Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes
Abstract
1. Introduction
2. Results
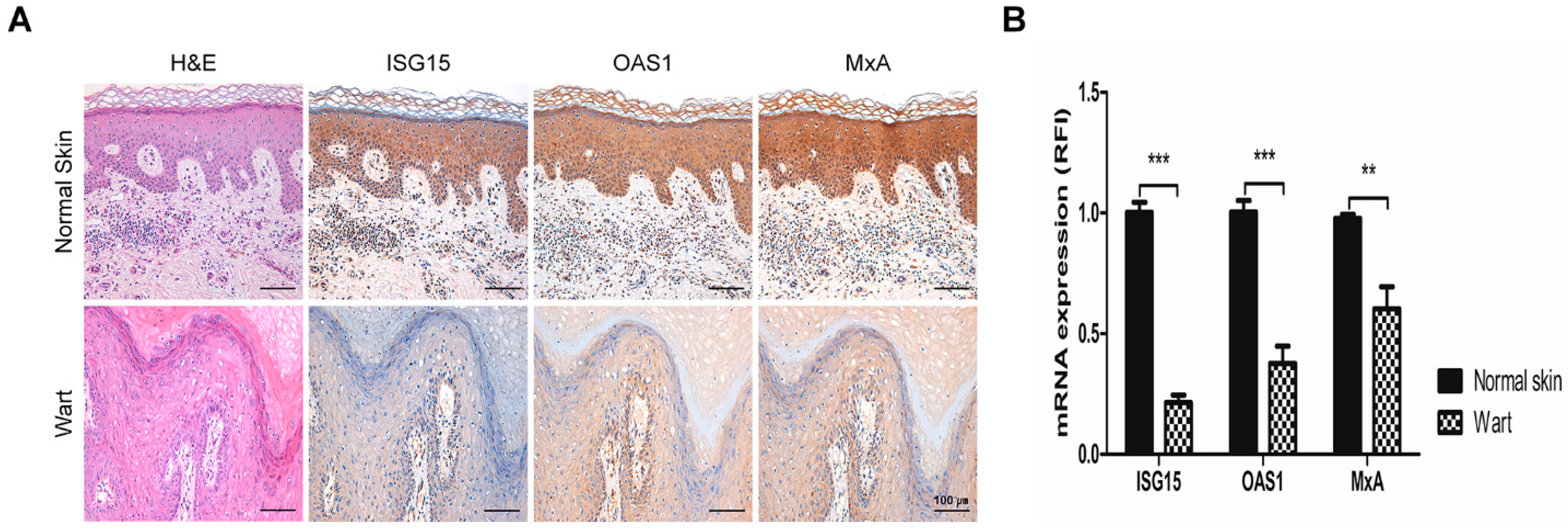
2.1. The Expression of Interferon-Stimulated Genes Declined in HPV-2 Wart Samples
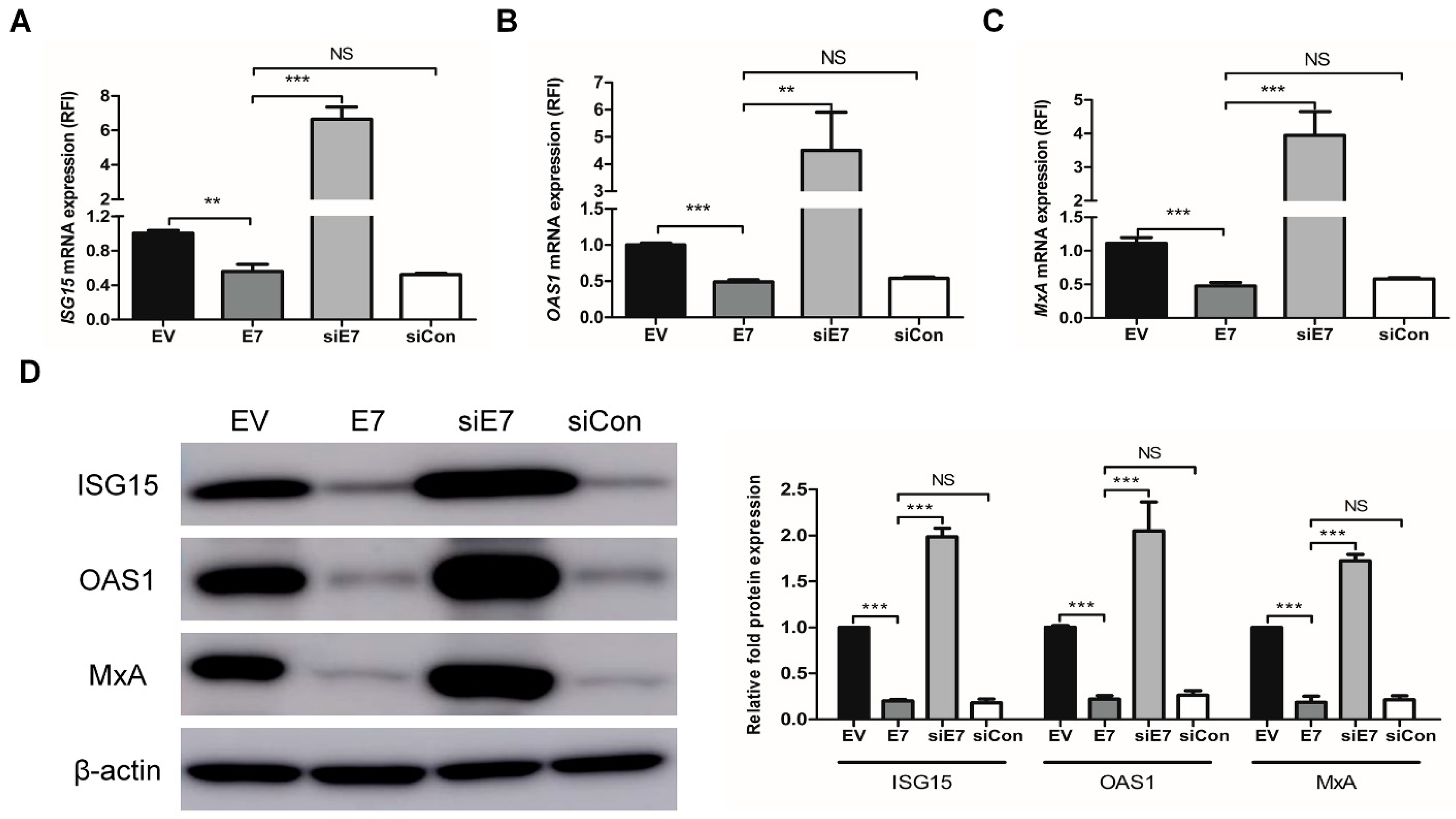
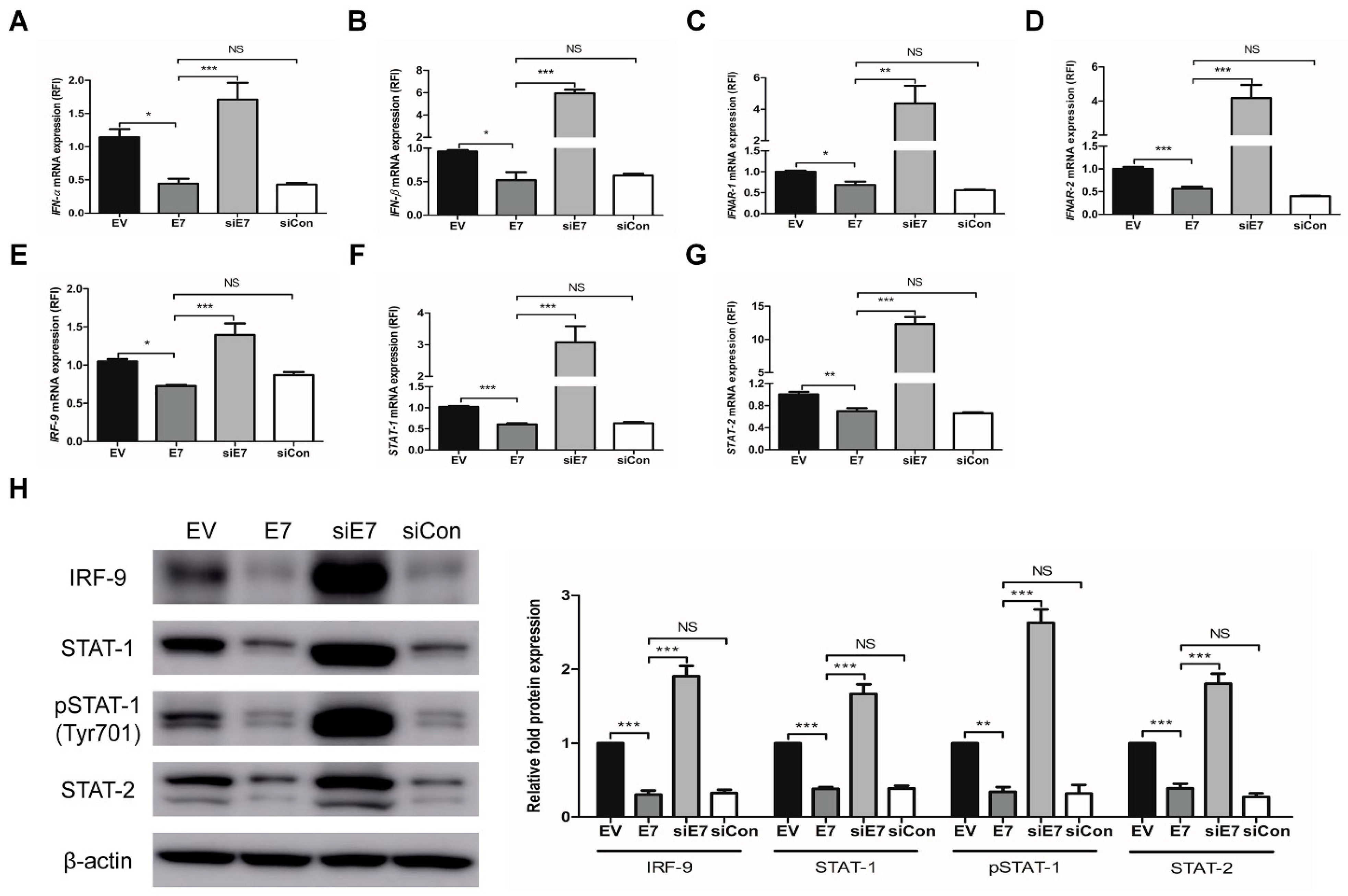
2.2. HPV-2 E7 Suppressed the Expression Of Interferon-Stimulated Genes by Down-Regulating the Type I Interferon Signaling pathway
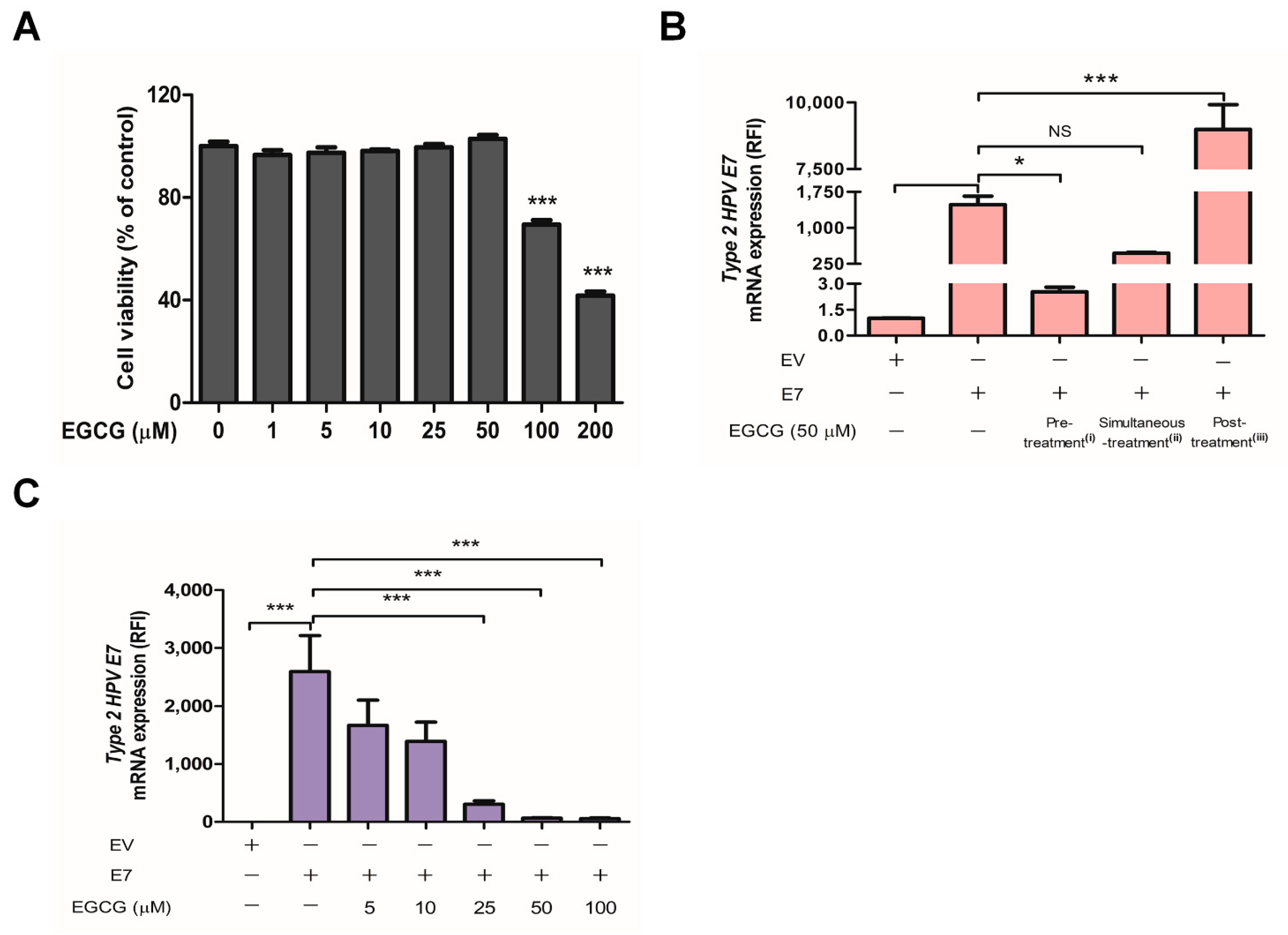
2.3. EGCG Pretreatment Inhibited HPV-2 E7 Transfection
2.4. EGCG Pretreatment Sustained the Expression of Interferon-Stimulated Genes by Blocking HPV-2 E7 Expression
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Immunohistochemistry
4.3. Cell Culture and Reagents
4.4. Cell Transfection
4.5. Cell Viability Assay
4.6. EGCG Treatment
4.7. β-Galactosidase Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.9. Western Blot
4.10. Immunofluorescence
4.11. TUNEL Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGCG | Epigallocatechin-3-gallate |
| EV | Empty vector |
| HPV | Human Papillomavirus |
| IFN | Interferon |
| IRF-9 | Interferon regulatory factors-9 |
| ISG | Interferon-stimulated gene |
| ISGF-3 | Interferon-stimulated gene factor-3 |
| ISG15 | Interferon-stimulated gene 15 |
| MxA | Myxovirus-resistance protein A |
| OAS1 | 2’-5’-oligoadenylate synthetase 1 |
| STAT | Signal Transducer and Activator of Transcription |
References
- Woodby, B.; Scott, M.; Bodily, J. Chapter Five—The Interaction Between Human Papillomaviruses and the Stromal Microenvironment. In Progress in Molecular Biology and Translational Science; Pruitt, K., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 144, pp. 169–238. [Google Scholar] [CrossRef]
- Hausen, H.Z. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. Manipulation of the innate immune response by human papillomaviruses. Virus Res. 2017, 231, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kanodia, L.M.F.A.W.M.K.S.; Fahey, L.M.; Kast, W.M. Mechanisms Used by Human Papillomaviruses to Escape the Host Immune Response. Curr. Cancer Drug Targets 2007, 7, 79–89. [Google Scholar] [CrossRef]
- Le Bon, A.; Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002, 14, 432–436. [Google Scholar] [CrossRef]
- Fensterl, V.; Chattopadhyay, S.; Sen, G.C. No Love Lost Between Viruses and Interferons. Annu. Rev. Virol. 2015, 2, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Evans, M.R.; James, C.D.; Bristol, M.L.; Nulton, T.J.; Wang, X.; Kaur, N.; White, E.A.; Windle, B.; Morgan, I.M. Human Papillomavirus 16 E2 Regulates Keratinocyte Gene Expression Relevant to Cancer and the Viral Life Cycle. J. Virol. 2018, 93. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Chang, Y.E.; Laimins, L.A. Microarray Analysis Identifies Interferon-Inducible Genes and Stat-1 as Major Transcriptional Targets of Human Papillomavirus Type 31. J. Virol. 2000, 74, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Labrecque, S.; Gauzzi, M.C.; Cuddihy, A.R.; Wong, A.H.; Pellegrini, S.; Matlashewski, G.J.; Koromilas, A.E. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-α. Oncogene 1999, 18, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Barnard, P.; McMillan, N.A. The Human Papillomavirus E7 Oncoprotein Abrogates Signaling Mediated by Interferon-α. Virology 1999, 259, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.H.; Park, Y.M. E7 protein of cutaneous human papillomavirus attenuates viperin expression in human keratinocytes. J. Dermatol. Sci. 2017, 87, 91–94. [Google Scholar] [CrossRef][Green Version]
- Lambert, J.D.; Yang, C.S. Mechanisms of Cancer Prevention by Tea Constituents. J. Nutr. 2003, 133, 3262S–3267S. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Song, S.; Wang, Y.; Gu, H. Integrity of a HPV11 infection cell model and identification of (-)-Epigallocatechin-3-gallate as a potential HPV11 inhibitor. Oncotarget 2016, 7, 37092–37102. [Google Scholar] [CrossRef]
- Nance, C.L.; Siwak, E.B.; Shearer, W.T. Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV-1 therapy. J. Allergy Clin. Immunol. 2009, 123, 459–465. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, X.; Peña, J.C.; Li, K.; Zhang, T.; Ho, W. (−)-Epigallocatechin-3-Gallate Enhances Hepatitis C Virus Double-Stranded RNA Intermediates-Triggered Innate Immune Responses in Hepatocytes. Sci. Rep. 2016, 6, 21595. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar]
- Howcroft, T.; Kirshner, S.L.; Singer, D.S. Measure of Transient Transfection Efficiency Using β-Galactosidase Protein. Anal. Biochem. 1997, 244, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, D.; Kurban, M.; Abbas, O. Plasmacytoid dendritic cells and type I interferon in the immunological response against warts. Clin. Exp. Dermatol. 2017, 42, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.; Li, C. The role of TLRs in cervical cancer with HPV infection: A review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef]
- Beglin, M.; Melar-New, M.; Laimins, L. Human Papillomaviruses and the Interferon Response. J. Interf. Cytokine Res. 2009, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ronco, L.V.; Karpova, A.Y.; Vidal, M.; Howley, P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998, 12, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kim, E.-J.; Kwon, H.-J.; Hwang, E.-S.; Namkoong, S.-E.; Um, S.-J. Inactivation of Interferon Regulatory Factor-1 Tumor Suppressor Protein by HPV E7 Oncoprotein. J. Biol. Chem. 2000, 275, 6764–6769. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Orozco, B.; Halec, G.; Rosenberger, S.; Muschik, D.; Nindl, I.; Bachmann, A.; Ritter, T.M.; Dondog, B.; Ly, R.; Bosch, F.X.; et al. Epigenetic Silencing of Interferon- in Human Papillomavirus Type 16-Positive Cells. Cancer Res. 2009, 69, 8718–8725. [Google Scholar] [CrossRef]
- Castro-Muñoz, L.J.; Manzo-Merino, J.; Muñoz-Bello, J.O.; Olmedo-Nieva, L.; Cedro-Tanda, A.; Alfaro-Ruiz, L.A.; Hidalgo-Miranda, A.; Madrid-Marina, V.; Lizano, M. The Human Papillomavirus (HPV) E1 protein regulates the expression of cellular genes involved in immune response. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Sunthamala, N.; Thierry, F.; Teissier, S.; Pientong, C.; Kongyingyoes, B.; Tangsiriwatthana, T.; Sangkomkamhang, U.; Ekalaksananan, T. E2 Proteins of High Risk Human Papillomaviruses Down-Modulate STING and IFN-κ Transcription in Keratinocytes. PLoS ONE 2014, 9, e91473. [Google Scholar] [CrossRef]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef]
- Kaihatsu, K.; Yamabe, M.; Ebara, Y. Antiviral Mechanism of Action of Epigallocatechin-3-O-gallate and Its Fatty Acid Esters. Molecules 2018, 23, 2475. [Google Scholar] [CrossRef]
- Vázquez-Calvo, Á.; De Oya, N.J.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef]
- Kesic, M.J.; Simmons, S.O.; Bauer, R.; Jaspers, I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free. Radic. Biol. Med. 2011, 51, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, C.C.; Schang, L.M. A Small Molecule Inhibits Virion Attachment to Heparan Sulfate- or Sialic Acid-Containing Glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ciesek, S.; Von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hattori, T.; Kodama, E.N. Epigallocatechin Gallate Inhibits the HIV Reverse Transcription Step. Antivir. Chem. Chemother. 2011, 21, 239–243. [Google Scholar] [CrossRef]
- Berger, S.J.; Guptab, S.; Belfi, C.A.; Gosky, D.M.; Mukhtarb, H. Green Tea Constituent (−)-Epigallocatechin-3-gallate Inhibits Topoisomerase I Activity in Human Colon Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001, 288, 101–105. [Google Scholar] [CrossRef]
- Ahn, W.S.; Huh, S.W.; Bae, S.-M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.-I. A Major Constituent of Green Tea, EGCG, Inhibits the Growth of a Human Cervical Cancer Cell Line, CaSki Cells, through Apoptosis, G1 Arrest, and Regulation of Gene Expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef]
- Jacob, J.; Cabarcas, S.; Veras, I.; Zaveri, N.; Schramm, L. The green tea component EGCG inhibits RNA polymerase III transcription. Biochem. Biophys. Res. Commun. 2007, 360, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-Y.; Lim, J.-H.; Kim, E.-J.; Kim, H.-S.; Kim, B.-J.; Kang, H.; Park, Y.-M. Detection and Typing of Human Papillomavirus in Cutaneous Common Warts by Multiplex Polymerase Chain Reaction. J. Life Sci. 2011, 21, 947–952. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Target | Sequence (5′ – 3′) | |
|---|---|---|
| HPV-2 E7 | Forward | GACCTACATTGCGACGAGCA |
| Reverse | GGACGGTTCTGCCACACTTA | |
| IFNAR-1 | Forward | TTGTGTGAAAGCCAGAGCAC |
| Reverse | TCAAGAAGACTTTCGCAGCA | |
| IFNAR-2 | Forward | CACCAGAGTTTGAGATTGTTGG |
| Reverse | GCTTGCTCATCACTGTGCTC | |
| IFN-α | Forward | CTGAATGACTTGGAAGCCTG |
| Reverse | ATTTCTGCTCTGACAACCTC | |
| IFN-β | Forward | GCAGCAGTTCCAGAAGGAG |
| Reverse | GCCAGGAGGTTCTCAACAAT | |
| IRF-9 | Forward | GTCCTGGGATGATACAGCTAAG |
| Reverse | CAGGCGAGTCTTCCAGACAG | |
| STAT-1 | Forward | AGGAAAAGCAAGCGTAATCTTCA |
| Reverse | TATTCCCCGACTGAGCCTGAT | |
| STAT-2 | Forward | GAGGAGAAGCAATGGGTCTTAG |
| Reverse | GGTCCACAACCAACGAATAGA | |
| ISG15 | Forward | ACTCATCTTTGCCAGTACAGGAG |
| Reverse | CAGCATCTTCACCGTCAGGTC | |
| OAS1 | Forward | CGGACCCTACAGGAAACTTG |
| Reverse | TGATACCTCCTGGGATCGTC | |
| MxA | Forward | ATCGGAATCTTGACGAAGCC |
| Reverse | CCCTTCTTCAGGTGGAACAC | |
| GAPDH | Forward | GAAGGTGAAGGTCGGAGTCAA |
| Reverse | GCTCCTGGAAGATGGTGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.Y.; Han, J.H.; Song, Y.; Lee, J.H.; Choi, S.Y.; Park, Y.M. Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes. Int. J. Mol. Sci. 2021, 22, 2418. https://doi.org/10.3390/ijms22052418
Song JY, Han JH, Song Y, Lee JH, Choi SY, Park YM. Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes. International Journal of Molecular Sciences. 2021; 22(5):2418. https://doi.org/10.3390/ijms22052418
Chicago/Turabian StyleSong, Ji Young, Ju Hee Han, Yumee Song, Ji Hyun Lee, Soon Yong Choi, and Young Min Park. 2021. "Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes" International Journal of Molecular Sciences 22, no. 5: 2418. https://doi.org/10.3390/ijms22052418
APA StyleSong, J. Y., Han, J. H., Song, Y., Lee, J. H., Choi, S. Y., & Park, Y. M. (2021). Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes. International Journal of Molecular Sciences, 22(5), 2418. https://doi.org/10.3390/ijms22052418






