Abstract
Early or primary injury due to brain aggression, such as mechanical trauma, hemorrhage or is-chemia, triggers the release of damage-associated molecular patterns (DAMPs) in the extracellular space. Some DAMPs, such as S100B, participate in the regulation of cell growth and survival but may also trigger cellular damage as their concentration increases in the extracellular space. When DAMPs bind to pattern-recognition receptors, such as the receptor of advanced glycation end-products (RAGE), they lead to non-infectious inflammation that will contribute to necrotic cell clearance but may also worsen brain injury. In this narrative review, we describe the role and ki-netics of DAMPs and RAGE at the acute phase of brain injury. We searched the MEDLINE database for “DAMPs” or “RAGE” or “S100B” and “traumatic brain injury” or “subarachnoid hemorrhage” or “stroke”. We selected original articles reporting data on acute brain injury pathophysiology, from which we describe DAMPs release and clearance upon acute brain injury, and the implication of RAGE in the development of brain injury. We will also discuss the clinical strategies that emerge from this overview in terms of biomarkers and therapeutic perspectives
1. Introduction
The cells of the innate immune system search the extracellular environment for exogenous pathogens or self-molecules—either modified (e.g., oxidized lipids) or usually confined within cells via pattern recognition and scavenger receptors (PRRs) [1,2,3]. In the central nervous system these receptors are mainly expressed by microglial cells but also by astrocytes, neurons, endothelial cells, and infiltrating leukocytes upon vessel injury or blood–brain barrier (BBB) opening [1,3,4]. Nevertheless, resident microglial cells have a larger repertoire of Toll-like receptors (TLRs) and a greater amount of the receptor for advanced glycation end-products (RAGE) [1,5,6]. According to the context, PRRs may polarize resident microglial cells and infiltrating leukocytes toward a panel of pro- or anti-inflammatory phenotypes, playing a role in tissue damage clearance and repair but which may also trigger neuronal and glial degeneration [1,3,5,7,8]. Although acute brain injuries encompass a wide variety of mechanisms, they share a common feature as they all lead to acute cellular necrosis with the release of intracellular ions, adenosine di- or triphosphate (ATP), proteins, and nucleic acids in the extracellular space, and eventually the extravasation of red blood cells and hemoglobin [9,10,11,12]. Collectively, this wide variety of endogenous molecules released upon tissue injury are termed damage or danger-associated molecular patterns (DAMPs).
Neurological recovery following acute brain injury depends on the patient’s prior medical condition and primary injuries, but also on secondary damage [13,14]. Among other secondary insults, the inflammation that is triggered by the release of DAMPs plays a crucial role in the development of brain lesions which will be discussed in the present review. The levels of inflammatory markers released in the systemic blood, such as the C-reactive protein, or interleukin (IL) 6 and 12, are actually predictors of post-stroke neurological dysfunction [14,15,16]. Moreover, the concomitant occurrence of a systemic inflammation, such as during sceptic or anaphylactic shock, increases neurological damage at the acute phase of stroke [17].
The acute increase of extracellular potassium after ischemia or hemolysis leads to the depolarization and swelling of neighboring cells thereby starting propagating waves of spreading depolarization [18] (Figure 1A,B). Potassium and ATP release from dying cells also activate the inflammasome in neurons and astrocytes via pannexin1, purinoreceptor, and nucleotide oligomerization domain receptors (NOD-like receptors) [1,19]. Larger molecules, such as S100 proteins, hemoglobin derivatives or the high mobility group protein 1 (HMGB1), bind mainly to TLRs and RAGE [1,10]; the ligation of TLRs and RAGE triggers a series of cellular signaling events, including the activation of nuclear factor-kappa B (NF-κB), leading to the production of pro-inflammatory cytokines, and causing non-sceptic inflammation [1,20,21]. However, the consequences of DAMPs ligation to PRRs depends on the context and their concentration in the extracellular space. For instance, S100B, a calcium binding protein that has several intracellular actions in astrocytes, can promote cell growth in the nM extracellular range (i.e., physiological conditions) [22,23,24]; whereas at higher concentration S100B activates astrocytes with a pro-inflammatory phenotype and facilitate neuronal death [24,25]. HMGB1 expression may also promote neuroinflammation related to brain injury but the deletion of the encoding gene is not able to prevent such consequences [26,27]. Furthermore, sustained activation and up-regulation of RAGE on neurons has been reported to cause death via stimulating the production of reactive oxygen species [28], but also regulates neurite growth and cell survival [24], as well as apoptosis and autophagy [20,29,30]. However, some extracellular soluble truncated receptors, such as sRAGE, act as decoys for ligands, and thus have a cytoprotective effect against advanced glycation end-products (AGE) and RAGE interactions; serum levels of sRAGE have been investigated in pathological process and proposed as a biomarker of their intensity and severity of outcome [31]. It seems that the net effect of DAMPs and PRRs, such as RAGE, depends on the context, cell type, and the number of DAMPs and the level of expression of PRRs.
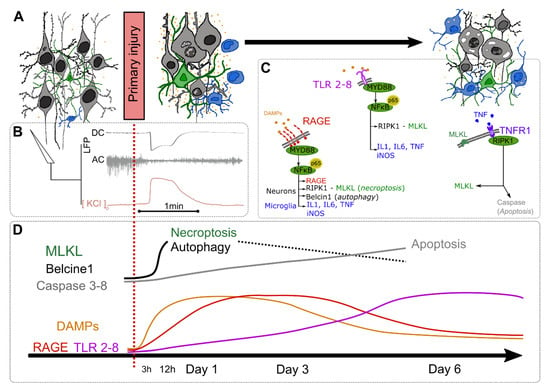
Figure 1.
Damage-associated molecular patterns (DAMPs) and pattern-recognition receptors (PRRs) changes at the acute phase of brain injury. (A) Morphological changes of neurons (grey), astrocytes (green), microglial and infiltrating leukocytes (blue), and DAMPs release (orange); (B) local field potential (LFP) and extracellular KCl recordings during the spreading depolarization triggered by the primary injury: the direct current (DC; 0–0.5Hz) shift is associated with a decrease of neuronal activity (AC; >0.5 Hz) and a KCl release; (C) Pattern-recognition receptors (PRRs) activation pathways; (D) kinetics of DAMPs and PRR expression as well as the course of cell death mechanisms. DAMPs: Damage-associated molecular patterns; IL: Interleukin; iNOS: inducible nitric oxide synthase; LFP: local field potential; MLKL: Mixed-lineage kinase domain-like pseudokinase; MyD88: Myeloid differentiation primary response 88; NFκB: nuclear factor-kappa B; RAGE: Receptor for advanced glycation end-products; RIPK1: receptor-interacting protein kinase 1; TLR: Toll-like receptor; TNF: Tumor necrosis factor; TNFR1: Tumor necrosis factor receptor 1.
In this review, we aimed to describe the current knowledge of the implication of DAMPs and their receptors, in particular RAGE, in acute brain injury pathophysiology. We will also discuss the clinical strategies that emerge from this overview in terms of biomarkers and therapeutic perspectives.
2. Acute Lesion Progression Pathophysiology
2.1. Kinetics of DAMPs and Consequences after the Primary Insult
In addition to mechanical cell destruction, necroptosis is the main cell death pathway at the acute phase of traumatic brain injury (TBI), intracerebral or subarachnoid hemorrhage (SAH), and ischemic stroke (IS) [9,20,32]. Necroptosis is a morphologically lytic form of cell death implicating the receptor-interacting protein kinase 1 and 3 (RIPK1-RIPK3) and the mixed-lineage kinase domain-like pseudokinase (MLKL) pathway, resulting in the release of the contents of the cell into the extracellular space [33] (Figure 1A). The subsequent release of DAMPs peaks around 24 h after the primary insult and decreases thereafter over several days [4,12,20,34,35,36,37,38]. There is a spillover of DAMPs into the core of the primary injury, with a drastic decrease of intracellular HMGB1 [37] while HMGB1 is translocated from the nucleus to the cytoplasm of neurons but not glial cells at the periphery [4,9,37,39]. This extracellular release of HMGB1 may also act as a chemoattractant to monocytes [40] that infiltrate the core of the lesion from the first hours following the injury [4,37]. After several days, HMGB1-positive cells are mainly phagocytic microglial cells [4] (Figure 1D). Some authors have also described a biphasic expression of HMGB1 and S100 proteins with a second peak 14 days later [34,35]. The cellular consequences of DAMPs depend on the expression of PRRs but also on their post-translational modification. For instance, HMGB1 contains three highly conserved cysteines that are readily oxidized by reactive oxygen species, forming an intramolecular disulfide bridge thereby changing its conformation [41,42]. The reduced form released upon the primary injury binds the chemokine ligands (CXCL) 2 and 4 on monocytes and RAGE but not TLR-4, thereby promoting autophagy and acting as a chemoattractant for monocytes [40,43]. Conversely, the oxidized form may promote cell death and, at the late phase, vascular remodeling and progenitor cell migration via TLR signaling [35,43].
The expression of RAGE on neurons and microglial cells follows the same time course, with a biphasic pattern peaking on day 1 and 14 [12,20,34,35,36,44,45]. The amount of TLRs, which are predominantly expressed on microglial cells, increases at the subacute phase (i.e., after day 1) when activated microglia are present [34] (Figure 1D). The ligation of DAMPs to RAGE in turn activates the nuclear factor-kappa B (NF-κB) which in a positive feedback loop will increase the expression of RAGE. The activation of PRRs is related to different signaling pathways in neurons and glial cells. In neurons RAGE promotes the expression of proteins involved in necroptosis (i.e., MLKL) and autophagy (i.e., Becline-1); accordingly, blocking RAGE reduces autophagy, but also increases neuronal sensitivity to injury and apoptosis [20]. Later, at the subacute phase, TLRs and RAGE activation will polarize microglial cells and infiltrating leukocytes toward a pro-inflammatory phenotype, termed M1, with the release of cytokines such as tumor necrosis factor (TNF), IL-6 or IL-1 and the up-regulation of the inducible NO synthase [5,11,21,34,37,39]. Experimental overactivation of RAGE or TLRs by DAMPs injection or an increase in glucose degradation products, such as during hyperglycemia, will in turn increase the cytokine levels and worsen neuronal injuries [5,11,37]. The progression of secondary lesions follows different pathways. The ligation of TNF to the TNF receptor 1 (TNFR1) expressed by neurons will induce both necroptosis (via RIP1-3 and MLKL) and apoptosis (via caspase 3 and 8) [9,33]. Moreover, the pro-inflammatory cocktail can also activate astrocytes with a destructive phenotype (termed A1) leading to neuronal death and fewer synapses [7] (Figure 1C).
2.2. DAMPs Clearence
Under normal conditions, there is a continuous flow of cerebrospinal fluid (CSF) allowing the renewal of extracellular medium and the clearance of solutes such as amyloid-β (Aβ), also called the glymphatic system [46]. the subarachnoid CSF circulates in para-vascular spaces and enters the brain along penetrating arteries reaching the capillary bed; the interstitial fluid is then cleared along a perivenous drainage pathway and cervical lymphatic structures [46,47,48]. The glymphatic flux is driven by arterial pulse [47], water flux through aquaporin-4 expressed on astrocytes [46,48], as well as sleep cycles [30]. The CSF convection in the glymphatic system participates in the clearance of DAMPs to the peripheral blood [48], but also exhibits several changes following brain injury. The spreading depolarization that occurs at the very beginning of brain injuries triggers transient cell swelling, vasoconstriction [49,50] (Figure 1B), and increases the CSF flux in the glymphatic system, participating in the increase in brain water content [51]. However, the glymphatic flux is then impaired from 30 min to several days after the injury thereby leading to an accumulation of extracellular proteins such as DAMPs and Aβ [52,53,54,55]. DAMPs are also actively cleared from the extracellular space by infiltrating myeloid cells from the peripheral blood and activated resident glial cells. Infiltrating myeloid cells penetrate the lesion then differentiate into phagocytes that express scavenger receptors such as the macrophage scavenger receptor 1 (MSR1) that can bind and internalize HMGB1 and S100 proteins, as well as peridoxine [56]. These MSR1+ cells are present up to several days after the injury and exhibit an M2 phenotype, as opposed to the pro-inflammatory M1 phenotype of activated microglia that also internalize DAMPs [3,4,56]. Astrocytes are also able to internalize S100B into lysosomes by a RAGE-dependent mechanism [57]. Activated phagocytes may release HMGB1 from secretory lysosomes when modified lipids such as lysophosphatidylcholine are present at the subacute phase of acute brain injury, which may explain its biphasic release [58,59,60]. DAMPs can also traffic across the BBB by transcytosis in endothelial cells via RAGE and eventually reenter the brain from the blood compartment [61,62].
Increased expression of RAGE has been associated with the promotion of neuroinflammation in the main brain injuries, downstream HMGB1 release and NF-κB pathway activation [36]. Such a positive feedback loop of neuroinflammation may be blocked by intervention with antagonist of RAGE or the release of a truncated soluble form of RAGE (sRAGE) which may act as a decoy receptor to mitigate the inherent consequences of the inflammatory cascade. sRAGE is released upon brain injury [12], either secreted by astrocytes or monocytes [63] or from the cleavage of the membrane-bound RAGE [64]. sRAGE can scavenge extracellular and circulating DAMPs thus preventing their refilling into the brain [61] and reduce brain lesion progression [12,37,65]. The protective effect of recombinant sRAGE in cerebral parenchyma has been recently depicted in an animal model of SAH [65]. Although the characteristics of sRAGE sound interesting for clinical objectives, it has been little studied in brain injuries, with inconsistent results for outcomes such as in acute IS [66], or aSAH [31,67]. These discrepencies in data may result from different types of sRAGE studied and timing of measurements. We can speculate that the plasma level of sRAGE may be either positively associated with the intensity of injury, or negatively because of the consumption by its ligants (i.e., HMGB1 or S100B) possibly reflecting the healing process. Sometimes differences in plasma sRAGE may result from previous patient conditions such as diabetes or renal dysfunction [68]. In such a complex context it might be particularly interesting to combine more than one of these biomarker measurements to characterize the entire process.
3. A Biomarker Approach
A biomarker is a defined physiological characteristic that is measured and monitored objectively and reproducibly. It must be an indicator of biological processes or reactions to an exposure or an intervention, including therapeutic interventions. The ideal biomarker should be sensitive and specific to a pathological condition such as processes involved in early brain injury progression. The clearance of cerebral molecules from the extracellular space to the blood compartment as described in the previous section allows us to measure them from accessible biofluids such as CSF, serum, plasma, and/or whole blood [47,48,69].
A biomarker is considered to be clinically useful if it provides information in addition to the clinical assessment; it can shorten the time to diagnosis or therapeutic decision, or allow a therapeutic follow-up if the kinetics of change are rapid and if the concentration in biological fluid is correlated with the intensity of the disease [70,71]. The biomarker and its analytical method must be adapted to the needs of physicians to shorten the time of a therapeutic decision. Physicians face several challenges at the acute phase of brain injury. The type and severity of brain injury (e.g., ischemic of hemorrhagic) is hard to diagnose when access to brain imaging is not possible, such as during out of hospital triage or in pediatric emergencies when general anesthesia could be required to perform cerebral imaging [72,73,74,75]. The early prognostication of future complication and long-term outcome is also uncertain using clinical data. For instance, neurological score and radiological assessment are not always reliable at the early phase of TBI, IS, and stroke mimics such as migraine or epileptic seizures [76,77]. However, they remain the gold standard to guide clinical decision. During this early phase of brain injury management, several applications of DAMPs as biomarkers have been investigated, such as early injury severity rating [78], distinguishing ischemic or hemorrhagic lesions [79], or prognostication of long-term outcome [80].
The most extensively studied biomarkers are those associated with damage to neurons (neuron-specific enolase, NSE) and glial cells (S100B and glial fibrillary acidic protein—GFAP, mainly expressed in astrocytes) [81]. S100B and GFAP allow the diagnosis of intracranial hemorrhage in patients with cerebral ischemia [78,79,80,82,83,84,85,86,87,88] (Table 1). They are also accurate diagnostic biomarkers that facilitate the triage and the need for brain imaging within a few hours following mild and moderate TBI [89,90,91] (Table 2). Moreover, NSE and S100B have demonstrated a prognostic potential on early and long-term functional recovery after ischemic or hemorrhagic strokes and TBI [80,84,85,86,87,88,92,93,94,95]. Likewise, elevated HMGB1 and sRAGE are predictors of poor prognosis after an IS [67,96] (Table 1 and Table 2). Changes in HMGB1, S100B, and sRAGE concentrations during the hospital stay can also help to diagnose delayed injury such as proximal vasospasm and delayed cerebral ischemia after SAH [10,31,97] (Table 1). Nevertheless, concomitant extracerebral injuries may bias the interpretation of DAMPs and sRAGE levels as they induce a similar increase [98]. S100B is also located extracerebrally, such as in chondrocytes, Schwann cells, adipocytes and malignant melanoma cells, which influences S100B serum levels [81]. As described in Table 1 and Table 2, there is a large variability in the sensitivity and specificity of DAMPs and sRAGE. This is likely to be in relation to the complexity of acute brain injury. Therefore, a single protein biomarker may not be sufficient, and it is reported that a panel of several biomarkers of different cellular origins and different kinetic profiles makes it possible to better predict the prognosis [99,100] and improve the diagnostic accuracy [101,102,103] (Table 3).

Table 1.
Biomarker approach of damage-associated molecular patterns following ischemic or hemorrhagic stroke.

Table 2.
Biomarker approach of damage-associated molecular patterns following traumatic brain injury.

Table 3.
Biomarkers panels approach.
Unfortunately, there is no standardized assays measuring these biomarkers, which constitutes a barrier to the dissemination of these measures in routine clinical practice with established and reproducible cutoffs. For example, S100B can be measure with several techniques such as enzyme-linked immunosorbent assay (ELISA; Sangtec, Saluggia Italy, canAG Diagnostics, Gothenburg Sweden), automated luminometric immunoassay (Diasorin, sangtec, Italy) or automated electrochemiluminescence immunoassay (Cobas, Roche Diagnostics, Mannheim Germany); each method has its own normal and pathological ranges [104,105]. Pre-analytical steps could also be a source of heterogeneity, although S100B measurement have been reported to be unaffected by hemolysis [106], storing, changes in temperature or freeze-thaw cycles [107]. Depending on the clinical and analytics challenges one technique should be preferred over the others. For instance, when rapid decision making is more relevant, rapid quantitative immunoassays are faster and available at any time compared to the traditional ELISA assays (i.e., less than one hour vs. 4 to 5 h). Some biomarkers are at very low concentration in biofluids with sensitivity issues; therefore, new platforms using digital ELISA (e.g., Simoa, Quanterix, Billerica USA) have been developed to overcome this limitation and greatly improved the sensitivity of the tests (e.g., Tau protein, GFAP or Neurofilaments) [108].
4. Therapeutic Perspectives
The brain levels of DAMPs and RAGE have been used as indirect markers of anti-inflammatory drug efficacy following experimental acute brain injury [112,113], but these could also be therapeutic targets, either by blocking the receptors leading to cellular death or by improving clearance of DAMPs from the brain. For instance, blocking RAGE (e.g., with N-benzyl-4-chloro-N-cyclohexylbenzamide, or glycyrrhizin that can be used in humans) can reduce cerebral expression of PRRs, HMGB1 release, and cerebral edema in models of TBI [39,114] or SAH [115,116]. Reducing cerebral edema following acute brain injury can be critical in cases of intracranial hypertension that worsens the prognosis [117]. However, while reducing cerebral edema and expression of PPRs, blocking RAGE might also increase neuronal apoptosis and down-regulate autophagy thereby worsening neuronal lesions [20]. Autophagy may serve as a major cytoprotective process by maintaining cellular homeostasis and recycling cytoplasmic contents [118]. Its role after acute brain injury remains controversial [119,120], and the safety and efficacy of a strategy resulting in autophagy down-regulation remains to be evaluated. Another cell death pathway involves the release of TNF that binds the TNFR1 leading to both necroptosis and apoptosis [9,33]. The intracellular recruitment of RIPK1 by TNFR1 starts a cascade that leads to cell death. Interestingly, the recruitment of RIPK1 is blocked by the endogenous TNF alpha-induced protein 3 (also known as A20) [9]. The level of A20 therefore represents an endogenous regulator of inflammation-induced cell death. Recently some authors described that the expression of A20 can be increased by the administration of melatonin, thereby reducing cell death and cerebral edema in experimental TBI or SAH models [9,121,122]. Melatonin is already approved for the treatment of primary insomnia in adults to promote sleep and could be a potent therapeutic agent to be used at the acute and subacute phase of brain injury. The effect of daily melatonin administration in the acute phase of stroke on inflammation (i.e., IL-6 systemic levels) is currently under investigation in a randomized controlled trial (NCT03843008).
The BBB forms a natural shield that prevents neuroprotective drugs from accessing brain tissue. However, small lipophilic molecules can cross the BBB and were therefore used to design nanoparticles carrying hydrophilic drugs to deliver them to the brain tissue [123]. The intranasal administration of nanoparticles can accumulate their cargo in the brain unlike systemic free administration [124]. Moreover, some nanoparticles release their cargo only in an acidic environment, which is a characteristic of ischemic tissue at risk of irreversible damage; this molecular design allows the enriching of acutely injured tissue without the risk of unfavorable off-target effects [125]. These new technological breakthroughs are a major step forward in specifically targeting injured cerebral tissue with drugs that interfere with DAMPs and PPRs. For instance, nanoparticles have been used to carry antibodies against TNF or IL-6, as well as short hairpin ribonucleic acid silencing the expression of TLRs thereby attenuating inflammation and improving tissue recovery [126,127].
The clearance of DAMPs is also critical to resolve brain inflammation. As described in the previous section the cleaning of DAMPs from the brain involves their scavenging by infiltrating monocytes and microglial cells, and their removal via CSF outflow or drainage. Infiltrating monocytes and macrophages can scavenge DAMPs via several receptors, such as MSR1 [56]. The expression of scavenger receptors is under the regulation of several transcription factors; MafB regulates the expression of MSR1. Vitamin A also has profound effects on cell proliferation, homeostasis, and metabolism through nuclear retinoid receptors [128]. Recently, agonists of the retinoid X receptor have been reported to induce MafB mRNA expression in macrophages [129]. Tamibarotene is an orally active, synthetic retinoid agonist that is more active than all-trans retinoic acid and improves the survival of patients with acute promyelocytic leukemia [130]. In an experimental stroke model tamibarotene enhanced expression of both MafB and MSR1, and in turn reduced the infarct volume [56]. It could therefore be a future therapeutic agent for acute brain injury; however, its tolerance and efficacy remain to be investigated in patients with acute brain injuries. Nevertheless, there is currently a phase 2 clinical trial in patients with Alzheimer’s disease investigating the administration of tamibarotene (NCT01120002). sRAGE can also act as a decoy receptor and scavenge DAMPs in the extracellular space and blood compartment. The administration of recombinant sRAGE reduced cell death in vitro [12,65] and at high dosage in in vivo experimental models of IS and SAH [12,37,65]. However, its efficacy and tolerance in humans is so far unknown. In addition, restoring the glymphatic system circulation after acute brain injury to enhance DAMPs clearance to the peripheral blood is also a future approach that should be investigated. CSF flux through the glymphatic system can be influenced by sleep or sedation. For instance, some data suggest that sevoflurane can enhance the clearance of Aβ by up-regulating the expression of aquaporin-4 in astrocytes thereby improving the CSF flux in the glymphatic system [69]. As low doses of sevoflurane are not supposed to increase the cerebral blood volume, this could be an additional tool to restore CSF clearance after acute brain injury and needs to be evaluated [131]. Once evacuated to the blood compartment, DAMPs can be further cleared using a hemadsorption extracorporeal circulation with porous polymer beads or nanotraps, thereby removing them from the whole body and preventing their refilling to the brain [132,133]. This strategy has yielded promising results in pre-clinical models of sepsis or TBI [133,134]. CSF can also be evacuated by an external ventricular drain (EVD), either when there is a hydrocephalus or to monitor intracranial pressure [135]; it can be used to measure DAMPs following acute brain injury [55], but has yet to be evaluated for the clearance of DAMPs. EVDs are also being used in several clinical trials investigating the effect of intraventricular fibrinolysis to restore the CSF circulation on patient outcome after SAH, when the subarachnoid space is covered with blood (e.g., the FIVHeMA trial, NCT03187405).
5. Materials and Methods
The methodological approach is a narrative review to summarize the main published findings in the field of DAMPs, RAGE, and acute brain injuries [116]. We convened a working group of experts in neurological intensive care medicine (B.B. and J.G.) and molecular biology and clinical chemistry (L.D. and A.P.L.). Section 2 is based on a searched of the MEDLINE database using the PubMed search engine, looking for original articles published in English language using the keywords: “Receptor for Advanced Glycation End-products”, “Damage-associated molecular patterns”, “traumatic brain injury”, “subarachnoid hemorrhage” and “stroke”; with the following Boolean operators: ((Receptor for Advanced Glycation End-products[Title/Abstract]) OR (Damage-associated molecular patterns[Title/Abstract]) OR (S100[Title/Abstract])) AND ((traumatic brain injury[Title/Abstract]) OR (subarachnoid hemorrhage[Title/Abstract]) OR (stroke[Title/Abstract])) NOT (Review[Publication Type]). We then excluded reviews and articles not related to acute brain injuries and selected articles reporting data on acute brain injury pathophysiology. We also searched clinicaltrails.gov for unpublished studies described in Section 4.
6. Conclusions
To conclude, the release of DAMPs upon acute brain injury triggers RAGE activation and steril inflammation, which is a major component of secondary damage progression. It promotes cerebral edema, triggers secondary vascular lesions such as vasospasm and microthrombi, and leads to cellular death via the release of inflammatory cytokines and the polarization of microglial cells toward an M1 phenotype. DAMPs are then cleared from the extracellular space through the glymphatic system to the peripheral blood, and also scavenge by the inflitration of circulating leukocytes followed by activated mciroglia. The concentration of DAMPs in the peripheral blood are therefore good biomarkers of the severity and type of the primary injury. DAMPs can also be used to predict the outcome and monitor the course of brain injury. The detection of a combination of different DAMPs may also improve their diangositic performance. However, the proinflamatory cytokine cocktail that has already been released upon the primary injury may worsen cerebral lesions, and targeting the downstream intracellular consequences could be a future therapeutic strategy. The recent developpment of nanoparticles may, in the near future, allow the administration of neuroprotective drugs specifically in the injured area where in may be most helpful. Clearing and scanvenging DAMPs from brain injury seems also to be critical to prevent lesion progression, and could repesent future theraptic targets.
Author Contributions
Conceptualization, B.B. and A.-C.L.; Methodology, B.B.; Investigation, Resources, Writing-Original Draft Preparation B.B., J.G., L.D.; Writing-Review & Editing: B.B., L.D., J.G., A-C.L., A.P-L.; Supervision, B.B., A-C.L., A.P-L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
B.B. was invited by ROCHE Diagnostics (France) as a member of the S100B in the intensive care unit expert board. The other authors declare no conflict of interest. ROCHE Diagnostics had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| Aβ | Peptide amyloid β |
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| CSF | Cerebrospinal fluid |
| CXCL | Chemokine (C-X-C motif) ligand |
| DAMPs | Damage or danger-associated molecular patterns |
| EVD | External ventricular drain |
| GFAP | Glial fibrillary acidic protein |
| HMGB1 | High mobility group protein 1 |
| IL | Interleukin |
| IS | Ischemic stroke |
| MLKL | Mixed-lineage kinase domain-like pseudokinase |
| MSR1 | Macrophage scavenger receptor 1 |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| NOD | Nucleotide oligomerization domain |
| NSE | Neuron-specific enolase |
| PRRs | Pattern-recognition receptors |
| RAGE | Receptor for advanced glycation end-products |
| sRAGE | Soluble form of the receptor for advanced glycation end-products |
| RIPK | Receptor-interacting protein kinase |
| SAH | Subarachnoid hemorrhage |
| SD | Spreading depolarization |
| TBI | Traumatic brain injury |
| TNF | Tumor necrosis factor |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TLRs | Toll-like receptors |
References
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Gao, T.-L.; Yuan, X.-T.; Yang, D.; Dai, H.-L.; Wang, W.-J.; Peng, X.; Shao, H.-J.; Jin, Z.-F.; Fu, Z.-J. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J. Trauma Acute Care Surg. 2012, 72, 643–649. [Google Scholar] [CrossRef]
- Khan, M.A.; Schultz, S.; Othman, A.; Fleming, T.; Lebrón-Galán, R.; Rades, D.; Clemente, D.; Nawroth, P.P.; Schwaninger, M. Hyperglycemia in Stroke Impairs Polarization of Monocytes/Macrophages to a Protective Noninflammatory Cell Type. J. Neurosci. 2016, 36, 9313–9325. [Google Scholar] [CrossRef]
- Bsibsi, M.; Ravid, R.; Gveric, D.; Noort, J.M. van Broad Expression of Toll-Like Receptors in the Human Central Nervous System. J. Neuropathol. Exp. Neurol. 2002, 61, 1013–1021. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Fan, L.; Zhao, L.; Xu, X.; Liu, Y.; Chao, H.; Liu, N.; You, Y.; Liu, Y.; Wang, X.; et al. Silencing of A20 Aggravates Neuronal Death and Inflammation After Traumatic Brain Injury: A Potential Trigger of Necroptosis. Front. Mol. Neurosci. 2019, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Hafez, A.; Jahromi, B.; Kinfe, T.; Lamprecht, A.; Niemelä, M.; Muhammad, S. Role of Damage Associated Molecular Pattern Molecules (DAMPs) in Aneurysmal Subarachnoid Hemorrhage (aSAH). Int. J. Mol. Sci. 2018, 19, 2035. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.-S.; Zhang, Z.-H.; Zhou, X.-M.; Gao, Y.-Y.; Liu, G.-J.; Wang, H.; Wu, L.-Y.; Li, W.; Hang, C.-H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef]
- Tang, S.-C.; Wang, Y.-C.; Li, Y.-I.; Lin, H.-C.; Manzanero, S.; Hsieh, Y.-H.; Phipps, S.; Hu, C.-J.; Chiou, H.-Y.; Huang, Y.-S.; et al. Functional Role of Soluble Receptor for Advanced Glycation End Products in Stroke. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 585–594. [Google Scholar] [CrossRef]
- Pinto, A.; Tuttolomondo, A.; Raimondo, D.D.; Fernandez, P.; Licata, G. Risk factors profile and clinical outcome of ischemic stroke patients admitted in a Department of Internal Medicine and classified by TOAST classification. Int. Angiol. J. Int. Union Angiol. 2006, 25, 261–267. [Google Scholar]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Mijajlović, M.D.; Pavlović, A.; Brainin, M.; Heiss, W.-D.; Quinn, T.J.; Ihle-Hansen, H.B.; Hermann, D.M.; Assayag, E.B.; Richard, E.; Thiel, A.; et al. Post-stroke dementia—A comprehensive review. BMC Med. 2017, 15, 11. [Google Scholar] [CrossRef]
- Kliper, E.; Bashat, D.B.; Bornstein, N.M.; Shenhar-Tsarfaty, S.; Hallevi, H.; Auriel, E.; Shopin, L.; Bloch, S.; Berliner, S.; Giladi, N.; et al. Cognitive Decline After Stroke. Stroke 2013, 44, 1433–1435. [Google Scholar] [CrossRef] [PubMed]
- Dénes, Á.; Ferenczi, S.; Kovács, K.J. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J. Neuroinflamm. 2011, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P.; Fabricius, M.; Ayata, C.; Sakowitz, O.W.; Shuttleworth, C.W.; Dohmen, C.; Graf, R.; Vajkoczy, P.; Helbok, R.; Suzuki, M.; et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: Review and recommendations of the COSBID research group. J. Cereb. Blood Flow Metab. 2017, 37, 1595–1625. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Z.; Limnuson, K.; Cheyuo, C.; Wang, P.; Ahn, C.H.; Narayan, R.K.; Hartings, J.A. Development and application of a microfabricated multimodal neural catheter for neuroscience. Biomed. Microdevices 2016, 18, 8. [Google Scholar] [CrossRef]
- Yao, X.; Liu, S.; Ding, W.; Yue, P.; Jiang, Q.; Zhao, M.; Hu, F.; Zhang, H. TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J. Neuroimmunol. 2017, 310, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kligman, D.; Marshak, D.R. Purification and characterization of a neurite extension factor from bovine brain. Proc. Natl. Acad. Sci. USA 1985, 82, 7136–7139. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Villarreal, A.; Seoane, R.; Torres, A.G.; Rosciszewski, G.; Angelo, M.F.; Rossi, A.; Barker, P.A.; Ramos, A.J. S100B protein activates a RAGE-dependent autocrine loop in astrocytes: Implications for its role in the propagation of reactive gliosis. J. Neurochem. 2014, 131, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wilson, G. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. HMGB1-Mediated Neuroinflammatory Responses in Brain Injuries: Potential Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 4609. [Google Scholar] [CrossRef]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after traumatic brain injury—The complex role of HMGB1 and neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for Advanced Glycation End Products Activation Injures Primary Sensory Neurons via Oxidative Stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef]
- Kang, R.; Tang, D.; Lotze, M.T.; III, H.J.Z. RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy 2011, 7, 442–444. [Google Scholar] [CrossRef]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Aida, Y.; Kamide, T.; Ishii, H.; Kitao, Y.; Uchiyama, N.; Nakada, M.; Hori, O. Soluble receptor for advanced glycation end products as a biomarker of symptomatic vasospasm in subarachnoid hemorrhage. J. Neurosurg. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, S.; Hu, H.; Zhang, X.; Xu, J.; Wang, R.; Zhang, P. Progranulin protects against cerebral ischemia-reperfusion (I/R) injury by inhibiting necroptosis and oxidative stress. Biochem. Bioph. Res. Commun. 2019, 521, 569–576. [Google Scholar] [CrossRef]
- Croker, B.A.; Rickard, J.A.; Shlomovitz, I.; Al-Obeidi, A.; D’Cruz, A.A.; Gerlic, M. Apoptosis and Beyond; Wiley: Hoboken, NJ, USA, 2018; pp. 99–128. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Jitsuishi, T.; Hozumi, T.; Iwanami, J.; Kitajo, K.; Yamaguchi, H.; Mori, Y.; Mogi, M.; Sawai, S. Temporal expression profiling of DAMPs-related genes revealed the biphasic post-ischemic inflammation in the experimental stroke model. Mol. Brain 2020, 13, 57. [Google Scholar] [CrossRef]
- Tian, X.; Sun, L.; Feng, D.; Sun, Q.; Dou, Y.; Liu, C.; Zhou, F.; Li, H.; Shen, H.; Wang, Z.; et al. HMGB1 promotes neurovascular remodeling via Rage in the late phase of subarachnoid hemorrhage. Brain Res. 2017, 1670, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, W.; Sun, Q.; Liu, M.; Li, W.; Zhang, X.; Zhou, M.; Hang, C. Expression and cell distribution of receptor for advanced glycation end-products in the rat cortex following experimental subarachnoid hemorrhage. Brain Res. 2014, 1543, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Barakat, W.; Stoyanov, S.; Murikinati, S.; Yang, H.; Tracey, K.J.; Bendszus, M.; Rossetti, G.; Nawroth, P.P.; Bierhaus, A.; et al. The HMGB1 Receptor RAGE Mediates Ischemic Brain Damage. J. Neurosci. 2008, 28, 12023–12031. [Google Scholar] [CrossRef]
- Pleines, U.E.; Morganti-Kossmann, M.C.; Rancan, M.; Joller, H.; Trentz, O.; Kossmann, T. S-100 reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J. Neurotrauma 2001, 18, 491–498. [Google Scholar] [CrossRef]
- Okuma, Y.; Liu, K.; Wake, H.; Liu, R.; Nishimura, Y.; Hui, Z.; Teshigawara, K.; Haruma, J.; Yamamoto, Y.; Yamamoto, H.; et al. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1–RAGE interaction. Neuropharmacology 2014, 85, 18–26. [Google Scholar] [CrossRef]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; Marchis, F.D.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4The HMGB1–CXCL12 heterocomplex acts via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef]
- Hoppe, G.; Talcott, K.E.; Bhattacharya, S.K.; Crabb, J.W.; Sears, J.E. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp. Cell Res. 2006, 312, 3526–3538. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciute, V.; Meister, S.; Fürnrohr, B.G.; Frey, B.; Gückel, E.; Schett, G.; Herrmann, M.; Voll, R.E. Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis. Autoimmunity 2009, 42, 305–307. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Cheh, C.-W.; Livesey, K.M.; Liang, X.; Schapiro, N.E.; Benschop, R.; Sparvero, L.J.; Amoscato, A.A.; Tracey, K.J.; et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010, 29, 5299–5310. [Google Scholar] [CrossRef]
- Hassid, B.G.; Nair, M.N.; Ducruet, A.F.; Otten, M.L.; Komotar, R.J.; Pinsky, D.J.; Schmidt, A.M.; Yan, S.F.; Connolly, E.S. Neuronal RAGE expression modulates severity of injury following transient focal cerebral ischemia. J. Clin. Neurosci. 2009, 16, 302–306. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, S.; Cao, T.; Tao, W.; Liu, M.; Wu, B. HMGB1 may act via RAGE to promote angiogenesis in the later phase after intracerebral hemorrhage. Neuroscience 2015, 295, 39–47. [Google Scholar] [CrossRef]
- Iliff, J.; Wang, M.; Liao, Y.; Plogg, B.; Peng, W.; Gundersen, G.; Benveniste, H.; Vates, G.; Deane, R.; Goldman, S.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid B. Sci. Transl. Med. 2013, 4. [Google Scholar] [CrossRef]
- Iliff, J.; Wang, M.; Zeppenfeld, D.; Venkataraman, A.; Plog, B.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. J. Neurosci. 2019, 33. [Google Scholar] [CrossRef]
- Plog, B.A.; Dashnaw, M.L.; Hitomi, E.; Peng, W.; Liao, Y.; Lou, N.; Deane, R.; Nedergaard, M. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J. Neurosci. 2015, 35, 518–526. [Google Scholar] [CrossRef]
- Risher, W.; Ard, D.; Yuan, J. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J. Neurosci. 2010, 29, 9859–9868. [Google Scholar] [CrossRef]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011, 17, 2333. [Google Scholar] [CrossRef]
- Mestre, H.; Du, T.; Sweeney, A.M.; Liu, G.; Samson, A.J.; Peng, W.; Mortensen, K.N.; Stæger, F.F.; Bork, P.A.R.; Bashford, L.; et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 2020, 367, eaax7171. [Google Scholar] [CrossRef]
- Iliff, J.; Chen, M.; Plog, B.; Zeppenfeld, D.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34. [Google Scholar] [CrossRef]
- Gaberel, T.; Gakuba, C.; Goulay, R.; Lizarrondo, S.; Hanouz, J.-L.; Emery, E.; Touze, E.; Vivien, D.; Gauberti, M. Impaired Glymphatic Perfusion After Strokes Revealed by Contrast-Enhanced MRI. Stroke 2014, 45, 3092–3096. [Google Scholar] [CrossRef]
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R.; et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017, 48, 2301–2305. [Google Scholar] [CrossRef]
- Lindblad, C.; Nelson, D.W.; Zeiler, F.A.; Ercole, A.; Ghatan, P.H.; von Horn, H.; Risling, M.; Svensson, M.; Agoston, D.V.; Bellander, B.-M.; et al. Influence of Blood–Brain Barrier Integrity on Brain Protein Biomarker Clearance in Severe Traumatic Brain Injury: A Longitudinal Prospective Study. J. Neurotraum. 2020, 37, 1381–1391. [Google Scholar] [CrossRef]
- Shichita, T.; Ito, M.; Morita, R.; Komai, K.; Noguchi, Y.; Ooboshi, H.; Koshida, R.; Takahashi, S.; Kodama, T.; Yoshimura, A. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat. Med. 2017, 23, 723–732. [Google Scholar] [CrossRef]
- Lasič, E.; Galland, F.; Vardjan, N.; Šribar, J.; Križaj, I.; Leite, M.C.; Zorec, R.; Stenovec, M. Time-dependent uptake and trafficking of vesicles capturing extracellular S100B in cultured rat astrocytes. J. Neurochem. 2016, 139, 309–323. [Google Scholar] [CrossRef]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef]
- Pasvogel, A.E.; Miketova, P.; Moore, I.M. (Ki) Cerebrospinal Fluid Phospholipid Changes Following Traumatic Brain Injury. Biol. Res. Nurs. 2008, 10, 113–120. [Google Scholar] [CrossRef]
- Pasvogel, A.E.; Miketova, P.; Moore, I.M. Differences in CSF Phospholipid Concentration by Traumatic Brain Injury Outcome. Biol. Res. Nurs. 2010, 11, 325–331. [Google Scholar] [CrossRef]
- Deane, R.; Yan, S.D.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hasu, M.; Popov, D.; Zhang, J.H.; Chen, J.; Yan, S.D.; Brett, J.; Cao, R.; Kuwabara, K.; Costache, G. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl. Acad. Sci. 1994, 91, 8807–8811. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Yeon, S.I.; Youn, J.H.; Choi, J.E.; Sasaki, N.; Choi, I.-H.; Shin, J.-S. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol. Immunol. 2004, 40, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). Faseb J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Wang, W.; Lu, R.; Feng, D.; Zhang, H. Sevoflurane Inhibits Glutamate-Aspartate Transporter and Glial Fibrillary Acidic Protein Expression in Hippocampal Astrocytes of Neonatal Rats Through the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) Pathway. Anest. Analg. 2016, 123, 93. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yeh, S.-J.; Tsai, L.-K.; Hu, C.-J.; Lien, L.-M.; Peng, G.-S.; Yang, W.-S.; Chiou, H.-Y.; Jeng, J.-S. Cleaved but not endogenous secretory RAGE is associated with outcome in acute ischemic stroke. Neurology 2016, 86, 270–276. [Google Scholar] [CrossRef]
- Yang, D.-B.; Dong, X.-Q.; Du, Q.; Yu, W.-H.; Zheng, Y.-K.; Hu, W.; Wang, K.-Y.; Chen, F.-H.; Xu, Y.-S.; Wang, Y.; et al. Clinical relevance of cleaved RAGE plasma levels as a biomarker of disease severity and functional outcome in aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 2018, 486, 335–340. [Google Scholar] [CrossRef]
- Prasad, K. Low Levels of Serum Soluble Receptors for Advanced Glycation End Products, Biomarkers for Disease State: Myth or Reality. Int. J. Angiol. 2014, 23, 011–016. [Google Scholar] [CrossRef]
- Gao, X.; Ming, J.; Liu, S.; Lai, B.; Fang, F.; Cang, J. Sevoflurane enhanced the clearance of Aβ1-40 in hippocampus under surgery via up-regulating AQP-4 expression in astrocyte. Life Sci. 2019, 221, 143–151. [Google Scholar] [CrossRef]
- Group, B.D.W. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Opal, S.M. Clinical review: The role of biomarkers in the diagnosis and management of community-acquired pneumonia. Crit. Care 2010, 14, 203. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Niogi, S.N.; Whitlow, C.J.; Tsiouris, A.J. Traumatic Brain Injury: Imaging Patterns and Complications. Radiographics 2019, 39, 1571–1595. [Google Scholar] [CrossRef]
- Clement, M.O. Imaging of Brain Trauma. Radiol. Clin. N. Am. 2019, 57, 733–744. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Desai, S.M.; Liebeskind, D.S.; Wechsler, L.R. Neuroimaging of Acute Stroke. Neurol. Clin. 2019, 38, 185–199. [Google Scholar] [CrossRef]
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med. Chir. 2016, 57, ra.2016-0191. [Google Scholar] [CrossRef]
- Madsen, T.E.; Khoury, J.; Cadena, R.; Adeoye, O.; Alwell, K.A.; Moomaw, C.J.; McDonough, E.; Flaherty, M.L.; Ferioli, S.; Woo, D.; et al. Potentially Missed Diagnosis of Ischemic Stroke in the Emergency Department in the Greater Cincinnati/Northern Kentucky Stroke Study. Acad. Emerg. Med. 2016, 23, 1128–1135. [Google Scholar] [CrossRef]
- Liberman, A.L.; Prabhakaran, S. Stroke Chameleons and Stroke Mimics in the Emergency Department. Curr. Neurol. Neurosci. 2017, 17, 15. [Google Scholar] [CrossRef]
- Balança, B.; Ritzenthaler, T.; Gobert, F.; Richet, C.; Bodonian, C.; Carrillon, R.; Terrier, A.; Desmurs, L.; Perret-Liaudet, A.; Dailler, F. Significance and Diagnostic Accuracy of Early S100B Serum Concentration after Aneurysmal Subarachnoid Hemorrhage. J. Clin. Med. 2020, 9, 1746. [Google Scholar] [CrossRef]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y.; et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci. Rep. 2016, 6, 24588. [Google Scholar] [CrossRef]
- Quintard, H.; Leduc, S.; Ferrari, P.; Petit, I.; Ichai, C. Early and persistent high level of PS 100β is associated with increased poor neurological outcome in patients with SAH: Is there a PS 100β threshold for SAH prognosis? Crit. Care 2016, 20, 33. [Google Scholar] [CrossRef]
- Kawata, K.; Liu, C.Y.; Merkel, S.F.; Ramirez, S.H.; Tierney, R.T.; Langford, D. Blood biomarkers for brain injury: What are we measuring? Neurosci. Biobehav. Rev. 2016, 68, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.; Jæger, H.S.; Dixon, J.; Bohmann, F.O.; Schaefer, J.; Richieri, S.P.; Larsen, K.; Hov, M.R.; Bache, K.G.; Foerch, C.; et al. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit. Care 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Makris, K.; Stefani, D.; Koniari, K.; Gialouri, E.; Lelekis, M.; Chondrogianni, M.; Zompola, C.; Dardiotis, E.; Rizos, I.; et al. Plasma Glial Fibrillary Acidic Protein in the Differential Diagnosis of Intracerebral Hemorrhage. Stroke 2018, 48, 2586–2588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Bao, J.; Wang, Y.; Pan, S. S100β as a biomarker for differential diagnosis of intracerebral hemorrhage and ischemic stroke. Neurol. Res. 2016, 38, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.P.; Oliveira, S.; Sargento-Freitas, J.; Galego, O.; Cordeiro, G.; Cunha, L.; Gonçalves, A.F.; Pinheiro, J. Neuroimaging, serum biomarkers, and patient characteristics as predictors of upper limb functioning 12 weeks after acute stroke: An observational, prospective study. Top. Stroke Rehabil. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Kedziora, J.; Burzynska, M.; Gozdzik, W.; Kübler, A.; Kobylinska, K.; Adamik, B. Biomarkers of Neurological Outcome After Aneurysmal Subarachnoid Hemorrhage as Early Predictors at Discharge from an Intensive Care Unit. Neurocrit. Care 2020, 1–11. [Google Scholar] [CrossRef]
- Kiiski, H.; Långsjö, J.; Tenhunen, J.; Ala-Peijari, M.; Huhtala, H.; Hämäläinen, M.; Moilanen, E.; Peltola, J. S100B, NSE and MMP-9 fail to predict neurologic outcome while elevated S100B associates with milder initial clinical presentation after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2018, 390, 129–134. [Google Scholar] [CrossRef]
- Abboud, T.; Mende, K.C.; Jung, R.; Czorlich, P.; Vettorazzi, E.; Priefler, M.; Kluge, S.; Westphal, M.; Regelsberger, J. Prognostic Value of Early S100 Calcium Binding Protein B and Neuron-Specific Enolase in Patients with Poor-Grade Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. World Neurosurg. 2017, 108, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Mahan, M.Y.; Thorpe, M.; Ahmadi, A.; Abdallah, T.; Casey, H.; Sturtevant, D.; Judge-Yoakam, S.; Hoover, C.; Rafter, D.; Miner, M.J.; et al. GFAP Outperforms S100B and UCH-L1 as Predictor for Positive Head CT in Trauma Subjects. World Neurosurg. 2019, 128, e434–e444. [Google Scholar] [CrossRef]
- Meier, T.B.; Nelson, L.D.; Huber, D.L.; Bazarian, J.J.; Hayes, R.L.; McCrea, M.A. Prospective Assessment of Acute Blood Markers of Brain Injury in Sport-Related Concussion. J. Neurotraum. 2017, 34, 3134–3142. [Google Scholar] [CrossRef]
- Çevik, S.; Özgenç, M.M.; Güneyk, A.; Evran, Ş.; Akkaya, E.; Çalış, F.; Katar, S.; Soyalp, C.; Hanımoğlu, H.; Kaynar, M.Y. NRGN, S100B and GFAP levels are significantly increased in patients with structural lesions resulting from mild traumatic brain injuries. Clin. Neurol. Neurosurg. 2019, 183, 105380. [Google Scholar] [CrossRef]
- Kellermann, I.; Kleindienst, A.; Hore, N.; Buchfelder, M.; Brandner, S. Early CSF and Serum S100B Concentrations for Outcome Prediction in Traumatic Brain Injury and Subarachnoid Hemorrhage. Clin. Neurol. Neurosurg. 2016, 145, 79–83. [Google Scholar] [CrossRef]
- Park, D.-W.; Park, S.-H.; Hwang, S.-K. Serial measurement of S100B and NSE in pediatric traumatic brain injury. Child. Nerv. Syst. 2019, 35, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Hwang, S.-K. Prognostic Value of Serum Levels of S100 Calcium-Binding Protein B, Neuron-Specific Enolase, and Interleukin-6 in Pediatric Patients with Traumatic Brain Injury. World Neurosurg. 2018, 118, e534–e542. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Jeppsson, E.; Frostell, A.; Svensson, M.; Mondello, S.; Bellander, B.-M.; Nelson, D.W. Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Crit. Care 2016, 20, 285. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Zeng, D.; Zhou, W.; Hong, X. Prognostic value of plasma HMGB1 in ischemic stroke patients with cerebral ischemia-reperfusion injury after intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105055. [Google Scholar] [CrossRef]
- Lai, P.M.; Du, R. Association between S100B Levels and Long-Term Outcome after Aneurysmal Subarachnoid Hemorrhage: Systematic Review and Pooled Analysis. PLoS ONE 2016, 11, e0151853. [Google Scholar] [CrossRef]
- Vourc’h, M.; Roquilly, A.; Asehnoune, K. Trauma-Induced Damage-Associated Molecular Patterns-Mediated Remote Organ Injury and Immunosuppression in the Acutely Ill Patient. Front. Immunol. 2018, 9, 1330. [Google Scholar] [CrossRef]
- Thelin, E.; Nimer, F.A.; Frostell, A.; Zetterberg, H.; Blennow, K.; Nyström, H.; Svensson, M.; Bellander, B.-M.; Piehl, F.; Nelson, D.W. A Serum Protein Biomarker Panel Improves Outcome Prediction in Human Traumatic Brain Injury. J. Neurotraum. 2019, 36, 2850–2862. [Google Scholar] [CrossRef]
- Battista, A.P.D.; Buonora, J.E.; Rhind, S.G.; Hutchison, M.G.; Baker, A.J.; Rizoli, S.B.; Diaz-Arrastia, R.; Mueller, G.P. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front. Neurol. 2015, 6, 110. [Google Scholar] [CrossRef]
- Posti, J.P.; Takala, R.S.K.; Raj, R.; Luoto, T.M.; Azurmendi, L.; Lagerstedt, L.; Mohammadian, M.; Hossain, I.; Gill, J.; Frantzén, J.; et al. Admission Levels of Interleukin 10 and Amyloid β 1–40 Improve the Outcome Prediction Performance of the Helsinki Computed Tomography Score in Traumatic Brain Injury. Front. Neurol. 2020, 11, 549527. [Google Scholar] [CrossRef]
- Posti, J.P.; Takala, R.S.K.; Lagerstedt, L.; Dickens, A.M.; Hossain, I.; Mohammadian, M.; Ala-Seppälä, H.; Frantzén, J.; van Gils, M.; Hutchinson, P.J.; et al. Correlation of Blood Biomarkers and Biomarker Panels with Traumatic Findings on Computed Tomography after Traumatic Brain Injury. J. Neurotraum. 2019, 36, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Soosaipillai, A.; Fraser, D.D.; Diamandis, E.P. Discovery of novel plasma biomarker ratios to discriminate traumatic brain injury. F1000Research 2019, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Smit, L.H.M.; Korse, C.M.; Bonfrer, J.M.G. Comparison of four different assays for determination of serum S-100B. Int. J. Biol. Mark. 2005, 20, 34–42. [Google Scholar] [CrossRef]
- Einav, S.; Itshayek, E.; Kark, J.D.; Ovadia, H.; Weiniger, C.F.; Shoshan, Y. Serum S100B levels after meningioma surgery: A comparison of two laboratory assays. BMC Clin. Pathol. 2008, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Beaudeux, J.L.; Léger, P.; Dequen, L.; Gandjbakhch, I.; Coriat, P.; Foglietti, M.J. Influence of hemolysis on the measurement of S-100beta protein and neuron-specific enolase plasma concentrations during coronary artery bypass grafting. Clin. Chem. 2000, 46, 989–990. [Google Scholar] [CrossRef]
- Raabe, A.; Kopetsch, O.; Groß, U.; Zimmermann, M.; Gebhart, P. Measurements of Serum S-100B Protein: Effects of Storage Time and Temperature on Pre-Analytical Stability. Clin. Chem. Lab. Med. 2003, 41, 700–703. [Google Scholar] [CrossRef]
- Kawata, K.; Steinfeldt, J.A.; Huibregtse, M.E.; Nowak, M.K.; Macy, J.T.; Kercher, K.; Rettke, D.J.; Shin, A.; Chen, Z.; Ejima, K.; et al. Association Between Proteomic Blood Biomarkers and DTI/NODDI Metrics in Adolescent Football Players: A Pilot Study. Front. Neurol. 2020, 11, 581781. [Google Scholar] [CrossRef]
- Tsukagawa, T.; Katsumata, R.; Fujita, M.; Yasui, K.; Akhoon, C.; Ono, K.; Dohi, K.; Aruga, T. Elevated Serum High-Mobility Group Box-1 Protein Level Is Associated with Poor Functional Outcome in Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 2404–2411. [Google Scholar] [CrossRef]
- Kiiski, H.; Långsjö, J.; Tenhunen, J.; Ala-Peijari, M.; Huhtala, H.; Hämäläinen, M.; Moilanen, E.; Öhman, J.; Peltola, J. Time-courses of plasma IL-6 and HMGB-1 reflect initial severity of clinical presentation but do not predict poor neurologic outcome following subarachnoid hemorrhage. Eneurologicalsci 2017, 6, 55–62. [Google Scholar] [CrossRef]
- Osier, N.D.; Conley, Y.P.; Okonkwo, D.O.; Puccio, A.M. Variation in Candidate Traumatic Brain Injury Biomarker Genes Are Associated with Gross Neurological Outcomes after Severe Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2684–2690. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Genrikhs, E.E.; Stelmashook, E.V.; Voronkov, D.N.; Novikova, S.V.; Alexandrova, O.P.; Fedorov, A.V.; Isaev, N.K. The single intravenous administration of methylene blue after traumatic brain injury diminishes neurological deficit, blood-brain barrier disruption and decrease in the expression of S100 protein in rats. Brain Res. 2020, 1740, 146854. [Google Scholar] [CrossRef]
- Gu, X.-J.; Xu, J.; Ma, B.-Y.; Chen, G.; Gu, P.-Y.; Wei, D.; Hu, W.-X. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin. J. Traumatol. 2014, 17, 1–7. [Google Scholar]
- Li, Y.; Sun, F.; Jing, Z.; Wang, X.; Hua, X.; Wan, L. Glycyrrhizic acid exerts anti-inflammatory effect to improve cerebral vasospasm secondary to subarachnoid hemorrhage in a rat model. Neurol. Res. 2017, 39, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Ieong, C.; Sun, H.; Wang, Q.; Ma, J. Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates inflammative effect after acute subarachnoid hemorrhage in rat model. J. Clin. Neurosci. 2018, 47, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Güiza, F.; Depreitere, B.; Piper, I.; Citerio, G.; Chambers, I.; Jones, P.A.; Lo, T.-Y.; Enblad, P.; Nillson, P.; Feyen, B.; et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015, 41, 1067–1076. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef]
- He, Y.; Wan, S.; Hua, Y.; Keep, R.F.; Xi, G. Autophagy after Experimental Intracerebral Hemorrhage. J. Cereb. Blood Flow Metab. 2007, 28, 897–905. [Google Scholar] [CrossRef]
- Zille, M.; Karuppagounder, S.S.; Chen, Y.; Gough, P.J.; Bertin, J.; Finger, J.; Milner, T.A.; Jonas, E.A.; Ratan, R.R. Neuronal Death After Hemorrhagic Stroke In Vitro and In Vivo Shares Features of Ferroptosis and Necroptosis. Stroke 2018, 48, 1033–1043. [Google Scholar] [CrossRef]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Hou, L.; Liu, N.; Ji, J. Melatonin attenuates traumatic brain injury-induced inflammation: A possible role for mitophagy. J. Pineal Res. 2016, 61, 177–186. [Google Scholar] [CrossRef]
- Lu, J.; Sun, Z.; Fang, Y.; Zheng, J.; Xu, S.; Xu, W.; Shi, L.; Mei, S.; Wu, H.; Liang, F.; et al. Melatonin Suppresses Microglial Necroptosis by Regulating Deubiquitinating Enzyme A20 After Intracerebral Hemorrhage. Front. Immunol. 2019, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Thangudu, S.; Cheng, F.-Y.; Su, C.-H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Soler, Y.; Karuppan, M.K.M.; Zhao, Y.; Batrakova, E.V.; El-Hage, N. Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles. Pharmaceutics 2021, 13, 223. [Google Scholar] [CrossRef]
- Tóth, O.M.; Menyhárt, Á.; Frank, R.; Hantosi, D.; Farkas, E.; Bari, F. Tissue Acidosis Associated with Ischemic Stroke to Guide Neuroprotective Drug Delivery. Biology 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.; Amorim, D.; Laranjeira, I.; Almeida, A.; Reis, R.L.; Ferreira, H.; Pinto-Ribeiro, F.; Neves, N.M. Modulating inflammation through the neutralization of Interleukin-6 and tumor necrosis factor-α by biofunctionalized nanoparticles. J. Control. Release 2021. [Google Scholar] [CrossRef]
- Nalamolu, K.R.; Challa, S.R.; Fornal, C.A.; Grudzien, N.A.; Jorgenson, L.C.; Choudry, M.M.; Smith, N.J.; Palmer, C.J.; Pinson, D.M.; Klopfenstein, J.D.; et al. Attenuation of the Induction of TLRs 2 and 4 Mitigates Inflammation and Promotes Neurological Recovery After Focal Cerebral Ischemia. Transl. Stroke Res. 2021, 1–14. [Google Scholar] [CrossRef]
- Szanto, A.; Narkar, V.; Shen, Q.; Uray, I.P.; Davies, P.J.A.; Nagy, L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004, 11, S126–S143. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Nakamura, M.; Tran, M.T.N.; Moriguchi, T.; Hong, C.; Ohsumi, T.; Dinh, T.T.H.; Kusakabe, M.; Hattori, M.; Katsumata, T.; et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 2014, 5, 3147. [Google Scholar] [CrossRef]
- Takeshita, A.; Asou, N.; Atsuta, Y.; Sakura, T.; Ueda, Y.; Sawa, M.; Dobashi, N.; Taniguchi, Y.; Suzuki, R.; Nakagawa, M.; et al. Tamibarotene maintenance improved relapse-free survival of acute promyelocytic leukemia: A final result of prospective, randomized, JALSG-APL204 study. Leukemia 2019, 33, 358–370. [Google Scholar] [CrossRef]
- Oddo, M.; Crippa, I.; Mehta, S.; Menon, D.; Payen, J.-F.; Taccone, F.; Citerio, G. Optimizing sedation in patients with acute brain injury. Crit. Care 2016, 20, 128. [Google Scholar] [CrossRef]
- Shi, C.; Wang, X.; Wang, L.; Meng, Q.; Guo, D.; Chen, L.; Dai, M.; Wang, G.; Cooney, R.; Luo, J. A nanotrap improves survival in severe sepsis by attenuating hyperinflammation. Nat. Commun. 2020, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Gruda, M.C.; Ruggeberg, K.-G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- McKinley, T.O.; Lei, Z.; Kalbas, Y.; White, F.A.; Shi, Z.; Wu, F.; Xu, Z.C.; Rodgers, R.B. Blood purification by nonselective hemoadsorption prevents death after traumatic brain injury and hemorrhagic shock in rats. J. Trauma Acute Care 2018, 85, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Nelson, D.W.; Ghatan, P.H.; Bellander, B.-M. Microdialysis Monitoring of CSF Parameters in Severe Traumatic Brain Injury Patients: A Novel Approach. Front. Neurol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).