Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing
Abstract
1. Introduction
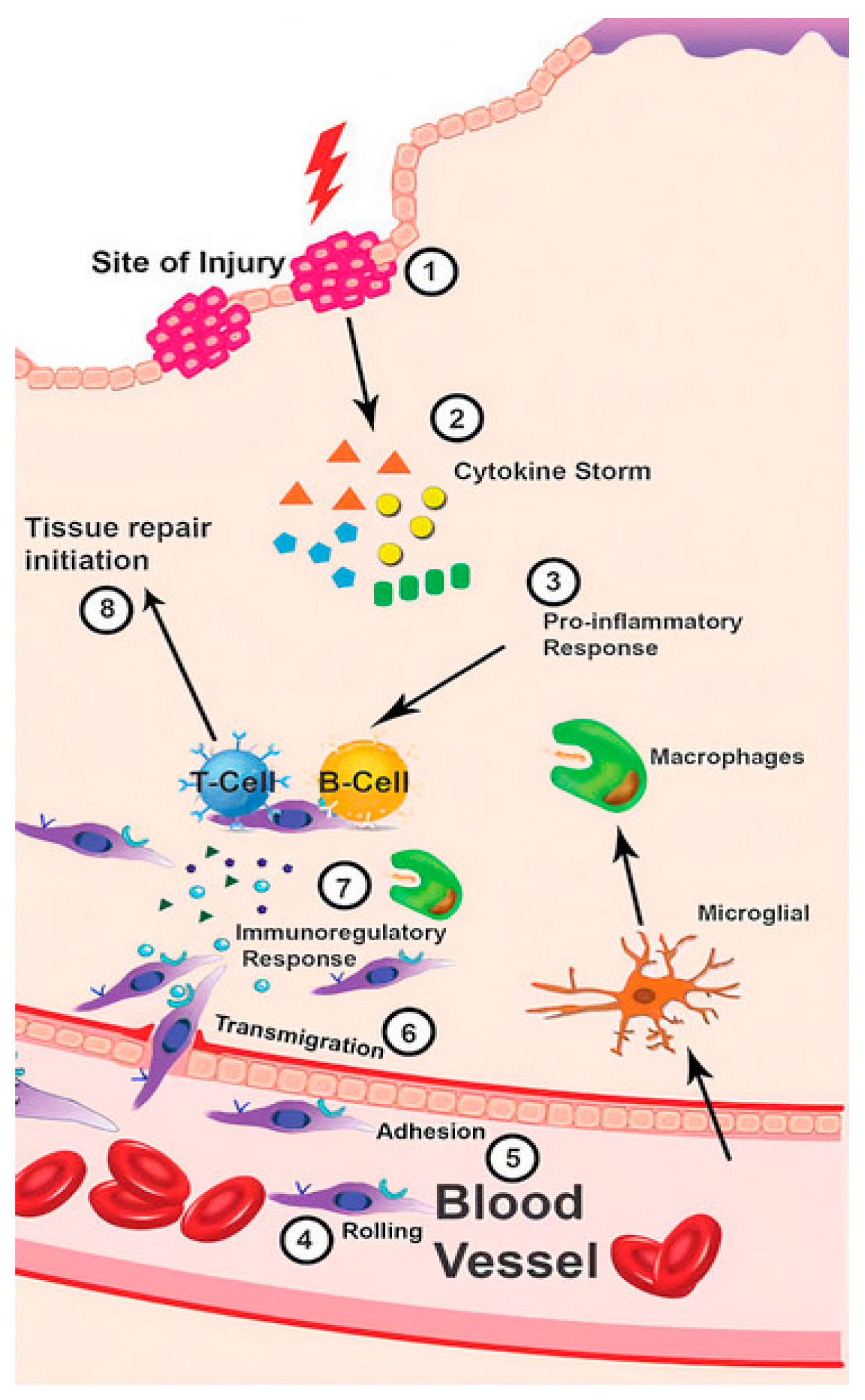
2. Immune Response in Wounds
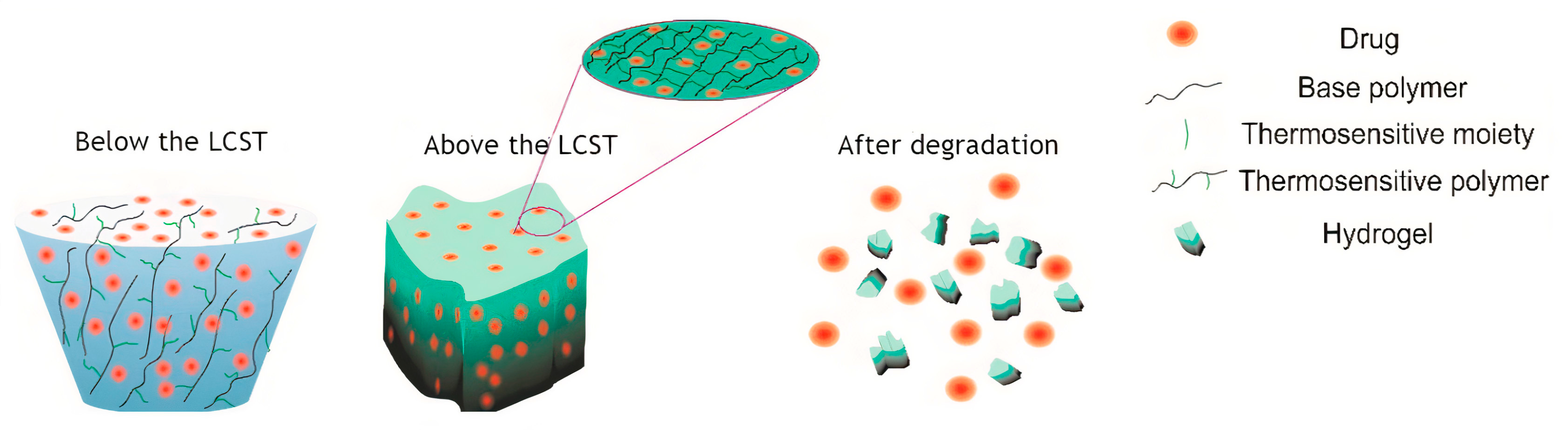
3. Thermo-Responsive Smart Polymers
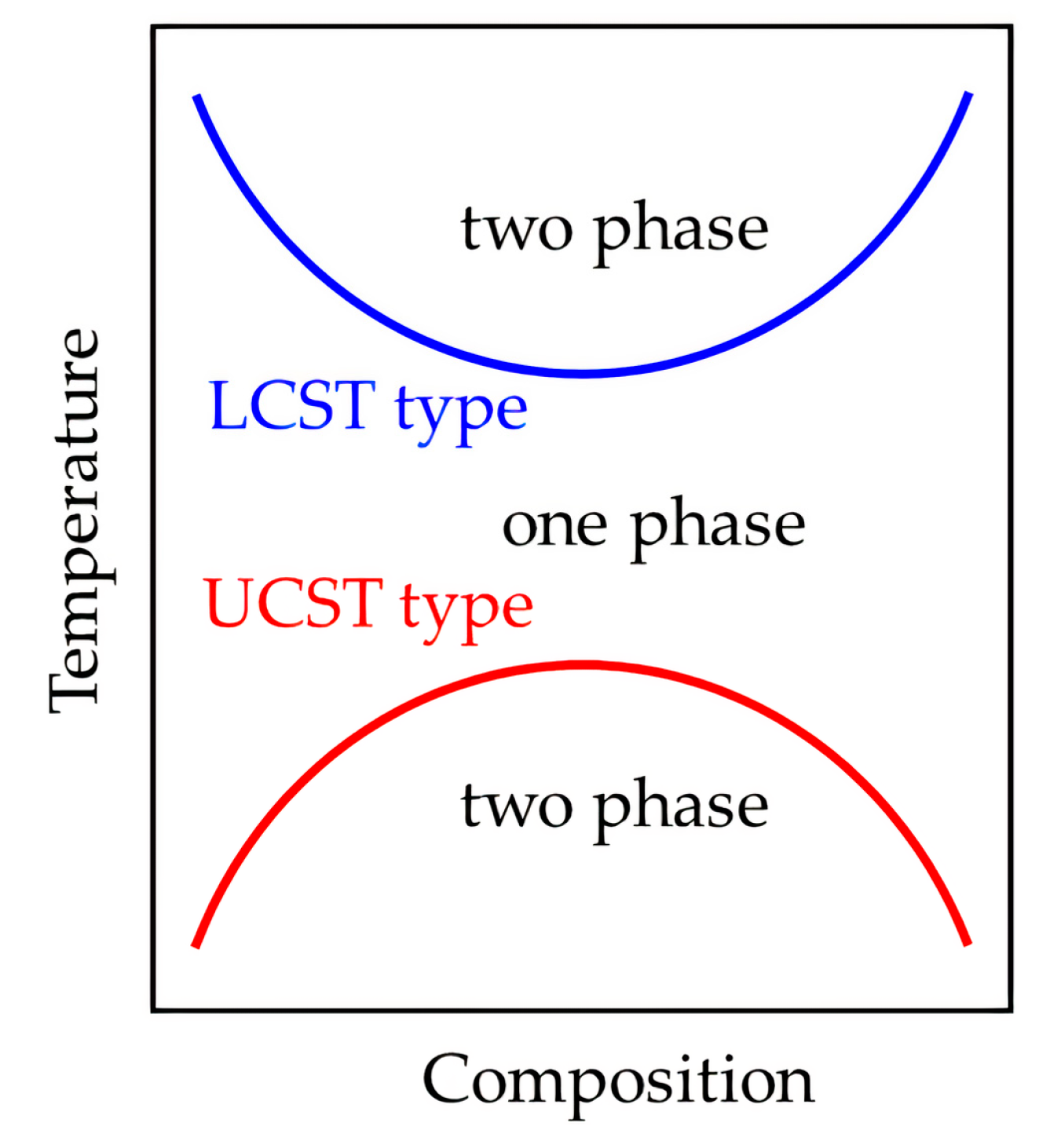
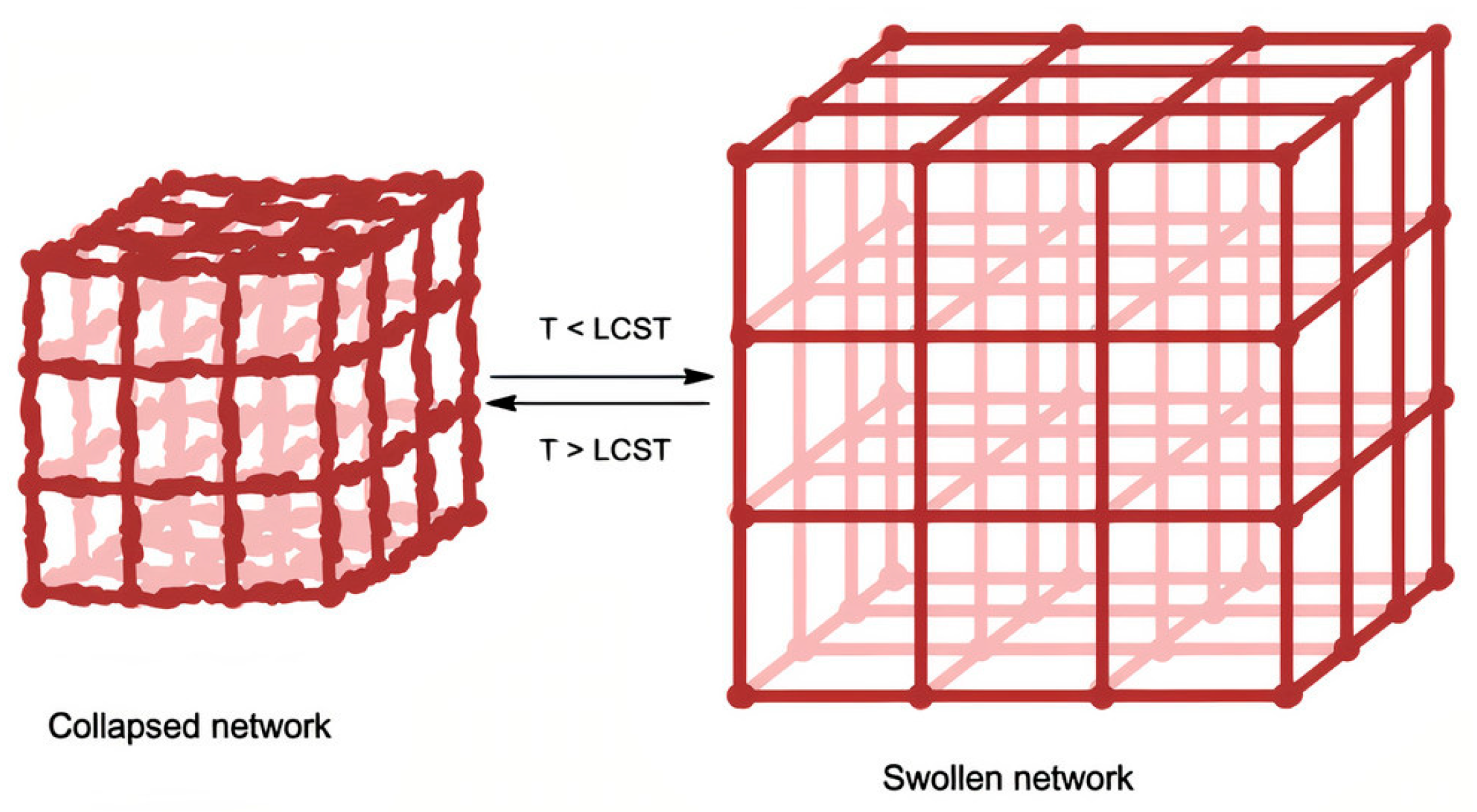
Phase Transition Thermodynamics and Critical Solution Temperature
4. Bioengineered Thermo-Responsive Scaffolds
4.1. Novel Manufacturing Techniques
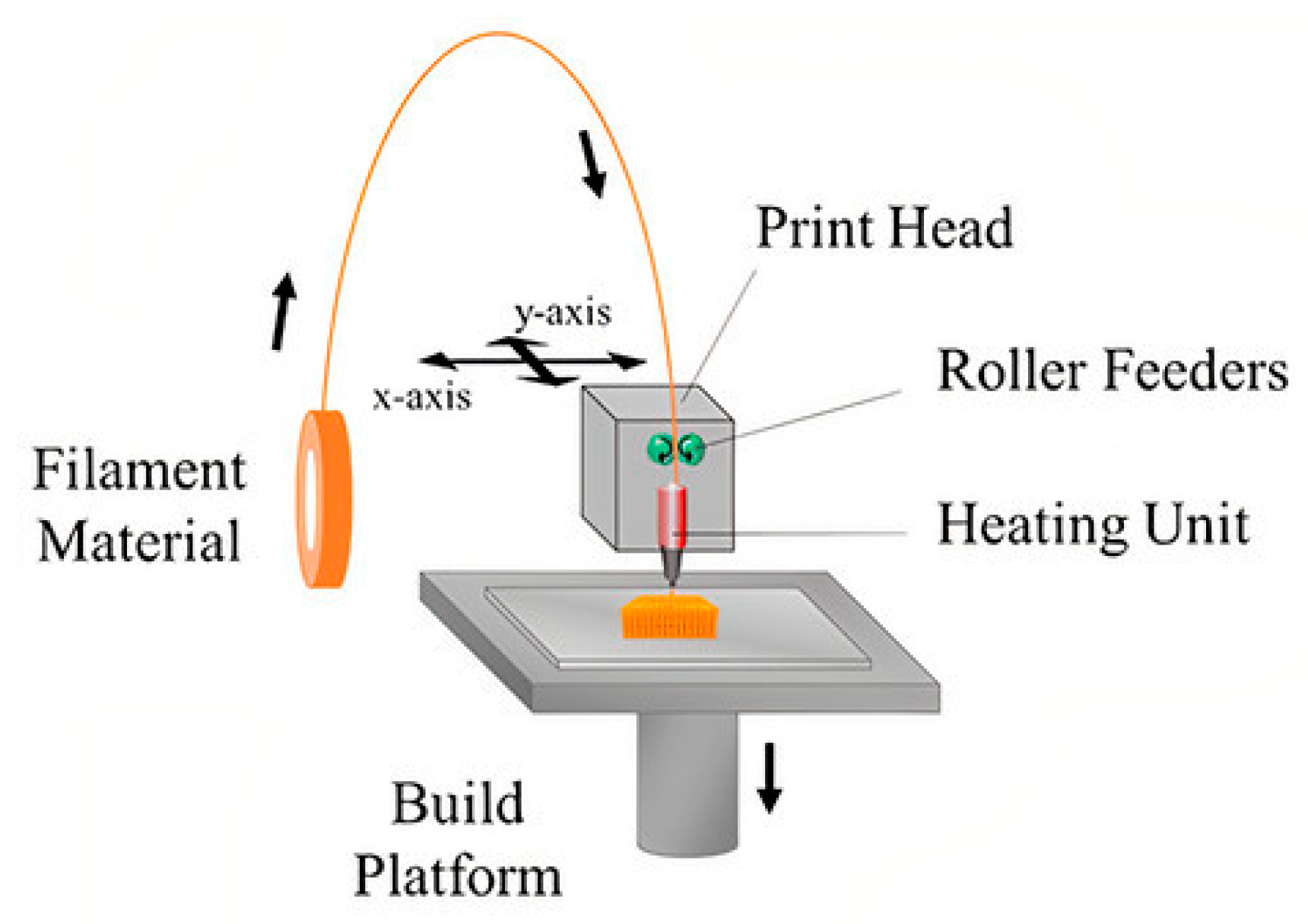
4.1.1. 3D Printing
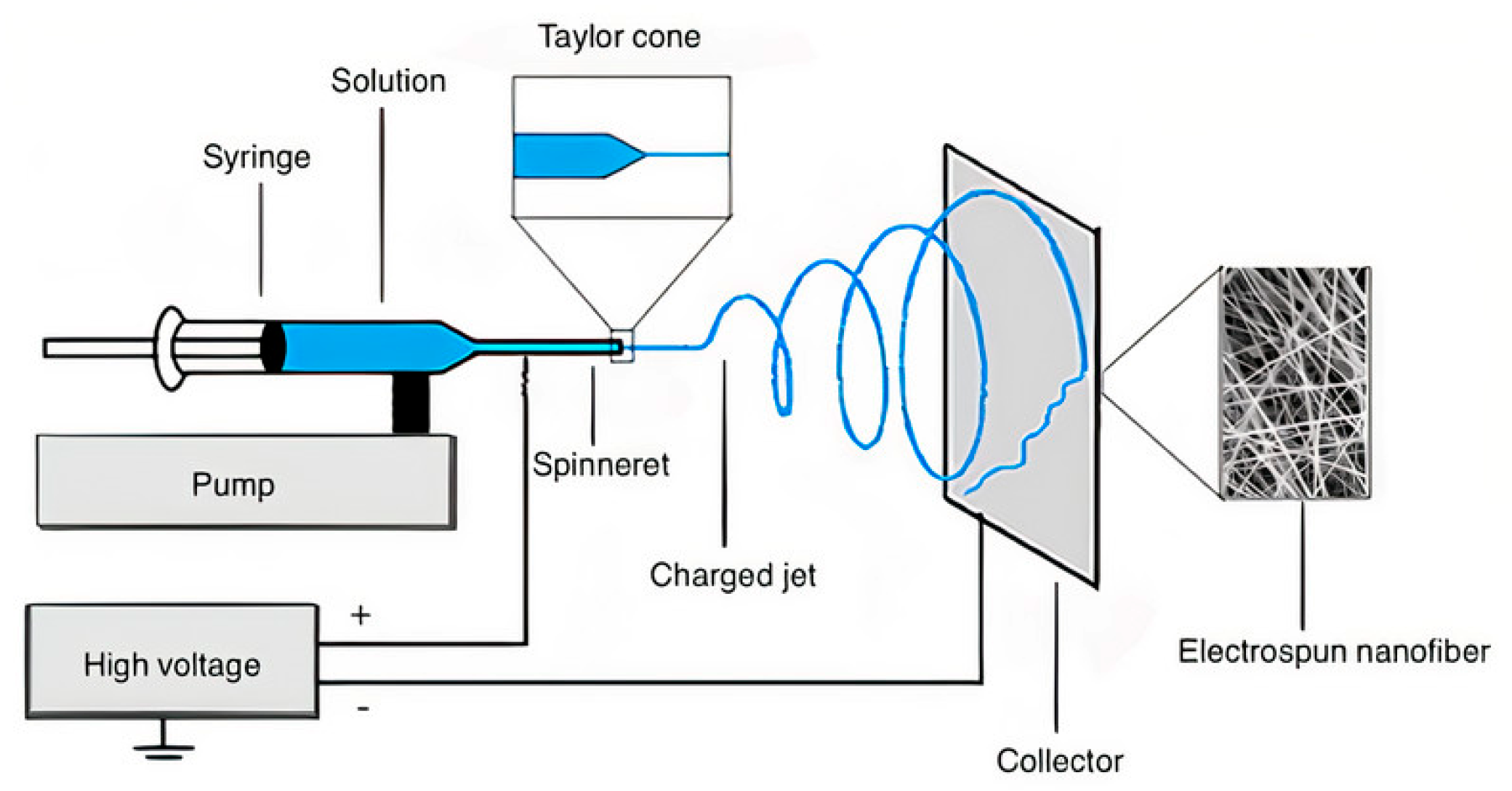
4.1.2. Electrospinning
4.2. Biocompatibility and Biodegradability
4.3. Biopharmaceutical Enhancement
5. Drug Delivery Applications of Bioengineered Thermo-Responsive Scaffolds in Wound Healing
6. Conclusions
Funding
Conflicts of Interest
References
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Singh, B.N.; Panda, N.N.; Mund, R.; Pramanik, K. Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr. Polym. 2016, 151, 335–347. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Wong, V.W.; Gurtner, G.C. Tissue engineering for the management of chronic wounds: Current concepts and future perspectives. Exp. Dermatol. 2012, 21, 729–734. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2016, 8, 127–143. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Teotia, A.K.; Sami, H.; Kumar, A. 1—Thermo-responsive polymers: Structure and design of smart materials. In Switchable and Responsive Surfaces and Materials for Biomedical Applications; Zhang, Z., Ed.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–43. ISBN 978-0-85709-713-2. [Google Scholar]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Fitzpatrick, S.D.; Fitzpatrick, L.E.; Thakur, A.; Mazumder, M.A.J.; Sheardown, H. Temperature-sensitive polymers for drug delivery. Expert Rev. Med. Devices 2012, 9, 339–351. [Google Scholar] [CrossRef]
- Dunne, M.; Hynynen, K.; Allen, C. Thermosensitive nanomedicines could revolutionize thermal therapy in oncology. Nano Today 2017, 16, 9–13. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Bedoya, D.A.; Figueroa, F.N.; Macchione, M.A.; Strumia, M.C. Stimuli-Responsive Polymeric Systems for Smart Drug Delivery. In A New Era for Microbial Corrosion Mitigation Using Nanotechnology; Springer: Cham, Switzerland, 2020; pp. 115–134. [Google Scholar]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. Part. A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Jyoti, K.; Malik, G.; Chaudhary, M.; Sharma, M.; Goswami, M.; Katare, O.P.; Singh, S.B.; Madan, J. Chitosan and phospholipid assisted topical fusidic acid drug delivery in burn wound: Strategies to conquer pharmaceutical and clinical challenges, opportunities and future panorama. Int. J. Biol. Macromol. 2020, 161, 325–335. [Google Scholar] [CrossRef]
- Benchaprathanphorn, K.; Sakulaue, P.; Siriwatwechakul, W.; Muangman, P.; Chinaroonchai, K.; Viravaidya-Pasuwat, K. Preparation and characterization of human keratinocyte–fibroblast cell sheets constructed using PNIAM-co-AM grafted surfaces for burn wound healing. J. Mater. Sci. Mater. Med. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Suntornnond, R.; An, J.; Chua, C.K. Bioprinting of Thermoresponsive Hydrogels for Next Generation Tissue Engineering: A Review. Macromol. Mater. Eng. 2017, 302, 1600266. [Google Scholar] [CrossRef]
- Dubsky, M.; Kubinová, Š.; Širc, J.; Voska, L.; Zajíček, R.; Zajícová, A.; Lesný, P.; Jirkovská, A.; Michálek, J.; Munzarová, M.; et al. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J. Mater. Sci. Mater. Med. 2012, 23, 931–941. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Nun, N.; Cruz, M.; Jain, T.; Tseng, Y.-M.; Menefee, J.; Jatana, S.; Patil, P.S.; Leipzig, N.D.; McDonald, C.; Maytin, E.; et al. Thread Size and Polymer Composition of 3D Printed and Electrospun Wound Dressings Affect Wound Healing Outcomes in an Excisional Wound Rat Model. Biomacromolecules 2020, 21, 4030–4042. [Google Scholar] [CrossRef]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef]
- Strbo, N.; Yin, N.; Stojadinovic, O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv. Wound Care 2014, 3, 492–501. [Google Scholar] [CrossRef]
- Mauri, C.; Bosma, A. Immune Regulatory Function of B Cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Nosbaum, A.; Prevel, N.; Truong, H.-A.; Mehta, P.; Ettinger, M.; Scharschmidt, T.C.; Ali, N.H.; Pauli, M.L.; Abbas, A.K.; Rosenblum, M. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016, 196, 2010–2014. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef]
- Bielefeld, K.A.; Amini-Nik, S.; Alman, B.A. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell. Mol. Life Sci. 2012, 70, 2059–2081. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Chen, S.-N. Apoptotic cell: Linkage of inflammation and wound healing. Front. Pharmacol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Tatler, A.L.; Jenkins, G. TGF-β Activation and Lung Fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Sampaio-Barros, P.; Stifano, G.; Borges, C.L.; De Carvalho, C.R.; Kairalla, R.; Parra, E.R.; Spira, A.; Simms, R.; Capellozzi, V.L.; et al. Association of Interferon- and Transforming Growth Factor β-Regulated Genes and Macrophage Activation with Systemic Sclerosis-Related Progressive Lung Fibrosis. Arthritis Rheumatol. 2014, 66, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Gupta, S.; Mohanty, S. Mesenchymal Stem Cells Modulate the Immune System in Developing Therapeutic Interventions. Immune Response Act. Immunomodul. 2019. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Ciaccia, L. Fundamentals of Inflammation. Yale J. Biol. Med. 2011, 84, 64–65. [Google Scholar]
- Oberyszyn, T.M. Inflammation and wound healing. Front. Biosci. 2007, 12, 2993–2999. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Babensee, J.E. 2.2.2—Inflammation, Wound Healing, the Foreign-Body Response, and Alternative Tissue Re-sponses. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 737–746. ISBN 978-0-12-816137-1. [Google Scholar]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Ling, Y.; Tang, H. Dual thermoresponsive homopolypeptide with LCST-type linkages and UCST-type pendants: Synthesis, characterization, and thermoresponsive properties. Polymers 2017, 132, 264–272. [Google Scholar] [CrossRef]
- Song, L.; Zhang, B.; Jin, E.; Xiao, C.; Li, G.; Zhu, X. A reduction-sensitive thermo-responsive polymer: Synthesis, characterization, and application in controlled drug release. Eur. Polym. J. 2018, 101, 183–189. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic Thermoresponsive Polymers in Water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 242, pp. 29–89. [Google Scholar] [CrossRef]
- Ng, W.S.; Connal, L.A.; Forbes, E.; Mohanarangam, K.; Franks, G.V. In situ investigation of aggregate sizes formed using thermo-responsive polymers: Effect of temperature and shear. J. Colloid Interface Sci. 2017, 494, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qub, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, J.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Pezeshki-Modaress, M.; Rajabi-Zeleti, S.; Zandi, M.; Mirzadeh, H.; Sodeifi, N.; Nekookar, A.; Aghdami, N. Cell-loaded gelatin/chitosan scaffolds fabricated by salt-leaching/lyophilization for skin tissue engineering:In vitroandin vivostudy. J. Biomed. Mater. Res. Part. A 2013, 102, 3908–3917. [Google Scholar] [CrossRef]
- González-Masís, J.; Cubero-Sesin, J.M.; Ureña, Y.R.C.; González-Camacho, S.; Mora-Ugalde, N.; Vega-Baudrit, J.R.; Loaiza, R.; Vega-Baudrit, J.R.; Gonzalez-Paz, R.J. Increased Fibroblast Metabolic Activity of Collagen Scaffolds via the Addition of Propolis Nanoparticles. Materials 2020, 13, 3118. [Google Scholar] [CrossRef]
- Song, F.; Wang, X.-L.; Wang, Y.-Z. Poly (N-isopropylacrylamide)/poly (ethylene oxide) blend nanofibrous scaffolds: Thermo-responsive carrier for controlled drug release. Colloids Surf. B Biointerfaces 2011, 88, 749–754. [Google Scholar] [CrossRef]
- Alf, M.E.; Hatton, T.A.; Gleason, K.K. Novel N-isopropylacrylamide based polymer architecture for faster LCST transition kinetics. Polymers 2011, 52, 4429–4434. [Google Scholar] [CrossRef]
- Sarwan, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Hybrid Thermo-Responsive Polymer Systems and Their Biomedical Applications. Front. Mater. 2020, 7, 73. [Google Scholar] [CrossRef]
- Kim, B.-R.; Nguyen, T.B.L.; Min, Y.-K.; Lee, B.-T. In Vitro and In Vivo Studies of BMP-2-Loaded PCL–Gelatin–BCP Electrospun Scaffolds. Tissue Eng. Part A 2014, 20, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, H. Biodegradability and Biocompatibility Study of Poly(Chitosan-g-lactic Acid) Scaffolds. Molecules 2012, 17, 3243–3258. [Google Scholar] [CrossRef] [PubMed]
- Boustta, M.; Colombo, P.-E.; Lenglet, S.; Poujol, S.; Vert, M. Versatile UCST-based thermoresponsive hydrogels for loco-regional sustained drug delivery. J. Control. Release 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Prudic, A.; Ji, Y.; Sadowski, G. Thermodynamic Phase Behavior of API/Polymer Solid Dispersions. Mol. Pharm. 2014, 11, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-sensitive polymers in medicine: A review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Li, Z.; Du, S. A phenomenological thermodynamic model for the chemo-responsive shape memory effect in polymers based on Flory–Huggins solution theory. Polym. Chem. 2014, 5, 1155–1162. [Google Scholar] [CrossRef]
- Donnelly, C.; Tian, Y.; Potter, C.; Jones, D.S.; Andrews, G.P. Probing the Effects of Experimental Conditions on the Character of Drug-Polymer Phase Diagrams Constructed Using Flory-Huggins Theory. Pharm. Res. 2015, 32, 167–179. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Sakai, T.; Katashima, T.; Matsushita, T.; Chung, U. Sol-Gel Transition Behavior near Critical Concentration and Connectivity. Polym. J. 2016, 48, 629–634. [Google Scholar] [CrossRef]
- Sun, W.; An, Z.; Wu, P. UCST or LCST? Composition-Dependent Thermoresponsive Behavior of Poly(N-acryloylglycinamide-co-diacetone acrylamide). Macromolecules 2017, 50, 2175–2182. [Google Scholar] [CrossRef]
- Käfer, F.; Liu, F.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Karg, M.; Agarwal, S. LCST and UCST in One: Double Thermoresponsive Behavior of Block Copolymers of Poly(ethylene glycol) and Poly(acrylamide-co-acrylonitrile). Langmuir 2015, 31, 8940–8946. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Lipson, J. LCST and UCST behavior in polymer solutions and blends. Polymer 2012, 53, 536–545. [Google Scholar] [CrossRef]
- Sugeno, K.; Kokubun, S.; Saito, H. UCST Type Phase Boundary and Accelerated Crystallization in PTT/PET Blends. Polymers 2020, 12, 2730. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Peng, Q.; Wang, B.; Xing, Y.; Hua, Z.; Wang, Z. Multiple Stimuli-Responsive Cellulose Hydrogels with Tunable LCST and UCST as Smart Windows. ACS Appl. Polym. Mater. 2020, 2, 3259–3266. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Heyda, J.; Dzubiella, J. Thermodynamic Description of Hofmeister Effects on the LCST of Thermosensitive Polymers. J. Phys. Chem. B 2014, 118, 10979–10988. [Google Scholar] [CrossRef]
- Heyda, J.; Soll, S.; Yuan, J.; Dzubiella, J. Thermodynamic Description of the LCST of Charged Thermoresponsive Copolymers. Macromolecules 2014, 47, 2096–2102. [Google Scholar] [CrossRef]
- Lee, C.H.; Bae, Y.C. Thermodynamic framework for switching the lower critical solution temperature of thermo-sensitive particle gels in aqueous solvent. Polymer 2020, 195, 122428. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Cai, S.; Gu, S.; Li, X.; Wan, S.; Chen, S.; He, X. Controlled grafting modification of starch and UCST-type thermosensitive behavior in water. Colloid Polym. Sci. 2020, 298, 1053–1061. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2016, 8, 220–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, J.; Yu, X.; Li, M.-H.; Hu, J. Tunable UCST thermoresponsive copolymers based on natural glycyrrhetinic acid. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Asadujjaman, A.; De Oliveira, T.E.; Mukherji, D.; Bertin, A. Polyacrylamide “revisited”: UCST-type reversible thermoresponsive properties in aqueous alcoholic solutions. Soft Matter 2018, 14, 1336–1343. [Google Scholar] [CrossRef]
- Mäkinen, L.; Varadharajan, D.; Tenhu, H.; Hietala, S. Triple Hydrophilic UCST–LCST Block Copolymers. Macromolecules 2016, 49, 986–993. [Google Scholar] [CrossRef]
- Yin, J.; Hu, J.; Zhang, G.; Liu, S. Schizophrenic Core–Shell Microgels: Thermoregulated Core and Shell Swelling/Collapse by Combining UCST and LCST Phase Transitions. Langmuir 2014, 30, 2551–2558. [Google Scholar] [CrossRef]
- Ieong, N.S.; Hasan, M.; Phillips, D.J.; Saaka, Y.; O’Reilly, R.K.; Gibson, M.I. Polymers with molecular weight dependent LCSTs are essential for cooperative behaviour. Polym. Chem. 2012, 3, 794–799. [Google Scholar] [CrossRef]
- Rajan, R.; Matsumura, K. Tunable Dual-Thermoresponsive Core-Shell Nanogels Exhibiting UCST and LCST Behavior. Macromol. Rapid Commun. 2017, 38, 1700478. [Google Scholar] [CrossRef] [PubMed]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Natural and Synthetic Biodegradable Polymers: Different Scaffolds for Cell Expansion and Tissue Formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar]
- Zhao, C.; Tian, S.; Liu, Q.; Xiu, K.; Lei, I.; Wang, Z.; Ma, P.X. Biodegradable Nanofibrous Temperature-Responsive Gelling Microspheres for Heart Regeneration. Adv. Funct. Mater. 2020, 30, 2000776. [Google Scholar] [CrossRef]
- Zhu, Y.; Wood, N.A.; Fok, K.; Yoshizumi, T.; Park, D.W.; Jiang, H.; Schwartzman, D.S.; Zenati, M.A.; Uchibori, T.; Wagner, W.R.; et al. Design of a Coupled Thermoresponsive Hydrogel and Robotic System for Postinfarct Biomaterial Injection Therapy. Ann. Thorac. Surg. 2016, 102, 780–786. [Google Scholar] [CrossRef]
- Deng, G.; Tang, C.; Li, F.; Jiang, H.; Chen, Y. Covalent Cross-Linked Polymer Gels with Reversible Sol−Gel Transition and Self-Healing Properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Guo, Z.; Yin, H.; Feng, Y.; He, S. Functionalization of single-walled carbon nanotubes with thermo-responsive poly(N-isopropylacrylamide): Effect of the polymer architecture. RSC Adv. 2016, 6, 37953–37964. [Google Scholar] [CrossRef]
- Porsch, C.; Hansson, S.; Nordgren, N.; Malmström, E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly(ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114–1123. [Google Scholar] [CrossRef]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Shah, T.V.; Vasava, D. A glimpse of biodegradable polymers and their biomedical applications. e-Polymers. [CrossRef]
- Moghaddam, S.Z.; Thormann, E. Surface forces and friction tuned by thermo-responsive polymer films. Curr. Opin. Colloid Interface Sci. 2020, 47, 27–45. [Google Scholar] [CrossRef]
- Amado, S.; Morouço, P.; Pascoal-Faria, P.; Alves, N. Tailoring Bioengineered Scaffolds for Regenerative Medicine. In Biomaterials in Regenerative Medicine; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, X.; Zhu, X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Rahmani Del Bakhshayesh, A.R.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Flanagan, C.L.; Zopf, D.A.; Morrison, R.J.; Nasser, H.; Wheeler, M.B.; Green, G.E. Chapter 3—Design and Quality Control for Translating 3D-Printed Scaffolds. In Essentials of 3D Biofabrication and Translation; Atala, A., Yoo, J.J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 43–59. ISBN 978-0-12-800972-7. [Google Scholar]
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D Printing Technology as Innovative Solutions for Biomedical Applications. Drug Discov. Today 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang-Lee, I.; Salas-Sánchez, F.; Pomares-Wauters, G.; Ramos-Gamboa, M.F.; Godfrey-Lowis, M.; Mora-Román, J.J. Bioimpresión de órganos y tejidos en tercera dimensión: Técnicas, aplicaciones y limitaciones. Revista Tecnología en Marcha 2018, 31, 41–51. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D Printing Promotes the Development of Drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef]
- Prabhakar, M.M.; Saravanan, A.K.; Lenin, A.H.; Mayandi, K.; Ramalingam, P.S. A Short Review on 3D Printing Methods, Process Parameters and Materials. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Vazquez-Vazquez, F.C.; Chanes-Cuevas, O.A.; Masuoka, D.; Alatorre, J.A.; Chavarria-Bolaños, D.; Vega-Baudrit, J.R.; Serrano-Bello, J.; Alvarez-Perez, M.A. Biocompatibility of Developing 3D-Printed Tubular Scaffold Coated with Nanofibers for Bone Applications. J. Nanomater. 2019, 2019, 6105818. [Google Scholar] [CrossRef]
- Spontak, R.J.; Ryan, J.J. Chapter 3—Polymer blend compatibilization by the addition of block copolymers. In Compatibilization of Polymer Blends; Ajitha, A., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–102. ISBN 978-0-12-816006-0. [Google Scholar]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer Structure-Property Requirements for Stereolithographic 3D Printing of Soft Tissue Engineering Scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and Its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the New Asset for the Production of Tailored Medicines. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Donderwinkel, I.; Van Hest, J.C.M.; Cameron, N.R. Bio-Inks for 3D Bioprinting: Recent Advances and Future Prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Patel, V.; Shah, J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Lu, Z.; Chester, S.A.; Lee, H. Micro 3D Printing of a Temperature-Responsive Hydrogel Using Projection Micro-Stereolithography. Sci. Rep. 2018, 8, 1963. [Google Scholar] [CrossRef]
- Fischetti, T.; Celikkin, N.; Contessi Negrini, N.; Farè, S.; Swieszkowski, W. Tripolyphosphate-Crosslinked Chitosan/Gelatin Biocomposite Ink for 3D Printing of Uniaxial Scaffolds. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Kuiper, R.V.; de Bruin, A.; van Nostrum, C.F.; Vermonden, T.; Dhert, W.J.A.; Hennink, W.E. In Vivo Biocompatibility and Biodegradation of 3D-Printed Porous Scaffolds Based on a Hydroxyl-Functionalized Poly(ε-Caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B: Biointerfaces, 71. [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Fundamentals of Electrospinning and Processing Technologies. Part. Sci. Technol. 2016, 34, 72–82. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Vargas-Zúñiga, R.; Pacheco-Molina, J.; Vega-Baudrit, J. Electrospun Nanofibers: A Nanotechnological Approach for Drug Delivery and Dissolution Optimization in Poorly Water-Soluble Drugs. ADMET DMPK 2020, 8, 325–353. [Google Scholar] [CrossRef]
- Morad, M.R.; Rajabi, A.; Razavi, M.; Sereshkeh, S.R.P. A Very Stable High Throughput Taylor Cone-Jet in Electrohydrodynamics. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, W.; Yang, Y.; Wang, K.; Yu, D.-G. From Taylor Cone to Solid Nanofiber in Tri-Axial Electrospinning: Size Relationships. Results Phys. 2019, 15, 102770. [Google Scholar] [CrossRef]
- He, J.-H. On the Height of Taylor Cone in Electrospinning. Results Phys. 2020, 17, 103096. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ndreu, A.; Yang, Y.; Ylikauppila, H.; Nikkola, L. Nanofiber-Based Scaffolds for Tissue Engineering. Eur. J. Plast. Surg. 2012, 35, 135–149. [Google Scholar] [CrossRef]
- Har-el, Y.; Gerstenhaber, J.A.; Brodsky, R.; Huneke, R.B.; Lelkes, P.I. Electrospun Soy Protein Scaffolds as Wound Dressings: Enhanced Reepithelialization in a Porcine Model of Wound Healing. Wound Med. 2014, 5, 9–15. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent Advances in Electrospun Polycaprolactone Based Scaffolds for Wound Healing and Skin Bioengineering Applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Mohammadian, F.; Eatemadi, A. Drug Loading and Delivery Using Nanofibers Scaffolds. Artif. Cells Nanomed. Biotechnol. 2017, 45, 881–888. [Google Scholar] [CrossRef]
- Mahalingam, S.; Raimi-Abraham, B.T.; Craig, D.Q.M.; Edirisinghe, M. Solubility–Spinnability Map and Model for the Preparation of Fibres of Polyethylene (Terephthalate) Using Gyration and Pressure. Chem. Eng. J. 2015, 280, 344–353. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Meng, Z.X.; Zheng, W.; Li, L.; Zheng, Y.F. Fabrication, Characterization and in Vitro Drug Release Behavior of Electrospun PLGA/Chitosan Nanofibrous Scaffold. Mater. Chem. Phys. 2011, 125, 606–611. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; van den Beucken, J.J.J.P.; Bian, Z.; Fan, M.; Chen, Z.; Jansen, J.A. Fibrous Scaffolds Loaded with Protein Prepared by Blend or Coaxial Electrospinning. Acta Biomater. 2010, 6, 4199–4207. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular Matrix Revisited: Roles in Tissue Engineering. Int. Neurourol. J. 2016, 20, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Lehn, J.-M.; Meijer, E.W.; Matyjaszewski, K. From Precision Polymers to Complex Materials and Systems. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.A.; Mishra, N. Polymers in Drug Delivery. J. Biosci. Med. 2015, 4, 69–84. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Murakami, D.; Kitahara, Y.; Kobayashi, S.; Tanaka, M. Thermosensitive Polymer Biocompatibility Based on Interfacial Structure at Biointerface. ACS Biomater. Sci. Eng. 2018, 4, 1591–1597. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of Polymer-Based Biomaterials and Medical Devices—Regulations, in Vitro Screening and Risk-Management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Leng, J.; Zhang, L.G. Four-Dimensional Printing Hierarchy Scaffolds with Highly Biocompatible Smart Polymers for Tissue Engineering Applications. Tissue Eng. Part C Methods 2016, 22, 10. [Google Scholar] [CrossRef]
- Cui, Z.; Lee, B.H.; Pauken, C.; Vernon, B.L. Degradation, Cytotoxicity, and Biocompatibility of NIPAAm-Based Thermosensitive, Injectable, and Bioresorbable Polymer Hydrogels. J. Biomed. Mater. Res. Part. A 2011, 98A, 159–166. [Google Scholar] [CrossRef]
- Anderson, J.M.; Shive, M.S. Biodegradation and Biocompatibility of PLA and PLGA Microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound Healing and the Role of Fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of Hydrogel-Based Scaffolds for Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Ratanavaraporn, J.; Furuya, H.; Tabata, Y. Local Suppression of Pro-Inflammatory Cytokines and the Effects in BMP-2-Induced Bone Regeneration. Biomaterials 2012, 33, 304–316. [Google Scholar] [CrossRef] [PubMed]
- González Masís, J.; Cubero Sesin, J.M.; Vega Baudrit, J.; González Paz, R.J. Development and Characterization of Biomaterials for Biomimetic Tissues Applications. J. Eng. Med. Devices 2017, 1. [Google Scholar]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Nasrabadi, H.T.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I.P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol Res. 2018, S335–S348. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable Thermoresponsive Polymers: Applications in Drug Delivery and Tissue Engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Ma, J.; Meng, J.; Simonet, M.; Stingelin, N.; Peijs, T.; Sukhorukov, G.B. Biodegradable Fibre Scaffolds Incorporating Water-Soluble Drugs and Proteins. J. Mater. Sci. Mater. Med. 2015, 26, 205. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Design Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Biodegradable Polymers as Scaffolds for Tissue Engineering. In Handbook of Biodegradable Polymers; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2011; pp. 341–362. ISBN 978-3-527-63581-8. [Google Scholar]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of Hydrophilic PCL Nanofiber Scaffolds via Electrospinning of PCL/PVP-b-PCL Block Copolymers for Enhanced Cell Biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W.E.; Anderson, J.M.; van den Beucken, J.J.J.P.; Jansen, J.A. Biocompatibility and Degradation Characteristics of PLGA-Based Electrospun Nanofibrous Scaffolds with Nanoapatite Incorporation. Biomaterials 2012, 33, 6604–6614. [Google Scholar] [CrossRef]
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D Printing of Nanocellulose Hydrogel Scaffolds with Tunable Mechanical Strength towards Wound Healing Application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Li, M.; Dong, Q.; Xiao, Y.; Du, Q.; Huselsteind, C.; Zhang, T.; He, X.; Tian, W.; Chen, Y. A Biodegradable Soy Protein Isolate-Based Waterborne Polyurethane Composite Sponge for Implantable Tissue Engineering. J. Mater. Sci. Mater. Med. 2020, 31, 120. [Google Scholar] [CrossRef]
- Ichanti, H.; Sladic, S.; Kalies, S.; Haverich, A.; Andrée, B.; Hilfiker, A. Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels 2020, 6, 27. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Rashidipour, M.; Khosravi, P.; Shahryarhesami, S.; Ashrafi, B.; Kaviani, M.; Sarabi, M.M. Biocompatibility, Cytotoxicity, Antimicrobial and Epigenetic Effects of Novel Chitosan-Based Quercetin Nanohydrogel in Human Cancer Cells. Int. J. Nanomed. 2020, 15, 5963–5975. [Google Scholar] [CrossRef]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel Strategies for the Formulation and Processing of Poorly Water-Soluble Drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Dressman, J.B. The Developability Classification System: Application of Biopharmaceutics Concepts to Formulation Development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J. Drug Nanocrystals—Versatile Option for Formulation of Poorly Soluble Materials. Int. J. Pharm 2018, 537, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed]
- Charalabidis, A.; Sfouni, M.; Bergström, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond Guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A Novel Carrier for Cell and Drug Delivery. Crit. Rev. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Zhao, L.; Mustapha, O.; Shafique, S.; Jamshaid, T.; ud Din, F.; Mehmood, Y.; Anwer, K.; ul Ain, Y.Q.; Hussain, T.; Khan, I.U.; et al. Electrospun Gelatin Nanocontainers for Enhanced Biopharmaceutical Performance of Piroxicam: In Vivo and In Vitro Investigations. Int. J. Nanomed. 2020, 15, 8819–8828. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; Prasad, L.K.; Brough, C.; Miller, D.A.; McGinity, J.W.; Williams, R.O. Thermal Processing of PVP- and HPMC-Based Amorphous Solid Dispersions. AAPS PharmSciTech 2015, 17, 120–132. [Google Scholar] [CrossRef]
- Luo, C.; Wu, W.; Lou, S.; Zhao, S.; Yang, K. Improving the in Vivo Bioavailability and in Vitro Anti-Inflammatory Activity of Tanshinone IIA by Alginate Solid Dispersion. J. Drug Deliv. Sci. Technol. 2020, 60, 101966. [Google Scholar] [CrossRef]
- Bedell, M.L.; Guo, J.L.; Xie, V.Y.; Navara, A.M.; Mikos, A.G. Chapter 17—Polymer scaffold fabrication. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 295–315. ISBN 978-0-12-818422-6. [Google Scholar]
- Llorens, E.; del Valle, L.J.; Ferrán, R.; Rodríguez-Galán, A.; Puiggalí, J. Scaffolds with Tuneable Hydrophilicity from Electrospun Microfibers of Polylactide and Poly(Ethylene Glycol) Mixtures: Morphology, Drug Release Behavior, and Biocompatibility. J. Polym. Res. 2014, 21, 360. [Google Scholar] [CrossRef]
- Llorens, E.; Ibañez, H.; del Valle, L.J.; Puiggalí, J. Biocompatibility and Drug Release Behavior of Scaffolds Prepared by Coaxial Electrospinning of Poly(Butylene Succinate) and Polyethylene Glycol. Mater. Sci. Eng. C 2015, 49, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Jennotte, O.; Koch, N.; Lechanteur, A.; Evrard, B. Three-Dimensional Printing Technology as a Promising Tool in Bioavailability Enhancement of Poorly Water-Soluble Molecules: A Review. Int. J. Pharm. 2020, 580, 119200. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.P.; Shabab, T.; Shafiee, A.; Peiffer, Q.C.; Fox, K.; Tran, N.; Dargaville, T.R.; Hutmacher, D.W.; Tran, P.A. 3D Printed Dual Macro-, Microscale Porous Network as a Tissue Engineering Scaffold with Drug Delivering Function. Biofabrication 2019, 11, 035014. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, Functionalization Strategies and Biomedical Applications of Targeted Biodegradable/Biocompatible Polymer-Based Nanocarriers for Drug Delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Kutlu, B.; Tiğlı Aydın, R.S.; Akman, A.C.; Gümüşderelioglu, M.; Nohutcu, R.M. Platelet-rich Plasma-loaded Chitosan Scaffolds: Preparation and Growth Factor Release Kinetics. J. Biomed. Mater. Res. Part B 2013, 101, 28–35. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-Responsive Polymers: Applications of Smart Materials in Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.-G. Influence of Hydrophilic Polymers on Functional Properties and Wound Healing Efficacy of Hydrocolloid Based Wound Dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef]
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High Surface Area Polypyrrole Scaffolds for Tunable Drug Delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Behl, T.; Chadha, S. A Rationalized and Innovative Perspective of Nanotechnology and Nanobiotechnology in Chronic Wound Management. J. Drug Deliv. Sci. Technol. 2020, 60, 101930. [Google Scholar] [CrossRef]
- Abudula, T.; Gauthaman, K.; Mostafavi, A.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Tamayol, A.; Memic, A. Sustainable Drug Release from Polycaprolactone Coated Chitin-Lignin Gel Fibrous Scaffolds. Sci. Rep. 2020, 10, 20428. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Frauenlob, M.; Shibata, Y.; Wang, L.; Nakajima, T.; Nonoyama, T.; Tsuda, M.; Tanaka, S.; Kurokawa, T.; Gong, J.P. Chitin-Based Double-Network Hydrogel as Potential Superficial Soft-Tissue-Repairing Materials. Biomacromolecules 2020, 21, 4220–4230. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, H.; Wu, R.; Patil, A.; Hou, C.; Lin, Z.; Meng, Z.; Ma, L.; Yu, R.; Yu, W.; et al. Programing Performance of Silk Fibroin Superstrong Scaffolds by Mesoscopic Regulation among Hierarchical Structures. Biomacromolecules 2020, 21, 4169–4179. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(Lactic Acid) Nanofibrous Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled Local Drug Delivery Strategies from Chitosan Hydrogels for Wound Healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef]
- Castro-Piedra, S.E.; Calvo-Castro, L.A.; AlvarengaVenutolo, S.; Centeno-Cerdas, C.; Ramos-Madrigal, M.; Vega-Baudrit, J.; Zamora-Mora, V.; Rojas-Chaves, M. Membranas de colágeno y quitosano de fuentes alternativas: Evaluación para su uso potencial en ingeniería de tejidos. Revista Tecnología en Marcha 2015, 28, 58–68. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Rosca, I.; Pinteala, M.; Mititelu-Tartau, L. Chitosan Derivatives in Macromolecular Co-Assembly Nanogels with Potential for Biomedical Applications. Biomacromolecules 2020, 21, 4231–4243. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Zuo, D.-Y.; Xu, W.-L.; Liu, H.-T. Effects of Polyvinylpyrrolidone on Structure and Performance of Composite Scaffold of Chitosan Superfine Powder and Polyurethane. Adv. Polym. Technol. 2012, 31, 310–318. [Google Scholar] [CrossRef]
- Román-Doval, R.; Tellez-Cruz, M.M.; Rojas-Chávez, H.; Cruz-Martínez, H.; Carrasco-Torres, G.; Vásquez-Garzón, V.R. Enhancing Electrospun Scaffolds of PVP with Polypyrrole/Iodine for Tissue Engineering of Skin Regeneration by Coating via a Plasma Process. J. Mater. Sci. 2019, 54, 3342–3353. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Kiran, V.; Hasnain, M.S.; Nayak, A.K. Chapter 13—Alginate-based hydrogel systems for drug releasing in wound healing. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 323–358. ISBN 978-0-12-817640-5. [Google Scholar]
- Hasnain, M.S.; Ray, P.; Nayak, A.K. Chapter 5—Alginate-based interpenetrating polymer networks for sustained drug release. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press; Boston, MA, USA, 2020; pp. 101–128. ISBN 978-0-12-817640-5.
- Nayak, A.K.; Ansari, M.T.; Sami, F.; Singh, H.K.B.; Hasnain, M.S. Chapter 2—Alginates as drug delivery excipients. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 19–39. ISBN 978-0-12-817640-5. [Google Scholar]
- Buyana, B.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-Pluronic Topical Gels Loaded with Thymol, Norfloxacin and ZnO Nanoparticles as Potential Wound Dressings. J. Drug Deliv. Sci. Technol. 2020, 60, 101960. [Google Scholar] [CrossRef]
- Ghosh Dastidar, D.; Chakrabarti, G. Chapter 6—Thermoresponsive Drug Delivery Systems, Characterization and Application. In Applications of Targeted Nano Drugs and Delivery Systems; Micro and Nano Technologies, Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–155. ISBN 978-0-12-814029-1. [Google Scholar]
- Wu, Y.; Zhou, F.; Yang, L.; Liu, J. A Shrinking Strategy for Creating Dynamic SERS Hot Spots on the Surface of Thermosensitive Polymer Nanospheres. Chem. Commun. 2013, 49, 5025–5027. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Hein, S.; Li, P.; Nygaard, J.V.; Kassem, M.; Kjems, J.; Besenbacher, F.; Bünger, C. Fabrication and Characterization of a Rapid Prototyped Tissue Engineering Scaffold with Embedded Multicomponent Matrix for Controlled Drug Release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef]
- Asadian-Ardakani, V.; Saber-Samandari, S.; Saber-Samandari, S. The Effect of Hydroxyapatite in Biopolymer-Based Scaffolds on Release of Naproxen Sodium. J. Biomed. Mater. Res. Part. A 2016, 104, 2992–3003. [Google Scholar] [CrossRef]
- Chogan, F.; Mirmajidi, T.; Rezayan, A.H.; Sharifi, A.M.; Ghahary, A.; Nourmohammadi, J.; Kamali, A.; Rahaie, M. Design, Fabrication, and Optimization of a Dual Function Three-Layer Scaffold for Controlled Release of Metformin Hydrochloride to Alleviate Fibrosis and Accelerate Wound Healing. Acta Biomater. 2020, 113, 144–163. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Davachi, S.M.; Bagher, Z.; Heraji Esfahani, A.; Jenabi, N.; Atoufi, Z.; Khanmohammadi, M.; Abbaspourrad, A.; Rashedi, H.; Jalessi, M. Fabrication of Chitosan/Polyvinylpyrrolidone Hydrogel Scaffolds Containing PLGA Microparticles Loaded with Dexamethasone for Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 356–370. [Google Scholar] [CrossRef]
- Biswas, A.; Amarajeewa, M.; Senapati, S.; Sahu, M.; Maiti, P. Sustained Release of Herbal Drugs Using Biodegradable Scaffold for Faster Wound Healing and Better Patient Compliance. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2131–2141. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Razmi Bagtash, H.R.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive Hydrogels in Biomedical Applications: A Seven-Year Update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Q.Y.; Cheng, H.; Liow, S.S.; Dou, Q.; Wu, Y.L.; Loh, X.J.; Li, Z. Poly(Carbonate Urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications. Polymers (Basel) 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-Responsive Hydrogels in Drug Delivery and Tissue Engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nichols, B.L.B.; Norris, A.M.; Frazier, C.E.; Edgar, K.J. All-Polysaccharide, Self-Healing Injectable Hydrogels Based on Chitosan and Oxidized Hydroxypropyl Polysaccharides. Biomacromolecules 2020, 21, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent Progress of Highly Adhesive Hydrogels as Wound Dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef] [PubMed]
- Bakaic, E.; Smeets, N.M.B.; Badv, M.; Dodd, M.; Barrigar, O.; Siebers, E.; Lawlor, M.; Sheardown, H.; Hoare, T. Injectable and Degradable Poly(Oligoethylene Glycol Methacrylate) Hydrogels with Tunable Charge Densities as Adhesive Peptide-Free Cell Scaffolds. ACS Biomater. Sci. Eng. 2018, 4, 3713–3725. [Google Scholar] [CrossRef]
- Baldassari, S.; Solari, A.; Zuccari, G.; Drava, G.; Pastorino, S.; Fucile, C.; Marini, V.; Daga, A.; Pattarozzi, A.; Ratto, A.; et al. Development of an Injectable Slow-Release Metformin Formulation and Evaluation of Its Potential Antitumor Effects. Sci Rep. 2018, 8. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Huynh, D.P.; Park, J.H.; Lee, D.S. Injectable Polymeric Hydrogels for the Delivery of Therapeutic Agents: A Review. Eur. Polym. J. 2015, 72, 602–619. [Google Scholar] [CrossRef]
- Andrgie, A.T.; Darge, H.F.; Mekonnen, T.W.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.-Y.; Wang, C.-F.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Ibuprofen-Loaded Heparin Modified Thermosensitive Hydrogel for Inhibiting Excessive Inflammation and Promoting Wound Healing. Polymers 2020, 12, 2619. [Google Scholar] [CrossRef]
- Dong, Y.; Zhuang, H.; Hao, Y.; Zhang, L.; Yang, Q.; Liu, Y.; Qi, C.; Wang, S. Poly(N-Isopropyl-Acrylamide)/Poly(γ-Glutamic Acid) Thermo-Sensitive Hydrogels Loaded with Superoxide Dismutase for Wound Dressing Application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Keshel, S.H. The Healing Effect of Stem Cells Loaded in Nanofibrous Scaffolds on Full Thickness Skin Defects. J. Biomed. Nanotechnol. 2013, 9, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in Drug Delivery Systems (DDSs) to Release Growth Factors for Wound Healing and Skin Regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Qiao, W.; Zhang, Y.; Wu, H.; Miao, S.; Cheng, Z.; Gong, Q.; Liang, J.; Zhu, A. A Gelatin Composite Scaffold Strengthened by Drug-Loaded Halloysite Nanotubes. Mater. Sci. Eng. C 2017, 78, 362–369. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, J.; Lin, Z.Y.W.; Pan, G.; Zhu, Y.; Cheng, Y.; Cui, W. Self-Coated Interfacial Layer at Organic/Inorganic Phase for Temporally Controlling Dual-Drug Delivery from Electrospun Fibers. Colloids Surf. B Biointerfaces 2015, 130, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Bai, S.; Yang, H.; Li, S.; Quan, J.; Zhu, L.; Nie, H. Controlled Release from Thermo-Sensitive PNVCL-Co-MAA Electrospun Nanofibers: The Effects of Hydrophilicity/Hydrophobicity of a Drug. Mater. Sci. Eng. C 2016, 67, 581–589. [Google Scholar] [CrossRef]
- Li, H.; Liu, K.; Sang, Q.; Williams, G.R.; Wu, J.; Wang, H.; Wu, J.; Zhu, L.-M. A Thermosensitive Drug Delivery System Prepared by Blend Electrospinning. Colloids Surf. B Biointerfaces 2017, 159, 277–283. [Google Scholar] [CrossRef]
- Kordjamshidi, A.; Saber-Samandari, S.; Ghadiri Nejad, M.; Khandan, A. Preparation of Novel Porous Calcium Silicate Scaffold Loaded by Celecoxib Drug Using Freeze Drying Technique: Fabrication, Characterization and Simulation. Ceram. Int. 2019, 45, 14126–14135. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of Chitosan-Polycaprolactone Composite Nanofibrous Scaffold for Simultaneous Delivery of Ferulic Acid and Resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline Hydrochloride-Loaded Electrospun Nanofibers Mats Based on PVA and Chitosan for Wound Dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef]
- Sinha, M.; Banik, R.M.; Haldar, C.; Maiti, P. Development of Ciprofloxacin Hydrochloride Loaded Poly(Ethylene Glycol)/Chitosan Scaffold as Wound Dressing. J. Porous Mater. 2013, 20, 799–807. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/Alginate Hydrogels Containing Alpha-Tocopherol for Wound Healing in Rat Model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Ramteke, P.W. Nanofibre Based Smart Pharmaceutical Scaffolds for Wound Repair and Regenerations. Curr. Pharm. Design 2016, 22, 1460–11471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, G.; Brey, E.M. Dual Delivery of Chlorhexidine and Platelet-Derived Growth Factor-BB for Enhanced Wound Healing and Infection Control. Acta Biomater. 2013, 9, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, Y.; Shen, A.; Yang, Y.; Diao, L.; Wang, L.; Cai, D.; Hu, Y. Injectable Chitosan-Based Thermosensitive Hydrogel/Nanoparticle-Loaded System for Local Delivery of Vancomycin in the Treatment of Osteomyelitis. Int. J. Nanomed. 2020, 15, 5855–5871. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Tang, R. Electrospun Scaffold with Sustained Antibacterial and Tissue-Matched Mechanical Properties for Potential Application as Functional Mesh. Int. J. Nanomed. 2020, 15, 4991–5004. [Google Scholar] [CrossRef]
- Feng, K.; Sun, H.; Bradley, M.A.; Dupler, E.J.; Giannobile, W.V.; Ma, P.X. Novel Antibacterial Nanofibrous PLLA Scaffolds. J. Control. Release 2010, 146, 363–369. [Google Scholar] [CrossRef]
- Abdoli, M.; Sadrjavadi, K.; Arkan, E.; Zangeneh, M.M.; Moradi, S.; Zangeneh, A.; Shahlaei, M.; Khaledian, S. Polyvinyl Alcohol/Gum Tragacanth/Graphene Oxide Composite Nanofiber for Antibiotic Delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102044. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in Wound Healing and Skin Tissue Engineering. Mol. Biol Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Chu, J.; Shi, P.; Yan, W.; Fu, J.; Yang, Z.; He, C.; Deng, X.; Liu, H. PEGylated Graphene Oxide-Mediated Quercetin-Modified Collagen Hybrid Scaffold for Enhancement of MSCs Differentiation Potential and Diabetic Wound Healing. Nanoscale 2018, 10, 9547–9560. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hung, K.-C.; Hsieh, M.-J.; Chang, S.-H.; Juang, J.-H.; Hsieh, I.-C.; Wen, M.-S.; Liu, S.-J. Core-Shell Insulin-Loaded Nanofibrous Scaffolds for Repairing Diabetic Wounds. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102123. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Tian, R.; Lv, K.; Liu, Z.; Ni, J.; Yuan, P.; Bai, Y.; Chen, X. Stimuli Responsive Co-Delivery of Celecoxib and BMP2 from Micro-Scaffold for Periodontal Disease Treatment. J. Mater. Sci. Technol. 2021, 75, 216–224. [Google Scholar] [CrossRef]
- Zehra, M.; Mehmood, A.; Yar, M.; Shahzadi, L.; Riazuddin, S. Development of NSAID-Loaded Nano-Composite Scaffolds for Skin Tissue Engineering Applications. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sun, X.; Zhao, X.; Wang, L.; Yu, J.; Pan, G.; Li, B.; Yang, H.; Zhang, Y.; Cui, W. Surface Biofunctional Drug-Loaded Electrospun Fibrous Scaffolds for Comprehensive Repairing Hypertrophic Scars. Biomaterials 2016, 83, 169–181. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous In Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Rhim, J.-W.; Adhikari, B. Switchable Dual-Function and Bioresponsive Materials to Control Bacterial Infections. ACS Appl. Mater. Interfaces 2019, 11, 22897–22914. [Google Scholar] [CrossRef]
- Najafloo, R.; Behyari, M.; Imani, R.; Nour, S. A Mini-Review of Thymol Incorporated Materials: Applications in Antibacterial Wound Dressing. J. Drug Deliv. Sci. Technol. 2020, 60, 101904. [Google Scholar] [CrossRef]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun Anti-Inflammatory Patch Loaded with Essential Oils for Wound Healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Salama, A.H. Norfloxacin-Loaded Collagen/Chitosan Scaffolds for Skin Reconstruction: Preparation, Evaluation and in-Vivo Wound Healing Assessment. Eur. J. Pharm. Sci. 2016, 83, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn Injury: Challenges and Advances in Burn Wound Healing, Infection, Pain and Scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Li, W.; Jiao, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. Therapeutic Efficacy of Antibiotic-Loaded Gelatin Microsphere/Silk Fibroin Scaffolds in Infected Full-Thickness Burns. Acta Biomater. 2014, 10, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Ahovan, Z.A.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-Responsive Chitosan Hydrogel for Healing of Full-Thickness Wounds Infected with XDR Bacteria Isolated from Burn Patients: In Vitro and in Vivo Animal Model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef]
| Polymer System | Delivered Drug | Application | Release Time | Ref |
|---|---|---|---|---|
| Gelatin | Ibuprofen | Inflammation and bone regeneration | 100 h | [219] |
| PLGA | Ibuprofen | Inflammation | 30 h | [220] |
| Poly(N-vinylcaprolactam-co-methacrylic acid) | Ketoprofen | Inflammation | 50 h | [221] |
| Poly(di(ethylene glycol) methyl ether methacrylate), Ethyl cellulose | Ketoprofen | Inflammation | 100 h (80%) | [222] |
| Sodium alginate | Celecoxib | Hyperthermia | - | [223] |
| Chitosan, PCL | Ferulic acid, resveratrol | Inflammation, pro-angiogenic | 120 h (55% of ferulic acid and 48% of resveratrol) | [224] |
| PVA, chitosan | Tetracycline HCl | Bacterial infection | 4 h (80%) | [225] |
| Chitosan, PEG | Ciprofloxacin HCl | Bacterial infection | 20 h (30%) | [226] |
| Chitosan, alginate | Alpha-tocoferol | Skin injuries, oxidative process | 14 days (77%) | [227] |
| Eudragit | Gentamicin sulphate | Bacterial infection in diabetic ulcer | 12 h (90% at acid pH) | [228] |
| PLGA | Clorhexidine | Infection treatment | 50 days | [229] |
| Chitosan | Vamcomycin | Bacterial infection, osteomyelitis | 26 days | [230] |
| PCL, silk fibroin | Amoxicillin | Bacterial infection | 14 days | [231] |
| PLGA, poly(L-lactic acid) | Doxycycline | Bacterial infection | 6 weeks | [232] |
| PVA | Tetracycline | Bacterial infection | 24 h (82% at pH 7.4) | [233] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci. 2021, 22, 1408. https://doi.org/10.3390/ijms22031408
Castillo-Henríquez L, Castro-Alpízar J, Lopretti-Correa M, Vega-Baudrit J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. International Journal of Molecular Sciences. 2021; 22(3):1408. https://doi.org/10.3390/ijms22031408
Chicago/Turabian StyleCastillo-Henríquez, Luis, Jose Castro-Alpízar, Mary Lopretti-Correa, and José Vega-Baudrit. 2021. "Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing" International Journal of Molecular Sciences 22, no. 3: 1408. https://doi.org/10.3390/ijms22031408
APA StyleCastillo-Henríquez, L., Castro-Alpízar, J., Lopretti-Correa, M., & Vega-Baudrit, J. (2021). Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. International Journal of Molecular Sciences, 22(3), 1408. https://doi.org/10.3390/ijms22031408








