Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity
Abstract
1. Introduction
2. Results
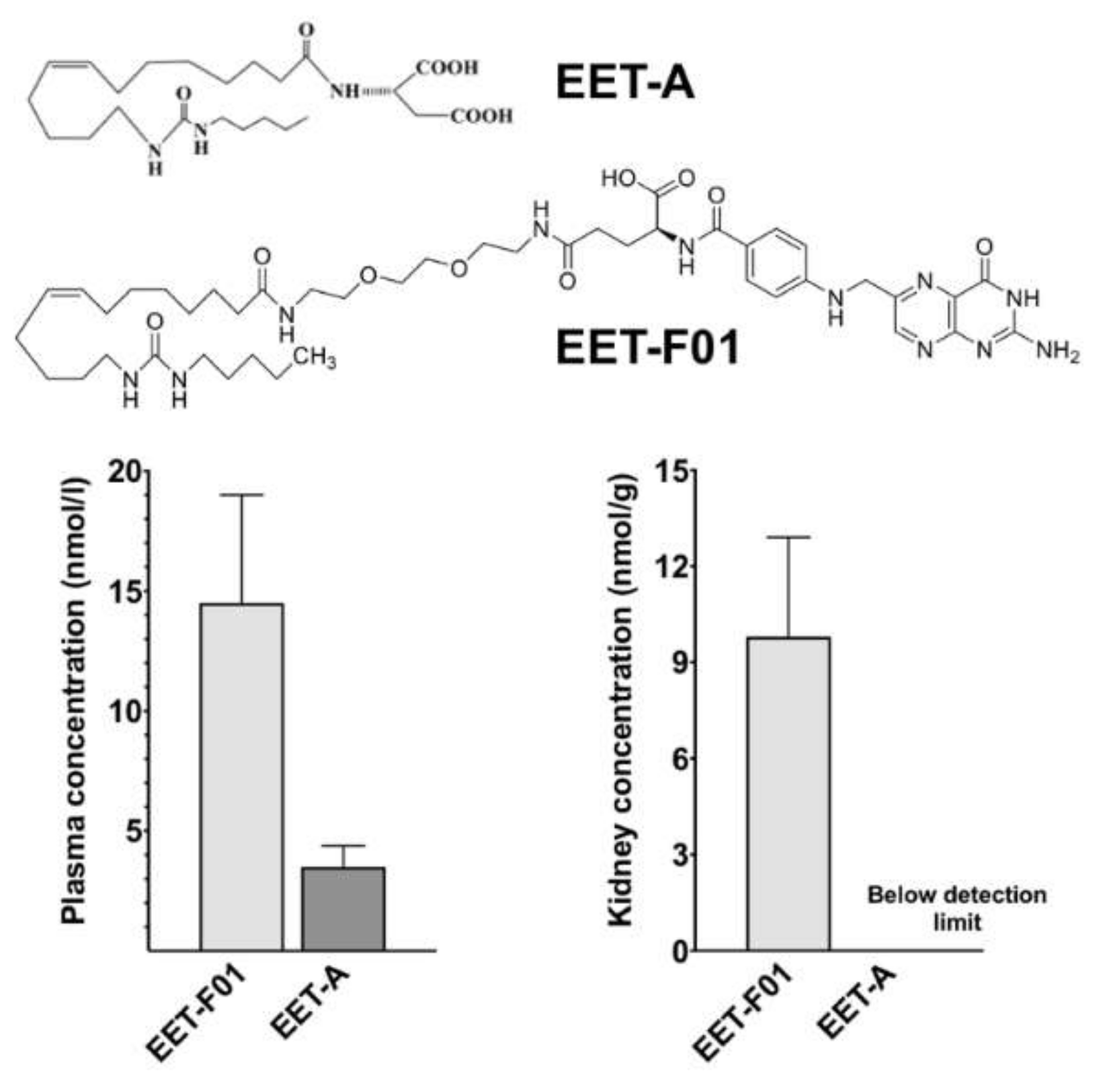
2.1. EET-F01 Targets Kidney Tissue
2.2. EET-F01 Protects against Cisplatin Nephrotoxicity
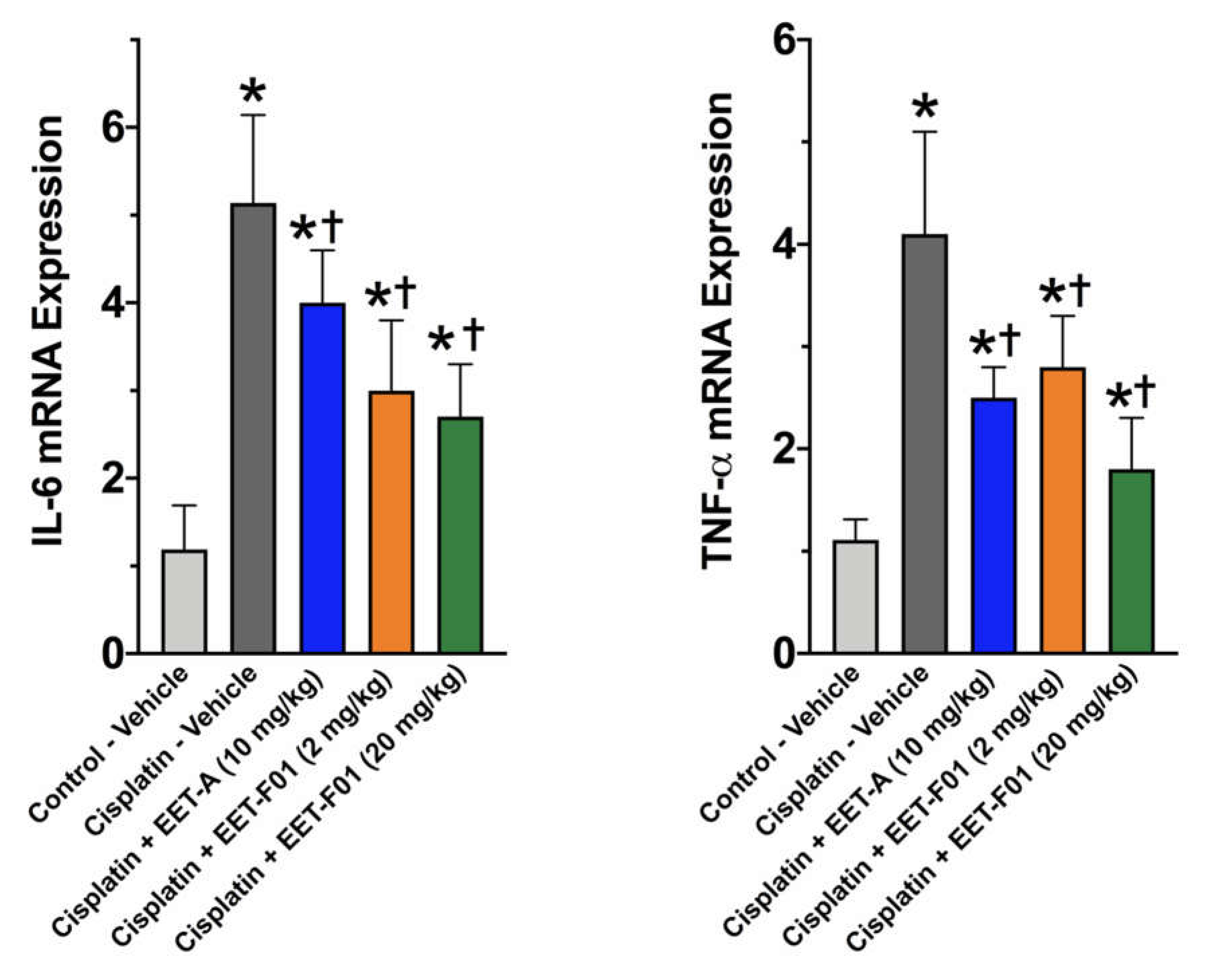
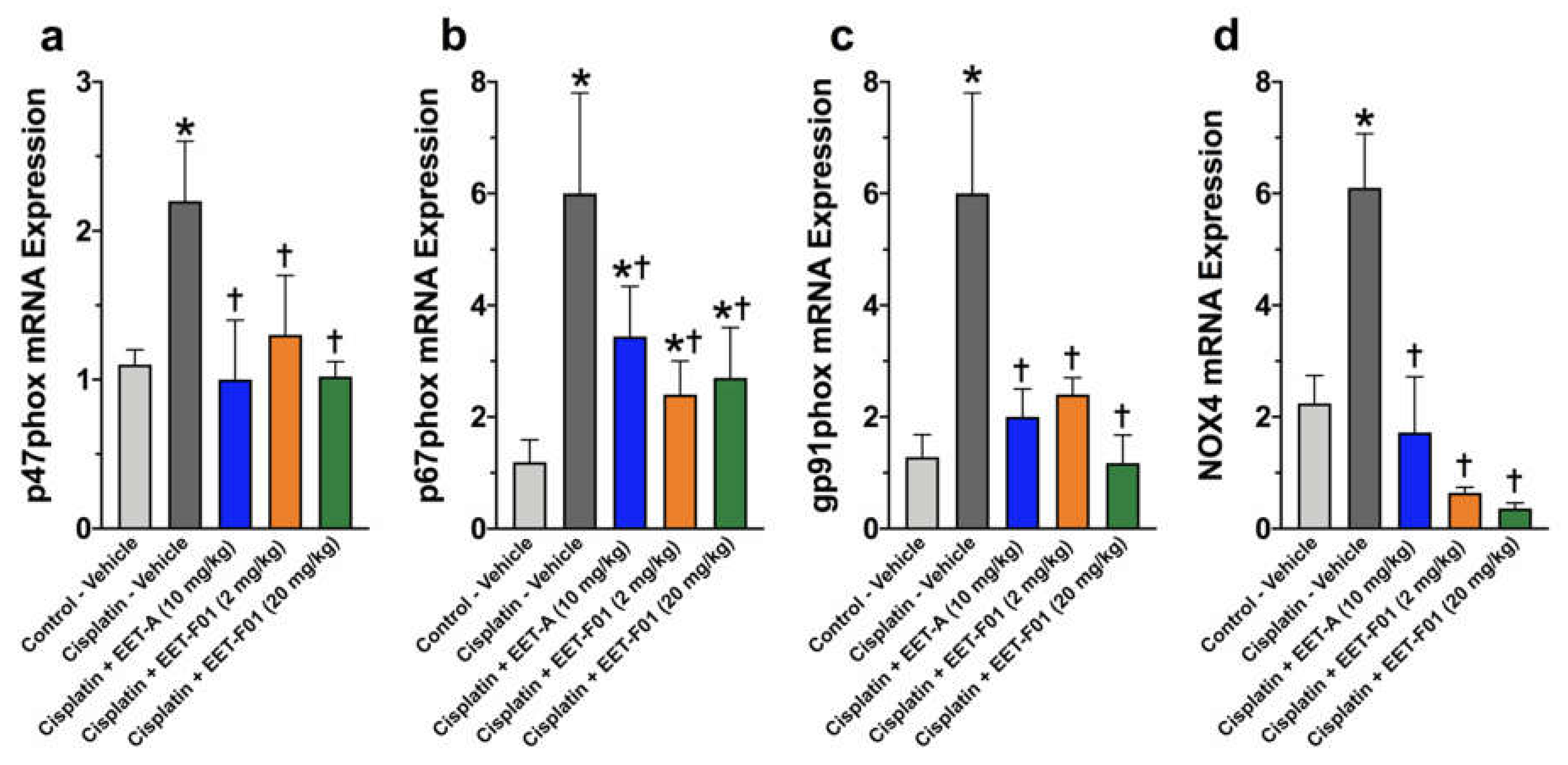
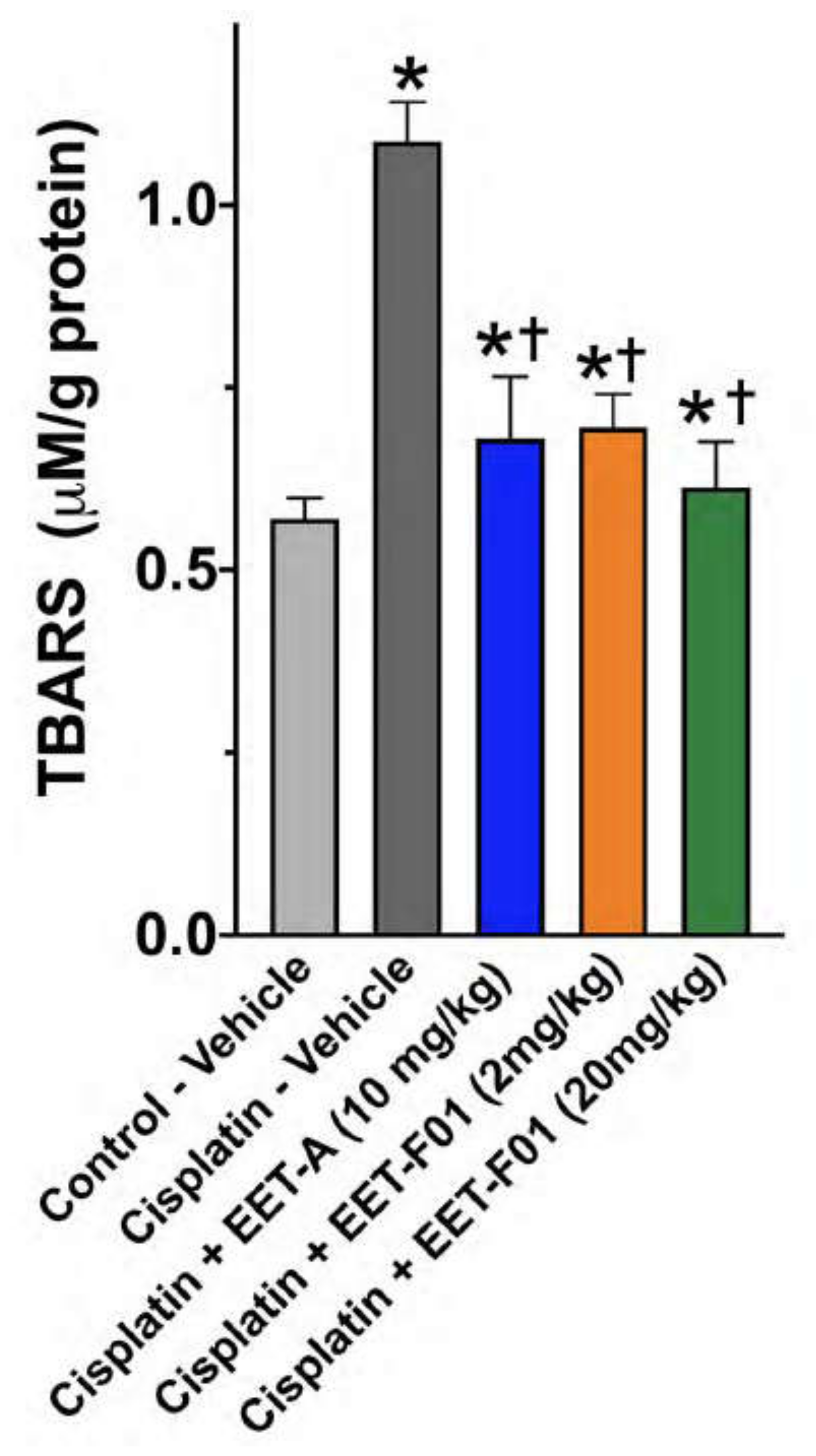
2.3. EET-F01 and EET-A Reduce Renal Inflammation and Oxidative Stress in Cisplatin Nephrotoxicity
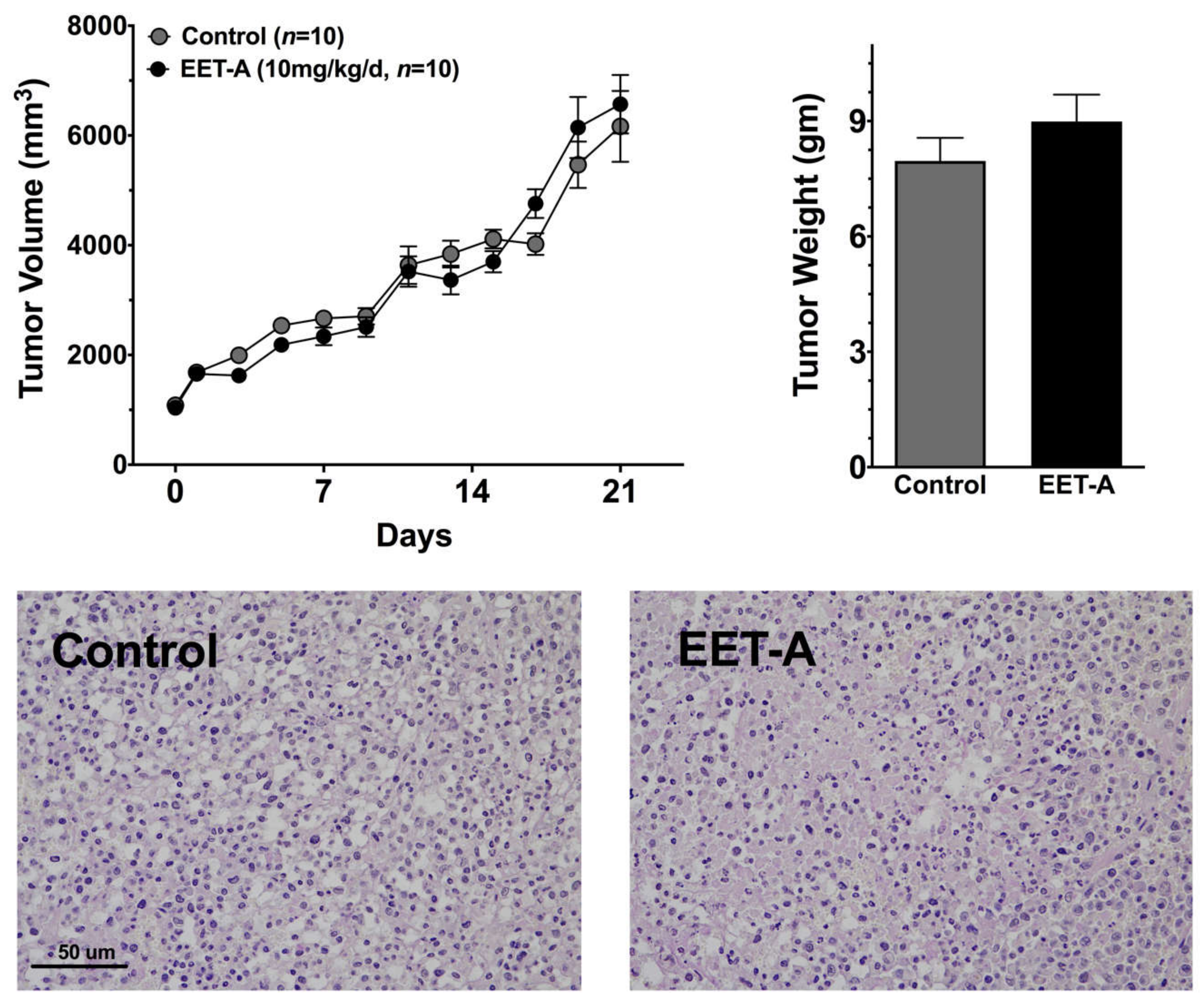
2.4. EET-A Does Not Enhance Tumor Growth
3. Discussion
4. Materials and Methods
4.1. Chemicals
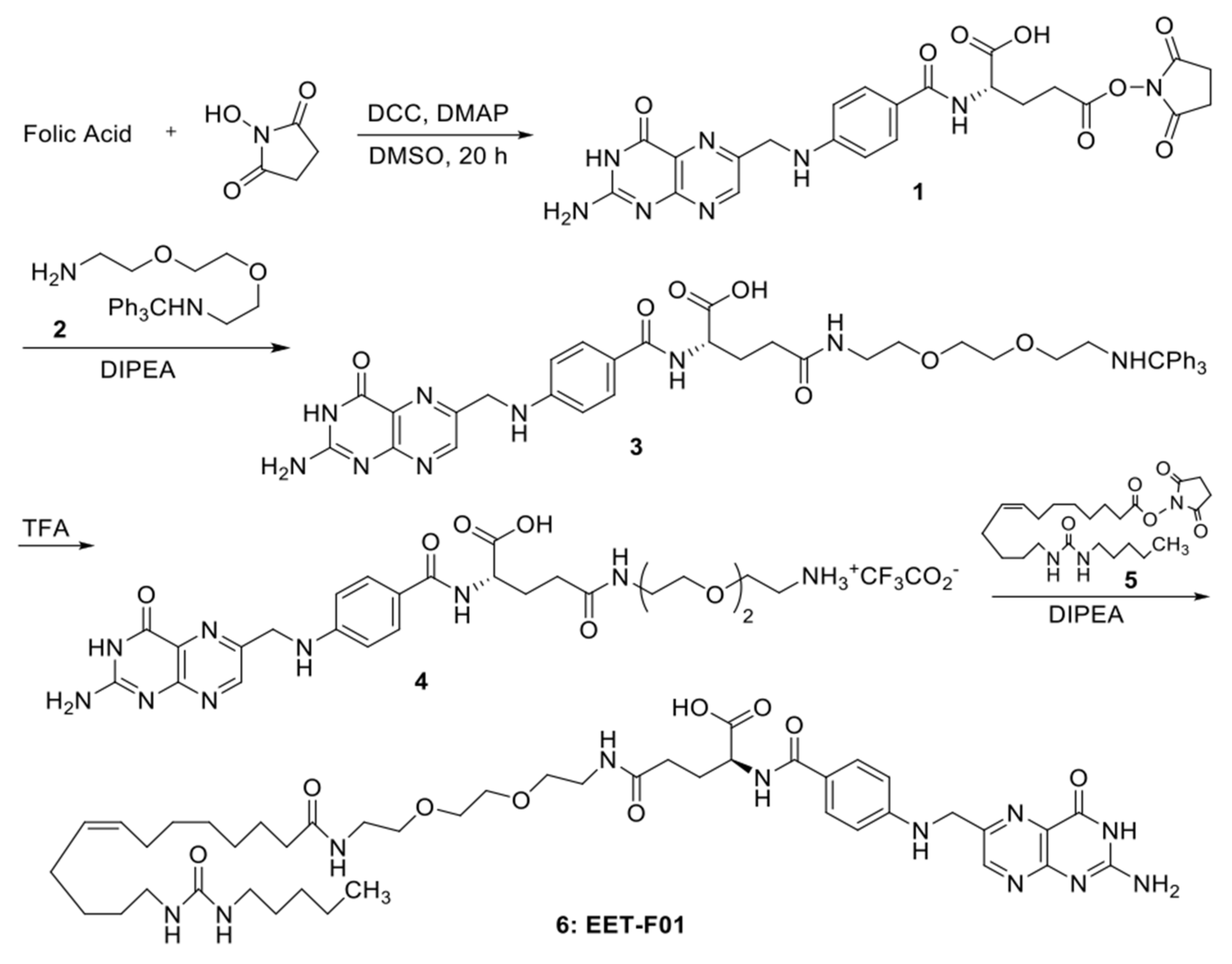
4.2. EET-F01 Synthesis
4.3. Animals
4.4. Kidney Targeting In Vivo Animal Studies
4.5. Mass Spectrometric Analysis
4.6. Cisplatin-Induced Nephrotoxicity Animal Study
4.7. Biochemical Analysis
4.8. Histopathology
4.9. Real-Time PCR Analyses
4.10. Tumor Bearing Studies
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bongers, M.L.; Coupé, V.M.H.; Jansma, E.P.; Smit, E.F.; Groot, C.A.U.-D. Cost Effectiveness of Treatment with New Agents in Advanced Non-Small-Cell Lung Cancer. PharmacoEconomics 2012, 30, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Kintzel, P.E. Anticancer Drug-Induced Kidney Disorders. Drug Saf. 2001, 24, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A.; Moeckel, G.W. Nephrotoxicity from Chemotherapeutic Agents: Clinical Manifestations, Pathobiology, and Prevention/Therapy. Semin. Nephrol. 2010, 30, 570–581. [Google Scholar] [CrossRef]
- Sánchez-González, P.D.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011, 41, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.B.; Imig, J.D.; Schmitz, J.M.; Falck, J.R. Orally Active Epoxyeicosatrienoic Acid Analogs. J. Cardiovasc. Pharmacol. 2017, 70, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Jankiewicz, W.K.; Khan, A.H. Epoxy Fatty Acids: From Salt Regulation to Kidney and Cardiovascular Therapeutics: 2019 Lewis K. Dahl Memorial Lecture. Hypertension 2020, 76, 3–15. [Google Scholar] [CrossRef]
- Imig, J.D.; Elmarakby, A.; Nithipatikom, K.; Wei, S.; Capdevila, J.H.; Tuniki, V.R.; Sangras, B.; Anjaiah, S.; Manthati, V.L.; Reddy, D.S.; et al. Development of Epoxyeicosatrienoic Acid Analogs with in Vivo Anti-Hypertensive Actions. Front. Physiol. 2010, 1, 157. [Google Scholar] [CrossRef]
- Khan, A.H.; Neckář, J.; Manthati, V.; Errabelli, R.; Pavlov, T.S.; Staruschenko, A.; Falck, J.R.; Imig, J.D. Orally Active Epoxyeicosatrienoic Acid Analog Attenuates Kidney Injury in Hypertensive Dahl Salt–Sensitive Rat. Hypertension 2013, 62, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Stavniichuk, A.; Sattar, M.A.; Falck, J.R.; Imig, J.D. Epoxyeicosatrienoic Acid Analog EET-A Blunts Development of Lupus Nephritis in Mice. Front. Pharmacol. 2019, 10, 2512. [Google Scholar] [CrossRef]
- Khan, A.H.; Pavlov, T.S.; Christain, S.V.; Neckář, J.; Staruschenko, A.; Gauthier, K.M.; Capdevila, J.H.; Falck, J.R.; Campbell, W.B.; Imig, J.D. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin. Sci. 2014, 127, 463–474. [Google Scholar] [CrossRef]
- Yeboah, M.M.; Khan, A.H.; Chesnik, M.A.; Sharma, A.; Paudyal, M.P.; Falck, J.R.; Imig, J.D. The epoxyeicosatrienoic acid analog PVPA ameliorates cyclosporine-induced hypertension and renal injury in rats. Am. J. Physiol. Physiol. 2016, 311, F576–F585. [Google Scholar] [CrossRef]
- Panigrahy, D.; Edin, M.L.; Lee, C.R.; Huang, S.; Bielenberg, D.R.; Butterfield, C.E.; Barnés, C.M.; Mammoto, A.; Mammoto, T.; Luria, A.; et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Investig. 2012, 122, 178–191. [Google Scholar] [CrossRef]
- Pozzi, A.; Popescu, V.; Yang, S.; Mei, S.; Shi, M.; Puolitaival, S.M.; Caprioli, R.M.; Capdevila, J.H. The Anti-tumorigenic Properties of Peroxisomal Proliferator-activated Receptor α Are Arachidonic Acid Epoxygenase-mediated. J. Biol. Chem. 2010, 285, 12840–12850. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Al Za’Abi, M.; Al Salam, S.; Al Suleimani, Y.; Ashique, M.; Manoj, P.; Nemmar, A.; Ali, B.H. Effects of repeated increasing doses of cisplatin as models of acute kidney injury and chronic kidney disease in rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 394, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.F.; Kundu, K.; Joseph, G.; Dikalov, S.; Weiss, D.; Murthy, N.; Taylor, W.R. Folate Receptor–Targeted Antioxidant Therapy Ameliorates Renal Ischemia–Reperfusion Injury. J. Am. Soc. Nephrol. 2012, 23, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Leamon, C.P.; Vlahov, I.R.; Weimbs, T. Folate-Conjugated Rapamycin Slows Progression of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2012, 23, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Sun, X.; Zhang, Z. Kidney–targeted drug delivery systems. Acta Pharm. Sin. B 2014, 4, 37–42. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, H.; Zhang, C. Advances in kidney-targeted drug delivery systems. Int. J. Pharm. 2020, 587, 119679. [Google Scholar] [CrossRef]
- Kipp, K.R.; Kruger, S.L.; Schimmel, M.F.; Parker, N.; Shillingford, J.M.; Leamon, C.P.; Weimbs, T. Comparison of folate-conjugated rapamycin versus unconjugated rapamycin in an orthologous mouse model of polycystic kidney disease. Am. J. Physiol. Physiol. 2018, 315, F395–F405. [Google Scholar] [CrossRef] [PubMed]
- Leamon, C.P. Folate-targeted drug strategies for the treatment of cancer. Curr. Opin. Investig. Drugs 2008, 9, 19037834. [Google Scholar]
- Leamon, C.P.; Jackman, A.L. Chapter 7 Exploitation of the Folate Receptor in the Management of Cancer and Inflammatory Disease. Vitam. Horm. 2008, 79, 203–233. [Google Scholar] [CrossRef]
- Birn, H.; Spiegelstein, O.; Christensen, E.I.; Finnell, R.H. Renal Tubular Reabsorption of Folate Mediated by Folate Binding Protein 1. J. Am. Soc. Nephrol. 2005, 16, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Liu, J.; Kumar, G.; Skapek, S.X.; Falck, J.R.; Imig, J.D. Novel orally active epoxyeicosatrienoic acid (EET) analogs attenuate cisplatin nephrotoxicity. FASEB J. 2014, 27, 2946–2956, Erratum in 2014, 28, 520. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Fish, B.; Wahl, G.; Sharma, A.; Falck, J.R.; Paudyal, M.P.; Moulder, J.E.; Imig, J.D.; Cohen, E.P. Epoxyeicosatrienoic acid analogue mitigates kidney injury in a rat model of radiation nephropathy. Clin. Sci. 2016, 130, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol. Ther. 2018, 192, 1–19. [Google Scholar] [CrossRef]
- Sodhi, K.; Inoue, K.; Gotlinger, K.H.; Canestraro, M.; Vanella, L.; Kim, D.H.; Manthati, V.L.; Koduru, S.R.; Falck, J.R.; Schwartzman, M.L.; et al. Epoxyeicosatrienoic Acid Agonist Rescues the Metabolic Syndrome Phenotype of HO-2-Null Mice. J. Pharmacol. Exp. Ther. 2009, 331, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Skibba, M.; Khan, A.H.; Kolb, L.L.; Yeboah, M.M.; Falck, J.R.; Amaradhi, R.; Imig, J.D. Epoxyeicosatrienoic Acid Analog Decreases Renal Fibrosis by Reducing Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Un, H.; Ugan, R.A.; Gurbuz, M.A.; Bayir, Y.; Kahramanlar, A.; Kaya, G.; Cadirci, E.; Halici, Z. Phloretin and phloridzin guard against cisplatin-induced nephrotoxicity in mice through inhibiting oxidative stress and inflammation. Life Sci. 2021, 266, 118869. [Google Scholar] [CrossRef]
- Aljuhani, N.; Ismail, R.S.; El-Awady, M.S.; Hassan, M.H. Modulatory effects of perindopril on cisplatin-induced nephrotoxicity in mice: Implication of inflammatory cytokines and caspase-3 mediated apoptosis. Acta Pharm. 2020, 70, 515–525. [Google Scholar] [CrossRef]
- Younis, N.N.; Elsherbiny, N.M.; Shaheen, M.A.; Elseweidy, M.M. Modulation of NADPH oxidase and Nrf2/HO-1 pathway by vanillin in cisplatin-induced nephrotoxicity in rats. J. Pharm. Pharmacol. 2020, 72, 1546–1555. [Google Scholar] [CrossRef]
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodríguez-Muñoz, R.; Reyes, J.L.; Barrera, D.; Pedraza-Chaverri, J. Superoxide Anion Production and Expression of gp91phoxand p47phoxAre Increased in Glomeruli and Proximal Tubules of Cisplatin-Treated Rats. J. Biochem. Mol. Toxicol. 2014, 29, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.; Wang, W.; Yang, H.; Yang, J.; Hallisey, V.M.; Deng, J.; Verheul, S.M.L.; Hwang, S.H.; Gartung, A.; Wang, Y.; et al. Resolution of eicosanoid/cytokine storm prevents carcinogen and inflammation-initiated hepatocellular cancer progression. Proc. Natl. Acad. Sci. USA 2020, 117, 21576–21587. [Google Scholar] [CrossRef]
- Jones, R.D.; Liao, J.; Tong, X.; Xu, D.; Sun, L.; Li, H.; Yang, G.-Y. Epoxy-Oxylipins and Soluble Epoxide Hydrolase Metabolic Pathway as Targets for NSAID-Induced Gastroenteropathy and Inflammation-Associated Carcinogenesis. Front. Pharmacol. 2019, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Sun, L.; Liao, J.; Li, H.; You, X.; Xu, D.; Yang, J.; Hwang, S.H.; Jones, R.D.; Hammock, B.; et al. Inhibition of Pancreatic Carcinoma Growth Through Enhancing ω-3 Epoxy Polyunsaturated Fatty Acid Profile by Inhibition of Soluble Epoxide Hydrolase. Anticancer. Res. 2019, 39, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, H.; Dong, H.; Liao, J.; Hammock, B.D.; Yang, G.Y. Soluble epoxide hydrolase deficiency inhibits dextran sulfate so-dium-induced colitis and carcinogenesis in mice. Anticancer Res. 2013, 33, 5261–5271. [Google Scholar] [PubMed]
- Holm, J.; Hansen, S.I. Characterization of soluble folate receptors (folate binding proteins) in humans. Biological roles and clinical potentials in infection and malignancy. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140466. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Dolman, M.; Harmsen, S.; Storm, G.; Hennink, W.; Kok, R. Drug targeting to the kidney: Advances in the active targeting of therapeutics to proximal tubular cells. Adv. Drug Deliv. Rev. 2010, 62, 1344–1357. [Google Scholar] [CrossRef]
- Trindade, A.F.; Frade, R.F.M.; Maçôas, E.M.S.; Graça, C.; Rodrigues, C.A.B.; Martinho, J.M.G.; Afonso, C.A.M. “Click and go”: Simple and fast folic acid conjugation. Org. Biomol. Chem. 2014, 12, 3181–3190. [Google Scholar] [CrossRef]
- Pasc, A.; Gizzi, P.; Dupuy, N.; Parant, S.; Ghanbaja, J.; Gérardin, C. Microscopic and macroscopic anisotropy in supramolecular hydrogels of histidine-based surfactants. Tetrahedron Lett. 2009, 50, 6183–6186. [Google Scholar] [CrossRef]
- Falck, J.R.; Kodela, R.; Manne, R.; Atcha, K.R.; Puli, N.; Dubasi, N.; Manthati, V.L.; Capdevila, J.H.; Yi, X.-Y.; Goldman, D.H.; et al. 14,15-Epoxyeicosa-5,8,11-trienoic Acid (14,15-EET) Surrogates Containing Epoxide Bioisosteres: Influence upon Vascular Relaxation and Soluble Epoxide Hydrolase Inhibition. J. Med. Chem. 2009, 52, 5069–5075. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Borowicz, S.; Pramanik, R.; Schultz, R.M.; Han, J.; Chen, G. Estrogen Receptor Inhibits c-Jun-dependent Stress-induced Cell Death by Binding and Modifying c-Jun Activity in Human Breast Cancer Cells. J. Biol. Chem. 2004, 279, 6769–6777. [Google Scholar] [CrossRef] [PubMed]

| TNFα | F- CGAGTGACAAGCCTGTAGCC R- GAGAACCTGGGAGTAGACAAGG |
| IL-6 | F- GAGACTTCCAGCCAGTTGCC R- TGAAGTCTCCTCTCCGGACTT |
| p47phox | F- TCGAGAAACGCTTCGTCCC R- GTAGACCACCTTCTCCGACA |
| gp91phox | F- GGCCCAACTGGGATAACGAG R- TTTTAGCCAAGGCTTCGGGG |
| Nox4 | F- GGCCCAACTGGGATAACGAG R- TTTTAGCCAAGGCTTCGGGG |
| p67phox | F- GCTTCGGAACATGGTGTCTAAGA R- AGAGTCAGGCAGTAGTTTTTCACTTG |
| Gpdh | F- AGTGCCAGCCTCGTCTCATA R- GGTAACCAGGCGTCCGATAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imig, J.D.; Hye Khan, M.A.; Burkhan, A.; Chen, G.; Adebesin, A.M.; Falck, J.R. Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2021, 22, 2793. https://doi.org/10.3390/ijms22062793
Imig JD, Hye Khan MA, Burkhan A, Chen G, Adebesin AM, Falck JR. Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity. International Journal of Molecular Sciences. 2021; 22(6):2793. https://doi.org/10.3390/ijms22062793
Chicago/Turabian StyleImig, John D., Md Abdul Hye Khan, Anna Burkhan, Guan Chen, Adeniyi Michael Adebesin, and John R. Falck. 2021. "Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity" International Journal of Molecular Sciences 22, no. 6: 2793. https://doi.org/10.3390/ijms22062793
APA StyleImig, J. D., Hye Khan, M. A., Burkhan, A., Chen, G., Adebesin, A. M., & Falck, J. R. (2021). Kidney-Targeted Epoxyeicosatrienoic Acid Analog, EET-F01, Reduces Inflammation, Oxidative Stress, and Cisplatin-Induced Nephrotoxicity. International Journal of Molecular Sciences, 22(6), 2793. https://doi.org/10.3390/ijms22062793







