Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia
Abstract
1. Critical Limb Ischemia
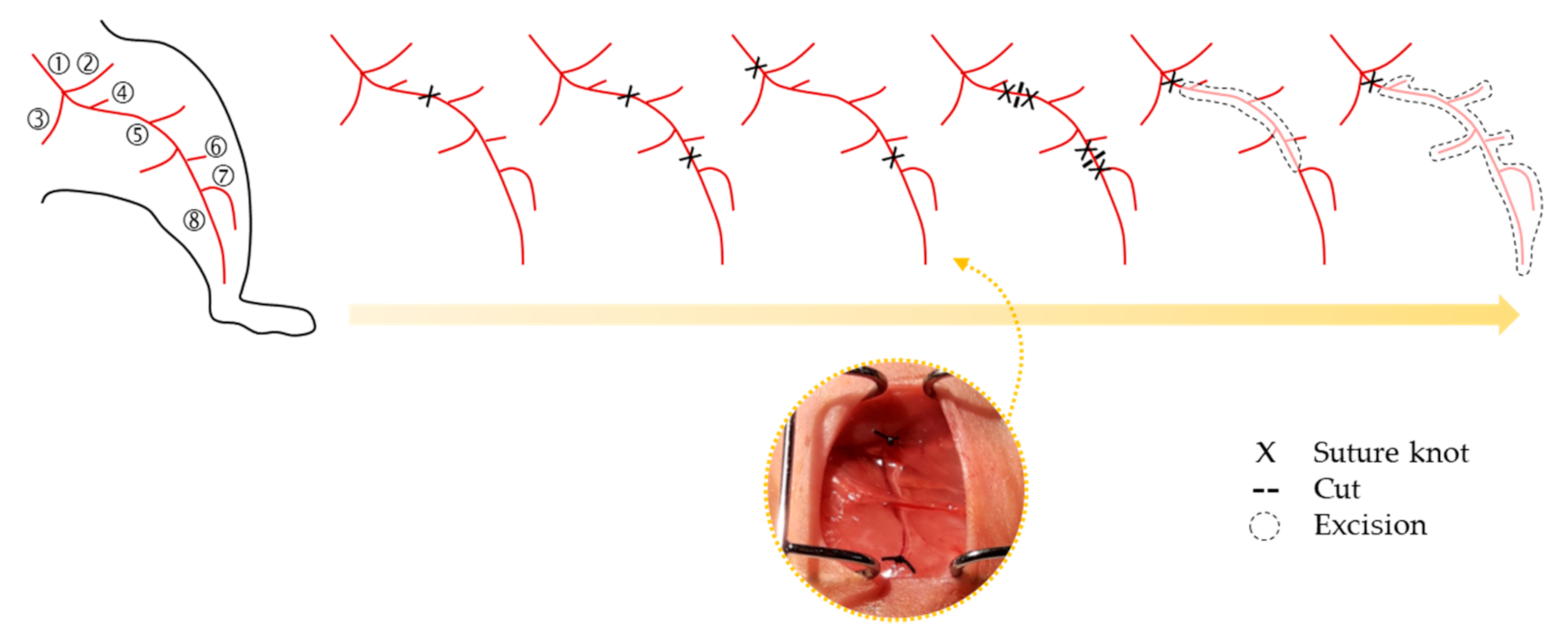
2. Animal Models of CLI
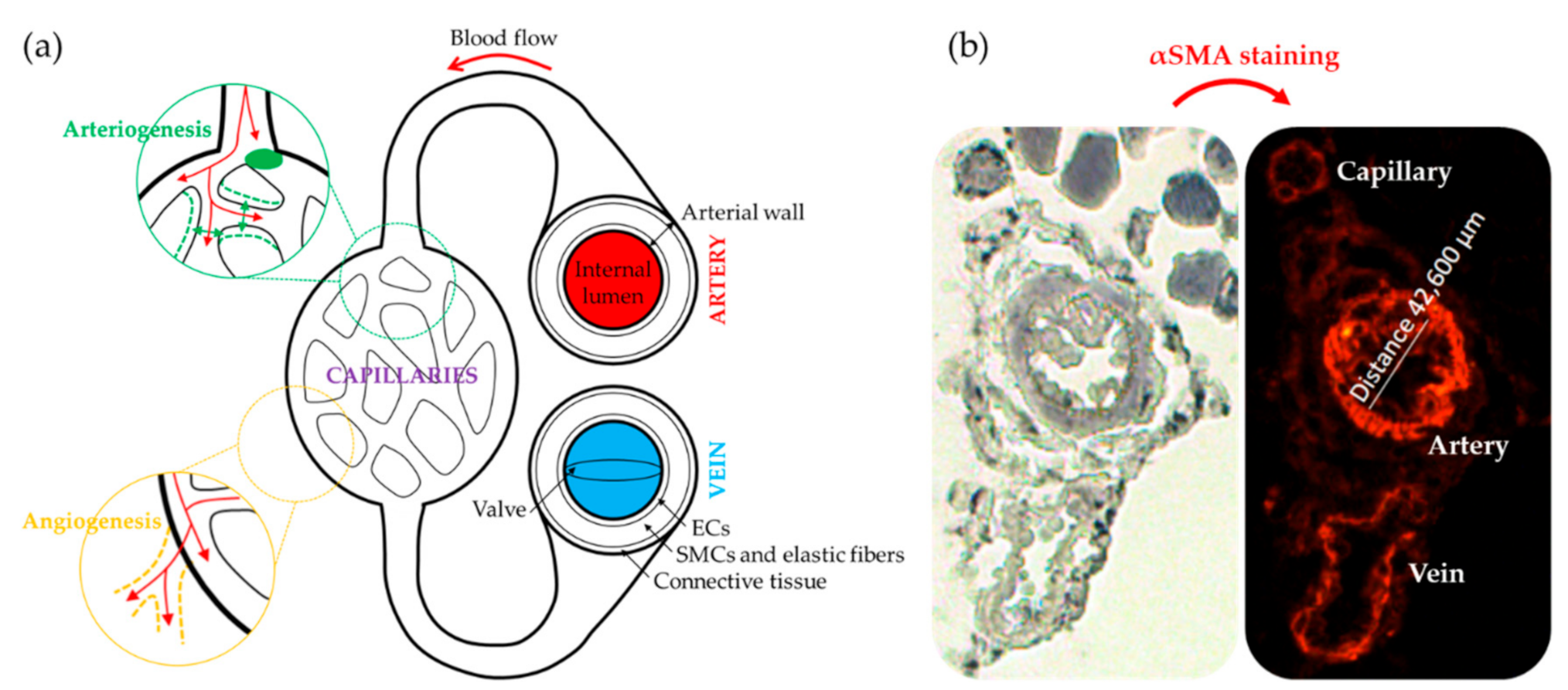
Strategies Followed to Assess Neovascularization in CLI
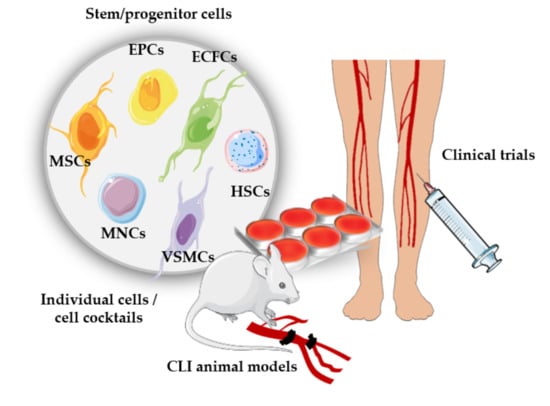
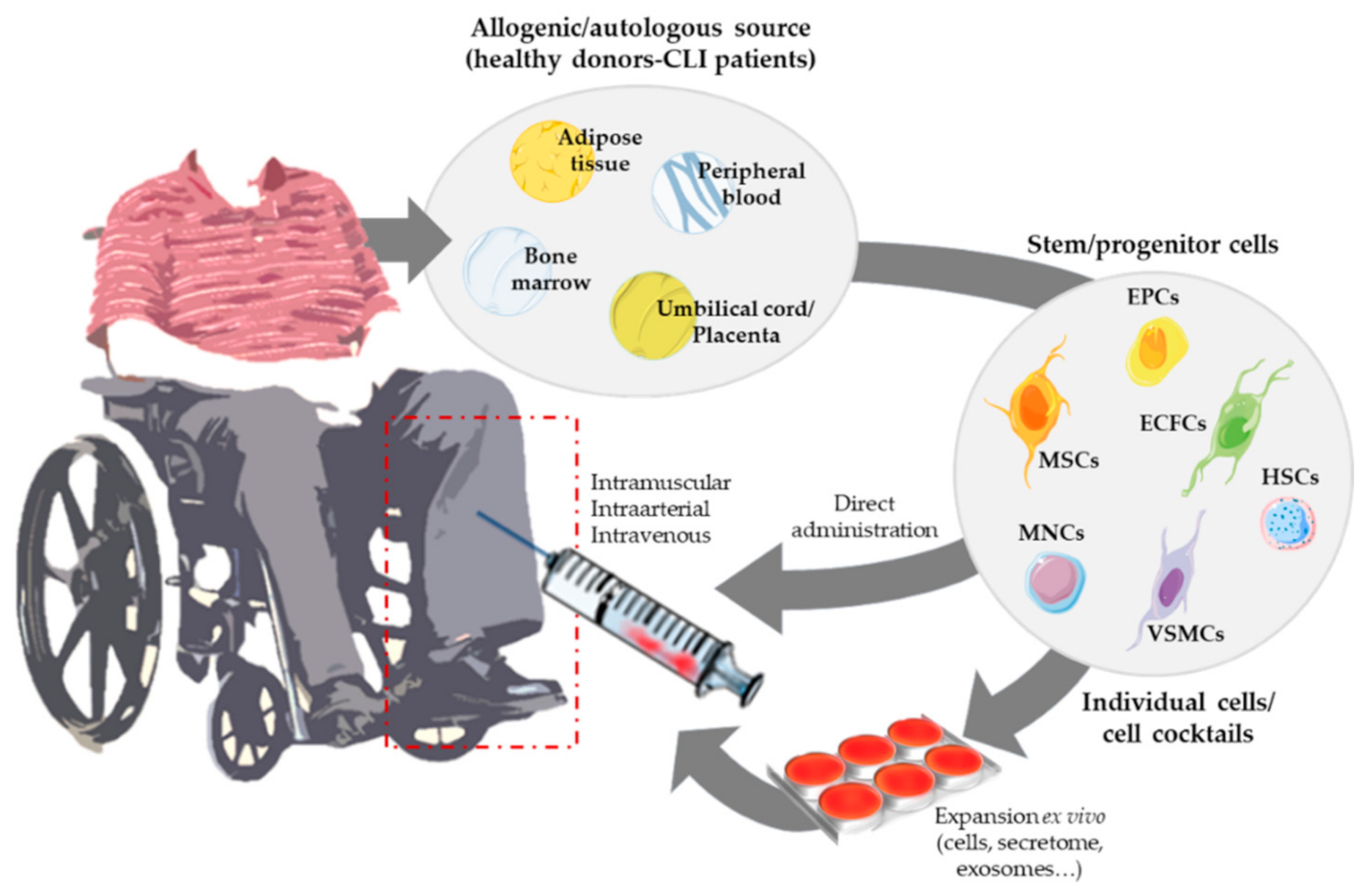
3. Angiogenic Cell Therapy
3.1. Cell Therapies Based on Single or Combined Isolated Cells
3.2. Cell Therapies Based on Cellular Cocktails
4. Clinical Trials
Limitations in Cell-based Clinical Trials
5. Strategies Derived from Cell Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a | Autologous |
| ABI | Ankle brachial index |
| aBMCs | Autologous bone marrow cell transplantation |
| AD | Arteriolar density |
| ADRCs | Adipose-derived regenerative cells |
| AFS | Amputation free survival |
| AFSCs | Amniotic fluid-derived stem cells |
| AI | Angiographic index |
| AR | Amputation rate |
| ASCs | Adipose tissue derived stem cells |
| ASO | Atherosclerosis obliterans |
| BM-MNCs | Bone marrow-derived mononuclear cells |
| BM-MSCs | Bone marrow mesenchymal stem cells |
| BM-TNCs | Bone marrow total nucleated cells |
| BFP | Blood flow perfusion |
| CACs | Circulating angiogenic cells |
| CBF | Calf blood pressure |
| CD | Capillary density |
| CM | Conditioned medium |
| CLI | Critical limb ischemia |
| CVDs | Cardiovascular diseases |
| CVF | Collateral vessel formation |
| DM | Diabetes mellitus |
| DR | Death rate |
| ECFCs | Endothelial colony forming cells |
| ECs | Endothelial cells |
| ECEPCs | Enriched circulating endothelial progenitor cells |
| EPCs | Endothelial progenitor cells |
| ESC-ECP | Stem cell-derived endothelial cell product |
| FAL | Femoral artery ligation |
| FGF1 | Fibroblast growth factor 1 |
| FS | Functional score |
| G-CSF | Granulocyte colony-stimulating factor |
| HD | High dose |
| HGF | Hepatocyte growth factor |
| HIF-1a | Hypoxia-inducible factor 1-alpha |
| HPCs | Hematopoietic progenitor cells |
| h | Human |
| IA | Intraarterial |
| IC | Intracardiac |
| IHC | Immunohistochemistry |
| IM | Intramuscular |
| IV | Intravenous |
| LD | Low dose |
| LDP | Laser Doppler Perfusion |
| MACs | Myeloid angioenic cells |
| MD | Medium dose |
| MMPs | Matrix metalloproteinases |
| MP | Matrigel plug |
| MSCs | Mesenchymal stem cells |
| NC | Non-controlled |
| NO | Nitric oxide |
| NR | Non-randomized |
| PAD | Peripheral arterial disease |
| PB-MNCs | Peripheral blood mononuclear cells |
| PFWD | Pain-free walking distance |
| PRP | Platelet-rich plasma |
| RCT | Randomized controlled trial |
| RPS | Rest pain score |
| SC | Subcutaneous |
| SMCs | Smooth muscle cells |
| SMPCs | Smooth muscle progenitor cells |
| SVF | Stromal vascular fraction |
| TAO | Thrombo-angiitis obliterans |
| TcPO2 | Transcutaneous oxygen pressure |
| TR | Tissue regeneration |
| UH | Ulcer healing |
| VAS | Visual analogue scale |
| VD | Vessel diameter |
| VEGF | Vascular endothelial growth factor |
| VIP | Vascular intersection percentage |
| VS | Visual Scale |
| VSEL | Very small embryonic-like stem cells |
References
- Conte, S.M.; Vale, P.R. Peripheral Arterial Disease. Heart Lung Circ. 2018, 27, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Maung, U.K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113 Pt A, 600–609. [Google Scholar] [CrossRef] [PubMed]
- van Weel, V.; van Tongeren, R.B.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Vascular growth in ischemic limbs: A review of mechanisms and possible therapeutic stimulation. Ann. Vasc. Surg. 2008, 22, 582–597. [Google Scholar] [CrossRef]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Hao, C.; Shintani, S.; Shimizu, Y.; Kondo, K.; Ishii, M.; Wu, H.; Murohara, T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: Comparison with bone marrow mononuclear cells. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H869–H879. [Google Scholar] [CrossRef]
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care 2001, 24, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Chusney, G.D.; Thomas, S.M.; Burt, D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000, 67, 291–300. [Google Scholar] [CrossRef]
- Nehler, M.R.; Duval, S.; Diao, L.; Annex, B.H.; Hiatt, W.R.; Rogers, K.; Zakharyan, A.; Hirsch, A.T. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J. Vasc. Surg. 2014, 60, 686–695 e2. [Google Scholar] [CrossRef]
- Suggested standards for reports dealing with lower extremity ischemia. Prepared by the Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery/North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1986, 4, 80–94.
- Fontaine, R.; Kim, M.; Kieny, R. [Surgical treatment of peripheral circulation disorders]. Helv. Chir. Acta. 1954, 21, 499–533. [Google Scholar]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Becker, F.; Robert-Ebadi, H.; Ricco, J.B.; Setacci, C.; Cao, P.; de Donato, G.; Eckstein, H.H.; De Rango, P.; Diehm, N.; Schmidli, J.; et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. 2), S4–S12. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 2000, 31 Pt 2, S1–S296. [Google Scholar] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Teraa, M.; Conte, M.S.; Moll, F.L.; Verhaar, M.C. Critical Limb Ischemia: Current Trends and Future Directions. J. Am. Heart Assoc. 2016, 5, e002938. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Pomposelli, F.B. Society for Vascular Surgery Practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J. Vasc. Surg. 2015, 61 (Suppl. 3), 1S. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar]
- Simpson, E.L.; Kearns, B.; Stevenson, M.D.; Cantrell, A.J.; Littlewood, C.; Michaels, J.A. Enhancements to angioplasty for peripheral arterial occlusive disease: Systematic review, cost-effectiveness assessment and expected value of information analysis. Health Technol. Assess. 2014, 18, 1–252. [Google Scholar] [CrossRef]
- Walter, D.H.; Krankenberg, H.; Balzer, J.O.; Kalka, C.; Baumgartner, I.; Schluter, M.; Tonn, T.; Seeger, F.; Dimmeler, S.; Lindhoff-Last, E.; et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA). Circ. Cardiovasc. Interv. 2011, 4, 26–37. [Google Scholar] [CrossRef]
- Lawall, H.; Zemmrich, C.; Bramlage, P.; Amann, B. Health related quality of life in patients with critical limb ischemia. Vasa 2012, 41, 78–88. [Google Scholar] [CrossRef]
- Patel, R.S. Team Approach to Critical Limb Ischemia Care and Research. Tech. Vasc Interv. Radiol. 2016, 19, 101–103. [Google Scholar] [CrossRef]
- Setacci, C.; de Donato, G.; Teraa, M.; Moll, F.L.; Ricco, J.B.; Becker, F.; Robert-Ebadi, H.; Cao, P.; Eckstein, H.H.; De Rango, P.; et al. Chapter IV: Treatment of critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 2011, 42 (Suppl. 2), S43–S59. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Schreve, M.A.; Ferraresi, R.; van den Heuvel, D.A.F.; Unlu, C.; Cabane, V.; Kum, S. Surgical and endovascular venous arterialization for treatment of critical limb ischaemia. Vasa 2018, 47, 17–22. [Google Scholar] [CrossRef]
- Duff, S.; Mafilios, M.S.; Bhounsule, P.; Hasegawa, J.T. The burden of critical limb ischemia: A review of recent literature. Vasc Health Risk Manag. 2019, 15, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Spreen, M.I.; Gremmels, H.; Teraa, M.; Sprengers, R.W.; Verhaar, M.C.; Statius van Eps, R.G.; de Vries, J.P.; Mali, W.P.; van Overhagen, H.; Padi; et al. Diabetes Is Associated with Decreased Limb Survival in Patients With Critical Limb Ischemia: Pooled Data From Two Randomized Controlled Trials. Diabetes Care 2016, 39, 2058–2064. [Google Scholar] [CrossRef]
- Howangyin, K.Y.; Silvestre, J.S. Diabetes mellitus and ischemic diseases: Molecular mechanisms of vascular repair dysfunction. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Ouma, G.O.; Zafrir, B.; Mohler, E.R., 3rd; Flugelman, M.Y. Therapeutic angiogenesis in critical limb ischemia. Angiology 2013, 64, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.; Hiatt, W.R.; Baumgartner, I.; Driver, I.V.; Nikol, S.; Norgren, L.; Van Belle, E.; TAMRIS Committees and Investigators. Effect of fibroblast growth factor NV1FGF on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011, 377, 1929–1937. [Google Scholar] [CrossRef]
- Matoba, S.; Tatsumi, T.; Murohara, T.; Imaizumi, T.; Katsuda, Y.; Ito, M.; Saito, Y.; Uemura, S.; Suzuki, H.; Fukumoto, S.; et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am. Heart J. 2008, 156, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.J.; Goodney, P.; Mendelsohn, F.O.; Moen, E.K.; Annex, B.H.; Investigators, H.G.F.T. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: Results of the HGF-0205 trial. J. Vasc. Surg. 2010, 52, 1525–1530. [Google Scholar] [CrossRef]
- van Royen, N.; Schirmer, S.H.; Atasever, B.; Behrens, C.Y.; Ubbink, D.; Buschmann, E.E.; Voskuil, M.; Bot, P.; Hoefer, I.; Schlingemann, R.O.; et al. START Trial: A pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation 2005, 112, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Bandyk, D.F. Therapeutic angiogenesis for critical limb ischemia. Semin. Vasc. Surg. 2014, 27, 23–31. [Google Scholar] [CrossRef]
- Lee, C.W.; Stabile, E.; Kinnaird, T.; Shou, M.; Devaney, J.M.; Epstein, S.E.; Burnett, M.S. Temporal patterns of gene expression after acute hindlimb ischemia in mice: Insights into the genomic program for collateral vessel development. J. Am. Coll. Cardiol. 2004, 43, 474–482. [Google Scholar] [CrossRef]
- Westvik, T.S.; Fitzgerald, T.N.; Muto, A.; Maloney, S.P.; Pimiento, J.M.; Fancher, T.T.; Magri, D.; Westvik, H.H.; Nishibe, T.; Velazquez, O.C.; et al. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J. Vasc. Surg. 2009, 49, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Brechot, N.; Gomez, E.; Bignon, M.; Khallou-Laschet, J.; Dussiot, M.; Cazes, A.; Alanio-Brechot, C.; Durand, M.; Philippe, J.; Silvestre, J.S.; et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS ONE 2008, 3, e3950. [Google Scholar] [CrossRef]
- Crawford, R.S.; Albadawi, H.; Robaldo, A.; Peck, M.A.; Abularrage, C.J.; Yoo, H.J.; Lamuraglia, G.M.; Watkins, M.T. Divergent systemic and local inflammatory response to hind limb demand ischemia in wild-type and ApoE-/- mice. J. Surg. Res. 2013, 183, 952–962. [Google Scholar] [CrossRef][Green Version]
- Rishi, M.T.; Selvaraju, V.; Thirunavukkarasu, M.; Shaikh, I.A.; Takeda, K.; Fong, G.H.; Palesty, J.A.; Sanchez, J.A.; Maulik, N. Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3) stabilizes hypoxia inducible factor-1 alpha, promotes neovascularization, and improves perfusion in a murine model of hind-limb ischemia. Microvasc. Res. 2015, 97, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tie, G.; Park, B.; Yan, Y.; Nowicki, P.T.; Messina, L.M. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: Roles of endothelial nitric oxide synthase and endothelial progenitor cells. J. Vasc. Surg. 2009, 50, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- MacAskill, M.G.; Saif, J.; Condie, A.; Jansen, M.A.; MacGillivray, T.J.; Tavares, A.A.S.; Fleisinger, L.; Spencer, H.L.; Besnier, M.; Martin, E.; et al. Robust Revascularization in Models of Limb Ischemia Using a Clinically Translatable Human Stem Cell-Derived Endothelial Cell Product. Mol. Ther. 2018, 26, 1669–1684. [Google Scholar] [CrossRef]
- Rojas-Torres, M.; Jiménez-Palomares, M.; Martín-Ramírez, J.; Beltrán-Camacho, L.; Sánchez-Gomar, I.; Eslava-Alcon, S.; Rosal-Vela, A.; Gavaldá, S.; Durán-Ruiz, M.C. REX-001, a BM-MNC Enriched Solution, Induces Revascularization of Ischemic Tissues in a Murine Model of Chronic Limb-Threatening Ischemia. Front. Cell Dev. Biol. 2020, 8, 1546. [Google Scholar] [CrossRef]
- Creane, M.; Howard, L.; O’Brien, T.; Coleman, C.M. Biodistribution and retention of locally administered human mesenchymal stromal cells: Quantitative polymerase chain reaction-based detection of human DNA in murine organs. Cytotherapy 2017, 19, 384–394. [Google Scholar] [CrossRef]
- Thomas, D.; Thirumaran, A.; Mallard, B.; Chen, X.; Browne, S.; Wheatley, A.M.; O’Brien, T.; Pandit, A. Variability in Endogenous Perfusion Recovery of Immunocompromised Mouse Models of Limb Ischemia. Tissue. Eng. Part C Methods 2016, 22, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fukuyama, N.; Aki, A.; Kanabuchi, K.; Kimura, K.; Taira, H.; Tanaka, E.; Wakana, N.; Mori, H.; Inoue, H. Search for appropriate experimental methods to create stable hind-limb ischemia in mouse. Tokai J. Exp. Clin. Med. 2006, 31, 128–132. [Google Scholar]
- Krishna, S.M.; Omer, S.M.; Golledge, J. Evaluation of the clinical relevance and limitations of current pre-clinical models of peripheral artery disease. Clin. Sci. 2016, 130, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Hellingman, A.A.; Bastiaansen, A.J.; de Vries, M.R.; Seghers, L.; Lijkwan, M.A.; Lowik, C.W.; Hamming, J.F.; Quax, P.H. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Camacho, L.; Jimenez-Palomares, M.; Rojas-Torres, M.; Sanchez-Gomar, I.; Rosal-Vela, A.; Eslava-Alcon, S.; Perez-Segura, M.C.; Serrano, A.; Antequera-Gonzalez, B.; Alonso-Pinero, J.A.; et al. Identification of the initial molecular changes in response to circulating angiogenic cells-mediated therapy in critical limb ischemia. Stem. Cell. Res. Ther. 2020, 11, 106. [Google Scholar] [CrossRef]
- Kochi, T.; Imai, Y.; Takeda, A.; Watanabe, Y.; Mori, S.; Tachi, M.; Kodama, T. Characterization of the arterial anatomy of the murine hindlimb: Functional role in the design and understanding of ischemia models. PLoS ONE 2013, 8, e84047. [Google Scholar] [CrossRef]
- Aref, Z.; de Vries, M.R.; Quax, P.H.A. Variations in Surgical Procedures for Inducing Hind Limb Ischemia in Mice and the Impact of These Variations on Neovascularization Assessment. Int. J. Mol. Sci. 2019, 20, 3704. [Google Scholar] [CrossRef]
- Fukino, K.; Sata, M.; Seko, Y.; Hirata, Y.; Nagai, R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res. Commun. 2003, 310, 143–147. [Google Scholar] [CrossRef]
- Nossent, A.Y.; Bastiaansen, A.J.; Peters, E.A.; de Vries, M.R.; Aref, Z.; Welten, S.M.; de Jager, S.C.; van der Pouw Kraan, T.C.; Quax, P.H. CCR7-CCL19/CCL21 Axis is Essential for Effective Arteriogenesis in a Murine Model of Hindlimb Ischemia. J. Am. Heart Assoc. 2017, 6, e005281. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Choquet, P.; Thaveau, F.; Singh, F.; Schlagowski, A.; Charles, A.L.; Laverny, G.; Metzger, D.; Zoll, J.; Chakfe, N.; et al. A new murine model of sustainable and durable chronic critical limb ischemia fairly mimicking human pathology. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Omer, S.M.; Li, J.; Morton, S.K.; Jose, R.J.; Golledge, J. Development of a two-stage limb ischemia model to better simulate human peripheral artery disease. Sci. Rep. 2020, 10, 3449. [Google Scholar] [CrossRef]
- Han, S.S.; Jin, Z.; Lee, B.S.; Han, J.S.; Choi, J.J.; Park, S.J.; Chung, H.M.; Mukhtar, A.S.; Moon, S.H.; Kang, S.W. Reproducible hindlimb ischemia model based on photochemically induced thrombosis to evaluate angiogenic effects. Microvasc. Res. 2019, 126, 103912. [Google Scholar] [CrossRef]
- Parikh, P.P.; Castilla, D.; Lassance-Soares, R.M.; Shao, H.; Regueiro, M.; Li, Y.; Vazquez-Padron, R.; Webster, K.A.; Liu, Z.J.; Velazquez, O.C. A Reliable Mouse Model of Hind limb Gangrene. Ann. Vasc. Surg. 2018, 48, 222–232. [Google Scholar] [CrossRef]
- Sligar, A.D.; Howe, G.; Goldman, J.; Felli, P.; Karanam, V.; Smalling, R.W.; Baker, A.B. Preclinical Model of Hind Limb Ischemia in Diabetic Rabbits. J. Vis. Exp. 2019, 148, e58964. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Kirton, J.P.; Xu, Q. Vascular repair by endothelial progenitor cells. Cardiovasc. Res. 2008, 78, 413–421. [Google Scholar] [CrossRef]
- van der Kwast, R.; Quax, P.H.A.; Nossent, A.Y. An Emerging Role for isomiRs and the microRNA Epitranscriptome in Neovascularization. Cells. 2019, 9, 61. [Google Scholar] [CrossRef]
- Cooke, J.P.; Meng, S. Vascular Regeneration in Peripheral Artery Disease. Arterioscler Thromb. Vasc. Biol. 2020, 40, 1627–1634. [Google Scholar] [CrossRef]
- Czapla, J.; Cichon, T.; Pilny, E.; Jarosz-Biej, M.; Matuszczak, S.; Drzyzga, A.; Krakowczyk, L.; Smolarczyk, R. Adipose tissue-derived stromal cells stimulated macrophages-endothelial cells interactions promote effective ischemic muscle neovascularization. Eur. J. Pharmacol. 2020, 883, 173354. [Google Scholar] [CrossRef]
- Lin, R.Z.; Lee, C.N.; Moreno-Luna, R.; Neumeyer, J.; Piekarski, B.; Zhou, P.; Moses, M.A.; Sachdev, M.; Pu, W.T.; Emani, S.; et al. Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat. Biomed. Eng. 2017, 1. [Google Scholar]
- Limbourg, A.; von Felden, J.; Jagavelu, K.; Krishnasamy, K.; Napp, L.C.; Kapopara, P.R.; Gaestel, M.; Schieffer, B.; Bauersachs, J.; Limbourg, F.P.; et al. MAP-Kinase Activated Protein Kinase 2 Links Endothelial Activation and Monocyte/macrophage Recruitment in Arteriogenesis. PLoS ONE 2015, 10, e0138542. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Chen, Y.C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef]
- Powell, R.J.; Simons, M.; Mendelsohn, F.O.; Daniel, G.; Henry, T.D.; Koga, M.; Morishita, R.; Annex, B.H. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 2008, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S.; Alitalo, K. Gene transfer as a tool to induce therapeutic vascular growth. Nat. Med. 2003, 9, 694–701. [Google Scholar] [CrossRef]
- Menasche, P. Cell therapy for peripheral arterial disease. Curr. Opin. Mol. Ther. 2010, 12, 538–545. [Google Scholar] [PubMed]
- Schmidt, C.A.; Amorese, A.J.; Ryan, T.E.; Goldberg, E.J.; Tarpey, M.D.; Green, T.D.; Karnekar, R.R.; Yamaguchi, D.J.; Spangenburg, E.E.; McClung, J.M. Strain-Dependent Variation in Acute Ischemic Muscle Injury. Am. J. Pathol. 2018, 188, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Kawamoto, A. Endothelial progenitor cells for postnatal vasculogenesis. Am. J. Physiol. Cell Physiol. 2004, 287, C572–C579. [Google Scholar] [CrossRef]
- Cunha, F.F.; Martins, L.; Martin, P.K.; Stilhano, R.S.; Han, S.W. A comparison of the reparative and angiogenic properties of mesenchymal stem cells derived from the bone marrow of BALB/c and C57/BL6 mice in a model of limb ischemia. Stem. Cell Res. Ther. 2013, 4, 86. [Google Scholar] [CrossRef]
- Garcia-Vazquez, M.D.; Herrero de la Parte, B.; Garcia-Alonso, I.; Morales, M.C. Analysis of Biological Properties of Human Adult Mesenchymal Stem Cells and Their Effect on Mouse Hind Limb Ischemia]. J. Vasc. Res. 2019, 56, 77–91. [Google Scholar] [CrossRef]
- Nammian, P.; Asadi-Yousefabad, S.L.; Daneshi, S.; Sheikhha, M.H.; Tabei, S.M.B.; Razban, V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem. Cell Res. Ther. 2021, 12, 58. [Google Scholar] [CrossRef]
- Rossi, E.; Smadja, D.; Goyard, C.; Cras, A.; Dizier, B.; Bacha, N.; Lokajczyk, A.; Guerin, C.L.; Gendron, N.; Planquette, B.; et al. Co-injection of mesenchymal stem cells with endothelial progenitor cells accelerates muscle recovery in hind limb ischemia through an endoglin-dependent mechanism. Thromb. Haemost. 2017, 117, 1908–1918. [Google Scholar] [CrossRef]
- Lian, W.; Hu, X.; Pan, L.; Han, S.; Cao, C.; Jia, Z.; Li, M. Human primary CD34(+) cells transplantation for critical limb ischemia. J. Clin. Lab. Anal. 2018, 32, e22569. [Google Scholar] [CrossRef] [PubMed]
- Kalka, C.; Masuda, H.; Takahashi, T.; Kalka-Moll, W.M.; Silver, M.; Kearney, M.; Li, T.; Isner, J.M.; Asahara, T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 2000, 97, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Heeschen, C.; Aicher, A.; Dernbach, E.; Zeiher, A.M.; Dimmeler, S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation 2003, 108, 2511–2516. [Google Scholar] [CrossRef]
- Zhao, W.N.; Xu, S.Q.; Liang, J.F.; Peng, L.; Liu, H.L.; Wang, Z.; Fang, Q.; Wang, M.; Yin, W.Q.; Zhang, W.J.; et al. Endothelial progenitor cells from human fetal aorta cure diabetic foot in a rat model. Metabolism 2016, 65, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Foubert, P.; Matrone, G.; Souttou, B.; Lere-Dean, C.; Barateau, V.; Plouet, J.; Le Ricousse-Roussanne, S.; Levy, B.I.; Silvestre, J.S.; Tobelem, G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ. Res. 2008, 103, 751–760. [Google Scholar] [CrossRef]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Sasaki, K.; Duan, J.; Imaizumi, T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001, 103, 897–903. [Google Scholar] [CrossRef]
- de Nigris, F.; Williams-Ignarro, S.; Sica, V.; D’Armiento, F.P.; Lerman, L.O.; Byrns, R.E.; Sica, G.; Fiorito, C.; Ignarro, L.J.; Napoli, C. Therapeutic effects of concurrent autologous bone marrow cell infusion and metabolic intervention in ischemia-induced angiogenesis in the hypercholesterolemic mouse hindlimb. Int. J. Cardiol. 2007, 117, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Song, S.J.; Bhang, S.H.; Choi, C.Y.; Kim, M.J.; Kim, B.S. Additive effect of endothelial progenitor cell mobilization and bone marrow mononuclear cell transplantation on angiogenesis in mouse ischemic limbs. J. Biomed. Sci. 2007, 14, 323–330. [Google Scholar] [CrossRef]
- Gan, L.; Matsuura, H.; Ichiki, T.; Yin, X.; Miyazaki, R.; Hashimoto, T.; Cui, J.; Takeda, K.; Sunagawa, K. Improvement of neovascularization capacity of bone marrow mononuclear cells from diabetic mice by ex vivo pretreatment with resveratrol. Hypertens. Res. 2009, 32, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Terry, T.; McNatt, J.M.; Willerson, J.T.; Zoldhelyi, P. Intra-arterial transplantation of adult bone marrow cells restores blood flow and regenerates skeletal muscle in ischemic limbs. Vasc. Endovascular. Surg. 2009, 43, 433–443. [Google Scholar] [CrossRef]
- Brenes, R.A.; Jadlowiec, C.C.; Bear, M.; Hashim, P.; Protack, C.D.; Li, X.; Lv, W.; Collins, M.J.; Dardik, A. Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis. J. Vasc. Surg. 2012, 56, 1669–1679; discussion 1679. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.E.; de Carvalho, L.P.; Yasumura, E.; da Silva, F.H.; Garcia, B.C.; Beutel, A.; Sacramento, C.B.; Baptista-Silva, J.C.; de Campos, R.R.; Takiya, C.M.; et al. Impact of angiogenic therapy in the treatment of critical lower limb ischemia in an animal model. Vasc. Endovascular. Surg. 2014, 48, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, G.; Nishinakamura, H.; Kojima, D.; Tashiro, T.; Kodama, S. GM-CSF treated F4/80+ BMCs improve murine hind limb ischemia similar to M-CSF differentiated macrophages. PLoS ONE 2014, 9, e106987. [Google Scholar] [CrossRef] [PubMed]
- Capoccia, B.J.; Robson, D.L.; Levac, K.D.; Maxwell, D.J.; Hohm, S.A.; Neelamkavil, M.J.; Bell, G.I.; Xenocostas, A.; Link, D.C.; Piwnica-Worms, D.; et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood 2009, 113, 5340–5351. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.; D’Ippolito, G.; Gomez, L.A.; Reiner, T.; Vazquez-Padron, R.I.; Perez-Stable, C.; Roos, B.A.; Pham, S.M.; Schiller, P.C. Human marrow-isolated adult multilineage-inducible (MIAMI) cells protect against peripheral vascular ischemia in a mouse model. Cytotherapy 2011, 13, 179–192. [Google Scholar] [CrossRef]
- Li, S.; Zhou, B.; Han, Z.C. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between CD34+ and CD34- mononuclear cells. Thromb. Haemost. 2006, 95, 301–311. [Google Scholar] [PubMed]
- Padilla, L.; Arguero-Sanchez, R.; Rodriguez-Trejo, J.M.; Carranza-Castro, P.H.; Suarez-Cuenca, J.A.; Polaco-Castillo, J.; DiSilvio-Lopez, M.; Lopez-Gutierrez, J.; Olguin-Juarez, H.; Hernandez-Patricio, A.; et al. Effect of autologous transplant of peripheral blood mononuclear cells in combination with proangiogenic factors during experimental revascularization of lower limb ischemia. J. Tissue. Eng. Regen. Med. 2020, 14, 600–608. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, P.; Qin, J.; Wu, X.; Wang, X.; Yang, X.; Li, B.; Zhang, W.; Ye, K.; Peng, Z.; et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1alpha/IL-10 pathway. Stem. Cells 2020, 38, 1307–1320. [Google Scholar] [PubMed]
- Rybalko, V.; Hsieh, P.L.; Ricles, L.M.; Chung, E.; Farrar, R.P.; Suggs, L.J. Therapeutic potential of adipose-derived stem cells and macrophages for ischemic skeletal muscle repair. Regen. Med. 2017, 12, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Chae, D.S.; Son, M.; Kim, S.W. Angiogenic characteristics of human stromal vascular fraction in ischemic hindlimb. Int. J. Cardiol. 2017, 234, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Prather, W.R.; Toren, A.; Meiron, M.; Ofir, R.; Tschope, C.; Horwitz, E.M. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy 2009, 11, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zahavi-Goldstein, E.; Blumenfeld, M.; Fuchs-Telem, D.; Pinzur, L.; Rubin, S.; Aberman, Z.; Sher, N.; Ofir, R. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy 2017, 19, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Soria-Juan, B.; Escacena, N.; Capilla-Gonzalez, V.; Aguilera, Y.; Llanos, L.; Tejedo, J.R.; Bedoya, F.J.; Juan, V.; De la Cuesta, A.; Ruiz-Salmeron, R.; et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front Immunol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, L.; Lian, C.; Qi, Y.; Li, W.; Wang, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Relieve Hind Limb Ischemia by Promoting Angiogenesis in Mice. Stem. Cells Dev. 2019, 28, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.A.; Naik, C.; Cahill, P.A.; Bhonde, R.R. Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis. Cell Mol. Life Sci. 2020, 77, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Maione, C.; Botti, C.; Coppola, A.; Silvestroni, A.; Lillo, S.; Schiavone, V.; Molinari, A.M.; Sica, V. Beneficial effects of VEGF secreted from stromal cells in supporting endothelial cell functions: Therapeutic implications for critical limb ischemia. Cell Transplant. 2010, 19, 1425–1437. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.J.; Li, Y.; Gao, Y. Mesenchymal Stem Cells Decrease M1/M2 Ratio and Alleviate Inflammation to Improve Limb Ischemia in Mice. Med. Sci. Monit. 2020, 26, e923287. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Patel, J.; Donovan, P.; Khosrotehrani, K. Concise Review: Functional Definition of Endothelial Progenitor Cells: A Molecular Perspective. Stem Cells Transl. Med. 2016, 5, 1302–1306. [Google Scholar] [CrossRef]
- Edwards, N.; Langford-Smith, A.W.W.; Wilkinson, F.L.; Alexander, M.Y. Endothelial Progenitor Cells: New Targets for Therapeutics for Inflammatory Conditions with High Cardiovascular Risk. Front. Med. 2018, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.H.M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and prospects. Stem. Cell Int. 2018, 2018, 24. [Google Scholar] [CrossRef]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem. Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Stitt, A.W.; O’Neill, C.L.; O’Doherty, M.T.; Archer, D.B.; Gardiner, T.A.; Medina, R.J. Vascular stem cells and ischaemic retinopathies. Prog. Retin. Eye. Res. 2011, 30, 149–166. [Google Scholar] [CrossRef]
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ. Res. 2004, 94, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Eslava-Alcon, S.; Extremera-Garcia, M.J.; Sanchez-Gomar, I.; Beltran-Camacho, L.; Rosal-Vela, A.; Munoz, J.; Ibarz, N.; Alonso-Pinero, J.A.; Rojas-Torres, M.; Jimenez-Palomares, M.; et al. Atherosclerotic Pre-Conditioning Affects the Paracrine Role of Circulating Angiogenic Cells Ex-Vivo. Int. J. Mol. Sci. 2020, 21, 5256. [Google Scholar] [CrossRef]
- Le Ricousse-Roussanne, S.; Barateau, V.; Contreres, J.O.; Boval, B.; Kraus-Berthier, L.; Tobelem, G. Ex vivo differentiated endothelial and smooth muscle cells from human cord blood progenitors home to the angiogenic tumor vasculature. Cardiovasc. Res. 2004, 62, 176–184. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Zuba-Surma, E.K.; Machalinski, B.; Ratajczak, J.; Kucia, M. Very small embryonic-like (VSEL) stem cells: Purification from adult organs, characterization, and biological significance. Stem. Cell Rev. 2008, 4, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Amann, B.; Luedemann, C.; Ratei, R.; Schmidt-Lucke, J.A. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009, 18, 371–380. [Google Scholar] [CrossRef]
- Fadini, G.P.; Agostini, C.; Avogaro, A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010, 209, 10–17. [Google Scholar] [CrossRef]
- Guo, J.; Guo, L.; Cui, S.; Tong, Z.; Dardik, A.; Gu, Y. Autologous bone marrow-derived mononuclear cell therapy in Chinese patients with critical limb ischemia due to thromboangiitis obliterans: 10-year results. Stem. Cell Res. Ther. 2018, 9, 43. [Google Scholar] [CrossRef]
- Idei, N.; Soga, J.; Hata, T.; Fujii, Y.; Fujimura, N.; Mikami, S.; Maruhashi, T.; Nishioka, K.; Hidaka, T.; Kihara, Y.; et al. Limb ischemia: A comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ. Cardiovasc. Interv. 2011, 4, 15–25. [Google Scholar] [CrossRef]
- Liang, T.W.; Jester, A.; Motaganahalli, R.L.; Wilson, M.G.; G’Sell, P.; Akingba, G.A.; Fajardo, A.; Murphy, M.P. Autologous bone marrow mononuclear cell therapy for critical limb ischemia is effective and durable. J. Vasc. Surg. 2016, 63, 1541–1545. [Google Scholar] [CrossRef][Green Version]
- Murphy, M.P.; Lawson, J.H.; Rapp, B.M.; Dalsing, M.C.; Klein, J.; Wilson, M.G.; Hutchins, G.D.; March, K.L. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J. Vasc. Surg. 2011, 53, 1565–1574 e1. [Google Scholar] [CrossRef]
- Ruiz-Salmeron, R.; de la Cuesta-Diaz, A.; Constantino-Bermejo, M.; Perez-Camacho, I.; Marcos-Sanchez, F.; Hmadcha, A.; Soria, B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011, 20, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.S.A.; Ismail, N.A.; Wan Jamaludin, W.F.; Muhamad, N.A.; Mohamad Idris, M.A.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem. Cell Res. Ther. 2018, 13, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, F.M.; Kajikawa, M.; Matsui, S.; Hashimoto, H.; Kishimoto, S.; Maruhashi, T.; Chowdhury, M.; Noma, K.; Nakashima, A.; Kihara, Y.; et al. Review of the Long-term Effects of Autologous Bone-Marrow Mononuclear Cell Implantation on Clinical Outcomes in Patients with Critical Limb Ischemia. Sci. Rep. 2019, 9, 7711. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.; Jali, V.P.; Pandit, A.K.; Misra, S.; Kumar, P.; Chakravarty, K.; Kathuria, P.; Gulati, A. Bone marrow mononuclear cell therapy in ischaemic stroke: A systematic review. Acta. Neurol. Scand. 2017, 135, 496–506. [Google Scholar] [CrossRef]
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Yasui, T.; Kimura, T.; Gul, S.; Claussen, C.; et al. Bone Marrow Mononuclear Cells Activate Angiogenesis via Gap Junction-Mediated Cell-Cell Interaction. Stroke 2020, 51, 1279–1289. [Google Scholar] [CrossRef]
- Huang, P.; Li, S.; Han, M.; Xiao, Z.; Yang, R.; Han, Z.C. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes. Care 2005, 28, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Horie, T.; Tsuda, I.; Abe, Y.; Yamada, M.; Egawa, H.; Iida, J.; Sakata, H.; Onodera, K.; Tamaki, T.; et al. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J. Artif. Organs. 2006, 9, 226–233. [Google Scholar] [CrossRef]
- Mohammadzadeh, L.; Samedanifard, S.H.; Keshavarzi, A.; Alimoghaddam, K.; Larijani, B.; Ghavamzadeh, A.; Ahmadi, A.S.; Shojaeifard, A.; Ostadali, M.R.; Sharifi, A.M.; et al. Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Exp. Clin. Endocrinol. Diabetes 2013, 121, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Kucukardali, Y.; Tangi, F.; Erikci, A.; Uzun, G.; Bashekim, C.; Sen, H.; Terekeci, H.; Narin, Y.; Ozyurt, M.; et al. Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat. 2012, 26, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, M.; Jirkovska, A.; Bem, R.; Fejfarova, V.; Pagacova, L.; Sixta, B.; Varga, M.; Langkramer, S.; Sykova, E.; Jude, E.B. Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 2013, 29, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Toko, H.; Tateno, K.; Nagai, T.; Komuro, I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet 2002, 360, 2083–2084; author reply 2084. [Google Scholar] [CrossRef]
- Capiod, J.C.; Tournois, C.; Vitry, F.; Sevestre, M.A.; Daliphard, S.; Reix, T.; Nguyen, P.; Lefrere, J.J.; Pignon, B. Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: Towards a new cellular product. Vox. Sang. 2009, 96, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.D.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Choi, J.Y.; Lieberman, J.R.; Huang, J.; Zuk, P.A.; Zhang, J.; Hedrick, M.H.; Benhaim, P. Bone induction by BMP-2 transduced stem cells derived from human fat. J. Orthop. Res. 2003, 21, 622–629. [Google Scholar] [CrossRef]
- Zhi, K.; Gao, Z.; Bai, J.; Wu, Y.; Zhou, S.; Li, M.; Qu, L. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci. 2014, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene. Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef]
- Hong, S.J.; Traktuev, D.O.; March, K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr. Opin. Organ. Transplant. 2010, 15, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Kondo, K.; Shibata, R.; Hayashida, R.; Shintani, S.; Yamaguchi, S.; Shimizu, Y.; Unno, K.; Kikuchi, R.; Kodama, A.; et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci. Rep. 2020, 10, 16045. [Google Scholar] [CrossRef]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem. Cells 2018, 36, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, J.S. Pro-angiogenic cell-based therapy for the treatment of ischemic cardiovascular diseases. Thromb. Res. 2012, 130 (Suppl. 1), S90–S94. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Norgren, L.; Weiss, N.; Nikol, S.; Hinchliffe, R.J.; Lantis, J.C.; Patel, M.R.; Reinecke, H.; Ofir, R.; Rosen, Y.; Peres, D.; et al. PLX-PAD Cell Treatment of Critical Limb Ischaemia: Rationale and Design of the PACE Trial. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 538–545. [Google Scholar] [CrossRef]
- De Angelis, B.; Gentile, P.; Orlandi, F.; Bocchini, I.; Di Pasquali, C.; Agovino, A.; Gizzi, C.; Patrizi, F.; Scioli, M.G.; Orlandi, A.; et al. Limb rescue: A new autologous-peripheral blood mononuclear cells technology in critical limb ischemia and chronic ulcers. Tissue. Eng. Part C Methods 2015, 21, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Misao, Y.; Nagai, H.; Kawasaki, M.; Nagashima, K.; Suzuki, K.; Tsuchiya, K.; Otsuka, S.; Uno, Y.; Takemura, G.; et al. Granulocyte colony-stimulating factor: A noninvasive regeneration therapy for treating atherosclerotic peripheral artery disease. Circ. J. 2006, 70, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.P.; Yang, X.F.; Li, S.Z.; Wen, J.C.; Zhang, Y.; Han, Z.C. Randomised comparison of G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow-mononuclear cells for the treatment of patients with lower limb arteriosclerosis obliterans. Thromb. Haemost. 2007, 98, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Jin, X.; Wang, M.; Zhang, S.; Xu, L. Autologous bone marrow mononuclear cells transplant in patients with critical leg ischemia: Preliminary clinical results. Exp. Clin. Transplant 2013, 11, 435–439. [Google Scholar] [CrossRef]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.; Slaper-Cortenbach, I.C.; van der Graaf, Y.; Algra, A.; van der Tweel, I.; Doevendans, P.A.; Mali, W.P.; Moll, F.L.; et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: The randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation 2015, 131, 851–860. [Google Scholar]
- Pignon, B.; Sevestre, M.A.; Kanagaratnam, L.; Pernod, G.; Stephan, D.; Emmerich, J.; Clement, C.; Sarlon, G.; Boulon, C.; Tournois, C.; et al. Autologous Bone Marrow Mononuclear Cell Implantation and Its Impact on the Outcome of Patients With Critical Limb Ischemia- Results of a Randomized, Double-Blind, Placebo-Controlled Trial. Circ. J. 2017, 81, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Parakh, R.; Desai, S.; Das, A.; Gottipamula, S.; Krishnamurthy, S.; Anthony, N.; Pherwani, A.; Majumdar, A.S. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J. Transl. Med. 2013, 11, 143. [Google Scholar] [CrossRef]
- Szabo, G.V.; Kovesd, Z.; Cserepes, J.; Daroczy, J.; Belkin, M.; Acsady, G. Peripheral blood-derived autologous stem cell therapy for the treatment of patients with late-stage peripheral artery disease-results of the short- and long-term follow-up. Cytotherapy 2013, 15, 1245–1252. [Google Scholar] [CrossRef]
- Raval, A.N.; Schmuck, E.G.; Tefera, G.; Leitzke, C.; Ark, C.V.; Hei, D.; Centanni, J.M.; de Silva, R.; Koch, J.; Chappell, R.G.; et al. Bilateral administration of autologous CD133+ cells in ambulatory patients with refractory critical limb ischemia: Lessons learned from a pilot randomized, double-blind, placebo-controlled trial. Cytotherapy 2014, 16, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Lara-Hernandez, R.; Lozano-Vilardell, P.; Blanes, P.; Torreguitart-Mirada, N.; Galmes, A.; Besalduch, J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann. Vasc. Surg. 2010, 24, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Fujita, Y.; Katayama, M.; Baba, R.; Shibakawa, M.; Yoshikawa, K.; Katakami, N.; Furukawa, Y.; Tsukie, T.; Nagano, T.; et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis 2012, 224, 440–445. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, B.; Fu, W.; Wang, Y.; Guo, D.; Wei, Z.; Xu, X.; Mendelsohn, F.O. Transplantation of purified CD34+ cells in the treatment of critical limb ischemia. J. Vasc. Surg. 2013, 58, 404–411 e3. [Google Scholar] [CrossRef]
- Fujita, Y.; Kinoshita, M.; Furukawa, Y.; Nagano, T.; Hashimoto, H.; Hirami, Y.; Kurimoto, Y.; Arakawa, K.; Yamazaki, K.; Okada, Y.; et al. Phase II clinical trial of CD34+ cell therapy to explore endpoint selection and timing in patients with critical limb ischemia. Circ. J. 2014, 78, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.J.; Marston, W.A.; Berceli, S.A.; Guzman, R.; Henry, T.D.; Longcore, A.T.; Stern, T.P.; Watling, S.; Bartel, R.L. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: The randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol. Ther. 2012, 20, 1280–1286. [Google Scholar] [CrossRef]
- Losordo, D.W.; Kibbe, M.R.; Mendelsohn, F.; Marston, W.; Driver, V.R.; Sharafuddin, M.; Teodorescu, V.; Wiechmann, B.N.; Thompson, C.; Kraiss, L.; et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ. Cardiovasc. Interv. 2012, 5, 821–830. [Google Scholar] [CrossRef]
- Liotta, F.; Annunziato, F.; Castellani, S.; Boddi, M.; Alterini, B.; Castellini, G.; Mazzanti, B.; Cosmi, L.; Acquafresca, M.; Bartalesi, F.; et al. Therapeutic Efficacy of Autologous Non-Mobilized Enriched Circulating Endothelial Progenitors in Patients With Critical Limb Ischemia- The SCELTA Trial. Circ. J. 2018, 82, 1688–1698. [Google Scholar] [CrossRef]
- Fang, G.; Jiang, X.; Fang, Y.; Pan, T.; Liu, H.; Ren, B.; Wei, Z.; Gu, S.; Chen, B.; Jiang, J.; et al. Autologous peripheral blood-derived stem cells transplantation for treatment of no-option angiitis-induced critical limb ischemia: 10-year management experience. Stem. Cell Res. Ther. 2020, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandey, N.N.; Sinha, M.; Kumar, S.; Jagia, P.; Gulati, G.S.; Gond, K.; Mohanty, S.; Bhargava, B. Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate Safety and Therapeutic Efficacy of Angiogenesis Induced by Intraarterial Autologous Bone Marrow-Derived Stem Cells in Patients with Severe Peripheral Arterial Disease. J. Vasc. Interv. Radiol. 2021, 32, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Becker, G.J. Standards for evaluating and reporting the results of surgical and percutaneous therapy for peripheral arterial disease. Radiology 1991, 181, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kusumanto, Y.H.; van Weel, V.; Mulder, N.H.; Smit, A.J.; van den Dungen, J.J.; Hooymans, J.M.; Sluiter, W.J.; Tio, R.A.; Quax, P.H.; Gans, R.O.; et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: A double-blind randomized trial. Hum. Gene. Ther. 2006, 17, 683–691. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, T.; Fang, Y.; Wei, Z.; Gu, S.; Fang, G.; Liu, Y.; Luo, Y.; Liu, H.; Zhang, T.; et al. Purified CD34(+) cells versus peripheral blood mononuclear cells in the treatment of angiitis-induced no-option critical limb ischaemia: 12-Month results of a prospective randomised single-blinded non-inferiority trial. EBioMedicine 2018, 35, 46–57. [Google Scholar] [CrossRef]
- Carstens, M.H.; Zelaya, M.; Calero, D.; Rivera, C.; Correa, D. Adipose-derived stromal vascular fraction (SVF) cells for the treatment of non-reconstructable peripheral vascular disease in patients with critical limb ischemia: A 6-year follow-up showing durable effects. Stem. Cell Res. 2020, 49, 102071. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.L.; Loyer, X.; Vilar, J.; Cras, A.; Mirault, T.; Gaussem, P.; Silvestre, J.S.; Smadja, D.M. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: Evidence of vasculogenic potential. Thromb. Haemost. 2015, 113, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Ingram, D.A. Endothelial progenitor cell: Ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr. Opin. Hematol. 2009, 16, 269–273. [Google Scholar] [CrossRef]
- Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Higashide, S.; Ioji, T.; Fujita, Y.; et al. Autologous Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood CD34 Positive Cell Transplantation for Hemodialysis Patients with Critical Limb Ischemia: A Prospective Phase II Clinical Trial. Stem Cells Transl. Med. 2018, 7, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Povsic, T.J.; Zavodni, K.L.; Kelly, F.L.; Zhu, S.; Goldschmidt-Clermont, P.J.; Dong, C.; Peterson, E.D. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J. Am. Coll Cardiol. 2007, 50, 2243–2248. [Google Scholar] [CrossRef][Green Version]
- Gremmels, H.; Teraa, M.; Quax, P.H.; den Ouden, K.; Fledderus, J.O.; Verhaar, M.C. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol. Ther. 2014, 22, 1960–1970. [Google Scholar] [CrossRef]
- Fadini, G.P.; Sartore, S.; Schiavon, M.; Albiero, M.; Baesso, I.; Cabrelle, A.; Agostini, C.; Avogaro, A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia 2006, 49, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S.; Singh, U.; Huet, B.A. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: Implications for increased cardiovascular risk. Atherosclerosis 2010, 211, 297–302. [Google Scholar] [CrossRef]
- Sibal, L.; Aldibbiat, A.; Agarwal, S.C.; Mitchell, G.; Oates, C.; Razvi, S.; Weaver, J.U.; Shaw, J.A.; Home, P.D. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia 2009, 52, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Q.; Zhang, J.; Guo, L.R.; Qi, L.X.; Zhang, S.W.; Xu, J.; Li, J.X.; Luo, T.; Ji, B.X.; Li, X.F.; et al. Transplantation of autologous bone marrow mononuclear cells for patients with lower limb ischemia. Chin. Med. J. 2008, 121, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, R.B.; Hamming, J.F.; Fibbe, W.E.; Van Weel, V.; Frerichs, S.J.; Stiggelbout, A.M.; Van Bockel, J.H.; Lindeman, J.H. Intramuscular or combined intramuscular/intra-arterial administration of bone marrow mononuclear cells: A clinical trial in patients with advanced limb ischemia. J. Cardiovasc Surg. 2008, 49, 51–58. [Google Scholar]
- Madaric, J.; Klepanec, A.; Valachovicova, M.; Mistrik, M.; Bucova, M.; Olejarova, I.; Necpal, R.; Madaricova, T.; Paulis, L.; Vulev, I. Characteristics of responders to autologous bone marrow cell therapy for no-option critical limb ischemia. Stem Cell Res. Ther. 2016, 7, 116. [Google Scholar] [CrossRef]
- Jaluvka, F.; Ihnat, P.; Madaric, J.; Vrtkova, A.; Janosek, J.; Prochazka, V. Current Status of Cell-Based Therapy in Patients with Critical Limb Ischemia. Int. J. Mol. Sci. 2020, 21, 8999. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef]
- Gao, W.; Chen, D.; Liu, G.; Ran, X. Autologous stem cell therapy for peripheral arterial disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Peeters Weem, S.M.; Teraa, M.; de Borst, G.J.; Verhaar, M.C.; Moll, F.L. Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Makridakis, M.; Roubelakis, M.G.; Vlahou, A. Stem cells: Insights into the secretome. Biochim. Biophys. Acta 2013, 1834, 2380–2384. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Minamino, S.; Kuwabara, K.; Arai, S. Stem cell secretome as a new booster for regenerative medicine. Biosci. Trends. 2019, 13, 299–307. [Google Scholar] [CrossRef]
- Baberg, F.; Geyh, S.; Waldera-Lupa, D.; Stefanski, A.; Zilkens, C.; Haas, R.; Schroeder, T.; Stuhler, K. Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim. Biophys. Acta Proteins. Proteom. 2019, 1867, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Maffioli, E.; Nonnis, S.; Angioni, R.; Santagata, F.; Cali, B.; Zanotti, L.; Negri, A.; Viola, A.; Tedeschi, G. Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. J. Proteomics. 2017, 166, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Merfeld-Clauss, S.; Lupov, I.P.; Lu, H.; March, K.L.; Traktuev, D.O. Adipose Stromal Cell Contact with Endothelial Cells Results in Loss of Complementary Vasculogenic Activity Mediated by Induction of Activin A. Stem Cells 2015, 33, 3039–3051. [Google Scholar] [CrossRef]
- Rubina, K.; Kalinina, N.; Efimenko, A.; Lopatina, T.; Melikhova, V.; Tsokolaeva, Z.; Sysoeva, V.; Tkachuk, V.; Parfyonova, Y. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue. Eng. Part A 2009, 15, 2039–2050. [Google Scholar] [CrossRef]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Jang, H.K.; Kim, B.S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther. 2014, 22, 862–872. [Google Scholar] [CrossRef]
- Di Santo, S.; Yang, Z.; Wyler von Ballmoos, M.; Voelzmann, J.; Diehm, N.; Baumgartner, I.; Kalka, C. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE 2009, 4, e5643. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, C.; Zhao, L.; Chen, K.; He, H.; Mo, Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int. J. Endocrinol. 2013, 2013, 592454. [Google Scholar] [CrossRef]
- Felice, F.; Piras, A.M.; Rocchiccioli, S.; Barsotti, M.C.; Santoni, T.; Pucci, A.; Burchielli, S.; Chiellini, F.; Ucciferri, N.; Solaro, R.; et al. Endothelial progenitor cell secretome delivered by novel polymeric nanoparticles in ischemic hindlimb. Int. J. Pharm. 2018, 542, 82–89. [Google Scholar] [CrossRef]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.J.; Sahoo, S. Angiogenic Mechanisms of Human CD34(+) Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Kir, D.; Schnettler, E.; Modi, S.; Ramakrishnan, S. Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis 2018, 21, 699–710. [Google Scholar] [CrossRef]
- Welten, S.M.; Bastiaansen, A.J.; de Jong, R.C.; de Vries, M.R.; Peters, E.A.; Boonstra, M.C.; Sheikh, S.P.; La Monica, N.; Kandimalla, E.R.; Quax, P.H.; et al. Inhibition of 14q32 MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ Res. 2014, 115, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Author (Year) | Ref. | Cell Source | Animal (Strain) | Administration (×105 Cells) | Route of Administration | Follow-up (Weeks) | Outcome |
|---|---|---|---|---|---|---|---|---|
| aMSCs | Cunha (2013) | [69] | Bone marrow | Mice (Balb-C & C57/BL6) | 5 | IM | 4 | VS, CD, TR |
| hMSCs | García-Vazquez (2019) | [70] | Adipose tissue | Mice (Athymic nude) | 6 | IM | 3 | BFP, CD, VS |
| aMSCs | Nammian (2021) | [71] | Bone marrow & adipose tissue | Mice (C57/BL6) | 5 | IM | 4 | FS, CD |
| hMSCs + hECFCs | Rossi (2017) | [72] | Bone marrow & peripheral blood | Mice (Athymic nude) | N/A | IV | 2 | BFP, CD, VS |
| hCD34+ | Lian (2018) | [73] | Peripheral blood | Mice (Balb-C Nude) | 1 | IM | 3 | FS, VS |
| hEPCs | Kalka (2000) | [74] | Peripheral blood | Mice (Athymic nude) | 5 | IC | 4 | BFP, CD, VS |
| hEPCs | Urbich (2003) | [75] | Peripheral blood | Mice (Athymic NMRI Nude) | 5 | IV | 2 | BFP, CD |
| hEPCs | Zhao (2016) | [76] | Fetal aorta | Rat (Goto-Kakizaki) | 100 | IM | 8 | BFP, CD, VS |
| hCACs | Beltrán-Camacho (2020) | [47] | Peripheral blood | Mice (Balb-C Nude) | 5 | IM | 4 days | BFP, CD, FS, VD |
| hEPCs + hOECs | Yoon (2005) | [77] | Peripheral blood | Mice (Athymic nude) | 2 | IM | 3 | BFP, CD, VS, MP |
| hEPCs + hSMPCs | Foubert (2008) | [78] | Umbilical cord blood | Mice (Athymic nude) | 5 | IV | 2 | BFP, CD, AD |
| hESC-ECP | MacAskill (2018) | [40] | hESC line | Mice (CD1-STZ DM inductor) | 10 | IM | 3 | BFP, CD |
| aBM-MNCs | Shintani (2001) | [79] | Bone marrow | Rabbit (Male New Zealand White) | 5 | IM | 4 | BFP, CBP, CD, CVF |
| aBMCs | De Nigris (2007) | [80] | Bone marrow | Mice (ApoE−/−) | 20 | IV | 2 | BFP, CD, CVF |
| aBM-MNCs | Jeon (2007) | [81] | Bone marrow | Mice (C57/BL6) | 20 | IM | 4 | CD, CVF |
| aBM-MNCs | Gan (2009) | [82] | Bone marrow | Mice (C57/BL6) | 30 | IM | 2 | BFP, CD |
| hBM-NCs | Liu (2009) | [83] | Bone marrow | Mice (C57/BL6 ApoE−/−) | 250 | IA | 4 | BFP, CVF |
| aBM-MNCs | Brenes (2012) | [84] | Bone marrow | Mice (C57/BL6) | 5, 10 & 20 | IM | 4 | BFP, CD, FS |
| aBM-MNCs | Reis (2014) | [85] | Bone marrow | Mice (Balb-C) | 5 | IM | 4 | CD, TR, VS |
| hBM-MNCs | Rojas-Torres (2020) | [41] | Bone marrow | Mice (Balb-C Nude) | 10 | IM | 3 | BFP, CD, FS, VD |
| aBMC-derived macrophages | Kuwahara (2014) | [86] | Bone marrow | Mice (C57/BL6N) | 1 | IM | 4 | BFP, CD |
| hALDH high activity cells | Capoccia (2009) | [87] | Bone marrow | Mice (NOD/SCID b2M) | 1–2 | IM | 3 | BFP, CD |
| aMIAMI cells | Rahnemai-Azar (2011) | [88] | Bone marrow | Mice (Balb-C) | 10 | IM | 4 | BFP, CD, FS, VS |
| hPB-MNCs1 | Li (2006) | [89] | Peripheral blood | Mice (Athymic nude) | 10 | IM | 4 | BFP, AI, CD, VS |
| aPB-MNCs + PRP | Padilla (2020) | [90] | Peripheral blood | Rat (Wistar) | 15 | IM | 4 | AI, VIP |
| aASCs | Liu (2020) | [91] | Adipose tissue | Mice (C57/BL6) | 10 | IM | 3 | BFP, CD, VS |
| aASCs + macrophages | Rybalko (2017) | [92] | Adipose tissue | Mice (C57/BL6) | 2 | IM | 3 | BFP, CD |
| hSVF | Jin (2017) | [93] | Adipose tissue | Mice (Nude) | 10 | IM | 2 | BFP, VS, CD, MP |
| PDX-PAD (adherent stromal cells) | Prather (2009) | [94] | Placenta | Mice (Balb-C) | 10 | IM | 3 | BFP, CD, FS |
| PLX-PAD (MSC like stromal cells) | Zahavi-Goldstein (2017) | [95] | Placenta | Mice (C57/BL6) | 0.02–10 | IM & SC | 3 | BFP, VS |
| Author (year) | Ref. | Type of Cell Therapy | Type of Study | Cause of PAD/CLI | Disease Stage | Nº Patients (T/C) | Control | Administration (x106 cells) | Route of Administration | Follow-up (Months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang (2005) | [125] | PB-MNCs1 | RCT | ASO | Fontaine III–IV | 28 (14/14) | Blank | 3000 | IM | 3 | ABI, AR, DR, PFWD, RPS, UH |
| Ozturk (2012) | [128] | PB-MNCs1 | RCT | N/A | Fontaine III–IV | 40 (20/20) | Blank | 24.8/mL (CD34+) | IM | 3 | ABI, AR, PFWD, RPS, TcPO2, UH |
| Mohammadzadeh (2013) | [127] | PB-MNCs1 | RCT | N/A | Fontaine III–IV | 21 (7/14) | Blank | 900–1200 | IM | 3 | ABI, AR, UH, PFWD |
| De Angelis (2015) | [144] | PB-MNCs | NR | ASO | Fontaine IV | 86 (43/43) | Blank5 | 125.65 | IM | 4.5 | AFS, AR, DR, PFWD, RPS, UH |
| Tateishi-Yuyama (2002) TACT | [142] | BM-MNCs | NR | ASO | Fontaine III–IV | 252 | Blank | 700–2700 | IM | 6 | ABI, TcPO2, RPS |
| BM-MNCs | R | Fontaine III–IV | 223 | Placebo | 889–2800 | IM | 6 | ||||
| Arai (2006) | [145] | BM-MNCs | RCT | N/A | Fontaine III–IV | 26 (13/13) | Blank | 1000–3000 | IM | 1 | ABI, TcPO2, RPS |
| Dubsky (2013) | [129] | PB-MNCs | NR | N/A | Rutherford 4–6 | 33 (11/22)4 | Blank | 10400 | IM | 6 | AR, TcPO2, UH |
| BM-MNCs | 39 (17/22)4 | 1800 | |||||||||
| Huang (2007) | [146] | PB-MNCs | NC | ASO | N/A | 76 | N/A | 7200 | IM | 3 | ABI, AR, PFWD, RPS, TcPO2, UH |
| BM-MNCs | 74 | 580 | |||||||||
| Matoba (2008) | [30] | BM-MNCs | NC | ASO & TAO | Fontaine III–IV | 115 | N/A | N/A | IM | 25.3 | ABI, AFS, DR, PFWD, RPS, TcPO2, UH |
| Ruiz-Salmeron (2011) | [120] | BM-MNCs | NC | ASO & others | Rutherford 4–6 | 20 | N/A | 100–400 | IA | 12 | ABI, AR, DR, TcPO2 |
| Amann (2009) BONMONT-1 | [114] | BM-MNCs | NC | N/A | Rutherford 4–6 | 12 | N/A | 1100 | IM | 13.5 | ABI, AFS, PFWD, TcPO2 |
| BM-TNCs | 39 | 3000 | |||||||||
| Walter (2011) PROVASA | [20] | BM-MNCs | RCT | ASO & TAO | Fontaine III–IV | 40 (19/21) | Placebo | 153 | IA | 3 | ABI, AR, DR, RPS, TcPO2, UH |
| Li (2013) | [147] | BM-MNCs | RCT | ASO | Fontaine III–IV | 58 (29/29) | Placebo | 10/mL | IM | 6 | ABI, AFS, AR, DR, RPS, UH |
| Teraa (2015) JUVENTAS | [148] | BM-MNCs | RCT | ASO | Fontaine IIB–IV | 160 (81/79) | Placebo | 500 | IA | 6 | ABI, AR, DR, TcPO2, UH |
| Pignon (2017) BALI | [149] | BM-MNCs | RCT | ASO | Rutherford 4–5 | 36 (17/19) | Placebo | 1300 | IM | 12 | ABI, AR, RPS, TcPO2, UH |
| Guo (2018) | [116] | BM-MNCs | NR | TAO | N/A | 59 (40/19) | Blank | 3500 | IM | 129.5 | ABI, AFS, AR, RPS, TcPO2, UH |
| Lu (2011) | [150] | BM-MNCs | RCT | ASO | Fontaine IV | 212 | Blank | 930 | IM | 6 | ABI, AR, PFWT, RPS, TcPO2, UH |
| BM-MSCs | 202 | 960 | |||||||||
| Dash (2009) | [151] | BM-MSCs | RCT | ASO | N/A | 6 (3/3) | Blank | N/A | IM | 3 | PFWD, UH |
| Buerger | 18 (9/9) | ||||||||||
| Gupta (2013) | [152] | BM-MSCs (allogenic) | RCT | ASO & TAO | Rutherford 4–6 | 20 (10/10) | Placebo | 200 | IM | 6 | ABI, AR, RPS, UH |
| Szabò (2013) | [153] | Ves-Cell | RCT | N/A | Fontaine III–IV | 20 (10/10) | Blank | 66.4 | IM | 3 | ABI, AR, DR, PFWD, RPS, TcPO2, UH |
| NC | 22.6 | ABI, AFS, AR, DR, PFWD, RPS, TcPO2, UH | |||||||||
| Raval (2014) SCRIPT-CLI | [154] | CD133+1 | RCT | ASO | N/A | 10 (3/7) | Placebo | 50–400 | IM | 12 | AFS, AR, DR |
| Lara-Hernandez (2010) | [155] | EPCs1 | NC | ASO & TAO | Fontaine III–IV | 28 | N/A | N/A | IM | 14.7 | ABI, RPS, UH |
| Kinoshita (2012) | [156] | CD34+1 | NC | ASO & Buerger | Rutherford 4–5 | 17 | N/A | 0.1/kg (LD) 0.5/kg (MD) 1/kg (HD) | IM | 12 | AR, DR, PFWD, RPS, TcPO2, UH |
| Dong (2013) | [157] | CD34+1 | NC | ASO, TAO & others | Rutherford 4–5 | 25 | N/A | 0.1/kg (LD) 0.5/kg (MD) 1/kg (HD) | IM | 6 | ABI, AR, DR, PFWT, RPS, TcPO2, UH |
| Fujita (2014) | [158] | CD34+1 | NC | ASO & Buerger | Rutherford 4–5 | 11 | N/A | 1/kg | IM | 12 | AR, PFWD, RPS, TcPO2 |
| Powell (2012) RESTORE-CLI | [159] | Ixmyelocel-T | RCT | N/A | N/A | 72 (48/24) | Placebo | 35–295 | IM | 12 | AFS, AR, DR |
| Losordo (2015) | [160] | CD34+1 | RCT | N/A | Rutherford 4–5 | 28 (16/12) | Placebo | 0.1/kg (LD) 1/kg (HD) | IM | 12 | ABI, AR, DR, PFWD, UH |
| Liotta (2018) | [161] | BM-MNCs | R | N/A | Rutherford 4–6 | 17 | Blank5 | 506 | IM | 12 | ABI, PFWD, RPS, TcPO2, UH |
| ECEPCs | 23 | 2506 | |||||||||
| Fang (2020) | [162] | PB-MNCs1 | RCT | TAO | Rutherford 4–5 | 78 | PB-MNC | 70, 377 | IM | 46,6 | ABI, AFS, PFWT, RPS, TcPO2 |
| CD34+1 | 82 | 31, 957 | |||||||||
| Sharma (2021) | [163] | BM-MNCs | RCT | ASO & others | Fontaine IIC–IV | 81 (41/40) | Placebo | 71, 51 | IA | 6 | ABI, AR, PFWD, RPS, TcPO2, UH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Camacho, L.; Rojas-Torres, M.; Durán-Ruiz, M.C. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int. J. Mol. Sci. 2021, 22, 2335. https://doi.org/10.3390/ijms22052335
Beltrán-Camacho L, Rojas-Torres M, Durán-Ruiz MC. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. International Journal of Molecular Sciences. 2021; 22(5):2335. https://doi.org/10.3390/ijms22052335
Chicago/Turabian StyleBeltrán-Camacho, Lucía, Marta Rojas-Torres, and Mᵃ Carmen Durán-Ruiz. 2021. "Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia" International Journal of Molecular Sciences 22, no. 5: 2335. https://doi.org/10.3390/ijms22052335
APA StyleBeltrán-Camacho, L., Rojas-Torres, M., & Durán-Ruiz, M. C. (2021). Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. International Journal of Molecular Sciences, 22(5), 2335. https://doi.org/10.3390/ijms22052335