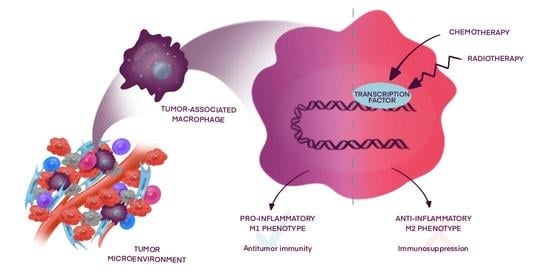
How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy
Abstract
1. Introduction
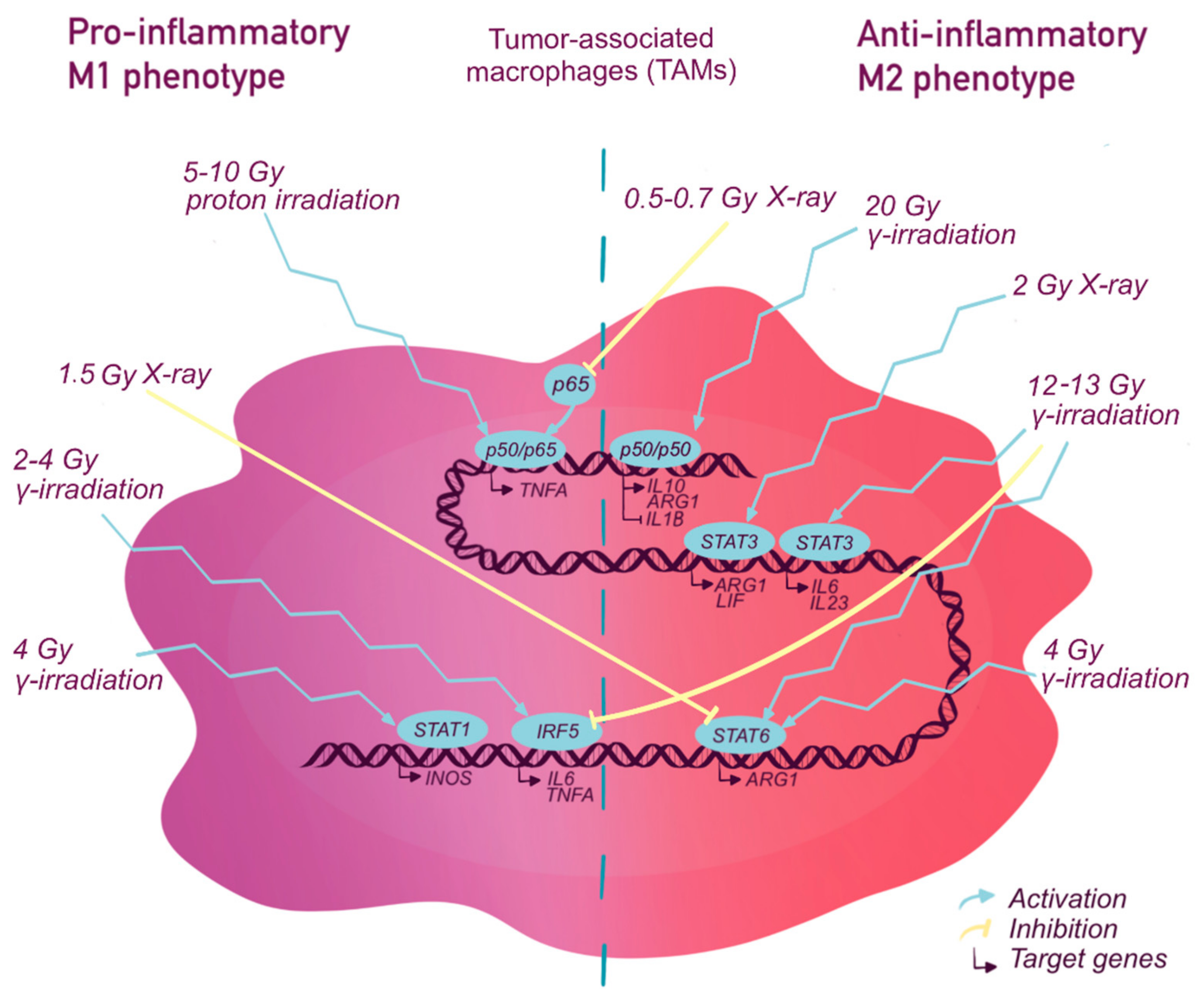
2. Macrophage Transcriptional Reprogramming during Chemo- and Radiotherapy
2.1. The Nuclear Factor Kappa B (NF-κb)
2.1.1. NF-κB and Chemotherapy
2.1.2. NF-κB and Radiotherapy
2.2. STAT Transcription Factor Family
2.2.1. STATs and Chemotherapy
2.2.2. STATs and Radiotherapy
2.3. Interferon Regulatory Factor (IRF)
2.3.1. IRFs and Chemotherapy
2.3.2. IRFs and Radiotherapy
2.4. P53
2.4.1. P53 and Chemotherapy
2.4.2. P53 and Radiotherapy
2.5. Other Transcription Factors Affected by Radio- and Chemotherapy
3. Macrophage Transcription Factors in Antitumor Therapy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADM | Adrenomedullin |
| Arg-1 | Arginase-1 |
| ATM | Ataxia telangiectasia mutated (serine/threonine kinase) |
| Bcl-xL | B-cell lymacrophageoma-extra large |
| CCL | Chemokine (C-C motif) ligand |
| CCR2 | C-C chemokine receptor 2 |
| CD40 | Cluster of differentiation 40 |
| CIITA | Class II, major histocompatibility complex, transactivator |
| COX2 | Prostaglandin-endoperoxide synthase 2 |
| CREB | cAMP response element-binding protein |
| CSF | Colony stimulating factor |
| CYP19A1 | Cytochrome P450 Family 19 Subfamily A Member 1 |
| CXCL1 | The chemokine (C-X-C motif) ligand |
| C/EBP | CCAAT/enhancer binding protein |
| DHFR | Dihydrofolate reductase |
| EGF | Epidermal growth factor |
| ERK | Mitogen-activated protein kinase |
| eIF4E | Eukaryotic translation initiation factor |
| FN1 | Fibronectin 1 |
| Fizz1 | Resistin-like beta |
| GBP6 | Guanylate binding protein |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| Gy | Gray |
| GDF-15 | Growth differentiation factor 15 |
| HDL | High-density lipoprotein |
| HLA | Human leukocyte antigens |
| HMGB1 | High-mobility group protein B1) |
| IFIT2 | Interferon-induced protein with tetratricopeptide repeats 2 |
| IFN | Interferon |
| IκB | Inhibitor of nuclear factor kappa B |
| IKK | IκB kinase |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IRF | Interferon regulatory factor |
| JAK | Janus kinase |
| LPS | Lipopolysaccharide |
| L-Arg | L-arginine |
| LIF | Leukemia inhibitory factor |
| MafB | V-maf musculoaponeurotic fibrosarcoma oncogene homolog B |
| MHC | Major histocompatibility complex |
| MMP | Matrix metallopeptidase |
| Mnk | MAP kinase-interacting kinase |
| MYD88 | Myeloid differentiation primary response 88 |
| NK-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NRF2 | The nuclear factor erythroid 2-related factor 2 |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| PARP | poly-ADP-ribose polymerase |
| PDTC | Pyrrolidine dithiocarbamate |
| PD-L1 | Programmed cell death 1 |
| PGE2 | Prostaglandin E2 |
| PPAR | Peroxisome proliferator-activated receptors |
| RHD | Rel homology domain |
| ROS | Reactive oxygen species |
| SDF-1 | Stromal cell-derived factor-1 |
| STAT | Signal transducer and activator of transcription |
| TAM | Tumor associated macrophage |
| TF | Transcription factor |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| TRAF | TNF receptor associated factor |
| TRAM | TRIF-related adaptor molecule |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| VEGF | Vascular endothelial growth factor |
| Ym1 | Chitinase 3-like 3 |
References
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Macciò, A.; Gramignano, G.; Cherchi, M.C.; Tanca, L.; Melis, L.; Madeddu, C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, C.W.; Colon, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1- profile in a TLR4-dependent manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Martin, E.M.; Mellows, T.W.P.; Clarke, J.; Ganesan, A.-P.; Wood, O.; Cazaly, A.; Seumois, G.; Chee, S.J.; Alzetani, A.; King, E.V.; et al. M1hot tumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. J. Immunother. Cancer 2020, 8, e000778. [Google Scholar] [CrossRef]
- Petrillo, M.; Zannoni, G.F.; Martinelli, E.; Pedone Anchora, L.; Ferrandina, G.; Tropeano, G.; Fagotti, A.; Scambia, G. Polarisation of Tumor-Associated Macrophages toward M2 Phenotype Correlates with Poor Response to Chemoradia-tion and Reduced Survival in Patients with Locally Advanced Cervical Cancer. PLoS ONE 2015, 10, e0136654. [Google Scholar] [CrossRef] [PubMed]
- Valeta-Magara, A.; Gadi, A.; Volta, V.; Walters, B.; Arju, R.; Giashuddin, S.; Zhong, H.; Schneider, R.J. Inflammatory Breast Cancer Promotes Development of M2 Tumor-Associated Macrophages and Cancer Mesenchymal Cells through a Complex Chemokine Network. Cancer Res. 2019, 79, 3360–3371. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, J.; Chen, W.; Chen, W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Zin, A.A.M.; Ang, K.C.; Ch’Ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Müller, E.; Christopoulos, P.F.; Halder, S.; Lunde, A.; Beraki, K.; Speth, M.; Øynebråten, I.; Corthay, A. Toll-Like Receptor Ligands and Interferon-γ Synergize for Induction of Antitumor M1 Macrophages. Front. Immunol. 2017, 8, 1383. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Levy, D.E.; Kessler, D.S.; Pine, R.; Reich, N.; Darnell, J.E. Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988, 2, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Schiarea, S.; Liguori, M.; Fabbri, M.; Pesce, S.; Zammataro, L.; Pasqualini, F.; Nebuloni, M.; Chiabrando, C.; Mantovani, A.; et al. Tumor-Conditioned Macrophages Secrete Migration-Stimulating Factor: A New Marker for M2-Polarization, Influencing Tumor Cell Motility. J. Immunol. 2010, 185, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Salinas, R.; Serafin-Lopez, J.; Ramos-Payan, R.; Mendez-Aragon, P.; Hernandez-Pando, R.; Van Soolingen, D.; Flores-Romo, L.; Estrada-Parra, S.; Estrada-Garcia, I. Differential pattern of cytokine expression by macrophages infected in vitro with different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 2005, 140, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, B.; Sara, A.; Ay, C.; Leung, W.Y.; Zhang, Y.; Dong, Y.; Liang, Q.; Zhang, X.; Weidner, P.; et al. Docking protein-1 promotes inflammatory macrophage signaling in gastric cancer. OncoImmunology 2019, 8, e1649961. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Ham, S.; Lima, L.G.; Lek, E.; Möller, A. The Impact of the Cancer Microenvironment on Macrophage Phenotypes. Front. Immunol. 2020, 11, 1308. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Huang, M.; Liu, T.; Wang, Y.; Xu, H.; Duan, H.; Ma, P.; Zhang, L.; Zamvil, S.S.; et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 2018, 9, 559. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010, 20, 701–712. [Google Scholar] [CrossRef]
- Li, H.; Jiang, T.; Li, M.-Q.; Zheng, X.-L.; Zhao, G.-J. Transcriptional Regulation of Macrophages Polarization by MicroRNAs. Front. Immunol. 2018, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFκB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nat. Cell Biol. 2008, 453, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Frede, S.; Stockmann, C.; Freitag, P.; Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κB. Biochem. J. 2006, 396, 517–527. [Google Scholar] [CrossRef]
- Hwang, D.; Jang, B.C.; Yu, G.; Boudreau, M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide. Biochem. Pharmacol. 1997, 54, 87–96. [Google Scholar] [CrossRef]
- Kim, J.-B.; Han, A.-R.; Park, E.-Y.; Kim, J.-Y.; Cho, W.; Lee, J.; Seo, E.-K.; Lee, K.-T. Inhibition of LPS-Induced iNOS, COX-2 and Cytokines Expression by Poncirin through the NF-.KAPPA.B Inactivation in RAW 264.7 Macrophage Cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef]
- Liu, C.-P.; Zhang, X.; Tan, Q.-L.; Xu, W.-X.; Zhou, C.-Y.; Luo, M.; Li, X.; Huang, R.-Y.; Zeng, X. NF-κB pathways are involved in M1 polarization of RAW 264.7 macrophage by polyporus polysaccharide in the tumor microenvironment. PLoS ONE 2017, 12, e0188317. [Google Scholar] [CrossRef]
- Shan, S.; Fang, B.; Zhang, Y.; Wang, C.; Zhou, J.; Niu, C.; Gao, Y.; Zhao, D.; He, J.; Wang, J.; et al. Mechanical stretch promotes tumoricidal M1 polarization via the FAK/NF-κB signaling pathway. FASEB J. 2019, 33, 13254–13266. [Google Scholar] [CrossRef] [PubMed]
- Wager, C.M.L.; Hole, C.R.; Campuzano, A.; Castro-Lopez, N.; Cai, H.; Van Dyke, M.C.C.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against Cryptococcus neoformans. PLoS Pathog. 2018, 14, e1007358. [Google Scholar] [CrossRef]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Van Der Vorst, E.P.; Theodorou, K.; Wu, Y.; Hoeksema, M.A.; Goossens, P.; Bursill, C.A.; Aliyev, T.; Huitema, L.F.; Tas, S.W.; Wolfs, I.M.; et al. High-Density Lipoproteins Exert Pro-inflammatory Effects on Macrophages via Passive Cholesterol Depletion and PKC-NF-κB/STAT1-IRF1 Signaling. Cell Metab. 2017, 25, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Ribechini, E.; Hutchinson, J.A.; Hergovits, S.; Heuer, M.; Lucas, J.; Schleicher, U.; Garrote, A.-L.J.; Potter, S.J.; Riquelme, P.; Brackmann, H.; et al. Novel GM-CSF signals via IFN-γR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Adv. 2017, 1, 947–960. [Google Scholar] [CrossRef]
- Xie, C.; Liu, C.; Wu, B.; Lin, Y.; Ma, T.; Xiong, H.; Wang, Q.; Changli, X.; Ma, C.; Tu, Z. Effects of IRF1 and IFN-β interaction on the M1 polarization of macrophages and its antitumor function. Int. J. Mol. Med. 2016, 38, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.; Matikainen, S.; Julkunen, I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J. Immunol. 1997, 159, 794. [Google Scholar]
- Cuesta, N.; Salkowski, C.A.; Thomas, K.E.; Vogel, S.N. Regulation of Lipopolysaccharide Sensitivity by IFN Regulatory Factor-2. J. Immunol. 2003, 170, 5739–5747. [Google Scholar] [CrossRef]
- Han, H.-S.; Shin, J.-S.; Lee, S.-B.; Park, J.C.; Lee, K.-T. Cirsimarin, a flavone glucoside from the aerial part of Cirsium japonicum var. ussuriense (Regel) Kitam. ex Ohwi, suppresses the JAK/STAT and IRF-3 signaling pathway in LPS-stimulated RAW 264.7 macrophages. Chem. Interact. 2018, 293, 38–47. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef]
- Li, C.; Ying, W.; Huang, Z.; Brehm, T.; Morin, A.; Vella, A.T.; Zhou, B. IRF6 Regulates Alternative Activation by Suppressing PPARγ in Male Murine Macrophages. Endocrinology 2017, 158, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Goubau, D.; Grandvaux, N.; Mesplede, T.; Julkunen, I.; Nardin, A.; Salcedo, M.; Hiscott, J.; Romieu-Mourez, R. Involvement of TBK1 and IKKε in lipopolysaccharide-induced activation of the interferon response in primary human macrophages. Eur. J. Immunol. 2007, 37, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Vera, M.; Xiong, Z.; Zhao, Y.; Zhao, J.; Donahoe, M.P.; Barge, S.; Horne, W.T.; Kolls, J.K.; McVerry, B.J.; Birukova, A.; et al. Full Spectrum of LPS Activation in Alveolar Macrophages of Healthy Volunteers by Whole Transcriptomic Profiling. PLoS ONE 2016, 11, e0159329. [Google Scholar] [CrossRef]
- Sol, V.V.-D.; Punzón, C.; Fresno, M. IFN-γ-Induced TNF-α Expression Is Regulated by Interferon Regulatory Factors 1 and 8 in Mouse Macrophages. J. Immunol. 2008, 181, 4461–4470. [Google Scholar] [CrossRef]
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 induces macrophages to M2-like phenotype via NF-κB. Cancer Manag. Res. 2018, 10, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Saccani, A.; Bottazzi, B.; Polentarutti, N.; Vecchi, A.; Van Damme, J.; Mantovani, A. Autocrine Production of IL-10 Mediates Defective IL-12 Production and NF-κB Activation in Tumor-Associated Macrophages. J. Immunol. 2000, 164, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, N.M.; Peterson, J.M.; Talbert, E.E.; Ladner, K.J.; Rajasekera, P.V.; Schmidt, C.R.; Dillhoff, M.E.; Swanson, B.J.; Haverick, E.; Kladney, R.D.; et al. NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J. Clin. Investig. 2017, 127, 3796–3809. [Google Scholar] [CrossRef]
- Giurisato, E.; Xu, Q.; Lonardi, S.; Telfer, B.; Russo, I.; Pearson, A.; Finegan, K.G.; Wang, W.; Wang, J.; Gray, N.S.; et al. Myeloid ERK5 deficiency suppresses tumor growth by blocking protumor macrophage polarization via STAT3 inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, E2801–E2810. [Google Scholar] [CrossRef]
- Yin, Z.; Ma, T.; Lin, Y.; Lu, X.; Zhang, C.; Chen, S.; Jian, Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 9419–9432. [Google Scholar] [CrossRef] [PubMed]
- Rahal, O.M.; Wolfe, A.R.; Mandal, P.K.; Larson, R.; Tin, S.; Jimenez, C.; Zhang, D.; Horton, J.; Reuben, J.M.; McMurray, J.S.; et al. Blocking Interleukin (IL)4- and IL13-Mediated Phosphorylation of STAT6 (Tyr641) Decreases M2 Polarization of Macrophages and Protects Against Macrophage-Mediated Radioresistance of Inflammatory Breast Cancer. Int. J. Radiat. Oncol. 2018, 100, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.J.; Farrar, M.A.; Schwertfeger, K.L. STAT5 deletion in macrophages alters ductal elongation and branching during mammary gland development. Dev. Biol. 2017, 428, 232–244. [Google Scholar] [CrossRef]
- Honma, K.; Udono, H.; Kohno, T.; Yamamoto, K.; Ogawa, A.; Takemori, T.; Kumatori, A.; Suzuki, S.; Matsuyama, T.; Yui, K. Interferon regulatory factor 4 negatively regulates the production of proinflammatory cytokines by macrophages in response to LPS. Proc. Natl. Acad. Sci. USA 2005, 102, 16001–16006. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ng, D.S.W.; Mah, W.-C.; Almeida, F.F.; Rahmat, S.A.; Rao, V.K.; Leow, S.C.; Laudisi, F.; Peh, M.T.; Goh, A.M.; et al. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ. 2014, 22, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, Y.; He, F.; Lv, S.; Zhang, X.; Fei, C. Abundance of CD163-Positive Tumor-Associated Macrophages in the Early Gastric Cancer Predicts the Recurrence after Curative Resection. Dig. Dis. 2020, 38, 458–465. [Google Scholar] [CrossRef]
- Curtis, L.T.; Leonard, F.; Godin, B.; Friebos, H.B. Modeling of the tumor microenvironment to highlight nonlinear interactions between chemotherapeutic response and macrophage polarization state. J. Clin. Oncol. 2018, 36, e24120. [Google Scholar] [CrossRef]
- Cao, L.; Che, X.; Qiu, X.; Li, Z.; Yang, B.; Wang, S.; Hou, K.; Fan, Y.; Qu, X.; Liu, Y. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag. Res. 2019, 11, 6125–6138. [Google Scholar] [CrossRef] [PubMed]
- Salmi, S.; Siiskonen, H.; Sironen, R.; Tyynelä-Korhonen, K.; Hirschovits-Gerz, B.; Valkonen, M.; Auvinen, P.; Pasonen-Seppänen, S. The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. 2019, 29, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Dou, R.; Xiong, B. Elevated CD163+/CD68+ Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int. J. Biol. Sci. 2019, 15, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, M.; Masieri, L.; Raspollini, M.R.; Minervini, A.; Mari, A.; Comito, G.; Giannoni, E.; Carini, M.; Chiarugi, P.; Serni, S. The Role of M1 and M2 Macrophages in Prostate Cancer in relation to Extracapsular Tumor Extension and Biochemical Recurrence after Radical Prostatectomy. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef]
- McMillan, D.C.; Elahi, M.M.; Sattar, N.; Angerson, W.J.; Johnstone, J.; McArdle, C.S. Measurement of the Systemic Inflammatory Response Predicts Cancer-Specific and Non-Cancer Survival in Patients with Cancer. Nutr. Cancer 2001, 41, 64–69. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Bottazzi, B.; Walter, S.; Govoni, D.; Colotta, F.; Mantovani, A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 1992, 148, 1280–1285. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1–M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A. NF-κB, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002, 2, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Mancino, A.; Lawrence, T. Nuclear Factor- B and Tumor-Associated Macrophages. Clin. Cancer Res. 2010, 16, 784–789. [Google Scholar] [CrossRef]
- Genard, G.; Wera, A.-C.; Huart, C.; Le Calve, B.; Penninckx, S.; Fattaccioli, A.; Tabarrant, T.; Demazy, C.; Ninane, N.; Heuskin, A.-C.; et al. Proton irradiation orchestrates macrophage reprogramming through NFκB signaling. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Lin, E.Y.; Li, J.-F.; Gnatovskiy, L.; Deng, Y.; Zhu, L.; Grzesik, D.A.; Qian, H.; Xue, X.-N.; Pollard, J.W. Macrophages Regulate the Angiogenic Switch in a Mouse Model of Breast Cancer. Cancer Res. 2006, 66, 11238–11246. [Google Scholar] [CrossRef]
- Lawrence, T. Macrophages and NF-κB in Cancer. Curr. Top. Microbiol. Immunol. 2010, 349, 171–184. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Mandinova, A.; Aaronson, S.A.; Lee, A.M.S.W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 2016, 16, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Purbey, P.K.; Scumpia, P.O.; Kim, P.J.; Tong, A.-J.; Iwamoto, K.S.; McBride, W.H.; Smale, S.T. Defined Sensing Mechanisms and Signaling Pathways Contribute to the Global Inflammatory Gene Expression Output Elicited by Ionizing Radiation. Immunology 2017, 47, 421–434.e3. [Google Scholar] [CrossRef]
- Wang, P.; Guo, F.; Han, L.; Wang, X.; Li, J.; Guo, Y.; Lu, Y. X-ray-Induced Changes in the Expression of Inflammation-Related Genes in Human Peripheral Blood. Int. J. Mol. Sci. 2014, 15, 19516–19534. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.-C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock. 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; Solis, M.; Nardin, A.; Goubau, D.; Baron-Bodo, V.; Lin, R.; Massie, B.; Salcedo, M.; Hiscott, J. Distinct Roles for IFN Regulatory Factor (IRF)-3 and IRF-7 in the Activation of Antitumor Properties of Human Macrophages. Cancer Res. 2006, 66, 10576–10585. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, C.; Li, C.; Jiao, X.; Griffin, B.B.; Dongol, S.; Wu, H.; Zhang, C.; Cao, W.; Dong, R.; et al. Upregulated MELK Leads to Doxorubicin Chemoresistance and M2 Macrophage Polarization via the miR-34a/JAK2/STAT3 Pathway in Uterine Leiomyosarcoma. Front. Oncol. 2020, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Takaishi, K.; Komohara, Y.; Tashiro, H.; Ohtake, H.; Nakagawa, T.; Katabuchi, H.; Takeya, M. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010, 101, 2128–2136. [Google Scholar] [CrossRef]
- Vasquez-Dunddel, D.; Pan, F.; Zeng, Q.; Gorbounov, M.; Albesiano, E.; Fu, J.; Blosser, R.L.; Tam, A.J.; Bruno, T.; Zhang, H.; et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013, 123, 1580–1589. [Google Scholar] [CrossRef]
- Buhtoiarov, I.N.; Sondel, P.M.; Wigginton, J.M.; Buhtoiarova, T.N.; Yanke, E.M.; Mahvi, D.A.; Rakhmilevich, A.L. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 2010, 132, 226–239. [Google Scholar] [CrossRef]
- Javeed, A.; Ashraf, M.; Riaz, A.; Ghafoor, A.; Afzal, S.; Mukhtar, M.M. Paclitaxel and immune system. Eur. J. Pharm. Sci. 2009, 38, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, K.; Szczepanik, M.; Ptak, M.; Zemelka, M.; Ptak, W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol. Rep. 2009, 61, 550–557. [Google Scholar] [CrossRef]
- Chauhan, P.; Sodhi, A.; Shrivastava, A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology 2009, 214, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, E.M.; Heusinkveld, M.; Tummers, B.; Vogelpoel, L.T.C.; Goedemans, R.; Jha, V.; Nortier, J.W.R.; Welters, M.J.P.; Kroep, J.R.; Van Der Burg, S.H. Chemotherapy Alters Monocyte Differentiation to Favor Generation of Cancer-Supporting M2 Macrophages in the Tumor Microenvironment. Cancer Res. 2013, 73, 2480–2492. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Chiba, S.; Yoshiyama, H.; Masutomi, K.; Kinoshita, I.; Dosaka-Akita, H.; Yagita, H.; Takaoka, A.; Tahara, H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12425–12430. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Van Ginderachter, J.A. Unsuspected allies: Chemotherapy teams up with immunity to fight cancer. Eur. J. Immunol. 2013, 43, 2538–2542. [Google Scholar] [CrossRef][Green Version]
- Stakheyeva, M.; Riabov, V.; Mitrofanova, I.; Litviakov, N.; Choynzonov, E.; Cherdyntseva, N.; Kzhyshkowska, J. Role of the Immune Component of Tumor Microenvironment in the Efficiency of Cancer Treatment: Perspectives for the Personalized Therapy. Curr. Pharm. Des. 2017, 23, 4807–4826. [Google Scholar] [CrossRef]
- Weizman, N.; Krelin, Y.; Shabtayorbach, A.; Amit, M.; Binenbaum, Y.; Wong, R.J.; Gil, Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 2013, 33, 3812–3819. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, S.; Hu, Y.; Feng, Y.; Li, M.; Bian, Z.; Zhang, J.; Qin, Y.; Qi, X.; Zhou, L.; et al. The Immune-microenvironment Confers Chemoresistance of Colorectal Cancer through Macrophage-Derived IL6. Clin. Cancer Res. 2017, 23, 7375–7387. [Google Scholar] [CrossRef]
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Qian, B.-Z.; Rowan, C.; Muthana, M.; Keklikoglou, I.; Olson, O.C.; Tazzyman, S.; Danson, S.; Addison, C.L.; Clemons, M.; et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015, 75, 3479–3491. [Google Scholar] [CrossRef]
- Anttila, J.V.; Shubin, M.; Cairns, J.; Borse, F.; Guo, Q.; Mononen, T.; Vázquez-García, I.; Pulkkinen, O.; Mustonen, V. Contrasting the impact of cytotoxic and cytostatic drug therapies on tumour progression. PLoS Comput. Biol. 2019, 15, e1007493. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Madani, J.; Puértolas, T.; Lvarez, M.; Hernández, A.; Pazo-Cid, R.; Artal, Á.; Torres, A.A. Liposomal Doxorubicin in the Treatment of Breast Cancer Patients: A Review. J. Drug Deliv. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Gallab, M.I.; Naddaf, L.A.; Kanan, M.R. The management of non-invasive bladder tumours with Doxorubicin in-travesical instillation after transurethral resection. Sultan Qaboos Univ. Med. J. 2009, 9, 53–58. [Google Scholar] [PubMed]
- James, N.; Coker, R.; Tomlinson, D.; Harris, J.; Gompels, M.; Pinching, A.; Stewart, J. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. 1994, 6, 294–296. [Google Scholar] [CrossRef]
- Lori, J.C.; Stein, T.J.; Thamm, D.H. Doxorubicin and cyclophosphamide for the treatment of canine lymphoma: A randomized, placebo-controlled study*. Veter. Comp. Oncol. 2010, 8, 188–195. [Google Scholar] [CrossRef]
- Lister, T.A.; Whitehouse, J.M.; Beard, M.E.; Brearley, R.L.; Wrigley, P.F.; Oliver, R.T.; Freeman, J.E.; Woodruff, R.K.; Malpas, J.S.; Paxton, A.M.; et al. Combination chemotherapy for acute lymphoblastic leukaemia in adults. BMJ 1978, 1, 199–203. [Google Scholar] [CrossRef][Green Version]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Baghdadi, M.; Wada, H.; Nakanishi, S.; Abe, H.; Han, N.; Putra, W.E.; Endo, D.; Watari, H.; Sakuragi, N.; Hida, Y.; et al. Chemotherapy-Induced IL34 Enhances Immunosuppression by Tumor-Associated Macrophages and Mediates Survival of Chemoresistant Lung Cancer Cells. Cancer Res. 2016, 76, 6030–6042. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Holmes, F.A.; Theriault, R.L.; Buzdar, A.U. Use of Taxol (Paclitaxel) in Breast Cancer. Oncology 1994, 51, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Chang, A.Y.; Ettinger, D.S. Taxol (paclitaxel) in the treatment of lung cancer: The Eastern Cooperative Oncology Group experience. Ann. Oncol. 1994, 5 (Suppl. S6), S45–S50. [Google Scholar]
- Ercolak, V.; Sahin, B.; Gunaldi, M.; Duman, B.B.; Afsar, C.U. Efficacy of paclitaxel in the treatment of Kaposi sarcoma. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4095–4100. [Google Scholar] [PubMed]
- Machida, H.; Moeini, A.; Ciccone, M.A.; Mostofizadeh, S.; Takiuchi, T.; Brunette, L.L.; Roman, L.D.; Matsuo, K. Efficacy of Modified Dose-dense Paclitaxel in Recurrent Cervical Cancer. Am. J. Clin. Oncol. 2018, 41, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Hidalgo, M. The Winning Formulation: The Development of Paclitaxel in Pancreatic Cancer. Clin. Cancer Res. 2013, 19, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.B. Taxol (paclitaxel): Mechanisms of action. Ann. Oncol. 1994, 5, 3–6. [Google Scholar]
- Yule, S.M.; Price, L.; McMahon, A.D.; Pearson, A.D.J.; Boddy, A.V. Cyclophosphamide Metabolism in Children with Non-Hodgkin’s Lymphoma. Clin. Cancer Res. 2004, 10, 455–460. [Google Scholar] [CrossRef][Green Version]
- Fairley, G.H.; Patterson, M.J.L.; Scott, R.B. Chemotherapy of Hodgkin’s Disease with Cyclophosphamide, Vinblastine, and Procarbazine. BMJ 1966, 2, 75–78. [Google Scholar] [CrossRef][Green Version]
- Scarisbrick, J.; Child, F.; Clift, A.; Sabroe, R.; Whittaker, S.; Spittle, M.; Russell-Jones, R. A trial of fludarabine and cyclophosphamide combination chemotherapy in the treatment of advanced refractory primary cutaneous T-cell lymphoma. Br. J. Dermatol. 2001, 144, 1010–1015. [Google Scholar] [CrossRef]
- Ito, S.; Oyake, T.; Murai, K.; Ishida, Y. Successful Use of Cyclophosphamide as an Add-On Therapy for Multiple Myeloma Patients with Acquired Resistance to Bortezomib or Lenalidomide. Case Rep. Hematol. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Song, H.-M.; Ni, L. Cyclophosphamide for the treatment of acute lymphoblastic leukemia. Med. 2019, 98, e14293. [Google Scholar] [CrossRef]
- Mustafa, M.M.; Jamshed, A.; Khafaga, Y.; Mourad, W.A.; Al-Mesfer, S.; Kofide, A.; El-Husseiny, G.; Gray, A. Adjuvant Chemotherapy with Vincristine, Doxorubicin, and Cyclophosphamide in the Treatment of Postenucleation High Risk Retinoblastoma. J. Pediatr. Hematol. 1999, 21, 364–369. [Google Scholar] [CrossRef]
- Ashraf, K.; Shaikh, F.; Gibson, P.; Baruchel, S.; Irwin, M.S. Treatment with topotecan plus cyclophosphamide in children with first relapse of neuroblastoma. Pediatr. Blood Cancer 2013, 60, 1636–1641. [Google Scholar] [CrossRef]
- Handolias, D.; Quinn, M.; Foo, S.; Mileshkin, L.; Grant, P.; Dutu, G.; Rischin, D. Oral cyclophosphamide in recurrent ovarian cancer. Asia Pac. J. Clin. Oncol. 2013, 12, e154–e160. [Google Scholar] [CrossRef]
- Mills, K.A.; Chess-Williams, R.; McDermott, C. Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde’s contribution to urothelial dysfunction in vitro. Arch. Toxicol. 2019, 93, 3291–3303. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Serkies, K.; Jassem, J. Concurrent weekly cisplatin and radiotherapy in routine management of cervical cancer: A report on patient compliance and acute toxicity. Int. J. Radiat. Oncol. 2004, 60, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ilson, D.H. Esophageal Cancer Chemotherapy: Recent Advances. Gastrointest Cancer Res. 2008, 2, 85–92. [Google Scholar]
- Sledge, G.W.; Roth, B.J. Cisplatin in the management of breast cancer. Semin. Oncol. 1989, 16, 110–115. [Google Scholar] [PubMed]
- Rodrigo, M.A.M.; Buchtelova, H.; Jimenez, A.M.J.; Adam, P.; Babula, P.; Heger, Z.; Adam, V. Transcriptomic Landscape of Cisplatin-Resistant Neuroblastoma Cells. Cells 2019, 8, 235. [Google Scholar] [CrossRef]
- Shani, J.; Wolf, W. Modalities of Cisplatin Administration to Brain Tumors. Cancer Investig. 1989, 7, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Sodhi, A.; Tarang, S. Cisplatin-treated murine peritoneal macrophages induce apoptosis in L929 cells: Role of Fas–Fas ligand and tumor necrosis factor–tumor necrosis factor receptor 1. Anti Cancer Drugs 2007, 18, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, G.; Cortese, N.; Castino, G.F.; Grizzi, F.; Gavazzi, F.; Ridolfi, C.; Capretti, G.; Mineri, R.; Todoric, J.; Zerbi, A.; et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut 2016, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared with Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S. The role of carboplatin in the treatment of small-cell lung cancer. Oncology 1998, 12, 36–43. [Google Scholar]
- Aisner, J.; Sinibaldi, V.; Eisenberger, M. Carboplatin in the treatment of squamous cell head and neck cancers. Semin. Oncol. 1992, 19, 60–65. [Google Scholar]
- Xia, Y.; Li, Y.-H.; Chen, Y.; Liu, Q.; Zhang, J.-H.; Deng, J.-Y.; Ai, T.-S.; Zhu, H.-T.; Badakhshi, H.; Zhao, K.-L. A phase II trial of concurrent chemoradiotherapy with weekly paclitaxel and carboplatin in advanced oesophageal carcinoma. Int. J. Clin. Oncol. 2018, 23, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Michener, C.M.; Peterson, G.; Kulp, B.; Webster, K.D.; Markman, M. Carboplatin plus paclitaxel in the treatment of advanced or recurrent endometrial carcinoma. J. Cancer Res. Clin. Oncol. 2005, 131, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Kimmey, G.; Bir, A.; Silbermins, D. Role of Carboplatin in the Treatment of Triple Negative Early- Stage Breast Cancer. Rev. Recent Clin. Trials 2015, 10, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, A.M.; Rocha, L.S.D.S.; Gimenez, R.D.; De Barros, L.A.B.; Fukushima, J.T.; Da Silva, S.C.S.; Miranda, V.D.C.; Caires, I.Q.D.S.; De Freitas, D.; Filho, E.A.; et al. Carboplatin-based chemoradiotherapy in advanced cervical cancer: An alternative to cisplatin-based regimen? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 161–165. [Google Scholar] [CrossRef]
- Hah, S.S.; Stivers, K.M.; White, R.W.D.V.; Henderson, P.T. Kinetics of Carboplatin−DNA Binding in Genomic DNA and Bladder Cancer Cells As Determined by Accelerator Mass Spectrometry. Chem. Res. Toxicol. 2006, 19, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Vogl, S.E.; Moukhtar, M.; Kaplan, B.H. Chemotherapy for advanced cervical cancer with methotrexate, bleomycin, and cis-dichlorodiammineplatinum(II). Cancer Treat. Rep. 1979, 63, 1005–1006. [Google Scholar]
- Yang, V.; Gouveia, M.J.; Santos, J.; Koksch, B.; Amorim, I.; Gärtner, F.; Vale, N. Breast cancer: Insights in disease and influence of drug methotrexate. RSC Med. Chem. 2020, 11, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Neijstrom, E.S.; Capizzi, R.L.; Rudnick, S.A.; Kirsch, M.; Delaney, D.; Kahn, L.; Lipper, S.; Carney, C. High-dose methotrexate in small cell lung cancer: Lack of efficacy in preventing CNS relapse. Cancer 1983, 51, 1056–1061. [Google Scholar] [CrossRef]
- Woods, R.L.; Fox, R.M.; Tattersall, M.H. Methotrexate treatment of squamous-cell head and neck cancers: Dose-response evaluation. BMJ 1981, 282, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Sakura, T.; for the Japan Adult Leukemia Study Group (JALSG); Hayakawa, F.; Sugiura, I.; Murayama, T.; Imai, K.; Usui, N.; Fujisawa, S.; Yamauchi, T.; Yujiri, T.; et al. High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: A phase III study by JALSG. Leukemia 2017, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.T.R.; Zhang, Z.; McCourt, L.; Dwyer, M.; Benkovic, S.J.; Hammes, G.G. Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics. Proc. Natl. Acad. Sci. USA 2002, 99, 13481–13486. [Google Scholar] [CrossRef]
- Municio, C.; Palacios, B.S.; Estrada-Capetillo, L.; Benguria, A.; Dopazo, A.; García-Lorenzo, E.; Fernández-Arroyo, S.; Joven, J.; Miranda-Carús, M.E.; González-Álvaro, I.; et al. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann. Rheum. Dis. 2016, 75, 2157–2165. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, S.E.; Davis, C.C.; Byers, K.F. Olaparib: A Novel Therapy for Metastatic Breast Cancer in Patients With a BRCA1/2 Mutation. J. Adv. Pract. Oncol. 2019, 10, 167–174. [Google Scholar]
- Dziadkowiec, K.N.; Gąsiorowska, E.; Nowak-Markwitz, E.; Jankowska, A. PARP inhibitors: Review of mechanisms of action and BRCA1/2 mutation targeting. Menopausal Rev. 2016, 15, 215–219. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Paoletti, A.; Leteur, C.; Mirjolet, C.; Martins, I.; Voisin, L.; Law, F.; Dakhli, H.; Mintet, E.; et al. NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ. 2017, 24, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.R.; Morgan, W.F. Gene expression profiling after irradiation: Clues to understanding acute and persistent responses? Cancer Metastasis Rev. 2004, 23, 259–268. [Google Scholar] [CrossRef]
- Bansal, A.; Neuhaus, R.; Izquierdo-Alvarez, E.; Vorholt, D.; Feldkötter, H.; Nolte, H.; Lohneis, P.; Büttner, R.; Krüger, M.; Pallasch, C.P.; et al. ATM-mediated DNA damage response in macrophages primes phagocytosis and immune checkpoint regulation. bioRxiv 2020, arXiv:2020.03.14.987438, 987438. [Google Scholar] [CrossRef]
- Pinto, A.T.; Pinto, M.L.; Cardoso, A.P.; Monteiro, C.; Maia, A.F.; Castro, P.; Figueira, R.C.S.; Monteiro, A.; Marques, M.; Mareel, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Pal, S.K. The dark side of Toll-like receptor signaling. OncoImmunology 2014, 3, e27894. [Google Scholar] [CrossRef]
- Chen, J.; Tian, X.; Mei, Z.; Wang, Y.; Yao, Y.; Zhang, S.; Li, X.; Wang, H.; Zhang, J.; Xie, C. The effect of the TLR9 ligand CpG-oligodeoxynucleotide on the protective immune response to radiation-induced lung fibrosis in mice. Mol. Immunol. 2016, 80, 33–40. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Ly, N.N.G.; Nguy, S.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Daley, D.; Barilla, R.; et al. Radiation Therapy Induces Macrophages to Suppress T-Cell Responses against Pancreatic Tumors in Mice. Gastroenterology 2016, 150, 1659–1672.e5. [Google Scholar] [CrossRef]
- Ayoub, M.; Shinde-Jadhav, S.; Mansure, J.J.; Alvarez, F.; Connell, T.; Seuntjens, J.; Piccirillo, C.A.; Kassouf, W. The immune mediated role of extracellular HMGB1 in a heterotopic model of bladder cancer radioresistance. Sci. Rep. 2019, 9, 6348. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, M.R.; Cottam, B.; Savage, T.; Nguyen, C.; Newell, P.; Gough, M.J. Expression of NF-κB p50 in Tumor Stroma Limits the Control of Tumors by Radiation Therapy. PLoS ONE 2012, 7, e39295. [Google Scholar] [CrossRef] [PubMed]
- Rovere-Querini, P.; Castiglioni, A. Adjuvant role for cell death during chemo- and radiotherapy of cancer? Expert Rev. Clin. Immunol. 2008, 4, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.; Todryk, S.; Hardwick, N.; Ford, M.; Jacobson, M.; Vile, R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998, 4, 581–587. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Ramapriyan, R.; Younes, A.I.; Caetano, M.S.; Menon, H.; Comeaux, N.I.; Cushman, T.R.; Schoenhals, J.E.; Cadena, A.P.; Reilly, T.P.; et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer 2020, 8, e000537. [Google Scholar] [CrossRef]
- Gong, D.; Shi, W.; Yi, S.-J.; Chen, H.; Groffen, J.; Heisterkamp, N. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012, 13, 31. [Google Scholar] [CrossRef]
- Moreira, D.; Sampath, S.; Won, H.; White, S.V.; Su, Y.-L.; Alcantara, M.; Wang, C.; Lee, P.P.; Maghami, E.; Massarelli, E.; et al. Myeloid cell–targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell–mediated immunity. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Kang, T.H.; Mao, C.-P.; Kim, Y.S.; Kim, T.W.; Yang, A.; Lam, B.; Tseng, S.-H.; Farmer, E.; Park, Y.-M.; Hung, C.-F. TLR9 acts as a sensor for tumor-released DNA to modulate anti-tumor immunity after chemotherapy. J. Immunother. Cancer 2019, 7, 260. [Google Scholar] [CrossRef]
- Oweida, A.J.; Mueller, A.C.; Piper, M.; Milner, D.; Van Court, B.; Bhatia, S.; Phan, A.; Bickett, T.; Jordan, K.; Proia, T.; et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol. Immunother. 2020, 1–12. [Google Scholar] [CrossRef]
- Farhood, B.; Khodamoradi, E.; Hoseini-Ghahfarokhi, M.; Motevaseli, E.; Mirtavoos-Mahyari, H.; Musa, A.E.; Najafi, M. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol. Res. 2020, 155, 104745. [Google Scholar] [CrossRef]
- Pinto, A.T.; Pinto, M.L.; Velho, S.; Pinto, M.T.; Cardoso, A.P.; Figueira, R.; Monteiro, A.; Marques, M.; Seruca, R.; Barbosa, M.A.; et al. Intricate Macrophage-Colorectal Cancer Cell Communication in Response to Radiation. PLoS ONE 2016, 11, e0160891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Yu, C.-F.; Hong, J.-H.; Tsai, C.-S.; Chiang, C.-S. Radiation Therapy-Induced Tumor Invasiveness Is Associated with SDF-1-Regulated Macrophage Mobilization and Vasculogenesis. PLoS ONE 2013, 8, e69182. [Google Scholar] [CrossRef]
- Kalbasi, A.; Komar, C.; Tooker, G.M.; Liu, M.; Lee, J.W.; Gladney, W.L.; Ben-Josef, E.; Beatty, G.L. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2017, 23, 137–148. [Google Scholar] [CrossRef]
- Shiao, S.L.; Ruffell, B.; DeNardo, D.G.; Faddegon, B.A.; Park, C.C.; Coussens, L.M. TH2-Polarized CD4+ T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol. Res. 2015, 3, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Stary, V.; Wolf, B.; Unterleuthner, D.; List, J.; Talic, M.; Längle, J.; Beer, A.; Strobl, J.; Stary, G.; Dolznig, H.; et al. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J. Immunother. Cancer 2020, 8, e000667. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- De Sousa, J.R.; Vasconcelos, P.F.D.C.; Quaresma, J.A.S. Functional aspects, phenotypic heterogeneity, and tissue immune response of macrophages in infectious diseases. Infect. Drug Resist. 2019, 12, 2589–2611. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Klug, F.; Nadella, V.; Mazumdar, V.; Schmitz-Winnenthal, H.; Umansky, L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: Lesson from insulinoma. Carcinogenesis 2016, 37, 301–313. [Google Scholar] [CrossRef]
- Nowosielska, E.M.; Cheda, A.; Wrembel-Wargocka, J.; Janiak, M.K. Effect of Low Doses of Low-Let Radiation on the Innate Anti-Tumor Reactions in Radioresistant and Radiosensitive Mice. Dose Response 2012, 10, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.J.; Rundle, J.K.; Lorimore, S.A.; Wright, E.G. Indirect Macrophage Responses to Ionizing Radiation: Implications for Genotype-Dependent Bystander Signaling. Cancer Res. 2008, 68, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Lödermann, B.; Wunderlich, R.; Frey, S.; Schorn, C.; Stangl, S.; Rödel, F.; Keilholz, L.; Fietkau, R.; Gaipl, U.S.; Frey, B. Low dose ionising radiation leads to a NF-κB dependent decreased secretion of active IL-1β by activated macrophages with a discontinuous dose-dependency. Int. J. Radiat. Biol. 2012, 88, 727–734. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.-W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKβ Links Inflammation and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, J.; Kocyk, M.; Nauman, P.; Gabrusiewicz, K.; Sielska, M.; Przanowski, P.; Maleszewska, M.; Rajan, W.D.; Pszczolkowska, D.; Tykocki, T.; et al. Down-regulation of IKKβ expression in glioma-infiltrating microglia/macrophages is associated with defective inflammatory/immune gene responses in glioblastoma. Oncotarget 2015, 6, 33077–33090. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Rimoldi, M.; Raes, G.; Brys, L.; Ghezzi, P.; Di Liberto, D.; Dieli, F.; Ghisletti, S.; Natoli, G.; De Baetselier, P.; et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor B. Proc. Natl. Acad. Sci. USA 2009, 106, 14978–14983. [Google Scholar] [CrossRef]
- Saccani, A.; Schioppa, T.; Porta, C.; Biswas, S.K.; Nebuloni, M.; Vago, L.; Bottazzi, B.; Colombo, M.P.; Mantovani, A.; Sica, A. p50 Nuclear Factor-κB Overexpression in Tumor-Associated Macrophages Inhibits M1 Inflammatory Responses and Antitumor Resistance. Cancer Res. 2006, 66, 11432–11440. [Google Scholar] [CrossRef]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; on behalf of the Australian Ovarian Cancer Study Group; Kim, M.K.; Noonan, A.M.; Sagher, E.; Kohlhammer, H.; Wright, G.L.; Lyle, L.T.; Steeg, P.S.; Anver, M.R.; et al. A dual role for Caspase8 and NF-κB interactions in regulating apoptosis and necroptosis of ovarian cancer, with correlation to patient survival. Cell Death Discov. 2015, 1, 15053. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Mandal, R.; Becker, S.; Strebhardt, K. The role of caspase-8 in the tumor microenvironment of ovarian cancer. Cancer Metastasis Rev. 2021, 40, 303–318. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Fiore, A.; Ugel, S.; De Sanctis, F.; Sandri, S.; Fracasso, G.; Trovato, R.; Sartoris, S.; Solito, S.; Mandruzzato, S.; Vascotto, F.; et al. Induction of immunosuppressive functions and NF-κB by FLIP in monocytes. Nat. Commun. 2018, 9, 5193. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, F.; Chen, Q.; Kang, A.; Lu, M.; Liu, W.; Zang, X.; Wang, G.; Zhang, J. Chronic inflammation up-regulates P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice. Sci. Rep. 2015, 5, 13558. [Google Scholar] [CrossRef]
- Martincuks, A.; Andryka, K.; Küster, A.; de Leur, H.S.-V.; Komorowski, M.; Müller-Newen, G. Nuclear translocation of STAT3 and NF-κB are independent of each other but NF-κB supports expression and activation of STAT3. Cell. Signal. 2017, 32, 36–47. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, J.; Zhang, B.; Liang, G.; Chen, X.; Yao, F.; Xu, X.; Wu, H.; He, Q.; Ding, L.; et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018, 133, 121–131. [Google Scholar] [CrossRef]
- Lee, E.-J.; Lee, S.J.; Kim, J.-H.; Kim, K.-J.; Yang, S.-H.; Jeong, K.-Y.; Seong, J. Radiation Inhibits Interleukin-12 Produc-tion via Inhibition of C-Rel through the Interleukin-6/ Signal Transducer and Activator of Transcription 3 Signaling Pathway in Dendritic Cells. PLoS ONE 2016, 11, e0146463. [Google Scholar]
- Xiao, X.; Luo, H.; Vanek, K.N.; LaRue, A.C.; Schulte, B.A.; Wang, G.Y. Catalase Inhibits Ionizing Radiation-Induced Apoptosis in Hematopoietic Stem and Progenitor Cells. Stem Cells Dev. 2015, 24, 1342–1351. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Tamura, T.; Taniguchi, T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 2008, 99, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Twum, D.Y.; Colligan, S.H.; Hoffend, N.C.; Katsuta, E.; Gomez, E.C.; Hensen, M.L.; Seshadri, M.; Nemeth, M.J.; Abrams, S.I. IFN regulatory factor–8 expression in macrophages governs an antimetastatic program. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Tarassishin, L.; Suh, H.-S.; Lee, S.C. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J. Neuroinflamm. 2011, 8, 187. [Google Scholar] [CrossRef]
- Nixon, B.; Kuo, F.; Liu, M.; Capistrano, K.; Do, M.; Franklin, R.; Wu, X.; Kansler, E.; Srivastava, R.; Purohit, T.; et al. IRF8 Governs Tumor-Associated Macrophage Control of T Cell Exhaustion. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Chen, T.-Z.; Li, G.-L.; Tan, C.-C.; Chen, Y.; Duan, S.-Q. Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway. Asian Pac. J. Trop. Med. 2016, 9, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Fu, X.; Duan, W.; Yu, P.; Zhao, Y. High density of CD68+ tumor-associated macrophages predicts a poor prognosis in gastric cancer mediated by IL-6 expression. Oncol. Lett. 2018, 15, 6217–6224. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Galietti, F.; Bollo, E.; Cappia, S.; Dondo, A.; Pregel, P.; Nicali, R.; Pozzi, E. p53 expression in cultured blood human monocytes infected with mycobacterial strains. Panminerva Med. 2001, 43, 249–255. [Google Scholar]
- Matas, D.; Milyavsky, M.; Shats, I.; Nissim, L.; Goldfinger, N.; Rotter, V. p53 is a regulator of macrophage differentiation. Cell Death Differ. 2004, 11, 458–467. [Google Scholar] [CrossRef]
- Mikhalkevich, N.; O’Carroll, I.P.; Tkavc, R.; Lund, K.; Sukumar, G.; Dalgard, C.L.; Johnson, K.R.; Li, W.; Wang, T.; Nath, A.; et al. Response of human macrophages to gamma radiation is mediated via expression of endogenous retro-viruses. PLoS Pathog. 2021, 17, e1009305. [Google Scholar] [CrossRef]
- Lowe, J.M.; Menendez, D.; Fessler, M.B. A new inflammatory role for p53 in human macrophages. Cell Cycle 2014, 13, 2983–2984. [Google Scholar] [CrossRef][Green Version]
- Murphy, S.H.; Suzuki, K.; Downes, M.; Welch, G.L.; De Jesus, P.; Miraglia, L.J.; Orth, A.P.; Chanda, S.K.; Evans, R.M.; Verma, I.M. Tumor suppressor protein (p)53, is a regulator of NF- B repression by the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 17117–17122. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Yang, Q. MiR-21 modulates the polarization of macrophages and increases the effects of M2 macrophages on promoting the chemoresistance of ovarian cancer. Life Sci. 2020, 242, 117162. [Google Scholar] [CrossRef] [PubMed]
- Saliba, D.G.; Heger, A.; Eames, H.L.; Oikonomopoulos, S.; Teixeira, A.; Blazek, K.; Androulidaki, A.; Wong, D.; Goh, F.G.; Weiss, M.; et al. IRF5:RelA Interaction Targets Inflammatory Genes in Macrophages. Cell Rep. 2014, 8, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nat. Cell Biol. 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Merched, A.J.; Williams, E.; Chan, L. Macrophage-Specific p53 Expression Plays a Crucial Role in Atherosclerosis Development and Plaque Remodeling. Arter. Thromb. Vasc. Biol. 2003, 23, 1608–1614. [Google Scholar] [CrossRef]
- Zheng, S.-J.; Lamhamedi-Cherradi, S.-E.; Wang, P.; Xu, L.; Chen, Y.H. Tumor Suppressor p53 Inhibits Autoimmune Inflammation and Macrophage Function. Diabetes 2005, 54, 1423–1428. [Google Scholar] [CrossRef]
- He, X.-Y.; Xiang, C.; Zhang, C.-X.; Xie, Y.-Y.; Chen, L.; Zhang, G.-X.; Lu, Y.; Liu, G. p53 in the Myeloid Lineage Modulates an Inflammatory Microenvironment Limiting Initiation and Invasion of Intestinal Tumors. Cell Rep. 2015, 13, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.M.; Menendez, D.; Bushel, P.R.; Shatz, M.; Kirk, E.L.; Troester, M.A.; Garantziotis, S.; Fessler, M.B.; Resnick, M.A. p53 and NF-κB Coregulate Proinflammatory Gene Responses in Human Macrophages. Cancer Res. 2014, 74, 2182–2192. [Google Scholar] [CrossRef]
- Lim, Y.-J.; Lee, J.; Choi, J.-A.; Cho, S.-N.; Son, S.-H.; Kwon, S.-J.; Son, J.-W.; Song, C.-H. M1 macrophage dependent-p53 regulates the intracellular survival of mycobacteria. Apoptosis 2020, 25, 42–55. [Google Scholar] [CrossRef]
- Rackov, G.; Hernández-Jiménez, E.; Shokri, R.; Carmona-Rodríguez, L.; Mañes, S.; Álvarez-Mon, M.; López-Collazo, E.; Martínez-A, C.; Balomenos, D. p21 mediates macrophage reprogramming through regulation of p50-p50 NF-κB and IFN-β. J. Clin. Investig. 2016, 126, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Hou, S.; Ricciardi, R.P. DNA Binding of Repressor Nuclear Factor-κB p50/p50 Depends on Phosphorylation of Ser337 by the Protein Kinase A Catalytic Subunit. J. Biol. Chem. 2005, 280, 9957–9962. [Google Scholar] [CrossRef]
- Li, Y.; Weng, Y.; Zhong, L.; Chong, H.; Chen, S.; Sun, Y.; Li, W.; Shi, Q. VEGFR3 inhibition chemosensitizes lung adenocarcinoma A549 cells in the tumor-associated macrophage microenvironment through upregulation of p53 and PTEN. Oncol. Rep. 2017, 38, 2761–2773. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, E.; Vorholt, D.; Sackey, B.; Nolte, J.L.; Blakemore, S.; Schmitz, J.; Barbarino, V.; Nickel, N.; Bachurski, D.; Lobastova, L.; et al. Loss of TP53 mediates suppression of Macrophage Effector Function via Extracellular Vesicles and PDL1 towards Resistance against Chemoimmunotherapy in B-cell malignancies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lorimore, S.A.; Rastogi, S.; Mukherjee, D.; Coates, P.J.; Wright, E.G. The Influence of p53 Functions on Radiation-Induced Inflammatory Bystander-Type Signaling in Murine Bone Marrow. Radiat. Res. 2013. [Google Scholar] [CrossRef]
- Kang, J.-H.; Woo, J.K.; Jang, Y.-S.; Oh, S.H. Radiation Potentiates Monocyte Infiltration into Tumors by Ninjurin1 Expression in Endothelial Cells. Cells 2020, 9, 1086. [Google Scholar] [CrossRef]
- Yoshino, H.; Konno, H.; Ogura, K.; Sato, Y.; Kashiwakura, I. Relationship between the Regulation of Caspase-8-Mediated Apoptosis and Radioresistance in Human THP-1-Derived Macrophages. Int. J. Mol. Sci. 2018, 19, 3154. [Google Scholar] [CrossRef]
- Tabraue, C.; Lara, P.C.; De Mirecki-Garrido, M.; De La Rosa, J.V.; López-Blanco, F.; Fernández-Pérez, L.; Boscá, L.; Castrillo, A. LXR Signaling Regulates Macrophage Survival and Inflammation in Response to Ionizing Radiation. Int. J. Radiat. Oncol. 2019, 104, 913–923. [Google Scholar] [CrossRef]
- Kamran, S.C.; Lennerz, J.K.; Margolis, C.A.; Liu, D.; Reardon, B.; Wankowicz, S.A.; Van Seventer, E.E.; Tracy, A.; Wo, J.Y.; Carter, S.L.; et al. Integrative Molecular Characterization of Resistance to Neoadjuvant Chemoradiation in Rectal Cancer. Clin. Cancer Res. 2019, 25, 5561–5571. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Morine, Y.; Ikemoto, T.; Imura, S.; Iwahashi, S.; Saito, Y.; Shimada, M. Nrf2 activation drive macrophages polarization and cancer cell epithelial-mesenchymal transition during interaction. Cell Commun. Signal. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Ji, Y.; Studzinski, G.P. Retinoblastoma Protein and CCAAT/Enhancer-Binding Protein β Are Required for 1,25-Dihydroxyvitamin D3-Induced Monocytic Differentiation of HL60 Cells. Cancer Res. 2004, 64, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Moriguchi, T.; Taguchi, K.; Takai, J.; Maher, J.M.; Suzuki, T.; Winnard, P.T., Jr.; Raman, V.; Ebina, M.; Nukiwa, T.; et al. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis 2010, 31, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Suzuki, T.; Yamamoto, M. Roles Nrf2 Plays in Myeloid Cells and Related Disorders. Oxidative Med. Cell. Longev. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Tamaishi, N.; Homma, T.; Kojima, S. Low-Dose Gamma-Ray Irradiation Induces Translocation of Nrf2 into Nuclear in Mouse Macrophage RAW264.7 Cells. J. Radiat. Res. 2010, 51, 349–353. [Google Scholar] [CrossRef]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Linghu, Y.; Sun, X.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Shu, Y.; Qin, M.; Song, Y.; Tang, Q.; Huang, Y.; Shen, P.; Lu, Y. M2 polarization of tumor-associated macrophages is dependent on integrin β 3 via peroxisome proliferator-activated receptor- γ up-regulation in breast cancer. Immunology 2020, 160, 345–356. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Huynh, H.; Chen, P.; Peña-Llopis, S.; Wan, Y. Macrophage PPARγ inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone. eLife 2016, 5, 557. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Ahn, Y.-H.; Jung, J.-H.; Lee, Y.-J.; Lee, J.-H.; Kang, J.L. Programming of macrophages by UV-irradiated apoptotic cancer cells inhibits cancer progression and lung metastasis. Cell. Mol. Immunol. 2019, 16, 851–867. [Google Scholar] [CrossRef]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.N.; Zhou, L.; Smale, S.T. C/EBPβ Regulation in Lipopolysaccharide-Stimulated Macrophages. Mol. Cell. Biol. 2003, 23, 4841–4858. [Google Scholar] [CrossRef] [PubMed]
- Screpanti, I.; Romani, L.; Musiani, P.; Modesti, A.; Fattori, E.; Lazzaro, D.; Sellitto, C.; Scarpa, S.; Bellavia, D.; Lattanzio, G. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995, 14, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, D.; Mourkioti, F.; Gambardella, A.; Kirstetter, P.; Lopez, R.G.; Rosenthal, N.; Nerlov, C. A CREB-C/EBP cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 2009, 106, 17475–17480. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. C/EBPβ LIP induces a tumor menagerie making it an oncogene. J. Mol. Med. 2014, 93, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ollivier, V.; Parry, G.C.; Cobb, R.R.; de Prost, D.; Mackman, N. Elevated Cyclic AMP Inhibits NF-κB-mediated Transcription in Human Monocytic Cells and Endothelial Cells. J. Biol. Chem. 1996, 271, 20828–20835. [Google Scholar] [CrossRef] [PubMed]
- Parry, G.C.; Mackman, N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J. Immunol. 1997, 159, 5450–5456. [Google Scholar] [PubMed]
- Shankar, D.B.; Cheng, J.C.; Kinjo, K.; Federman, N.; Moore, T.B.; Gill, A.; Rao, N.P.; Landaw, E.M.; Sakamoto, K.M. The role of CREB as a proto-oncogene in hematopoiesis and in acute myeloid leukemia. Cancer Cell 2005, 7, 351–362. [Google Scholar] [CrossRef]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional Control of Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Koschmieder, S.; Agrawal, S.; Radomska, S.H.; Huettner, S.C.; Tenen, G.D.; Ottmann, G.O.; Berdel, E.W.; Serve, L.H.; Müller-Tidow, C. Decitabine and Vitamin D3 differentially affect hematopoietic transcription factors to induce monocytic differentiation. Int. J. Oncol. 2007, 30, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Smink, J.J.; Leutz, A. Rapamycin and the transcription factor C/EBPβ as a switch in osteoclast differentiation: Implications for lytic bone diseases. J. Mol. Med. 2009, 88, 227–233. [Google Scholar] [CrossRef]
- Nakahara, T.; Morita, A.; Yagasaki, R.; Mori, A.; Sakamoto, K. Mammalian Target of Rapamycin (mTOR) as a Potential Therapeutic Target in Pathological Ocular Angiogenesis. Biol. Pharm. Bull. 2017, 40, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Delgado-López, F.; Perez-Castro, R.; Gonzalez, I.; Romero, J.; Rojas, I.; Araya, P.; Añazco, C.; Morales, E.; Llanos, J. HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumor Biol. 2016, 37, 3321–3329. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, J.; Hao, Q. HMGB1 combining with tumor-associated macrophages enhanced lymphangiogenesis in human epithelial ovarian cancer. Tumor Biol. 2014, 35, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, S.; Fu, S.; Wu, J. HMGB1 in Radiotherapy: A Two Headed Signal Regulating Tumor Radiosensitivity and Immunity. OncoTargets Ther. 2020, 13, 6859–6871. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Criollo, A.; Ortiz, C.; Lidereau, R.; Mariette, C.; Chaput, N.; Mira, J.-P.; Delaloge, S.; et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol. Rev. 2007, 220, 47–59. [Google Scholar] [CrossRef]
- Liu, M.; Tong, Z.; Ding, C.; Luo, F.; Wu, S.; Wu, C.; Albeituni, S.; He, L.; Hu, X.; Tieri, D.; et al. Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J. Clin. Investig. 2020, 130, 2081–2096. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Inoue, Y.; Nakane-Otani, A.; Tsunakawa, Y.; Jeon, H.; Samir, O.; Teramoto, A.; Kulathunga, K.; Kusakabe, M.; Nakamura, M.; et al. Transcription factor MafB is a marker of tumor-associated macrophages in both mouse and humans. Biochem. Biophys. Res. Commun. 2020, 521, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Hamada, M.; Jeon, H.; Imamura, Y.; Tran, M.T.N.; Takahashi, S. Differential expression patterns of MafB and c-Maf in macrophages in vivo and in vitro. Biochem. Biophys. Res. Commun. 2016, 473, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.F.; Baccarella, A.; Pancholi, N.; Pufall, M.A.; Herbert, D.R.; Kim, C.C. JUNB Is a Key Transcriptional Modulator of Macrophage Activation. J. Immunol. 2015, 194, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bartish, M.; Tong, D.; Pan, Y.; Wallerius, M.; Liu, H.; Ristau, J.; Ferreira, S.D.S.; Wallmann, T.; Van Hoef, V.; Masvidal, L.; et al. MNK2 governs the macrophage antiinflammatory phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 27556–27565. [Google Scholar] [CrossRef]
- Rocco, D.; Della Gravara, L.; Battiloro, C.; Gridelli, C. The role of combination chemo-immunotherapy in advanced non-small cell lung cancer. Expert Rev. Anticancer. Ther. 2019, 19, 561–568. [Google Scholar] [CrossRef]
- Vera Aguilera, J.; Paludo, J.; McWilliams, R.R.; Zhang, H.; Li, Y.; Kumar, A.B.; Failing, J.; Kottschade, L.A.; Block, M.S.; Markovic, S.N.; et al. Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients. Melanoma Res. 2020, 30, 364–375. [Google Scholar] [CrossRef]
- Ma, Y.; Adjemian, S.; Mattarollo, S.R.; Yamazaki, T.; Aymeric, L.; Yang, H.; Catani, J.P.P.; Hannani, D.; Duret, H.; Steegh, K.; et al. Anticancer Chemotherapy-Induced Intratumoral Recruitment and Differentiation of Antigen-Presenting Cells. Immun. 2013, 38, 729–741. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, Y.; Li, B.; Li, X.; Wang, Y.; Han, S.; Cheng, D.; Shuai, X. M2-Like Tumor-Associated Macrophage-Targeted Codelivery of STAT6 Inhibitor and IKKβ siRNA Induces M2-to-M1 Repolarization for Cancer Immunotherapy with Low Immune Side Effects. ACS Central Sci. 2020, 6, 1208–1222. [Google Scholar] [CrossRef]
- De Silva, S.; Fromm, G.; Shuptrine, C.W.; Johannes, K.; Patel, A.; Yoo, K.J.; Huang, K.; Schreiber, T.H. CD40 Enhances Type I Interferon Responses Downstream of CD47 Blockade, Bridging Innate and Adaptive Immunity. Cancer Immunol. Res. 2019, 8, 230–245. [Google Scholar] [CrossRef]
- Cabrales, P. RRx-001 Acts as a Dual Small Molecule Checkpoint Inhibitor by Downregulating CD47 on Cancer Cells and SIRP-α on Monocytes/Macrophages. Transl. Oncol. 2019, 12, 626–632. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.-P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; Mccarty, A.; et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef]
- Yan, D.; Kowal, J.; Akkari, L.; Schuhmacher, A.J.; Huse, J.T.; West, B.L.; Joyce, J.A. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 2017, 36, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.I.; Tiersma, J.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Buzzelli, J.; Im, J.H.; Muschel, R.J. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol. Med. 2018, 10, e9342. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.H.; Hirai, T.; Deng, L.; Chernikova, S.B.; Urata, K.; West, B.L.; Brown, J.M. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-Oncology 2016, 18, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation Exposure Induces Inflammasome Pathway Activation in Immune Cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Hong, J.-H.; Wu, Y.C.; McBride, W.H.; Dougherty, G.J. Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Ther. 2000, 7, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, Y.; Li, D.; Xiang, L.; Ou, Y.; Jiang, L.; Shu, P.; Liu, X.; Guo, F.; Qin, D.; et al. Low-Dose Radiation Therapy Promotes Radiation Pneumonitis by Activating NLRP3 Inflammasome. Int. J. Radiat. Oncol. 2020, 107, 804–814. [Google Scholar] [CrossRef]
- Qin, H.; Wilson, C.A.; Lee, S.J.; Zhao, X.; Benveniste, E.N. LPS induces CD40 gene expression through the activation of NF-κB and STAT-1α in macrophages and microglia. Blood 2005, 106, 3114–3122. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Sanchez, E.; Soof, C.M.; Bujarski, S.; Ng, N.; Cao, J.; Hekmati, T.; Zahab, B.; Nosrati, J.D.; et al. JAK1/2 pathway inhibition suppresses M2 polarization and overcomes resistance of myeloma to lenalidomide by reducing TRIB1, MUC1, CD44, CXCL12, and CXCR4 expression. Br. J. Haematol. 2020, 188, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Park-Min, K.-H.; Ho, H.H.; Ivashkiv, L.B. IFN-γ-Primed Macrophages Exhibit Increased CCR2-Dependent Migration and Altered IFN-γ Responses Mediated by Stat1. J. Immunol. 2005, 175, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kamata, H.; Luo, J.-L.; Leffert, H.; Karin, M. IKKβ Couples Hepatocyte Death to Cytokine-Driven Compensatory Proliferation that Promotes Chemical Hepatocarcinogenesis. Cell 2005, 121, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2019, 12, 130–144. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Wu, K.; Yan, W.; Tian, T.; Wang, Y.; Yang, H. Paraquat modulates microglia M1/M2 polarization via activation of TLR4-mediated NF-κB signaling pathway. Chem. Interact. 2019, 310, 108743. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, I.; Martinet, W.; Schrijvers, D.M.; Timmermans, J.-P.; Bult, H.; De Meyer, G.R.Y. Toll-like receptor 7 stimulation by imiquimod induces macrophage autophagy and inflammation in atherosclerotic plaques. Basic Res. Cardiol. 2012, 107, 1–13. [Google Scholar] [CrossRef]
- D’Acquisto, F. Inhibition of Nuclear Factor Kappa B (NF-B): An Emerging Theme in Anti-Inflammatory Therapies. Mol. Interv. 2002, 2, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-κB Activation by TNF Via the Activation of p38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S.; May, M.J. Cell penetrating peptide inhibitors of Nuclear Factor-kappa B. Cell. Mol. Life Sci. 2008, 65, 3564–3591. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Song, J.-Y.; Yun, Y.-S.; Yi, S.-Y. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. Int. J. Radiat. Biol. 2006, 82, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yuzhen, W.; Wang, J.; Iwanowycz, S.; Saaoud, F.; Wang, Y.; Hu, J.; Wang, Q.; Fan, D. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res. Treat. 2014, 148, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Zhang, J.-Q.; Liang, G.-K.; He, Q.-J.; Ding, L.; Yang, B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1501–1511. [Google Scholar] [CrossRef]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef] [PubMed]

| Transcription Factor | Activating or Inhibitory Stimuli | Target Genes (Up/Down Regulated) | Reference |
|---|---|---|---|
| NF-κB | Hypoxia ↑ | Up: HIF1A, COX2 | [24] |
| LPS ↑ | Up: ADM, HIF1A, COX2, INOS, TNFA, IL6 | [25,26,27] | |
| Fungal polysaccharide↑ | Up: INOS, IL6, TNFA, COX2 | [28] | |
| Mechanical stretch ↑ | Up: INOS, TNFA, IL1B, IL6 Down: ARG1, CD206, TGFB1 | [29] | |
| STAT1 | IFN-γ ↑ | Up: GBP6, CXCL10, CIITA, IRF1, CXCL11, IFIT2 | [30,31] |
| HDL ↑ | Up: IL12B | [32] | |
| STAT3 | LPS ↑ | Up: IL8 and TNFA | [33] |
| IRF1 | GM-CSF + IFN-γ ↑ | Up: INOS | [34] |
| IFN-γ ↑ IFN-ɑ ↑ | Up: IL12B, IL6, TNFA Down: IL10 | [35,36] | |
| IRF2 | LPS ↑ | Up: IL12B, IL12Rb1, IFNG, IL1B, IL6 Down: TNFA | [37] |
| IRF3 | LPS ↑ | Up: IFNB1 | [38] |
| IRF5 | IFN-γ + LPS/GM-CSF ↑ | Up: IL12B, IL23 Down: IL10 | [39] |
| IRF6 | IL-4 ↓ | Up: ARG1, IL10, PPARG | [40] |
| IRF7 | IFN-α, LPS ↑ | Down: IL10 | [41,42] |
| IRF8 | IFN-γ ↑ | Up: TNFA | [43] |
| Transcription Factor | Activating or Inhibitory Stimuli | Target Genes (Up/Down Regulated) | Reference |
|---|---|---|---|
| NF-κB | IL-17 ↑ | Up: ARG1, FIZZ1, YM1, CD206, CD163 | [44] |
| IL-10 ↑ | Down: IL12B | [45] | |
| GDF-15 ↑ | Down: INOS, TNFA | [46] | |
| STAT3 | IL-6 + LIF/ERK5 ↑ IL-6 ↑ | Up: ARG1, VEGFA, TGFB1 and IL10 Down: IL12B, INOS, TNFA Up: ARG1, FIZZ1, IL10 | [47,48] |
| STAT6 | IL-4, IL-13 ↑ | Up: FN1, CCL22 | [49] |
| STAT5 | IL-6 ↑ | Up: PDCD1LG2, CYP19A1 | [50] |
| IRF4 | LPS ↑ | Down: TNFA, IL12B | [51] |
| p53 | IL-4 ↓ | Up: MYC, ARG1, FIZZ1 | [52] |
| Therapeutic Intervention | Tumor Type | Mechanism of Action | Impact on Macrophages |
|---|---|---|---|
| Chemotherapy | |||
| Doxorubicin | Breast, bladder carcinoma, Kaposi sarcoma, lymphoma, acute lymphocytic leukemia [94,95,96,97,98] |
|
|
| Taxol (Paclitaxel) | Ovarian, breast, lung, sarcoma Kaposi, cervix, pancreas [101,102,103,104,105] | - Stimulates tubulin polymerization, anti-mitotic and proapoptotic activity [106] |
|
| Cyclophosphamide (CY) | Hodgkin, non-Hodgkin, cutaneous T-cell lymacrophageomas, multiple myeloma, leukemia, retinoblastoma, neuroblastoma, ovarian cancer, breast cancer [107,108,109,110,111,112,113,114] |
| |
| Cisplatin | Testicular, ovarian, cervical, breast, bladder, head and neck, esophageal, lung, mesothelioma, neuroblastoma [117,118,119,120,121,122] |
|
|
| Carboplatin | Ovarian, lung, head and neck, endometrial, esophageal, bladder, breast, cervical cancers; central nervous system or germ cell tumors; osteogenic sarcoma [126,127,128,129,130,131,132] |
| - Induces M2-like phenotype via STAT3 activation, STAT1 and STAT6 suppression, IL-10, IL-12 stimulation [85,86] |
| Methotrexate | Cervical, breast, lung, head and neck, lymacrophageoma, leukemia [134,135,136,137,138] | - Inhibits dihydrofolate reductase (DHFR) and nucleotide biosynthesis [139] | -Induces p53 activity in TAMs; contributes to the thymidylate synthase-dependent drug sensitivity [140] |
| Olaparib | Prostate, pancreatic, and breast cancer [141,142,143] |
| - Induces M1 polarization via IRF5 [145] |
| Radiotherapy, dose | |||
| 2 Gy | Rectal cancer [145] |
|
|
| 10 Gy | Colon carcinoma [148] | - Recruits ATM and activates Chk2 for DNA repair and checkpoint escape [146,147] |
|
| 12–13.3 Gy | Melanoma [149] Lung cancer [150] Pancreatic ductal adenocarcinoma [151] | - Induces HMGB1 production by tumor and subsequent macrophage remodeling [152] |
|
| 20 Gy | Breast cancer [153] | - Induces tumor antigens and endogenous adjuvants production (heat shock proteins) and subsequent macrophage remodeling [154,155] |
|
| 1.5 Gy X-ray | Lung cancer [156] | - Decreases TGF-β production in the tumor microenvironment [157] |
|
| 66–70 Gy total in 2–2.2 Gy fractions | Head and neck cancers [158] | - Induces tumor-derived mitochondrial DNA production, TLR9 signaling, and macrophage remodeling [159] |
|
| 8 Gy | Pancreatic ductal adenocarcinoma [160] | - Stimulates TGF-β production in the tumor microenvironment [161] |
|
| 5–10 Gy proton irradiation | Lung adenocarcinoma [68] | - Induces ATM recruitment and DNA repair |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedeva, G.F.; Kuzmina, D.O.; Nuzhina, J.; Shtil, A.A.; Dukhinova, M.S. How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy. Int. J. Mol. Sci. 2021, 22, 2662. https://doi.org/10.3390/ijms22052662
Medvedeva GF, Kuzmina DO, Nuzhina J, Shtil AA, Dukhinova MS. How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy. International Journal of Molecular Sciences. 2021; 22(5):2662. https://doi.org/10.3390/ijms22052662
Chicago/Turabian StyleMedvedeva, Galina F., Daria O. Kuzmina, Julia Nuzhina, Alexander A. Shtil, and Marina S. Dukhinova. 2021. "How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy" International Journal of Molecular Sciences 22, no. 5: 2662. https://doi.org/10.3390/ijms22052662
APA StyleMedvedeva, G. F., Kuzmina, D. O., Nuzhina, J., Shtil, A. A., & Dukhinova, M. S. (2021). How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy. International Journal of Molecular Sciences, 22(5), 2662. https://doi.org/10.3390/ijms22052662