Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis
Abstract
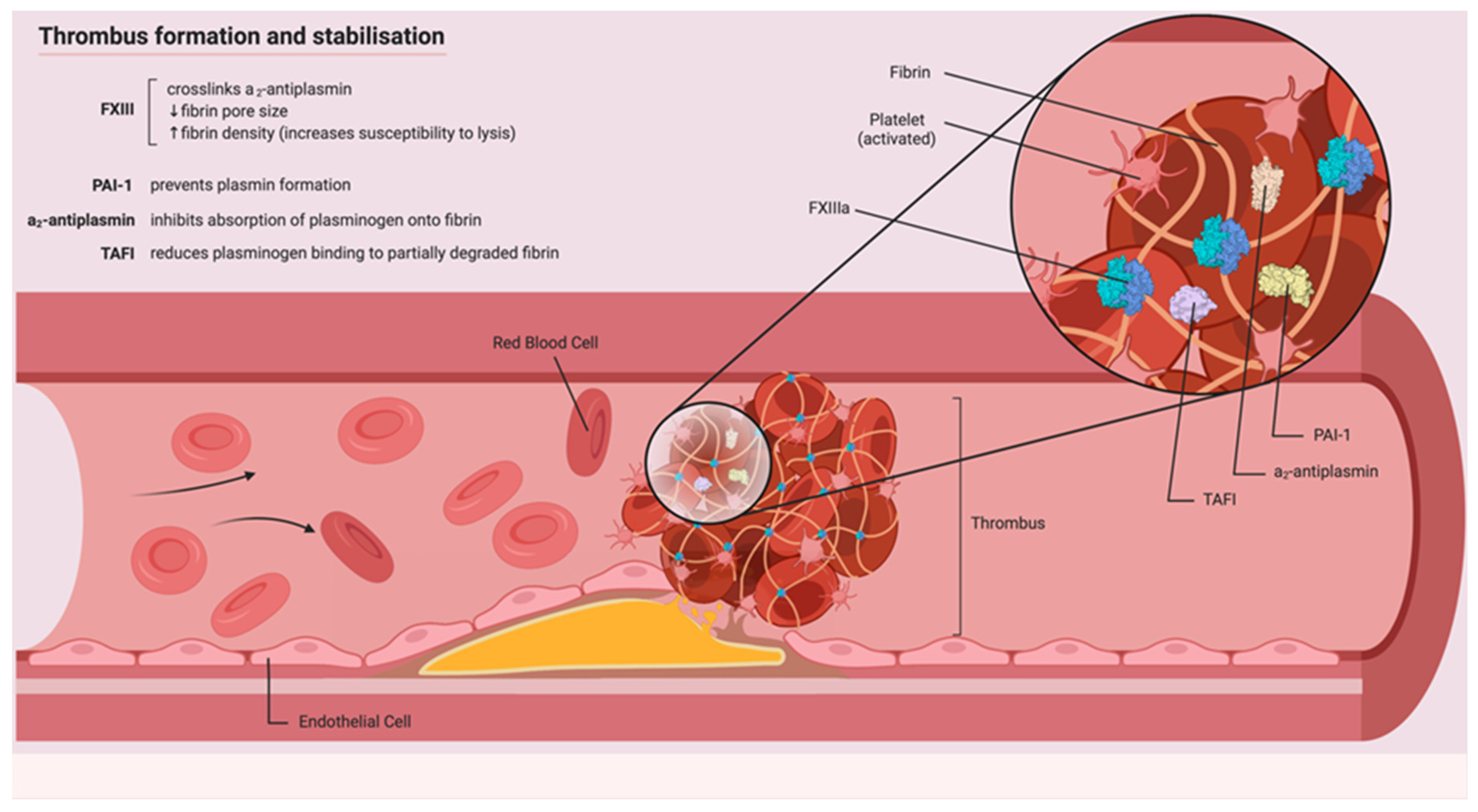
1. Introduction
1.1. Physiological Role of Fibrin
1.2. Assessment of Fibrin Clot Structure and Permeability
1.3. Involvement of Fibrin Clot Structure in Disease States
1.4. Physiological Role of FXIII
2. Measurement of FXIII
2.1. FXIII Activity Assays
2.1.1. Clot Solubility Test
2.1.2. Quantitative Activity Assays
- I.
- Ammonia assays (the most commonly used in clinical laboratories) quantify the ammonia released due to FXIII’s transglutaminase activity, utilising spectrophotometry. This acts as an indirect measurement of transglutaminase activity and is used to quantify overall FXIII activity.
- II.
- Amine-incorporation assays also quantify FXIII’s transglutaminase activity by assessing the isoamide bond formation, catalysed by FXIIIa. A labeled amine substrate, such as 5-biotinamidopentylamine, is paired with a glutamine-containing protein, such as casein. The activity of FXIIIa is quantified by measuring the residual unincorporated labeled amine. Importantly, amine incorporation assays may yield falsely elevated FXIII activity levels in subjects with the common Val34Leu polymorphism, which is present in ~25% of European Caucasians [107]. Thus, it is rarely used in the clinical setting.
- III.
- Isopeptidase assays measure the FXIIIa-dependent hydrolysis of γ:ε isopeptide bonds. A FXIII substrate (A101) with a fluorophore and a fluorescent quencher linked by γ:ε isopeptide bonds is formulated. The isopeptidase-dependent release of the quencher unmasks A101 fluorescence which is detected by a spectrofluorometer, allowing for dynamic measurement of FXIII enzyme activity.
3. FXIII Quantitative Antigen Assays
4. FXIII Genotyping
4.1. Involvement of FXIII in Disease States
4.2. Physiological Role of Fibrinolysis
5. Measurement of Fibrinolysis
5.1. Assessment of Level/Activity of Individual Molecules (Factors) Regulating Fibrinolysis
5.2. Global Assessment of Fibrinolysis
5.2.1. Plasma Clot Lysis Time (LT)
5.2.2. Global Thrombosis Test (GTT)
6. Involvement of Fibrinolysis in Disease States
7. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| A2AP | alpha 2-antiplasmin |
| ACS | acute coronary syndrome |
| CAD | coronary artery disease |
| FXIII/FXIIIa | coagulation factor XIII/activated coagulation factor XIII |
| GTT | Global Thrombosis Test |
| MACE | major cardiovascular adverse events |
| MI | myocardial infarction |
| PAI-1/-2 | Plasminogen activator inhibitor-1/-2 |
| ROTEM | Thromboelastometry |
| TAFI | Thrombin activatable fibrinolysis inhibitor |
| TEG | Thromboelastography |
| VTE | venous thromboembolism |
References
- Wolberg, A.S.; Aleman, M.M.; Leiderman, K.; Machlus, K.R. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth. Analg. 2012, 114, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Rosendaal, F.R.; Weitz, J.I.; Jaffer, I.H.; Agnelli, G.; Baglin, T.; Mackman, N. Venous thrombosis. Nat. Rev. Dis Primers 2015, 1, 15006. [Google Scholar] [CrossRef]
- Standeven, K.F.; Ariëns, R.A.; Grant, P.J. The molecular physiology and pathology of fibrin structure/function. Blood Rev. 2005, 19, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Kant, J.A.; Fornace, A.J.; Saxe, D.F.; I Simon, M.; McBride, O.W.; Crabtree, G.R. Evolution and organization of the fibrinogen locus on chromosome 4: Gene duplication accompanied by transposition and inversion. Proc. Natl. Acad. Sci. USA 1985, 82, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.W.; Rixon, M.W.; Que, B.G.; Davie, E.W. Cloning of fibrinogen genes and their cDNA. Ann. N. Y. Acad. Sci. 1983, 408, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.W.; Harris, J.E.; Davie, E.W. Nucleotide Sequences of the Three Genes Coding for Human Fibrinogen. Adv. Exp. Med. Biol. 1990, 281, 39–48. [Google Scholar] [CrossRef]
- Takeda, Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J. Clin. Investig. 1966, 45, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Macromol. Protein Complexes III Struct. Funct. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Wolberg, A.S. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin. Thromb. Hemost. 2016, 42, 445–454. [Google Scholar] [CrossRef]
- Budzynski, A.Z.; Olexa, S.A.; Pandya, B.V. Fibrin polymerization sites in fibrinogen and fibrin fragments. Ann. N. Y. Acad. Sci. 1983, 408, 301–314. [Google Scholar] [CrossRef]
- Lord, S.T. Molecular Mechanisms Affecting Fibrin Structure and Stability. Arter. Thromb. Vasc. Biol. 2011, 31, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Terasawa, F.; Haneishi, A.; Fujihara, N.; Hirota-Kawadobora, M.; Yamauchi, K.; Ota, H.; Lord, S.T. B:b interactions are essential for polymerization of variant fibrinogens with impaired holes ‘a’. J. Thromb. Haemost. 2007, 5, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Spraggon, G.; Everse, S.J.; Doolittle, R.F. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature 1997, 389, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mochalkin, I.; Doolittle, R.F. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc. Natl. Acad. Sci. USA 2000, 97, 14156–14161. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, I.N.; Nagaswami, C.; Purohit, P.K.; Weisel, J.W. Fibrin Clots Are Equilibrium Polymers That Can Be Remodeled Without Proteolytic Digestion. Sci. Rep. 2012, 2, srep00879. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef]
- Wolberg, A.S. Thrombin generation and fibrin clot structure. Blood Rev. 2007, 21, 131–142. [Google Scholar] [CrossRef]
- Blombäck, B.; Carlsson, K.; Fatah, K.; Hessel, B.; Procyk, R. Fibrin in human plasma: Gel architectures governed by rate and nature of fibrinogen activation. Thromb. Res. 1994, 75, 521–538. [Google Scholar] [CrossRef]
- Ryan, E.A.; Mockros, L.F.; Weisel, J.W.; Lorand, L. Structural Origins of Fibrin Clot Rheology. Biophys. J. 1999, 77, 2813–2826. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Monroe, D.M.; Roberts, H.R.; Hoffman, M. Elevated prothrombin results in clots with an altered fiber structure: A possible mechanism of the increased thrombotic risk. Blood 2003, 101, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Allen, G.A.; Monroe, D.M.; Hedner, U.; Roberts, H.R.; Hoffman, M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br. J. Haematol. 2005, 131, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, C.L.; Dyrlund, T.F.; Poulsen, E.T.; Enghild, J.J.; Scavenius, C. Coagulation factor XIIIa substrates in human plasma: Identification and incorporation into the clot. J. Biol. Chem. 2014, 289, 6526–6534. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Aoki, N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J. Clin. Investig. 1980, 65, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Lawrie, L.C.; Mosesson, M.W.; Booth, N.A. Characterization of Crosslinking Sites in Fibrinogen for Plasminogen Activator Inhibitor 2 (PAI-2). Ann. N. Y. Acad. Sci. 2006, 936, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Lawrie, L.C.; Crombie, P.W.; Booth, N.A.; Mosesson, M.W. Cross-linking of Plasminogen Activator Inhibitor 2 and α2-Antiplasmin to Fibrin(ogen). J. Biol. Chem. 2000, 275, 24915–24920. [Google Scholar] [CrossRef]
- Rijken, D.C.; Uitte de Willige, S. Inhibition of Fibrinolysis by Coagulation Factor XIII. Biomed Res. Int. 2017, 2017, 1209676. [Google Scholar] [CrossRef]
- Aleman, M.M.; Gardiner, C.; Harrison, P.; Wolberg, A.S. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J. Thromb. Haemost. 2011, 9, 2251–2261. [Google Scholar] [CrossRef]
- Campbell, R.A.; Overmyer, K.A.; Selzman, C.H.; Sheridan, B.C.; Wolberg, A.S. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood 2009, 114, 4886–4896. [Google Scholar] [CrossRef]
- Cines, D.B.; Lebedeva, T.; Nagaswami, C.; Hayes, V.; Massefski, W.; Litvinov, R.I.; Rauova, L.; Lowery, T.J.; Weisel, J.W. Clot contraction: Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 2014, 123, 1596–1603. [Google Scholar] [CrossRef]
- Zubairova, L.D.; Nabiullina, R.M.; Nagaswami, C.; Zuev, Y.F.; Mustafin, I.G.; Litvinov, R.I.; Weisel, J.W. Circulating Microparticles Alter Formation, Structure and Properties of Fibrin Clots. Sci. Rep. 2015, 5, 17611. [Google Scholar] [CrossRef] [PubMed]
- Wohner, N.; Sótonyi, P.; Machovich, R.; Szabó, L.; Tenekedjiev, K.; Silva, M.M.; Longstaff, C.; Kolev, K.N. Lytic Resistance of Fibrin Containing Red Blood Cells. Arter. Thromb. Vasc. Biol. 2011, 31, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 2009, 102, 1169–1175. [Google Scholar] [CrossRef]
- Campbell, R.A.; Overmyer, K.A.; Bagnell, C.R.; Wolberg, A.S. Cellular Procoagulant Activity Dictates Clot Structure and Stability as a Function of Distance from the Cell Surface. Arter. Thromb. Vasc. Biol. 2008, 28, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Jerome, W.G.; Handt, S.; Hantgan, R.R. Endothelial cells organize fibrin clots into structures that are more resistant to lysis. Microsc. Microanal. 2005, 11, 268–277. [Google Scholar] [CrossRef]
- Weisel, J.W. Structure of fibrin: Impact on clot stability. J. Thromb. Haemost. 2007, 5 (Suppl. 1), 116–124. [Google Scholar] [CrossRef]
- Collet, J.-P.; Montalescot, G.; Lesty, C.; Weisel, J. A Structural and Dynamic Investigation of the Facilitating Effect of Glycoprotein IIb/IIIa Inhibitors in Dissolving Platelet-Rich Clots. Circ. Res. 2002, 90, 428–434. [Google Scholar] [CrossRef]
- Varjú, I.; Sótonyi, P.; Machovich, R.; Szabo, L.; Tenekedjiev, K.; Silva, M.M.; Longstaff, C.; Kolev, K.N. Hindered dissolution of fibrin formed under mechanical stress. J. Thromb. Haemost. 2011, 9, 979–986. [Google Scholar] [CrossRef]
- Siudut, J.; Grela, M.; Wypasek, E.; Plens, K.; Undas, A. Reduced plasma fibrin clot permeability and susceptibility to lysis are associated with increased risk of postthrombotic syndrome. J. Thromb. Haemost. 2016, 14, 784–793. [Google Scholar] [CrossRef]
- Undas, A.; Zawilska, K.; Ciesla-Dul, M.; Lehmann-Kopydłowska, A.; Skubiszak, A.; Ciepłuch, K.; Tracz, W. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 2009, 114, 4272–4278. [Google Scholar] [CrossRef]
- Mills, J.D.; Ariëns, R.A.S.; Mansfield, M.W.; Grant, P.J. Altered Fibrin Clot Structure in the Healthy Relatives of Patients with Premature Coronary Artery Disease. Circulation 2002, 106, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Zabczyk, M.; Plens, K.; Wojtowicz, W.; Undas, A. Prothrombotic Fibrin Clot Phenotype Is Associated with Recurrent Pulmonary Embolism After Discontinuation of Anticoagulant Therapy. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.M.; Macrae, F.L.; Duval, C.; McPherson, H.R.; Bridge, K.I.; Ajjan, R.A.; Ridger, V.C.; Connell, S.D.; Philippou, H.; Ariëns, R.A.S. Thrombin and fibrinogen γ′ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood 2016, 127, 487–495. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Blombäck, M.; Ekman, G.J.; Hedner, U. The role of recombinant factor VIIa (FVIIa) in fibrin structure in the absence of FVIII/FIX. J. Thromb. Haemost. 2003, 1, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Ariëns, R.A.S. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- Korte, W.; Poon, M.-C.; Iorio, A.; Makris, M. Thrombosis in Inherited Fibrinogen Disorders. Transfus. Med. Hemotherapy 2017, 44, 70–76. [Google Scholar] [CrossRef]
- Fatah, K.; Hamsten, A.; Blombäck, B.; Blombäck, M. Fibrin gel network characteristics and coronary heart disease: Relations to plasma fibrinogen concentration, acute phase protein, serum lipoproteins and coronary atherosclerosis. Thromb. Haemost. 1992, 68, 130–135. [Google Scholar] [CrossRef]
- Fatah, K.; Silveira, A.; Tornvall, P.; Karpe, F.; Blombäck, M.; Hamsten, A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb. Haemost. 1996, 76, 535–540. [Google Scholar] [CrossRef]
- Meltzer, M.E.; Doggen, C.J.M.; De Groot, P.G.; Rosendaal, F.R.; Lisman, T. Plasma levels of fibrinolytic proteins and the risk of myocardial infarction in men. Blood 2010, 116, 529–536. [Google Scholar] [CrossRef]
- Collet, J.-P.; Allali, Y.; Lesty, C.; Tanguy, M.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered Fibrin Architecture Is Associated with Hypofibrinolysis and Premature Coronary Atherothrombosis. Arter. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [Google Scholar] [CrossRef]
- Undas, A.; Plicner, D.; Stępień, E.Ł.; Drwiła, R.; Sadowski, J. Altered fibrin clot structure in patients with advanced coronary artery disease: A role of C-reactive protein, lipoprotein(a) and homocysteine. J. Thromb. Haemost. 2007, 5, 1988–1990. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Zalewski, J.; Krochin, M.; Siudak, Z.; Sadowski, M.; Pregowski, J.; Dudek, D.; Janion, M.; Witkowski, A.; Zmudka, K. Altered Plasma Fibrin Clot Properties Are Associated with In-Stent Thrombosis. Arter. Thromb. Vasc. Biol. 2010, 30, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, J.; Undas, A.; Godlewski, J.; Stępień, E.Ł.; Zmudka, K. No-Reflow Phenomenon After Acute Myocardial Infarction Is Associated with Reduced Clot Permeability and Susceptibility to Lysis. Arter. Thromb. Vasc. Biol. 2007, 27, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Szułdrzynski, K.; Stepien, E.; Zalewski, J.; Godlewski, J.; Tracz, W.; Pasowicz, M.; Zmudka, K. Reduced clot permeability and susceptibility to lysis in patients with acute coronary syndrome: Effects of inflammation and oxidative stress. Atherosclerosis 2008, 196, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Slowik, A.; Wolkow, P.P.; Szczudlik, A.; Tracz, W. Fibrin clot properties in acute ischemic stroke: Relation to neurological deficit. Thromb. Res. 2010, 125, 357–361. [Google Scholar] [CrossRef]
- Rooth, E.; Wallen, N.; Blombäck, M.; He, S. Decreased fibrin network permeability and impaired fibrinolysis in the acute and convalescent phase of ischemic stroke. Thromb. Res. 2011, 127, 51–56. [Google Scholar] [CrossRef]
- Undas, A.; Podolec, P.; Zawilska, K.; Pieculewicz, M.; Jedliński, I.; Stępień, E.; Konarska-Kuszewska, E.; Węglarz, P.; Duszyńska, M.; Hanschke, E.; et al. Altered Fibrin Clot Structure/Function in Patients with Cryptogenic Ischemic Stroke. Stroke 2009, 40, 1499–1501. [Google Scholar] [CrossRef]
- Undas, A.; Nowakowski, T.; Cieśla-Dul, M.; Sadowski, J. Abnormal plasma fibrin clot characteristics are associated with worse clinical outcome in patients with peripheral arterial disease and thromboangiitis obliterans. Atherosclerosis 2011, 215, 481–486. [Google Scholar] [CrossRef]
- Bhasin, N.; Parry, D.J.; Scott, D.J.A.; Ariëns, R.; Grant, P.J.; West, R.M. Regarding “Altered fibrin clot structure and function in individuals with intermittent claudication. ” J. Vasc. Surg. 2009, 49, 1088–1089. [Google Scholar] [CrossRef]
- Guimarães, A.H.C.; De Bruijne, E.L.E.; Lisman, T.; Dippel, D.W.J.; Deckers, J.W.; Poldermans, N.; Rijken, D.C.; Leebeek, F.W. Hypofibrinolysis is a risk factor for arterial thrombosis at young age. Br. J. Haematol. 2009, 145, 115–120. [Google Scholar] [CrossRef]
- Bhasin, N.; Ariëns, R.; West, R.M.; Parry, D.J.; Grant, P.J.; Scott, D.J.A. Altered fibrin clot structure and function in the healthy first-degree relatives of subjects with intermittent claudication. J. Vasc. Surg. 2008, 48, 1497–1503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartlett, J.W.; De Stavola, B.; Meade, T.W. Assessing the contribution of fibrinogen in predicting risk of death in men with peripheral arterial disease. J. Thromb. Haemost. 2009, 7, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.J.; Al-Barjas, H.S.; Chappell, L.; Rashid, T.; Ariëns, R.A.S.; Scott, D.J.A. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. Br. J. Surg. 2009, 96, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mohler, E.R., III; Xie, D.; Shlipak, M.G.; Townsend, R.R.; Appel, L.J.; Raj, D.S.; Ojo, A.O.; Schreiber, M.J.; Strauss, L.F.; et al. Risk factors for peripheral arterial disease among patients with chronic kidney disease. Am. J. Cardiol. 2012, 110, 136–141. [Google Scholar] [CrossRef][Green Version]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef]
- Lisman, T.; de Groot, P.G.; Meijers, J.C.M.; Rosendaal, F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood 2005, 105, 1102–1105. [Google Scholar] [CrossRef]
- Undas, A.; Natorska, J. Improving fibrinolysis in venous thromboembolism: Impact of fibrin structure. Expert Rev. Hematol. 2019, 12, 597–607. [Google Scholar] [CrossRef]
- Sjøland, J.A.; Sidelmann, J.J.; Brabrand, M.; Pedersen, R.S.; Pedersen, J.H.; Esbensen, K.; Standeven, K.F.; Ariëns, R.A.; Gram, J. Fibrin clot structure in patients with end-stage renal disease. Thromb. Haemost. 2007, 98, 339–345. [Google Scholar]
- Undas, A.; Kolarz, M.; Kopeć, G.; Tracz, W. Altered fibrin clot properties in patients on long-term haemodialysis: Relation to cardiovascular mortality. Nephrol. Dial. Transplant. 2008, 23, 2010–2015. [Google Scholar] [CrossRef]
- Schütt, K.; Savvaidis, A.; Maxeiner, S.; Lysaja, K.; Jankowski, V.; Schirmer, S.H.; Dimkovic, N.; Boor, P.; Kaesler, N.; Dekker, F.W.; et al. Clot Structure: A Potent Mortality Risk Factor in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 1622–1630. [Google Scholar] [CrossRef]
- Kaczmarek, P.; Sladek, K.; Stepien, E.; Skucha, W.; Rzeszutko, M.; Gorkiewicz-Kot, I.; Tracz, W.; Undas, A. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Thromb. Haemost. 2009, 102, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Laki, K.; Lorand, L. On the Solubility of Fibrin Clots. Science 1948, 108, 280. [Google Scholar] [CrossRef] [PubMed]
- Duckert, F.; Jung, E.; Shmerling, D.H. Coagulation physiology research in a new coagulation disorder. Deficiency of a fibrin-stabilizing factor. Schweiz. Med. Wochenschr. 1961, 91, 1139–1140. [Google Scholar] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komáromi, I.; Katona, É. Factor XIII: A Coagulation Factor with Multiple Plasmatic and Cellular Functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Elgheznawy, A.; Shi, L.; Hu, J.; Wittig, I.; Laban, H.; Pircher, J.; Mann, A.; Provost, P.; Randriamboavonjy, V.; Fleming, I. Dicer Cleavage by Calpain Determines Platelet microRNA Levels and Function in Diabetes. Circ. Res. 2015, 117, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.S.; Shuman, M.A. The zymogen forms of blood coagulation factor XIII bind specifically to fibrinogen. J. Biol. Chem. 1982, 257, 6096–6101. [Google Scholar] [CrossRef]
- Takagi, T.; Doolittle, R.F. Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin. Biochemistry 1974, 13, 750–756. [Google Scholar] [CrossRef]
- Schroeder, V.; Vuissoz, J.-M.; Caflisch, A.; Kohler, H.P. Factor XIII activation peptide is released into plasma upon cleavage by thrombin and shows a different structure compared to its bound form. Thromb. Haemost. 2007, 97, 890–898. [Google Scholar] [CrossRef]
- Komáromi, I.; Bagoly, Z.; Muszbek, L. Factor XIII: Novel structural and functional aspects. J. Thromb. Haemost. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Credo, R.B.; Curtis, C.G.; Lorand, L. Alpha-chain domain of fibrinogen controls generation of fibrinoligase (coagulation factor XIIIa). Calcium ion regulatory aspects. Biochemistry 1981, 20, 3770–3778. [Google Scholar] [CrossRef]
- Credo, R.B.; Curtis, C.G.; Lorand, L. Ca2+-related regulatory function of fibrinogen. Proc. Natl. Acad. Sci. USA 1978, 75, 4234–4237. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, T.J.; Shafer, J.A. Interactions of factor XIII with fibrin as substrate and cofactor. Biochemistry 1992, 31, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Polgár, J.; Boda, Z. Platelet factor XIII becomes active without the release of activation peptide during platelet activation. Thromb. Haemost. 1993, 69, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Pease, R.J.; Avery, C.A.; Brown, J.M.; Adamson, P.J.; Cooke, E.J.; Neergaard-Petersen, S.; Cordell, P.A.; Ariëns, R.; Fishwick, C.W.; et al. The activation peptide cleft exposed by thrombin cleavage of FXIII-A2 contains a recognition site for the fibrinogen α chain. Blood 2013, 121, 2117–2126. [Google Scholar] [CrossRef][Green Version]
- Ortner, E.; Schroeder, V.; Walser, R.; Zerbe, O.; Kohler, H.P. Sensitive and selective detection of free FXIII activation peptide: A potential marker of acute thrombotic events. Blood 2010, 115, 5089–5096. [Google Scholar] [CrossRef]
- Durda, M.A.; Wolberg, A.S.; Kerlin, B.A. State of the art in factor XIII laboratory assessment. Transfus. Apher. Sci. 2018, 57, 700–704. [Google Scholar] [CrossRef]
- Collet, J.-P.; Moen, J.L.; Veklich, Y.I.; Gorkun, O.V.; Lord, S.T.; Montalescot, G.; Weisel, J.W. The αC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood 2005, 106, 3824–3830. [Google Scholar] [CrossRef]
- Standeven, K.F.; Carter, A.M.; Grant, P.J.; Weisel, J.W.; Chernysh, I.; Masova, L.; Lord, S.T.; Ariëns, R.A. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: Determination of a cross-linking pattern that maximizes clot stiffness. Blood 2007, 110, 902–907. [Google Scholar] [CrossRef]
- Hethershaw, E.L.; La Corte, A.L.C.; Duval, C.; Ali, M.; Grant, P.J.; Ariëns, R.A.S.; Philippou, H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J. Thromb. Haemost. 2014, 12, 197–205. [Google Scholar] [CrossRef]
- Kurniawan, N.N.; Grimbergen, J.; Koopman, J.; Koenderink, G.H. Factor XIII stiffens fibrin clots by causing fiber compaction. J. Thromb. Haemost. 2014, 12, 1687–1696. [Google Scholar] [CrossRef]
- Collet, J.-P.; Shuman, H.; Ledger, R.E.; Lee, S.; Weisel, J.W. The elasticity of an individual fibrin fiber in a clot. Proc. Natl. Acad. Sci. USA 2005, 102, 9133–9137. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jawerth, L.M.; Sparks, E.A.; Falvo, M.R.; Hantgan, R.R.; Superfine, R.; Lord, S.T.; Guthold, M. Fibrin Fibers Have Extraordinary Extensibility and Elasticity. Science 2006, 313, 634. [Google Scholar] [CrossRef] [PubMed]
- Houser, J.R.; Hudson, N.E.; Ping, L.; O’Brien, E.T., III; Superfine, R.; Lord, S.T.; Falvo, M.R. Evidence that αC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys. J. 2010, 99, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Glover, C.J.; McIntire, L.V.; Brown, C.H., III; Natelson, E.A. Rheological properties of fibrin clots. Effects of fibrinogen concentration, Factor XIII deficiency, and Factor XIII inhibition. J. Lab. Clin. Med. 1975, 86, 644–656. [Google Scholar] [PubMed]
- Van Giezen, J.J.; Minkema, J.; Bouma, B.N.; Jansen, J.W. Cross-linking of alpha 2-antiplasmin to fibrin is a key factor in regulating blood clot lysis: Species differences. Blood Coagul. Fibrinolysis 1993, 4, 869–875. [Google Scholar] [CrossRef]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of factor XIII is exclusively expressed through α2-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef]
- Valnickova, Z.; Enghild, J.J. Human procarboxypeptidase U, or thrombin-activable fibrinolysis inhibitor, is a substrate for transglutaminases. Evidence for transglutaminase-catalyzed cross-linking to fibrin. J. Biol. Chem. 1998, 273, 27220–27224. [Google Scholar] [CrossRef]
- Jensen, P.H.; Lorand, L.; Ebbesen, P.; Gliemann, J. Type-2 plasminogen-activator inhibitor is a substrate for trophoblast transglutaminase and Factor XIIIa. Transglutaminase-catalyzed cross-linking to cellular and extracellular structures. JBIC J. Biol. Inorg. Chem. 1993, 214, 141–146. [Google Scholar] [CrossRef]
- Sakata, Y.; Aoki, N. Significance of Cross-Linking of α2-Plasmin Inhibitor to Fibrin in Inhibition of Fibrinolysis and in Hemostasis. J. Clin. Investig. 1982, 69, 536–542. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Duval, C.; Wang, Y.; Hansen, C.E.; Ahn, B.; Mooberry, M.J.; Clark, M.A.; Johnsen, J.M.; Lord, S.T.; Lam, W.A.; et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin α-chain crosslinking. Blood 2015, 126, 1940–1948. [Google Scholar] [CrossRef]
- Aleman, M.M.; Byrnes, J.R.; Wang, J.-G.; Tran, R.; Lam, W.A.; Di Paola, J.; Mackman, N.; Degen, J.L.; Flick, M.J.; Wolberg, A.S. Factor XIII activity mediates red blood cell retention in venous thrombi. J. Clin. Investig. 2014, 124, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, S.; Myneni, V.D.; Hitomi, K.; Kaartinen, M.T. Transglutaminase activity arising from Factor XIIIA is required for stabilization and conversion of plasma fibronectin into matrix in osteoblast cultures. Bone 2014, 59, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Myneni, V.D.; Hitomi, K.; Kaartinen, M.T. Factor XIII-A transglutaminase acts as a switch between preadipocyte proliferation and differentiation. Blood 2014, 124, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Richardson, V.R.; Cordell, P.; Standeven, K.F.; Carter, A.M. Substrates of Factor XIII-A: Roles in thrombosis and wound healing. Clin. Sci. 2012, 124, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Soendergaard, C.; Kvist, P.H.; Seidelin, J.B.; Nielsen, O.H. Tissue-regenerating functions of coagulation factor XIII. J. Thromb. Haemost. 2013, 11, 806–816. [Google Scholar] [CrossRef]
- Mangla, A.; Hamad, H.; Kumar, A. Factor XIII Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Muszbek, L. Deficiency causing mutations and common polymorphisms in the factor XIII-A gene. Thromb. Haemost. 2000, 84, 524–527. [Google Scholar] [CrossRef]
- Kerlin, B.A.; Brand, B.; Inbal, A.; Halimeh, S.; Nugent, D.; Lundblad, M.; Tehranchi, R. Pharmacokinetics of recombinant factor XIII at steady state in patients with congenital factor XIII A-subunit deficiency. J. Thromb. Haemost. 2014, 12, 2038–2043. [Google Scholar] [CrossRef]
- World Federation of Hemophilia Report on the Annual Global Survey 2012. World Federation of Hemophilia: Montreal, QC, Canada, 2013.
- Biswas, A.; Ivaškevičius, V.; Seitz, R.; Thomas, A.; Oldenburg, J. An update of the mutation profile of Factor 13 A and B genes. Blood Rev. 2011, 25, 193–204. [Google Scholar] [CrossRef]
- Dorgalaleh, A.; Rashidpanah, J. Blood coagulation factor XIII and factor XIII deficiency. Blood Rev. 2016, 30, 461–475. [Google Scholar] [CrossRef]
- Muszbek, L.; Bagoly, Z.; Cairo, A.; Peyvandi, F. Novel aspects of factor XIII deficiency. Curr. Opin. Hematol. 2011, 18, 366–372. [Google Scholar] [CrossRef]
- Korte, W. Catridecacog: A breakthrough in the treatment of congenital factor XIII A-subunit deficiency? J. Blood Med. 2014, 5, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nugent, D.J. Prophylaxis in rare coagulation disorders—Factor XIII deficiency. Thromb. Res. 2006, 118, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Sharief, L.A.T.; Kadir, R.A. Congenital factor XIII deficiency in women: A systematic review of literature. Haemophilia 2013, 19, e349–e357. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bagoly, Z.; Bereczky, Z.; Katona, E. The involvement of blood coagulation factor XIII in fibrinolysis and thrombosis. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 190–205. [Google Scholar] [CrossRef]
- Bereczky, Z.; Muszbek, L. Factor XIII and Venous Thromboembolism. Semin. Thromb. Hemost. 2011, 37, 305–314. [Google Scholar] [CrossRef]
- Bagoly, Z.; Koncz, Z.; Hársfalvi, J.; Muszbek, L. Factor XIII, clot structure, thrombosis. Thromb. Res. 2012, 129, 382–387. [Google Scholar] [CrossRef]
- Wartiovaara, U.; Mikkola, H.; Szôke, G.; Haramura, G.; Kárpáti, L.; Balogh, I.; Lassila, R.; Muszbek, L.; Palotie, A. Effect of Val34Leu polymorphism on the activation of the coagulation factor XIII-A. Thromb. Haemost. 2000, 84, 595–600. [Google Scholar]
- Ariëns, R.A.; Philippou, H.; Nagaswami, C.; Weisel, J.W.; Lane, D.A.; Grant, P.J. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood 2000, 96, 988–995. [Google Scholar]
- Wells, P.S.; Anderson, J.L.; Scarvelis, D.K.; Doucette, S.P.; Gagnon, F. Factor XIII Val34Leu Variant Is Protective against Venous Thromboembolism: A HuGE Review and Meta-Analysis. Am. J. Epidemiol. 2006, 164, 101–109. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Sandhu, M.S.; Wareham, N.J.; Luben, R.N.; Reitsma, P.H.; Khaw, K.-T. Fibrinogen plasma levels modify the association between the factor XIII Val34Leu variant and risk of coronary artery disease: The EPIC-Norfolk prospective population study. J. Thromb. Haemost. 2006, 4, 2204–2209. [Google Scholar] [CrossRef]
- Bereczky, Z.; Balogh, E.; Katona, É.; Pocsai, Z.; Czuriga, I.; Széles, G.; Kárpáti, L.; Ádány, R.; Édes, I.; Muszbek, L. Modulation of the risk of coronary sclerosis/myocardial infarction by the interaction between factor XIII subunit A Val34Leu polymorphism and fibrinogen concentration in the high risk Hungarian population. Thromb. Res. 2007, 120, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.C.; Ariëns, R.; Carter, A.M.; Weisel, J.W.; Grant, P.J. Genetic regulation of fibrin structure and function: Complex gene-environment interactions may modulate vascular risk. Lancet 2003, 361, 1424–1431. [Google Scholar] [CrossRef]
- Jung, J.H.; Song, G.G.; Kim, J.-H.; Seo, Y.H.; Choi, S.J. Association of factor XIII Val34Leu polymorphism and coronary artery disease: A meta-analysis. Cardiol. J. 2017, 24, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Stępień, E.Ł.; Plicner, D.; Kapelak, B.; Wypasek, E.; Sadowski, J.; Undas, A. Factor XIII Val34Leu polymorphism as a modulator of fibrin clot permeability and resistance to lysis in patients with severe coronary artery disease. Kardiol. Pol. 2009, 67, 947–955. [Google Scholar] [PubMed]
- Vossen, C.Y.; Rosendaal, F.R. The protective effect of the factor XIII Val34Leu mutation on the risk of deep venous thrombosis is dependent on the fibrinogen level. J. Thromb. Haemost. 2005, 3, 1102–1103. [Google Scholar] [CrossRef][Green Version]
- Komanasin, N.; Catto, A.J.; Futers, T.S.; Vlieg, A.H.; Rosendaal, F.R.; Ariëns, R. A novel polymorphism in the factor XIII B-subunit (His95Arg): Relationship to subunit dissociation and venous thrombosis. J. Thromb. Haemost. 2005, 3, 2487–2496. [Google Scholar] [CrossRef]
- Pruissen, D.M.O.; Slooter, A.J.C.; Rosendaal, F.R.; Van Der Graaf, Y.; Algra, A. Coagulation factor XIII gene variation, oral contraceptives, and risk of ischemic stroke. Blood 2008, 111, 1282–1286. [Google Scholar] [CrossRef][Green Version]
- Siegerink, B.; Maino, A.; Algra, A.; Rosendaal, F.R. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. J. Thromb. Haemost. 2015, 13, 1568–1575. [Google Scholar] [CrossRef]
- Landau, M.B.; Renni, M.S.; Zalis, M.G.; Spector, N.; Gadelha, T. Coagulation factor XIII Tyr204Phe gene variant and the risk of ischemic stroke. J. Thromb. Haemost. 2013, 11, 1426–1427. [Google Scholar] [CrossRef]
- Mezei, Z.A.; Katona, É.; Kállai, J.; Bereczky, Z.; Somodi, L.; Molnár, É.; Kovács, B.; Miklós, T.; Ajzner, É.; Muszbek, L. Factor XIII levels and factor XIII B subunit polymorphisms in patients with venous thromboembolism. Thromb. Res. 2017, 158, 93–97. [Google Scholar] [CrossRef]
- Carpenter, S.L.; Mathew, P. Alpha2-antiplasmin and its deficiency: Fibrinolysis out of balance. Haemophilia 2008, 14, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Rijken, D.C.; Lijnen, H.R. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gurewich, V. Therapeutic Fibrinolysis: How Efficacy and Safety Can Be Improved. J. Am. Coll. Cardiol. 2016, 68, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Carmeliet, P.; Fay, W.P. Plasminogen Activator Inhibitor-1 Is a Major Determinant of Arterial Thrombolysis Resistance. Circulation 1999, 99, 3050–3055. [Google Scholar] [CrossRef]
- Kohler, H.P.; Grant, P.J. Plasminogen-Activator Inhibitor Type 1 and Coronary Artery Disease. N. Engl. J. Med. 2000, 342, 1792–1801. [Google Scholar] [CrossRef]
- Abdul, S.; Leebeek, F.W.; Rijken, D.C.; De Willige, S.U. Natural heterogeneity of α2-antiplasmin: Functional and clinical consequences. Blood 2016, 127, 538–545. [Google Scholar] [CrossRef]
- Plug, T.; Meijers, J.C.M. Structure-function relationships in thrombin-activatable fibrinolysis inhibitor. J. Thromb. Haemost. 2016, 14, 633–644. [Google Scholar] [CrossRef]
- Wang, W.; Boffa, M.B.; Bajzar, L.; Walker, J.B.; Nesheim, M.E. A study of the mechanism of inhibition of fibrinolysis by activated thrombin-activable fibrinolysis inhibitor. J. Biol. Chem. 1998, 273, 27176–27181. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Renucci, J.F.; Grimaux, M.; Morange, P.; Gouvernet, J.; Gourmelin, Y.; Alessi, M.-C. Thrombin-Activatable Fibrinolysis Inhibitor Antigen Levels and Cardiovascular Risk Factors. Arter. Thromb. Vasc. Biol. 2000, 20, 2156–2161. [Google Scholar] [CrossRef]
- Kokame, K.; Zheng, X.; Sadler, J.E. Activation of Thrombin-activable Fibrinolysis Inhibitor Requires Epidermal Growth Factor-like Domain 3 of Thrombomodulin and Is Inhibited Competitively by Protein C. J. Biol. Chem. 1998, 273, 12135–12139. [Google Scholar] [CrossRef]
- Deb, A.; Caplice, N.M. Lipoprotein(a): New insights into mechanisms of atherogenesis and thrombosis. Clin. Cardiol. 2004, 27, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Okafor, O.N.; Gorog, D.A. Endogenous Fibrinolysis: An Important Mediator of Thrombus Formation and Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 65, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Tipoe, T.L.; Wu, W.K.K.; Chung, L.; Gong, M.; Dong, M.; Liu, T.; Roever, L.; Ho, J.; Wong, M.C.S.; Chan, M.T.V.; et al. Plasminogen Activator Inhibitor 1 for Predicting Sepsis Severity and Mortality Outcomes: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Tofler, G.H.; Massaro, J.; O’Donnell, C.; Wilson, P.; Vasan, R.; Sutherland, P.; Meigs, J.; Levy, D.; D’Agostino, R. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb. Res. 2016, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Van der Bom, J.G.; de Knijff, P.; Haverkate, F.; Bots, M.L.; Meijer, P.; de Jong, P.T.; Hofman, A.; Kluft, C.; Grobbee, D.E. Tissue plasminogen activator and risk of myocardial infarction. The Rotterdam Study. Circulation 1997, 95, 2623–2627. [Google Scholar] [CrossRef]
- Larsen, J.B.; Hvas, A.-M. Fibrin Clot Formation and Lysis in Plasma. Methods Protoc. 2020, 3, 67. [Google Scholar] [CrossRef]
- Kuiper, G.J.A.J.M.; Kleinegris, M.-C.F.; van Oerle, R.; Spronk, H.M.H.; Lancé, M.D.; Ten Cate, H.; Henskens, Y.M. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb. J. 2016, 14, 1–3. [Google Scholar] [CrossRef]
- Panigada, M.; Zacchetti, L.; L’Acqua, C.; Cressoni, M.; Anzoletti, M.B.; Bader, R.; Protti, A.; Consonni, D.; D’Angelo, A.; Gattinoni, L. Assessment of Fibrinolysis in Sepsis Patients with Urokinase Modified Thromboelastography. PLoS ONE 2015, 10, e0136463. [Google Scholar] [CrossRef]
- Bainey, K.R.; Fu, Y.; Wagner, G.S.; Goodman, S.G.; Ross, A.; Granger, C.B.; Van De Werf, F.; Armstrong, P.W. Spontaneous reperfusion in ST-elevation myocardial infarction: Comparison of angiographic and electrocardiographic assessments. Am. Hear. J. 2008, 156, 248–255. [Google Scholar] [CrossRef]
- Fefer, P.; Hod, H.; Hammerman, H.; Boyko, V.; Behar, S.; Matetzky, S. Relation of Clinically Defined Spontaneous Reperfusion to Outcome in ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2009, 103, 149–153. [Google Scholar] [CrossRef]
- Saraf, S.; Christopoulos, C.; Ben Salha, I.; Stott, D.J.; Gorog, D.A. Impaired Endogenous Thrombolysis in Acute Coronary Syndrome Patients Predicts Cardiovascular Death and Nonfatal Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Spinthakis, N.; Gue, Y.X.; Srinivasan, M.; Sullivan, K.; Wellsted, D.; Gorog, D.A. Impaired endogenous fibrinolysis in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention is a predictor of recurrent cardiovascular events: The RISK PPCI study. Eur. Hear. J. 2019, 40, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Hear. J. 2018, 39, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, J.P.; Niewada, M.; Siudut, J.; Plens, K.; Członkowska, A.; Undas, A. Fibrin clot characteristics in acute ischaemic stroke patients treated with thrombolysis: The impact on clinical outcome. Thromb. Haemost. 2017, 117, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Drabik, L.; Konieczyńska, M.; Undas, A. Clot Lysis Time Predicts Stroke During Anticoagulant Therapy in Patients with Atrial Fibrillation. Can. J. Cardiol. 2020, 36, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Taomoto, K.; Ohnishi, H.; Kuga, Y.; Nakashima, K.; Ichioka, T.; Kodama, Y.; Kubota, H.; Tominaga, T.; Hirose, T.; Hayashi, M.; et al. Platelet Function and Spontaneous Thrombolytic Activity of Patients with Cerebral Infarction Assessed by the Global Thrombosis Test. Pathophysiol. Haemost. Thromb. 2009, 37, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Farrington, K.; Kozarski, R.; Christopoulos, C.; Niespialowska-Steuden, M.; Moffat, D.; Gorog, D.A. Impaired thrombolysis: A novel cardiovascular risk factor in end-stage renal disease. Eur. Hear. J. 2012, 34, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, A.; Wakabayashi, I.; Yamashita, T.; Yamamoto, J. Attenuation of spontaneous thrombolytic activity measured by the global thrombosis test in male habitual smokers. J. Thromb. Thrombolysis 2014, 37, 414–418. [Google Scholar] [CrossRef]
- Stanford, S.N.; Sabra, A.; Lawrence, M.; Morris, R.H.; Storton, S.; Wani, M.; Hawkins, K.; Williams, P.R.; Potter, J.F.; Evans, A. Prospective Evaluation of Blood Coagulability and Effect of Treatment in Patients with Stroke Using Rotational Thromboelastometry. J. Stroke Cerebrovasc. Dis. 2015, 24, 304–311. [Google Scholar] [CrossRef]
- McDonald, M.M.; Wetzel, J.; Fraser, S.; Elliott, A.; Bowry, R.; Kawano-Castillo, J.F.; Cai, C.; Sangha, N.; E Messier, J.; Hassler, A.; et al. Thrombelastography does not predict clinical response to rtPA for acute ischemic stroke. J. Thromb. Thrombolysis 2015, 41, 505–510. [Google Scholar] [CrossRef]
- Alessi, M.-C.; Gaudin, C.; Grosjean, P.; Martin, V.; Timsit, S.; Mahagne, M.-H.; Larrue, V.; Sibon, I.; Zuber, M.; Brouns, R.; et al. Changes in Activated Thrombin-Activatable Fibrinolysis Inhibitor Levels Following Thrombolytic Therapy in Ischemic Stroke Patients Correlate with Clinical Outcome. Cerebrovasc. Dis. 2016, 42, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.W.; Kim, E.H.; Kim, D.J.; Lee, K.; Heo, J.H.; Kim, D.I. Plasma Fibrinolysis Inhibitor Levels in Acute Stroke Patients with Thrombolysis Failure. J. Clin. Neurol. 2005, 1, 142–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karasu, A.; Baglin, T.P.; Luddington, R.; Baglin, C.A.; Vlieg, A. Prolonged clot lysis time increases the risk of a first but not recurrent venous thrombosis. Br. J. Haematol. 2016, 172, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.E.; Lisman, T.; Doggen, C.J.M.; De Groot, P.G.; Rosendaal, F.R. Synergistic Effects of Hypofibrinolysis and Genetic and Acquired Risk Factors on the Risk of a First Venous Thrombosis. PLoS Med. 2008, 5, e97. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T. Decreased Plasma Fibrinolytic Potential as a Risk for Venous and Arterial Thrombosis. Semin. Thromb. Hemost. 2016, 43, 178–184. [Google Scholar] [CrossRef]
- Traby, L.; Kollars, M.; Eischer, L.; Eichinger, S.; Kyrle, P.A. Prediction of Recurrent Venous Thromboembolism by Clot Lysis Time: A Prospective Cohort Study. PLoS ONE 2012, 7, e51447. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memtsas, V.P.; Arachchillage, D.R.J.; Gorog, D.A. Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis. Int. J. Mol. Sci. 2021, 22, 1472. https://doi.org/10.3390/ijms22031472
Memtsas VP, Arachchillage DRJ, Gorog DA. Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis. International Journal of Molecular Sciences. 2021; 22(3):1472. https://doi.org/10.3390/ijms22031472
Chicago/Turabian StyleMemtsas, Vassilios P., Deepa R. J. Arachchillage, and Diana A. Gorog. 2021. "Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis" International Journal of Molecular Sciences 22, no. 3: 1472. https://doi.org/10.3390/ijms22031472
APA StyleMemtsas, V. P., Arachchillage, D. R. J., & Gorog, D. A. (2021). Role, Laboratory Assessment and Clinical Relevance of Fibrin, Factor XIII and Endogenous Fibrinolysis in Arterial and Venous Thrombosis. International Journal of Molecular Sciences, 22(3), 1472. https://doi.org/10.3390/ijms22031472






