Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells
Abstract
1. Introduction
2. Results
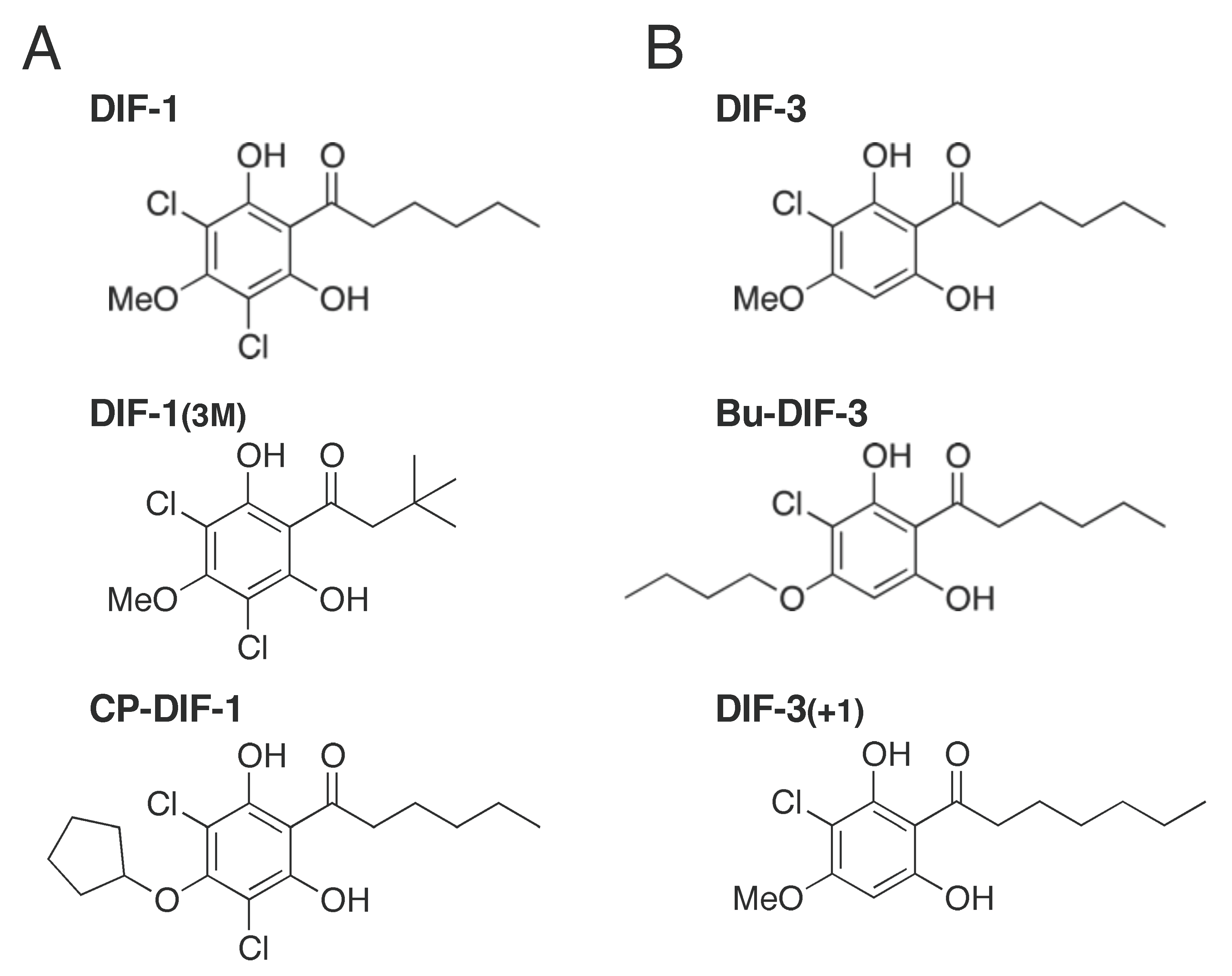
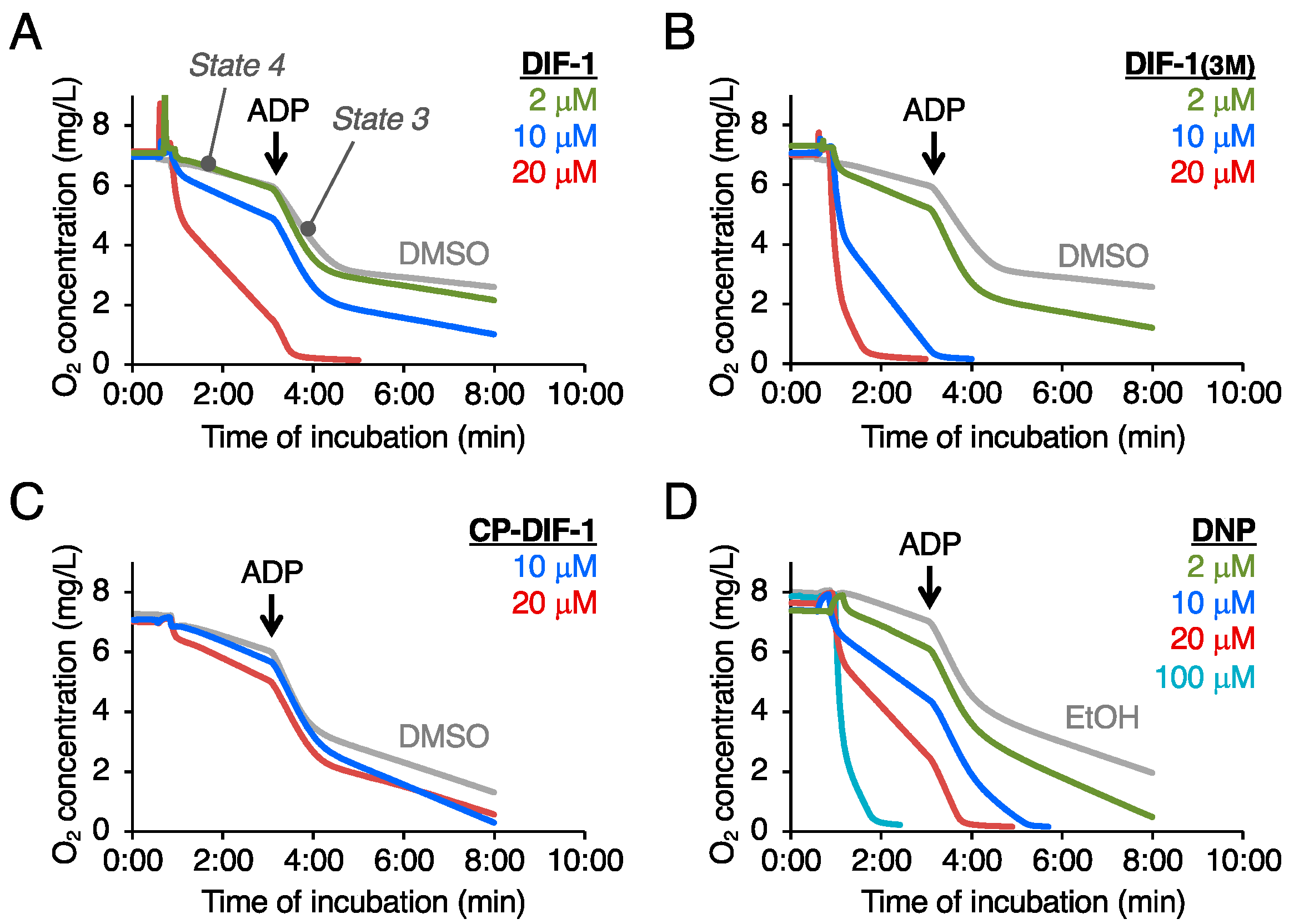
2.1. Effects of DIF Derivatives on Mitochondrial Oxygen Consumption (MOC)
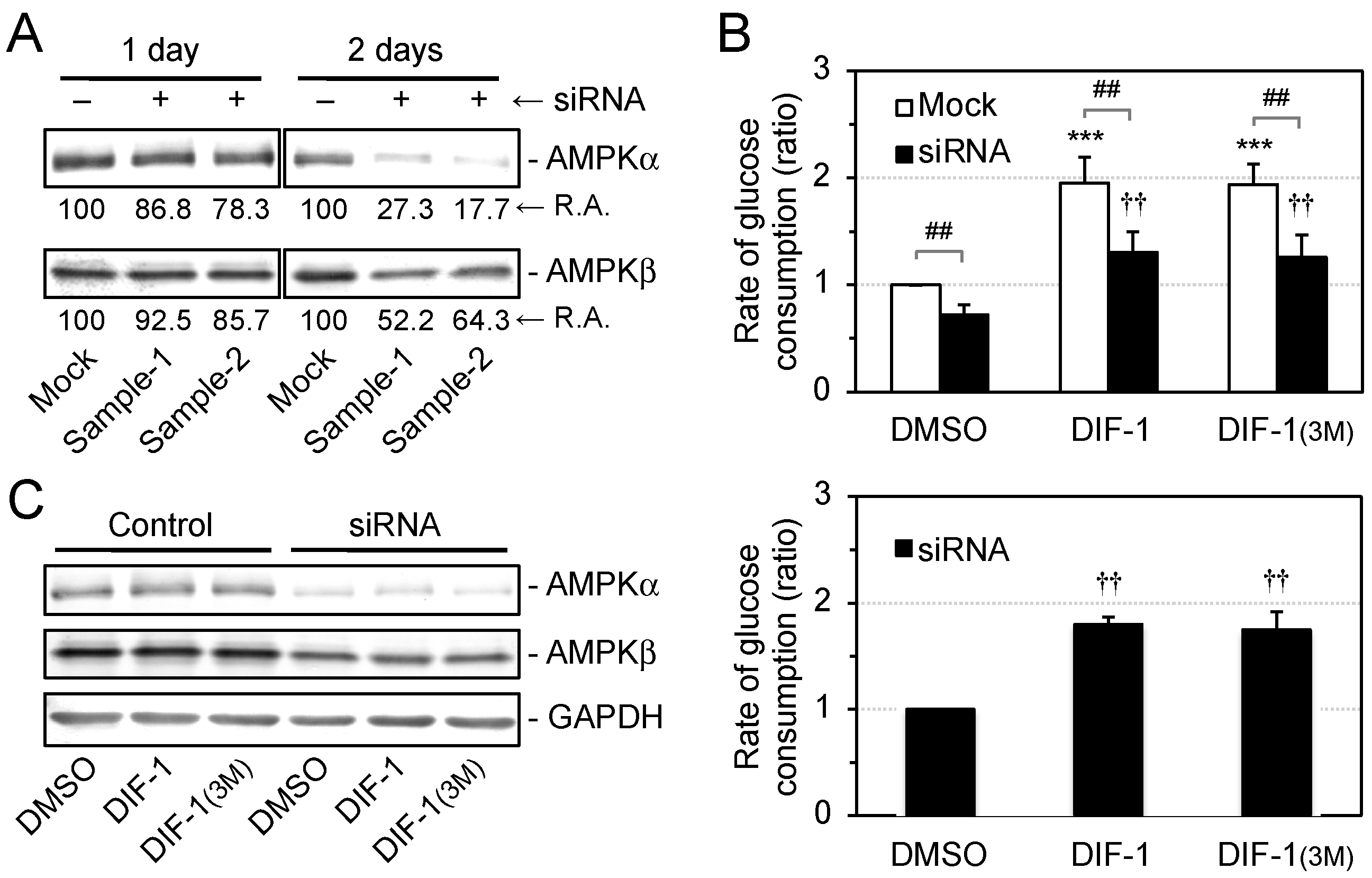
2.2. Effects of DIF Derivatives on AMPK Activity in 3T3-L1 Cells
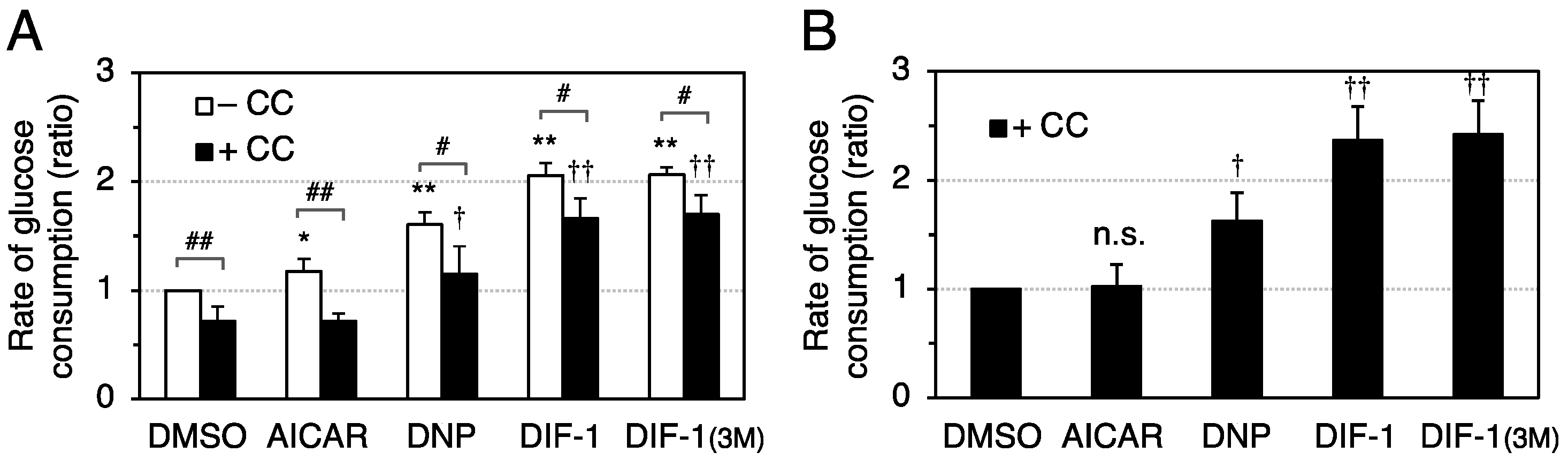
2.3. Effects of DIF Derivatives, DNP, and AICAR on Glucose Consumption in 3T3-L1 Cells
2.4. Effects of AMPK Inhibition on DIF-Promoted Glucose Uptake in 3T3-L1 Cells
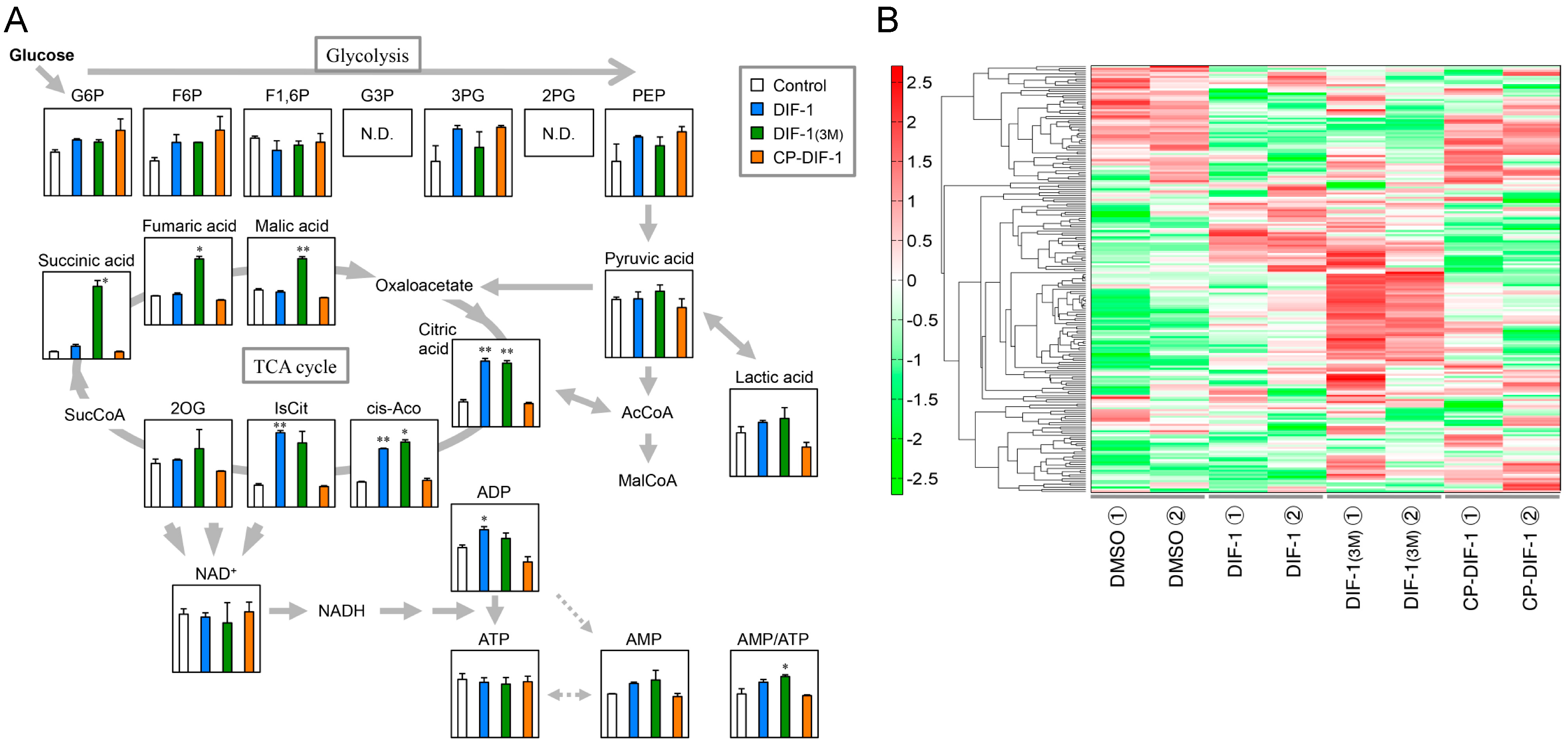
2.5. Effects of DIF Derivatives on Glucose Metabolism in 3T3-L1 Cells
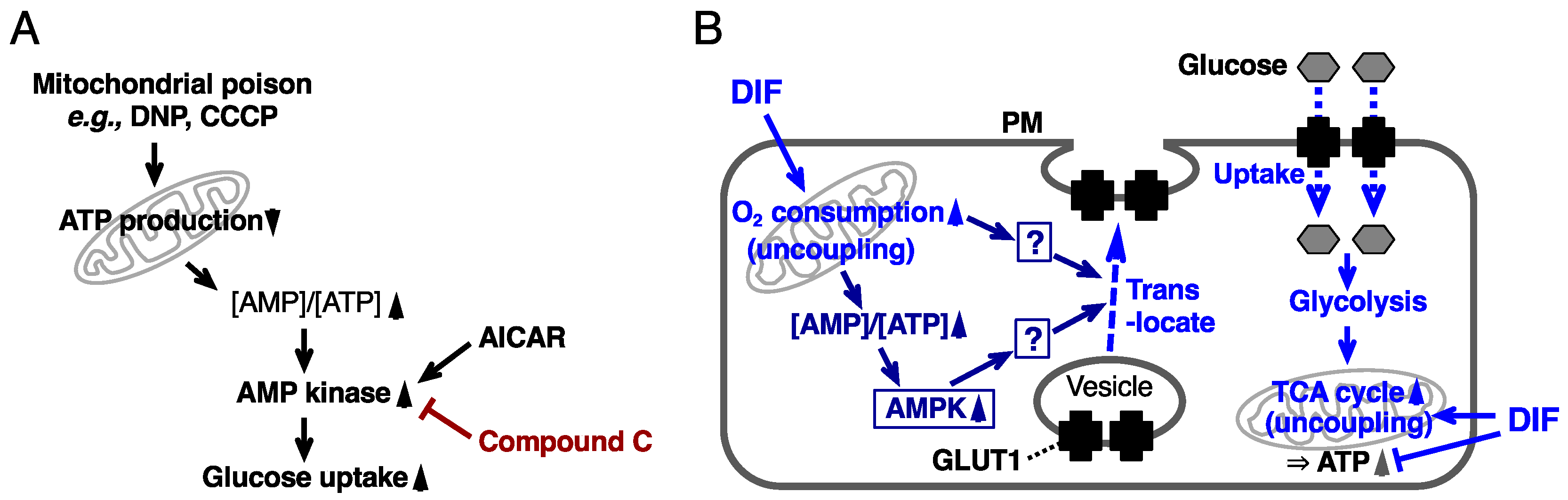
3. Discussion
3.1. DIF Derivatives That Promote Glucose Consumption (Uptake) in Mammalian Cells
3.2. Involvement of AMPK in the Actions of DIF-1 and DIF-1(3M)
4. Materials and Methods
4.1. Cells and Reagents
4.2. Measurement of Mitochondrial Oxygen Consumption
4.3. Assessment of Glucose Consumption (Uptake) in 3T3-L1 Cells
4.4. Western Blotting
4.5. RNA Interference (RNAi) Using Small Interfering RNA (siRNA)
4.6. Metabolome Analysis
4.7. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konijn, T.M.; van de Meene, J.G.C.; Bonner, J.T.; Barkley, D.S. The acrasin activity of adenosine-3′,5′-cyclic phosphate. Proc. Natl. Acad. Sci. USA 1967, 58, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Darmon, M.; Brachet, P.; Pereira da Silva, L.H. Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 1975, 72, 3163–3166. [Google Scholar] [CrossRef]
- Morris, H.R.; Taylor, G.W.; Masento, M.S.; Jermyn, K.A.; Kay, R.R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature 1987, 328, 811–814. [Google Scholar] [CrossRef]
- Kay, R.R.; Berks, M.; Traynor, D. Morphogen hunting in Dictyostelium discoideum. Development 1989, 107, 81–90. [Google Scholar]
- Kay, R.R.; Flatman, P.; Thompson, C.R.L. DIF signalling and cell fate. Semin. Cell Dev. Biol. 1999, 10, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, H.; Kubohara, Y. Differentiation-inducing factor-1 and -2 function also as modulators for Dictyostelium chemotaxis. PLoS ONE 2009, 4, e6658. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.R.; Masento, M.S.; Taylor, G.W.; Jermyn, K.A.; Kay, R.R. Structure elucidation of two differentiation inducing factors (DIF-2 and DIF-3) from the cellular slime mould Dictyostelium discoideum. Biochem. J. 1988, 249, 903–906. [Google Scholar] [CrossRef]
- Asahi, K.; Sakurai, A.; Takahashi, N.; Kubohara, Y.; Okamoto, K.; Tanaka, Y. DIF-1, morphogen of Dictyostelium discoideum, induces the erythroid differentiation in murine and human leukemia cells. Biochem. Biophys. Res. Commun. 1995, 208, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y.; Saito, Y.; Tatemoto, K. Differentiation-inducing factor of D. discoideum raises intracellular calcium concentration and suppresses cell growth in rat pancreatic AR42J cells. FEBS Lett. 1995, 359, 119–122. [Google Scholar] [CrossRef]
- Kubohara, Y.; Kimura, C.; Tatemoto, K. Putative morphogen, DIF, of Dictyostelium discoideum induces apoptosis in rat pancreatic AR42J cells. Dev. Growth Differ. 1995, 37, 711–716. [Google Scholar] [CrossRef]
- Kubohara, Y. DIF-1, putative morphogen of D. discoideum, suppresses cell growth and promotes retinoic acid-induced cell differentiation in HL-60. Biochem. Biophys. Res. Commun. 1997, 236, 418–422. [Google Scholar] [CrossRef]
- Kubohara, Y. Effects of differentiation-inducing factors (DIFs) of Dictyostelium discoideum on the human leukemia K562 cells: DIF-3 is the most potent anti-leukemic agent. Eur. J. Pharmacol. 1999, 381, 57–62. [Google Scholar] [CrossRef]
- Kanai, M.; Konda, Y.; Nakajima, T.; Izumi, Y.; Nanakin, A.; Kanda, N.; Kubohara, Y.; Chiba, T. Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity involved in gastric cancer cell proliferation via MEK-ERK dependent pathway. Oncogene 2003, 22, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Taba, Y.; Miwa, Y.; Kubohara, Y.; Watanabe, Y.; Hirata, M.; Morimoto, S.; Sasaguri, T. Dictyostelium differentiation-inducing factor-3 activates glycogen synthase kinase-3β and degrades cyclin D1 in mammalian cells. J. Biol. Chem. 2003, 278, 9663–9670. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Murata, T.; Tagawa, T.; Takahashi, K.; Ishikawa, R.; Abe, Y.; Hosaka, K.; Kubohara, Y. Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1) is a pharmacological target of differentiation-inducing factor-1, an anti-tumor agent isolated from Dictyostelium. Cancer Res. 2004, 64, 2568–2571. [Google Scholar] [CrossRef] [PubMed]
- Gokan, N.; Kikuchi, H.; Nakamura, K.; Oshima, Y.; Hosaka, K.; Kubohara, Y. Structural requirements of Dictyostelium differentiation-inducing factors for their stalk-cell-inducing activity in Dictyostelium cells and anti-proliferative activity in K562 human leukemic cells. Biochem. Pharmacol. 2005, 70, 676–685. [Google Scholar] [CrossRef]
- Kubohara, Y.; Kikuchi, H.; Matsuo, Y.; Oshima, Y.; Homma, Y. Mitochondria are the target organelle of differentiation-inducing factor-3, an anti-tumor agent isolated from Dictyostelium discoideum. PLoS ONE 2013, 8, e72118. [Google Scholar] [CrossRef]
- Kubohara, Y.; Komachi, M.; Homma, Y.; Kikuchi, H.; Oshima, Y. Derivatives of Dictyostelium differentiation-inducing factors inhibit lysophosphatidic acid–stimulated migration of murine osteosarcoma LM8 cells. Biochem. Biophys. Res. Commun. 2015, 463, 800–805. [Google Scholar] [CrossRef]
- Kubohara, Y.; Kikuchi, H. Dictyostelium: An important source of structural and functional diversity in drug discovery. Cells 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, K.; Makioka, Y.; Iizumi, K.; Takahashi, K.; Oshima, Y.; Kikuchi, H.; Kubohara, Y. Halogen-substituted derivatives of Dictyostelium differentiation-inducing factor-1 suppress serum-induced cell migration of human breast cancer MDA-MB-231 cells in vitro. Biomolecules 2019, 9, 256. [Google Scholar] [CrossRef]
- Omata, W.; Shibata, H.; Nagasawa, M.; Kojima, I.; Kikuchi, H.; Oshima, Y.; Hosaka, K.; Kubohara, Y. Dictyostelium differentiation-inducing factor-1 induces glucose transporter 1 translocation and promotes glucose uptake in mammalian cells. FEBS J. 2007, 274, 3392–3404. [Google Scholar] [CrossRef] [PubMed]
- Kubohara, Y.; Kikuchi, H.; Oshima, Y. Exploitation of the derivatives of Dictyostelium differentiation-inducing factor-1, which promote glucose consumption in mammalian cells. Life Sci. 2008, 83, 608–612. [Google Scholar] [CrossRef]
- Kawaharada, R.; Nakamura, A.; Takahashi, K.; Kikuchi, H.; Oshima, Y.; Kubohara, Y. Oral administration of Dictyostelium differentiation-inducing factor 1 lowers blood glucose levels in streptozotocin-induced diabetic rats. Life Sci. 2016, 155, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Corton, J.M.; Gillespie, J.G.; Hardie, D.G. Role of the AMP-activated protein kinase in the cellular stress response. Curr. Biol. 1994, 4, 315–324. [Google Scholar] [CrossRef]
- Abbud, W.; Habinowski, S.; Zhang, J.Z.; Kendrew, J.; Elkairi, F.S.; Kemp, B.E.; Witters, L.A.; Ismail-Beigi, F. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch. Biochem. Biophys. 2000, 380, 347–352. [Google Scholar] [CrossRef]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef]
- Barnes, K.; Ingram, J.C.; Porras, O.H.; Barros, L.F.; Hudson, E.R.; Fryer, L.G.; Foufelle, F.; Carling, D.; Hardie, D.G.; Baldwin, S.A. Activation of GLUT1 by metabolic and osmotic stress: Potential involvement of AMP-activated protein kinase (AMPK). J. Cell Sci. 2002, 115, 2433–2442. [Google Scholar] [PubMed]
- Ismail-Beigi, F. Metabolic regulation of glucose transport. J. Membr. Biol. 1993, 135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shetty, M.; Loeb, J.N.; Vikstrom, K.; Ismail-Beigi, F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. J. Biol. Chem. 1993, 268, 17225–17232. [Google Scholar] [CrossRef]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997, 273, E1107–E1112. [Google Scholar] [CrossRef] [PubMed]
- Asahi, Y.; Hayashi, H.; Wang, L.; Ebina, Y. Fluoromicroscopic detection of myc-tagged GLUT4 on the cell surface: Co-localization of the translocated GLUT4 with rearranged actin by insulin treatment in CHO cells and L6 myotubes. J. Med. Investig. 1999, 46, 192–199. [Google Scholar]
- Bashan, N.; Burdett, E.; Gumà, A.; Sargeant, R.; Tumiati, L.; Liu, Z.; Klip, A. Mechanisms of adaptation of glucose transporters to changes in the oxidative chain of muscle and fat cells. Am. J. Physiol. 1993, 264, C430–C440. [Google Scholar] [CrossRef]
- Taha, C.; Tsakiridis, T.; McCall, A.; Klip, A. Glucose transporter expression in L6 muscle cells: Regulation through insulin- and stress-activated pathways. Am. J. Physiol. 1997, 273, E68–E76. [Google Scholar] [CrossRef]
- Kang, J.; Heart, E.; Sung, C. Effects of cellular ATP depletion on glucose transport and insulin signaling in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E428–E435. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Khayat, Z.A.; Ruderman, N.B.; Klip, A. Dissociation of 5′ AMP-activated protein kinase activation and glucose uptake stimulation by mitochondrial uncoupling and hyperosmolar stress: Differential sensitivities to intracellular Ca2+ and protein kinase C inhibition. Biochem. Biophys. Res. Commun. 2001, 285, 1066–1070. [Google Scholar] [CrossRef]
- Pelletier, A.; Joly, E.; Prentki, M.; Coderre, L. Adenosine 5′-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology 2005, 146, 2285–2294. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Katahira, H.; Ozawa, S.; Nakamichi, Y.; Tanaka, T.; Shimoyama, T.; Takahashi, K.; Yoshimoto, K.; Imaizumi, M.O.; Nagamatsu, S.; et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. 2005, 289, E643–E649. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Ismail-Beigi, F. Critical role of 5′-AMP-activated protein kinase in the stimulation of glucose transport in response to inhibition of oxidative phosphorylation. Am. J. Physiol. 2007, 292, C477–C487. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Cheruvu, V.K.; Ismail-Beigi, F. Stimulation of glucose transport in response to activation of distinct AMPK signaling pathways. Am. J. Physiol. 2008, 295, C1071–C1082. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside: A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Takahashi, K.; Kikuchi, H.; Nguyen, V.H.; Oshima, Y.; Ishigaki, H.; Nakajima-Shimada, J.; Kubohara, Y. Biological activities of novel derivatives of differentiation-inducing factor-3 from Dictyostelium discoideum. Biol. Pharm. Bull. 2017, 40, 1941–1947. [Google Scholar] [CrossRef]
- Oladimeji, P.; Kubohara, Y.; Kikuchi, H.; Oshima, Y.; Rusch, C.; Skerl, R.; Diakonova, M. A derivative of differentiation-inducing factor-3 inhibits PAK1 activity and breast cancer cell proliferation. Int. J. Cancer Clinic. Res. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Alimova, I.N.; Liu, B.; Fan, Z.; Edgerton, S.M.; Dillon, T.; Lind, S.E.; Thor, A.D. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009, 8, 909–915. [Google Scholar] [CrossRef]
- Yung, M.M.; Chan, D.W.; Liu, V.W.; Yao, K.M.; Ngan, H.Y. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer 2013, 13, 327. [Google Scholar] [CrossRef]
- Zou, Y.F.; Xie, C.W.; Yang, S.X.; Xiong, J.P. AMPK activators suppress breast cancer cell growth by inhibiting DVL3-facilitated Wnt/β-catenin signaling pathway activity. Mol. Med. Rep. 2017, 15, 899–907. [Google Scholar] [CrossRef][Green Version]
- Jiralerspong, S.; Gonzalez-Angulo, A.M.; Hung, M.C. Expanding the arsenal: Metformin for the treatment of triple-negative breast cancer? Cell Cycle 2009, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.F.; Schmidt, M.C. AMPK-mediated regulation of alpha-arrestins and protein trafficking. Int. J. Mol. Sci. 2019, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, T.; Haucke, V.; Maritzen, T. Endocytosis in the adaptation to cellular stress. Cell Stress 2020, 4, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Kabuyama, Y.; Suzuki, T.; Nakazawa, N.; Yamaki, J.; Homma, M.K.; Homma, Y. Dysregulation of very long chain acyl-CoA dehydrogenase coupled with lipid peroxidation. Am. J. Physiol. 2010, 298, C107–C113. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Thompson, C.B. Mitochondrial respiratory control is lost during growth factor deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 12801–12806. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, J.; Potla, R.; Chwae, Y.J.; Sepuri, N.B.; Zhang, Q.; Koeck, T.; Derecka, M.; Szczepanek, K.; Szelag, M.; Gornicka, A.; et al. Function of mitochondrial Stat3 in cellular respiration. Science 2009, 323, 793–797. [Google Scholar] [CrossRef]
- Soga, T.; Heiger, D.N. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000, 72, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, D.; Horiuchi, Y.; Kano, F.; Noguchi, Y.; Sugawara, T.; Takemoto, I.; Kubota, N.; Kadowaki, T.; Murata, M. L-cysteine reversibly inhibits glucose-induced biphasic insulin secretion and ATP production by inactivating PKM2. Proc. Natl. Acad. Sci. USA 2015, 112, E1067–E1076. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubohara, Y.; Homma, Y.; Shibata, H.; Oshima, Y.; Kikuchi, H. Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells. Int. J. Mol. Sci. 2021, 22, 2293. https://doi.org/10.3390/ijms22052293
Kubohara Y, Homma Y, Shibata H, Oshima Y, Kikuchi H. Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells. International Journal of Molecular Sciences. 2021; 22(5):2293. https://doi.org/10.3390/ijms22052293
Chicago/Turabian StyleKubohara, Yuzuru, Yoshimi Homma, Hiroshi Shibata, Yoshiteru Oshima, and Haruhisa Kikuchi. 2021. "Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells" International Journal of Molecular Sciences 22, no. 5: 2293. https://doi.org/10.3390/ijms22052293
APA StyleKubohara, Y., Homma, Y., Shibata, H., Oshima, Y., & Kikuchi, H. (2021). Dictyostelium Differentiation-Inducing Factor-1 Promotes Glucose Uptake, at Least in Part, via an AMPK-Dependent Pathway in Mouse 3T3-L1 Cells. International Journal of Molecular Sciences, 22(5), 2293. https://doi.org/10.3390/ijms22052293





