett. 2016, 7, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129–3140. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Adjei, A.A. The Ras/Raf/MAPK Pathway. J. Thorac. Oncol. 2006, 1, 7–9. [Google Scholar] [CrossRef]
- Dale, T.C.; Clarke, P.A.; Esdar, C.; Waalboer, D.; Adeniji-Popoola, O.; Ortiz-Ruiz, M.-J.; Mallinger, A.; Samant, R.S.; Czodrowski, P.; Musil, D.; et al. A selective chemical probe for exploring the role of CDK8 and CDK19 in human disease. Nat. Chem. Biol. 2015, 11, 973–980. [Google Scholar] [CrossRef]
- Nagaria, T.S.; Williams, J.L.; LeDuc, C.; A Squire, J.; A Greer, P.; Sangrar, W. Flavopiridol Synergizes with Sorafenib to Induce Cytotoxicity and Potentiate Antitumorigenic Activity in EGFR/HER-2 and Mutant RAS/RAF Breast Cancer Model Systems. Neoplasia 2013, 15, 939-IN27. [Google Scholar] [CrossRef]
- Mancini, M.; Yarden, Y. Mutational and network level mechanisms underlying resistance to anti-cancer kinase inhibitors. Semin. Cell Dev. Biol. 2016, 50, 164–176. [Google Scholar] [CrossRef]
- Adrián, F.J.; Ding, Q.; Sim, T.; Velentza, A.V.; Sloan, C.; Liu, Y.; Zhang, G.; Hur, W.; Ding, S.; Manley, P.W.; et al. Allosteric inhibitors of Bcr-abl–dependent cell proliferation. Nat. Chem. Biol. 2006, 2, 95–102. [Google Scholar] [CrossRef]
- Sebolt-Leopold, J.S.; Dudley, D.T.; Herrera, R.; Van Becelaere, K.; Wiland, A.; Gowan, R.C.; Tecle, H.; Barrett, S.D.; Bridges, A.; Przybranowski, S.; et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 1999, 5, 810–816. [Google Scholar] [CrossRef]
- Gavrin, L.K.; Saiah, E. Approaches to discover non-ATP site kinase inhibitors. MedChemComm 2013, 4, 41–51. [Google Scholar] [CrossRef]
- Yueh, C.; Rettenmaier, J.; Xia, B.; Hall, D.R.; Alekseenko, A.; Porter, K.A.; Barkovich, K.; Keseru, G.M.; Whitty, A.; Wells, J.A.; et al. Kinase Atlas: Druggability Analysis of Potential Allosteric Sites in Kinases. J. Med. Chem. 2019, 62, 6512–6524. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Grove, L.E.; Hall, D.R.; Bohnuud, T.; E Mottarella, S.; Luo, L.; Xia, B.; Beglov, D.; Vajda, S. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 2015, 10, 733–755. [Google Scholar] [CrossRef]
- Betzi, S.; Alam, R.; Martin, M.; Lubbers, D.J.; Han, H.; Jakkaraj, S.R.; Georg, G.I.; Schönbrunn, E. Discovery of a Potential Allosteric Ligand Binding Site in CDK2. ACS Chem. Biol. 2011, 6, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Parang, K.; Till, J.H.; Ablooglu, A.J.; A Kohanski, R.; Hubbard, S.; A Cole, P. Mechanism-based design of a protein kinase inhibitor. Nat. Genet. 2001, 8, 37–41. [Google Scholar] [CrossRef]
- Poot, A.J.; Van Ameijde, J.; Slijper, M.; Berg, A.V.D.; Hilhorst, R.; Ruijtenbeek, R.; Rijkers, D.T.S.; Liskamp, R.M.J. Development of Selective Bisubstrate-Based Inhibitors Against Protein Kinase C (PKC) Isozymes By Using Dynamic Peptide Microarrays. ChemBioChem 2009, 10, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Gower, C.M.; Chang, M.E.K.; Maly, D.J. Bivalent inhibitors of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013, 49, 102–115. [Google Scholar] [CrossRef]
- Hill, Z.B.; Perera, B.G.K.; Maly, D.J. A Chemical Genetic Method for Generating Bivalent Inhibitors of Protein Kinases. J. Am. Chem. Soc. 2009, 131, 6686–6688. [Google Scholar] [CrossRef] [PubMed]
- Kedika, S.R.; Udugamasooriya, D.G. Converting a weaker ATP-binding site inhibitor into a potent hetero-bivalent ligand by tethering to a unique peptide sequence derived from the same kinase. Org. Biomol. Chem. 2018, 16, 6443–6449. [Google Scholar] [CrossRef]
- Wong, M.L.; Murphy, J.; Harrington, E.; Gower, C.M.; Jain, R.K.; Schirle, M.; Thomas, J.R. Examining the influence of specificity ligands and ATP-competitive ligands on the overall effectiveness of bivalent kinase inhibitors. Proteome Sci. 2016, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- McCrae, J.C.; Morrison, E.E.; MacIntyre, I.M.; Dear, J.W.; Webb, D.J. Long-term adverse effects of paracetamol—A review. Br. J. Clin. Pharmacol. 2018, 84, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, M.; Laufer, S.A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62, 5673–5724. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, N.P.; Zhang, T.; Rahl, P.B.; Abraham, B.J.; Reddy, J.; Ficarro, S.B.; Dastur, A.; Amzallag, A.; Ramaswamy, S.; Tesar, B.; et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nat. Cell Biol. 2014, 511, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Marineau, J.J.; Rajagopal, N.; Hamman, K.B.; Choi, Y.J.; Schmidt, D.R.; Ke, N.; Johannessen, L.; Bradley, M.J.; Orlando, D.A.; et al. Discovery and Characterization of SY-1365, a Selective, Covalent Inhibitor of CDK7. Cancer Res. 2019, 79, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kwiatkowski, N.; Olson, C.M.; E Dixon-Clarke, S.; Abraham, B.J.; Greifenberg, A.K.; Ficarro, S.B.; Elkins, J.M.; Liang, Y.; Hannett, N.M.; et al. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat. Chem. Biol. 2016, 12, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, T.; Terai, H.; Ficarro, S.B.; Kwiatkowski, N.; Hao, M.-F.; Sharma, B.; Christensen, C.L.; Chipumuro, E.; Wong, K.-K.; et al. Overcoming Resistance to the THZ Series of Covalent Transcriptional CDK Inhibitors. Cell Chem. Biol. 2018, 25, 135–142.e5. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Liu, R.; Yue, Z.; Tsai, C.-C.; Shen, J. Assessing Lysine and Cysteine Reactivities for Designing Targeted Covalent Kinase Inhibitors. J. Am. Chem. Soc. 2019, 141, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Coll-Martínez, B.; Delgado, A.; Crosas, B. The Potential of Proteolytic Chimeras as Pharmacological Tools and Therapeutic Agents. Molecules 2020, 25, 5956. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Wakeling, A.; I Nicholson, R. Fulvestrant: An oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer 2004, 90, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Sadok, A.; Collins, I. A critical evaluation of the approaches to targeted protein degradation for drug discovery. Drug Discov. Today Technol. 2019, 31, 5–13. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Fouad, S.; Wells, O.S.; Hill, M.A.; D’Angiolella, V. Cullin Ring Ubiquitin Ligases (CRLs) in Cancer: Responses to Ionizing Radiation (IR) Treatment. Front. Physiol. 2019, 10, 1144. [Google Scholar] [CrossRef]
- Bettayeb, K.; Oumata, N.; Echalier, A.; Ferandin, Y.; A Endicott, J.; Galons, H.; Meijer, L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene 2008, 27, 5797–5807. [Google Scholar] [CrossRef]
- Słabicki, M.; Kozicka, Z.; Petzold, G.; Li, Y.-D.; Manojkumar, M.; Bunker, R.D.; Donovan, K.A.; Sievers, Q.L.; Koeppel, J.; Suchyta, D.; et al. The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature 2020, 585, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Ohoka, N.; Shibata, N.; Tsukumo, Y. Targeted Protein Degradation by Chimeric Small Molecules, PROTACs and SNIPERs. Front. Chem. 2019, 7, 849. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Burgess, K. PROTACs suppression of CDK4/6, crucial kinases for cell cycle regulation in cancer. Chem. Commun. 2019, 55, 2704–2707. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, E.S.; Donovan, K.A.; Liang, Y.; Fischer, E.S.; Zhang, T.; Gray, N.S. Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew. Chem. Int. Ed. 2019, 58, 6321–6326. [Google Scholar] [CrossRef]
- Brand, M.; Jiang, B.; Bauer, S.; Donovan, K.A.; Liang, Y.; Wang, E.S.; Nowak, R.P.; Yuan, J.C.; Zhang, T.; Kwiatkowski, N.; et al. Homolog-Selective Degradation as a Strategy to Probe the Function of CDK6 in AML. Cell Chem. Biol. 2019, 26, 300–306.e9. [Google Scholar] [CrossRef]
- Su, S.; Yang, Z.; Gao, H.; Yang, H.; Zhu, S.; An, Z.; Wang, J.; Li, Q.; Chandarlapaty, S.; Deng, H.; et al. Potent and Preferential Degradation of CDK6 via Proteolysis Targeting Chimera Degraders. J. Med. Chem. 2019, 62, 7575–7582. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Z.; Bhatt, T.; Dickler, M.; Giri, D.; Scaltriti, M.; Baselga, J.; Rosen, N.; Chandarlapaty, S. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017, 36, 2255–2264. [Google Scholar] [CrossRef]
- Rzymski, T.; Mikula, M.; Wiklik, K.; Brzózka, K. CDK8 kinase—An emerging target in targeted cancer therapy. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2015, 1854, 1617–1629. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Wang, E.S.; Johannessen, L.; Kwiatkowski, N.; Sim, T.; Gray, N.S. Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8. ACS Med. Chem. Lett. 2018, 9, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bagella, L.; MacLachlan, T.K.; Buono, R.J.; Pisano, M.M.; Giordano, A.; De Luca, A. Cloning of murine CDK9/PITALRE and its tissue-specific expression in development. J. Cell. Physiol. 1998, 177, 206–213. [Google Scholar] [CrossRef]
- Vladimir, K.; Stjepan, U. Cyclin-Dependent Kinase Inhibitors as Anticancer Drugs. Curr. Drug Targets 2010, 11, 291–302. [Google Scholar] [CrossRef]
- Petzold, G.; Fischer, E.S.; Thomä, G.P.E.S.F.N.H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase. Nat. Cell Biol. 2016, 532, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Robb, C.M.; Contreras, J.I.; Kour, S.; Taylor, M.A.; Abid, M.; Sonawane, Y.A.; Zahid, M.; Murry, D.J.; Natarajan, A.; Rana, S. Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC). Chem. Commun. 2017, 53, 7577–7580. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M.; Jiang, B.; Erb, M.A.; Liang, Y.; Doctor, Z.M.; Zhang, Z.; Zhang, T.; Kwiatkowski, N.; Boukhali, M.; Green, J.L.; et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat. Chem. Biol. 2018, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Ren, J.; Li, Y.; Wang, J.; Xu, X.; Feng, Y.; Tang, H.; Wang, Y.; Li, Z. Discovery of Wogonin-based PROTACs against CDK9 and capable of achieving antitumor activity. Bioorganic Chem. 2018, 81, 373–381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).