The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro
Abstract
1. Introduction
2. Results
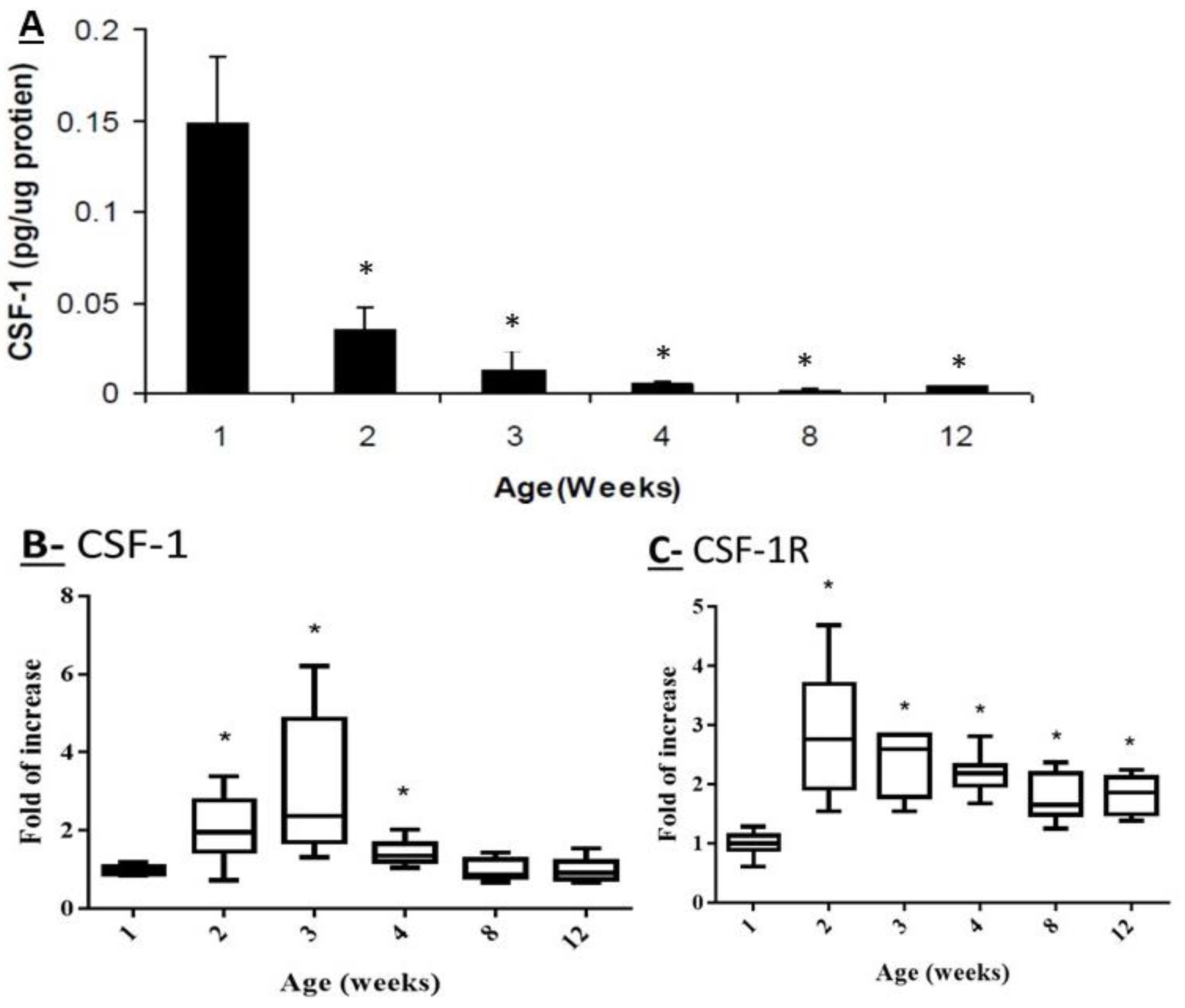
2.1. Effect of Mouse Age on the Protein Levels of CSF-1 and on the Transcriptome Levels of CSF-1 and CSF-1R in Testicular Homogenates
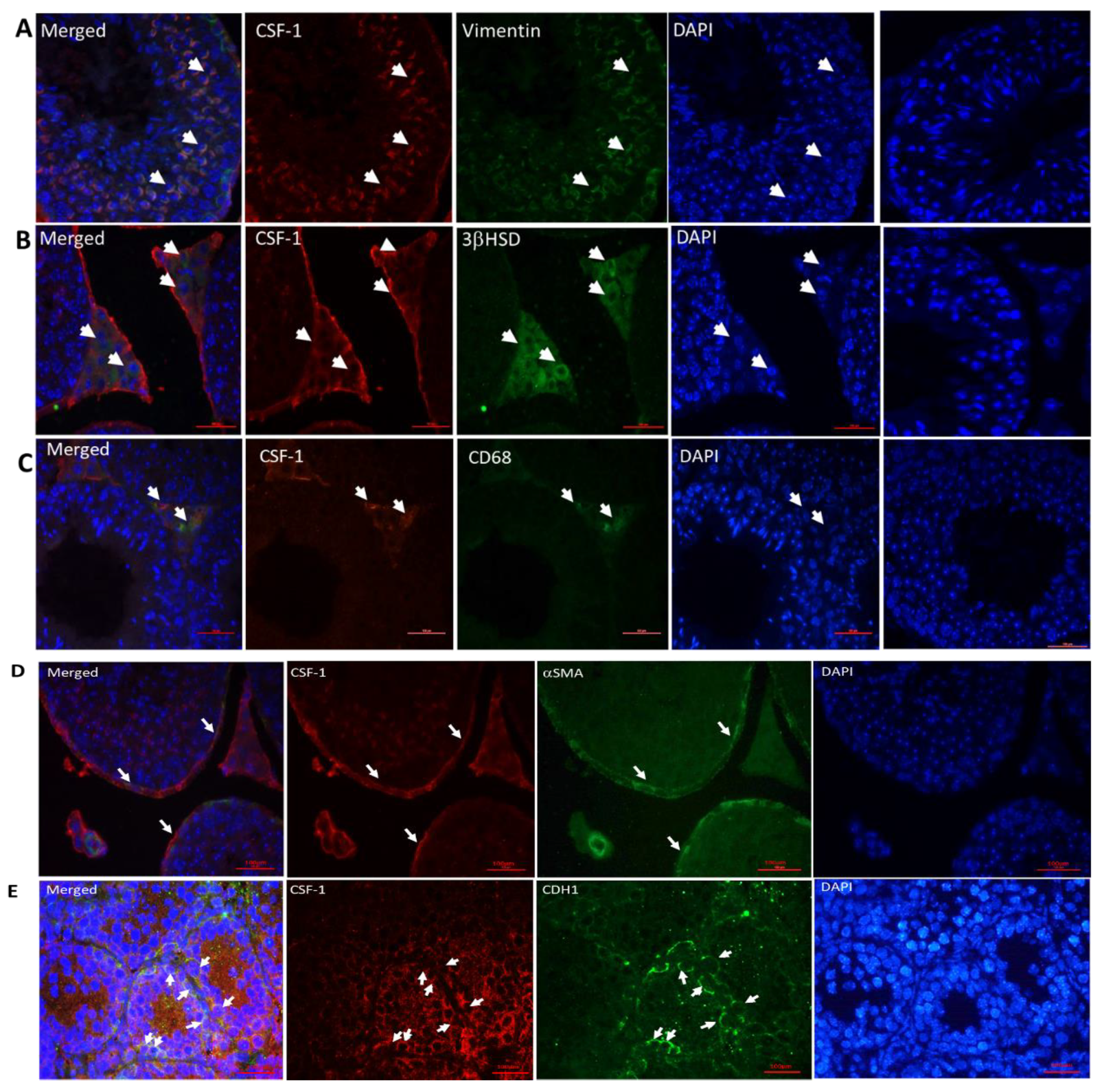
2.2. Cellular Localization of CSF-1 in Mouse Testicular Cells
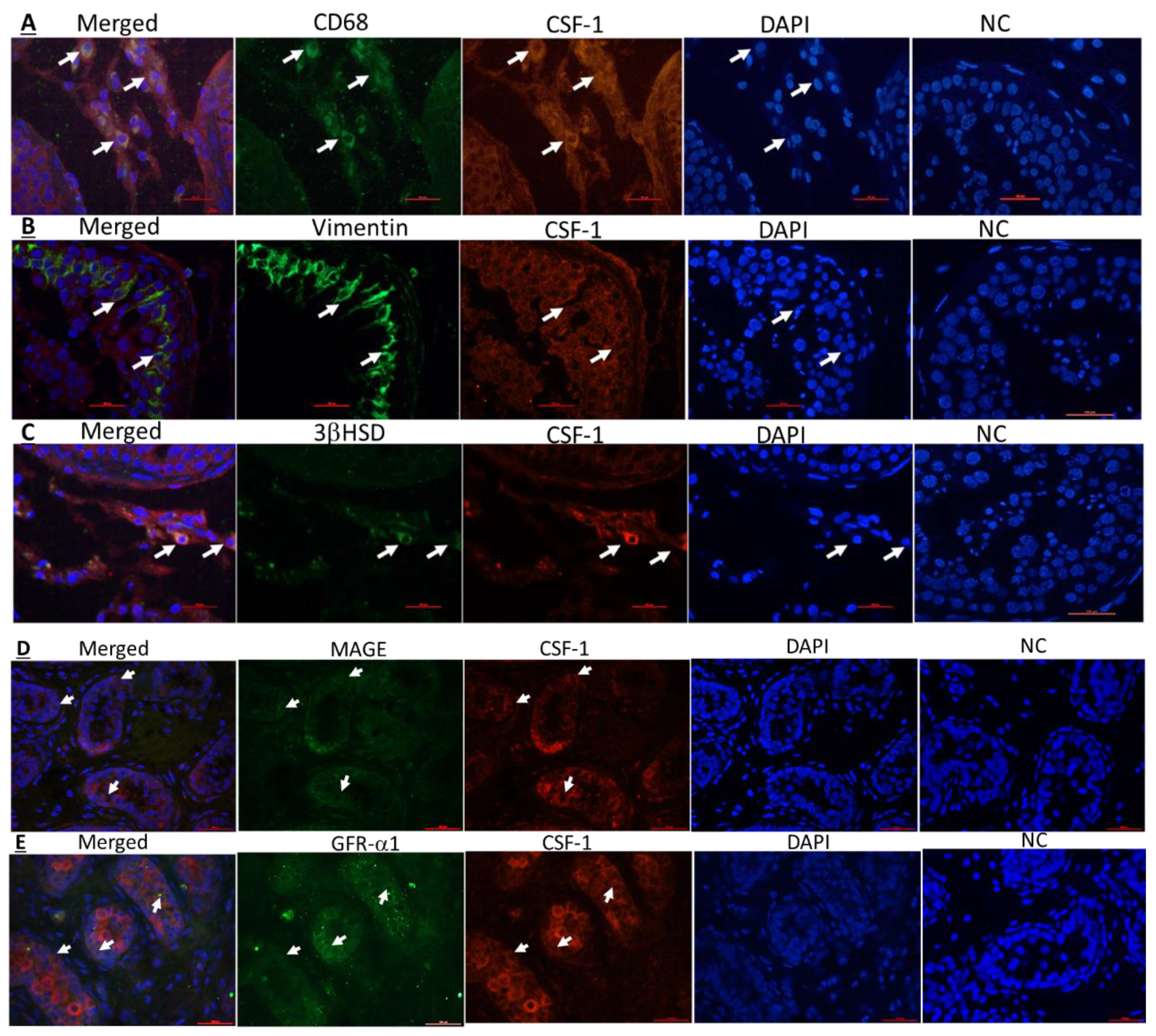
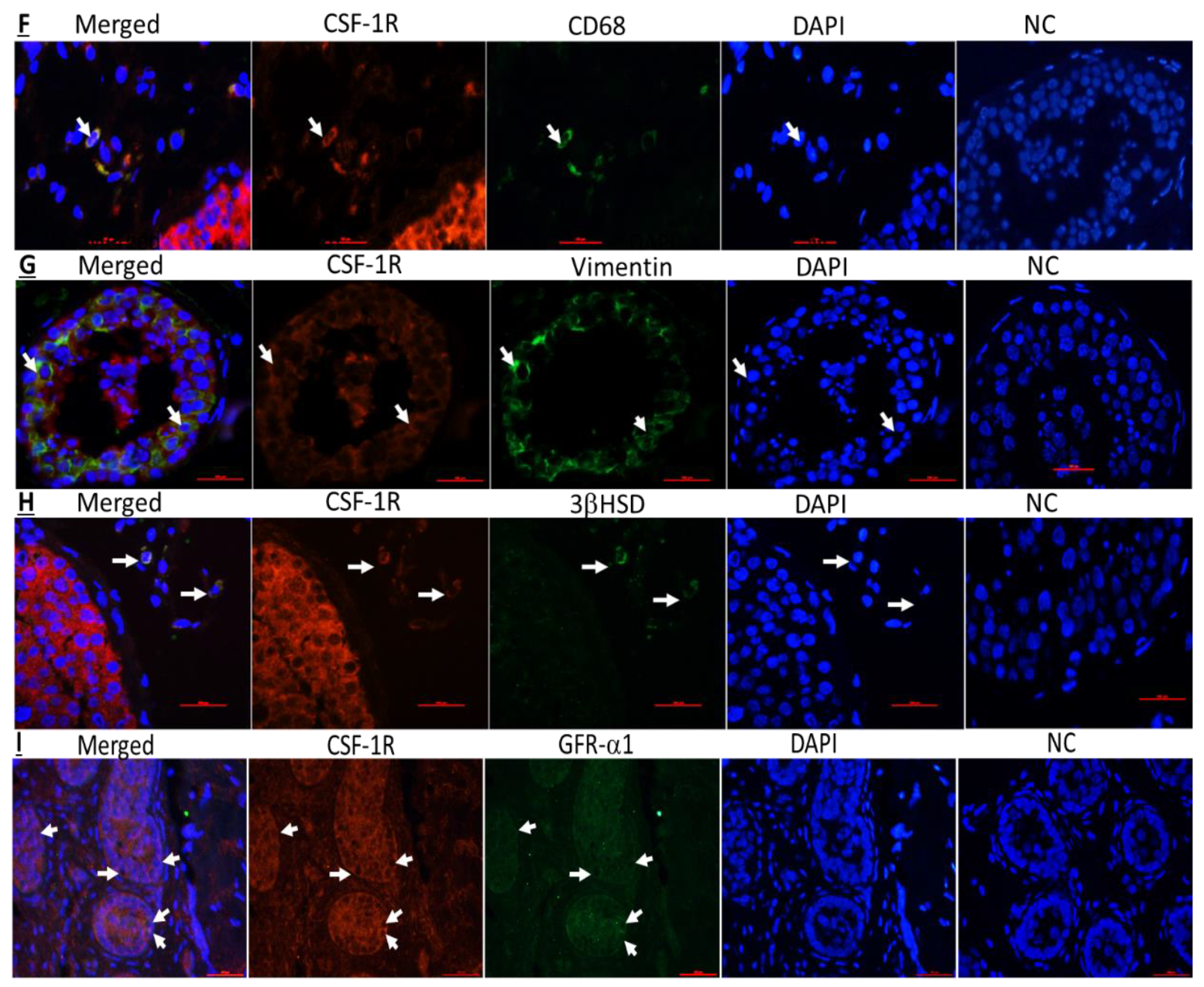
2.3. Cellular Localization of CSF-1 and CSF-1R in Human Testicular Cells
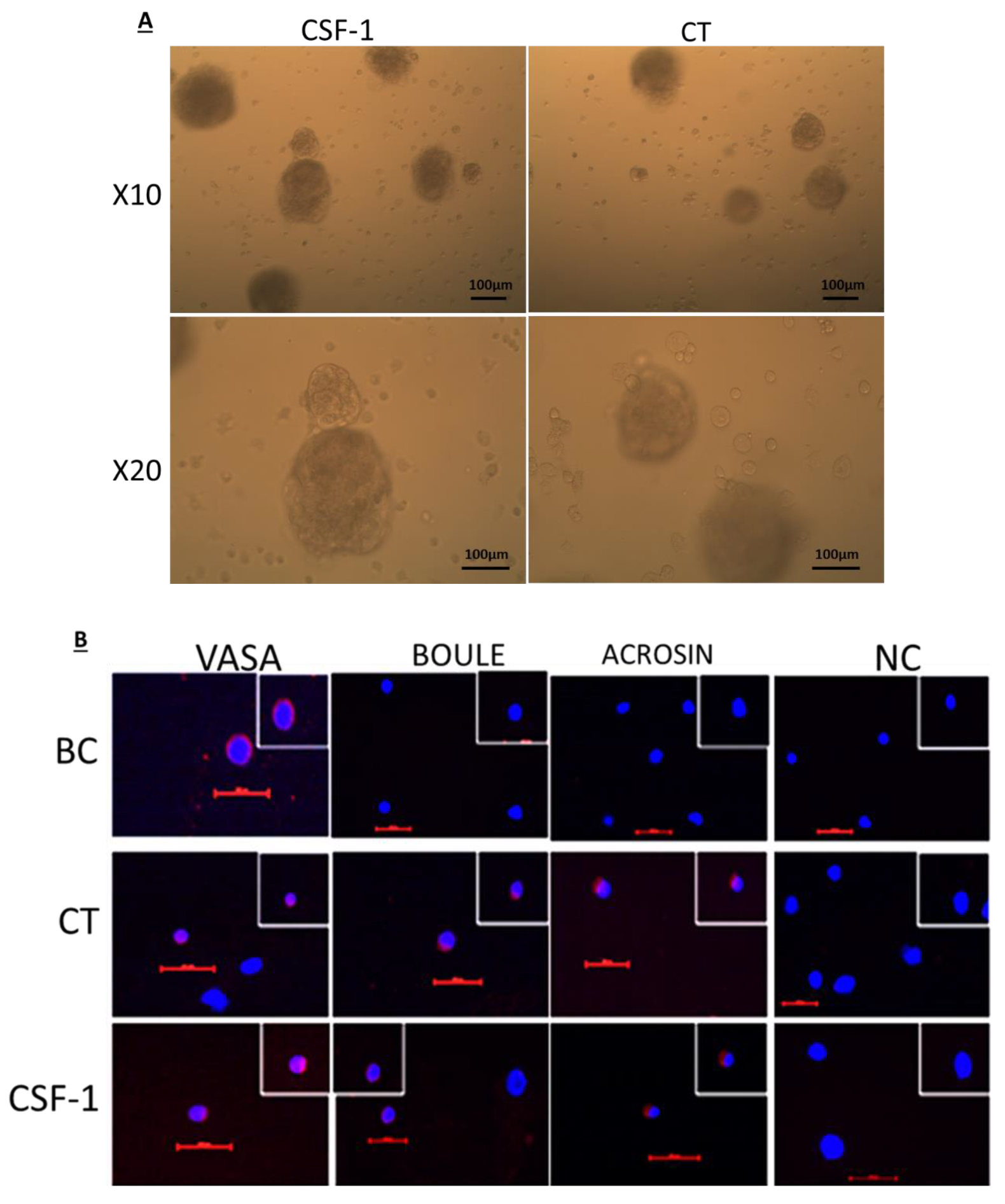
2.4. Development of Colonies and Different Stages of Spermatogenesis from Spermatogonial Cells In Vitro
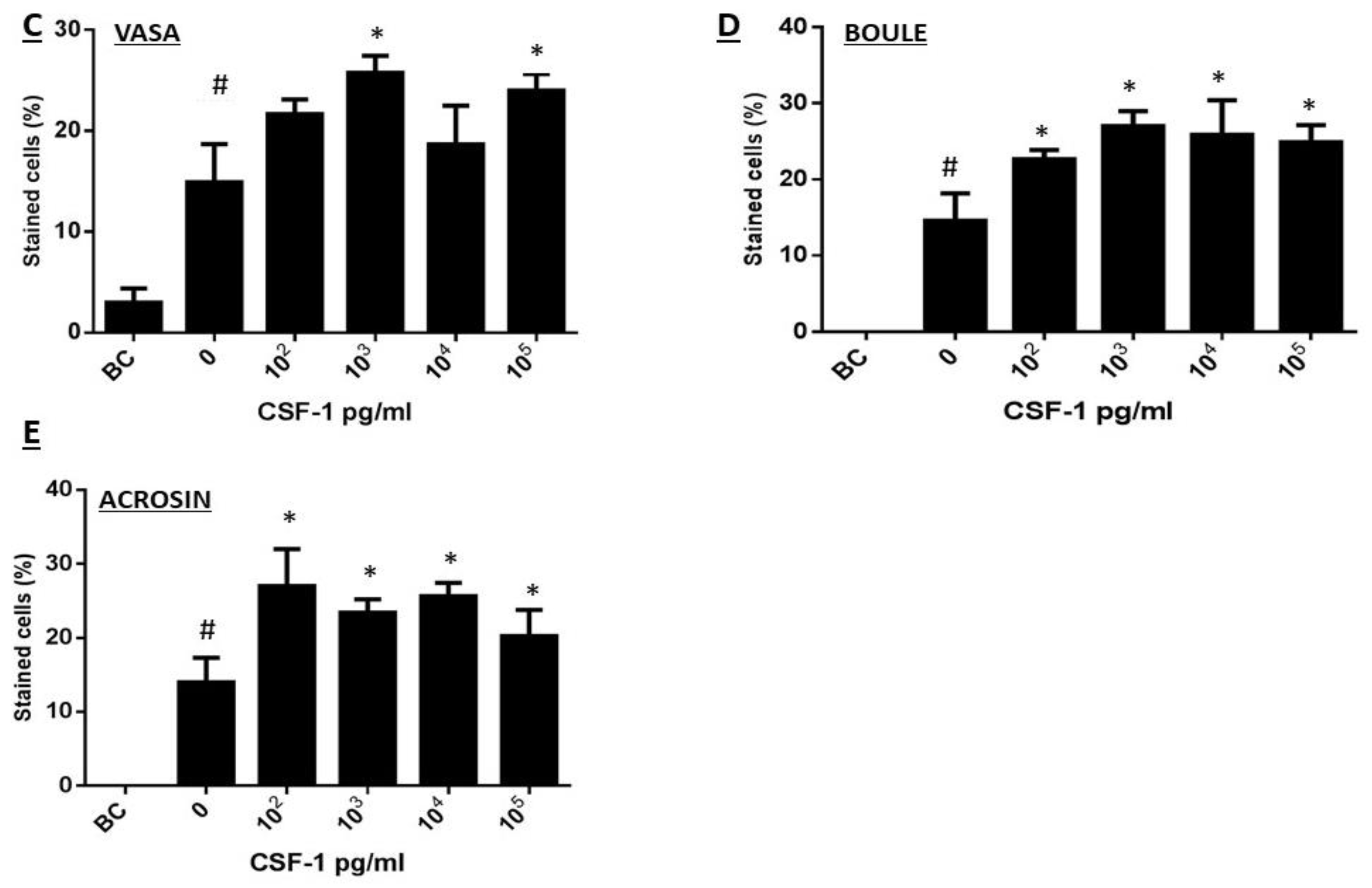
2.5. CSF-1 Induced the Development of Spermatogonial Cells to Different Stages of Spermatogenesis In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals and Human Materials
4.2. Chemicals and Reagents
4.3. Testicular Homogenates
4.4. Extraction of Total RNA for Real-Time PCR Analysis
4.5. Immunofluorescence Staining of Testicular Tissue
4.6. Isolation of Testicular Tubular/Spermatogonial Cells
4.7. Effect of CSF-1 on the Development of Isolated Spermatogonial Cells In Vitro in Methylcellulose Culture System (MCS)
4.8. Evaluating the Spermatogenic Types Developed in MCS by Immunofluorescence Staining
4.9. Data Handling and Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Oatley, M.J.; Avarbock, M.R.; Tobias, J.W.; Brinster, R.L. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009, 136, 1191–1199. [Google Scholar] [CrossRef]
- Takashima, A.; Edelbaum, D.; Kitajima, T.; Shadduck, R.K.; Gilmore, G.L.; Xu, S.; Taylor, R.S.; Bergstresser, P.R.; Ariizumi, K. Colony-stimulating factor-1 secreted by fibroblasts promotes the growth of dendritic cell lines (XS series) derived from murine epidermis. J. Immunol. 1995, 154, 5128–5135. [Google Scholar]
- Wang, Y.; Berezovska, O.; Fedoroff, S. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J. Neurosci. Res. 1999, 57, 616–632. [Google Scholar] [CrossRef]
- Byrne, P.V.; Guilbert, L.J.; Stanley, E.R. Distribution of cells bearing receptors for a colony-stimulating factor (csf-1) in murine tissues. J. Cell Biol. 1981, 91, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, L.J.; Stanley, E.R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J. Cell Biol. 1980, 85, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, W.; Wetterwald, A.; Cecchini, M.G.; Mueller, C.; Felix, R. Detection of transcripts and binding sites for colony-stimulating factor-1 during bone development. Bone 1995, 17, 145–151. [Google Scholar] [CrossRef]
- Arceci, R.J.; Shanahan, E.; Stanley, E.R.; Pollard, J.W. Temporal expression and location of colony-stimulating factor 1 (CSF-I) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc. Natl. Acad. Sci. USA 1989, 86, 8818–8822. [Google Scholar] [CrossRef]
- Regenstreif, L.J.; Rossant, J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev. Biol. 1989, 133, 284–294. [Google Scholar] [CrossRef]
- Johnston, D.S.; Jelinsky, S.A.; Zhi, Y.; Finger, J.N.; Kopf, G.S.; Wright, W.W. Identification of testis-specific male contraceptive targets: Insights from transcriptional profiling of the cycle of the rat seminiferous epithelium and purified testicular cells. Ann. N. Y. Acad. Sci. 2007, 1120, 36–46. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Lee, T.-L.; He, Z.; Jiang, J.; Golestaneh, N.; Hofmann, M.-C.; Chan, W.-Y.; Dym, M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol. Reprod. 2009, 80, 707–717. [Google Scholar] [CrossRef]
- Sawaied, A.; Lunenfeld, E.; Huleihel, M. Interleukin-34, a Novel Paracrine/Autocrine Factor in Mouse Testis, and Its Possible Role in the Development of Spermatogonial Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 8143. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.E.; Hardy, M.P.; Pollard, J.W. Colony-Stimulating Factor-1 Plays a Major Role in the Development of Reproductive Function in Male Mice. Mol. Endocrin. 1997, 11, 1636–1650. [Google Scholar] [CrossRef]
- Cohen, P.E.; Chisholm, O.; Arceci, R.J.; Stanley, E.R.; Pollard, J.W. Absence of Colony-Stimulating Factor-1 in Osteopetrotic (csfmoP/csfmOP) Mice Results in Male Fertility Defects. Biol. Reprod. 1996, 55, 310–317. [Google Scholar] [CrossRef]
- Pollard, J.W.; Dominguez, M.G.; Mocci, S.; Cohen, P.E.; Stanley, E.R. Effect of colony stimulating factor-1 (CSF-1) null mutation, osteopetrotic (csfmop), on the distribution of macrophages in the male reproductive tract. Biol. Reprod. 1997, 56, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H. Concise review: Spermatogenesis in an artificial three-dimensional system. Stem Cells 2012, 30, 2355–2360. [Google Scholar] [CrossRef]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Aandrol. 2015, 17, 972. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neuro. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Lunenfeld, E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004, 6, 259–268. [Google Scholar]
- França, L.R.; Hess, R.A.; Dufour, J.; Hofmann, M.-C.; Griswold, M. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Elhija, M.A.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J. Androl. 2012, 14, 285. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Schlatt, S.; Simoni, M.; Yeung, C.-H.; Elhija, M.A.; Luetjens, C.M.; Huleihel, M.; Wistuba, J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol. Hum. Reprod. 2009, 15, 521–529. [Google Scholar] [CrossRef]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of postmeiotic cells in vitro from spermatogonial cells of prepubertal cancer patients. Stem Cells Dev. 2018, 27, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of spermatogenesis in vitro in three-dimensional culture from spermatogonial cells of busulfan-treated immature mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef]
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.S.; Bar-Ami, S.; Huleihel, M. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies without Sperm from Sertoli Cell-Only Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-M.; Ryan, G.R.; Hapel, A.J.; Dominguez, M.J.; Russell, R.G.; Kapp, S.; Sylvestre, V.; Stanley, E.R. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002, 99, 111–120. [Google Scholar] [CrossRef]
- Sauter, K.A.; Pridans, C.; Sehgal, A.; Tsai, Y.T.; Bradford, B.M.; Raza, S.; Moffat, L.; Gow, D.J.; Beard, P.M.; Mabbott, N.A.; et al. Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J. Leukoc. Biol. 2014, 96, 265–274. [Google Scholar] [CrossRef]
- Cohen, P.E.; Nishimura, K.; Zhu, L.; Pollard, J.W. Macrophages: Important accessory cells for reproductive function. J. Leukoc. Biol. 1999, 66, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.C. Physiologic interactions between macrophages and Leydig cells. Exp. Biol. Med. 2006, 231, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wiktor-Jedrzejczak, W.; Bartocci, A.; Ferrante, A.W., Jr.; Ahmed-Ansari, A.; Sell, K.W.; Pollard, J.W.; Stanley, E.R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 1990, 87, 4828–4832. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Fadlon, E.; Abuelhija, A.; Haber, E.P.; Lunenfeld, E. Glial cell line-derived neurotrophic factor (GDNF) induced migration of spermatogonial cells in vitro via MEK and NF-kB pathways. Differentiation 2013, 86, 38–47. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Har-Vardi, I.; Harlev, A.; Levitas, E.; Zeadna, A.; Abofoul-Azab, M.; Dyomin, V.; Sheffield, V.C.; Lunenfeld, E.; Huleihel, M.; et al. Mutation in TDRD9 causes non-obstructive azoospermia in infertile men. J. Med. Genet. 2017, 54, 633–639. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawaied, A.; Arazi, E.; AbuElhija, A.; Lunenfeld, E.; Huleihel, M. The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro. Int. J. Mol. Sci. 2021, 22, 2325. https://doi.org/10.3390/ijms22052325
Sawaied A, Arazi E, AbuElhija A, Lunenfeld E, Huleihel M. The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro. International Journal of Molecular Sciences. 2021; 22(5):2325. https://doi.org/10.3390/ijms22052325
Chicago/Turabian StyleSawaied, Alaa, Eden Arazi, Ahmad AbuElhija, Eitan Lunenfeld, and Mahmoud Huleihel. 2021. "The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro" International Journal of Molecular Sciences 22, no. 5: 2325. https://doi.org/10.3390/ijms22052325
APA StyleSawaied, A., Arazi, E., AbuElhija, A., Lunenfeld, E., & Huleihel, M. (2021). The Presence of Colony-Stimulating Factor-1 and Its Receptor in Different Cells of the Testis; It Involved in the Development of Spermatogenesis In Vitro. International Journal of Molecular Sciences, 22(5), 2325. https://doi.org/10.3390/ijms22052325






