Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways
Abstract
1. Introduction
2. Results
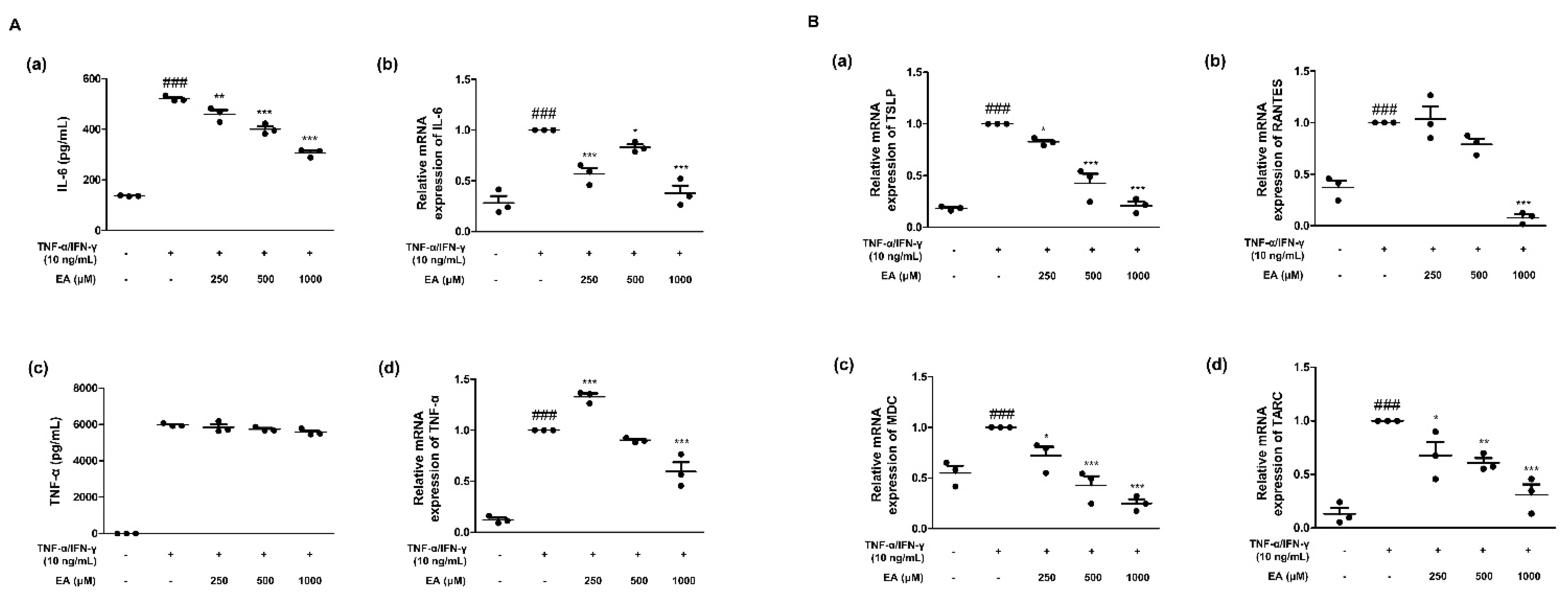
2.1. Effects of EA on Inflammatory Cytokines and Chemokines in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
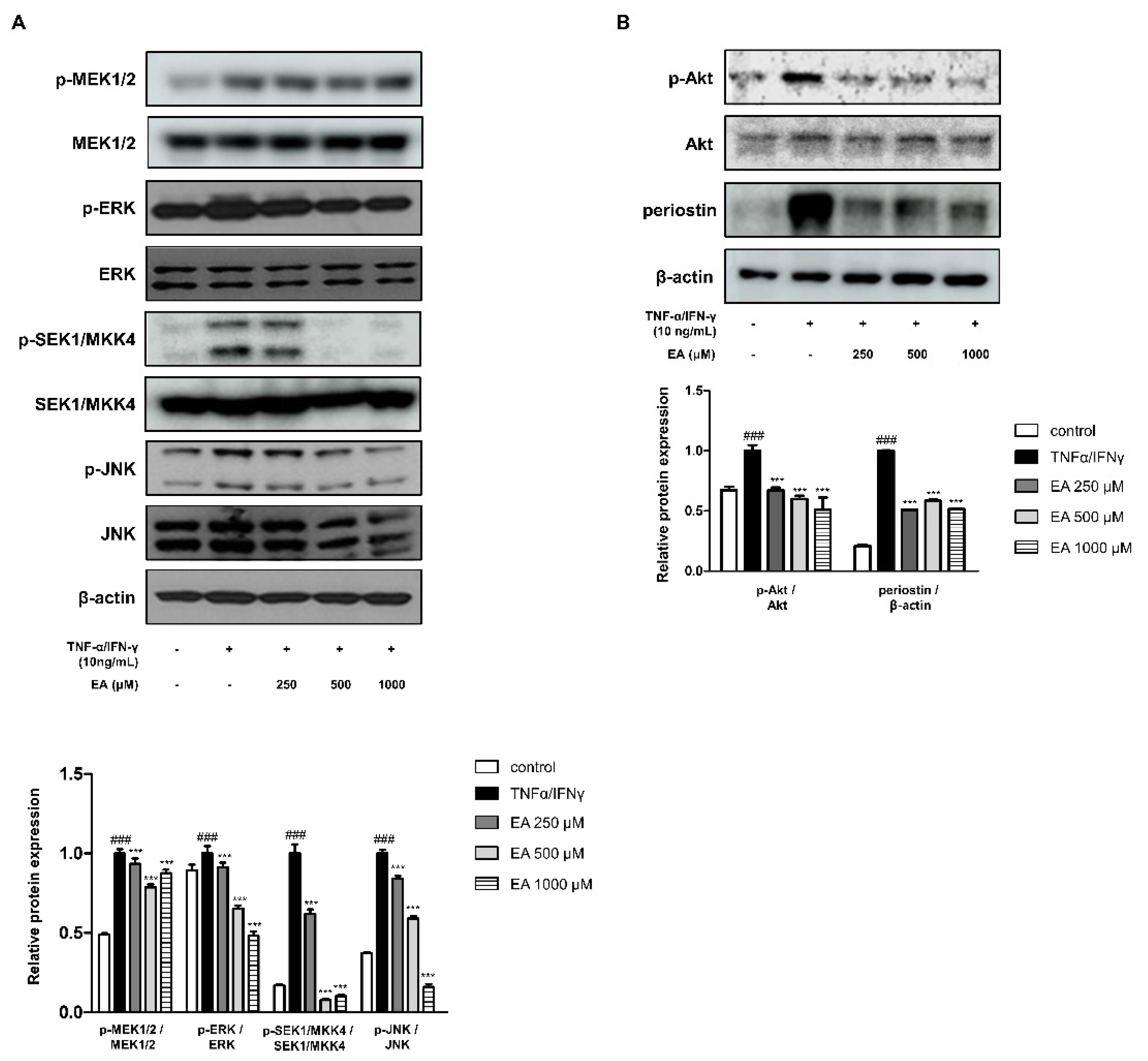
2.2. Effects of EA on Inflammatory Signaling Pathways in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
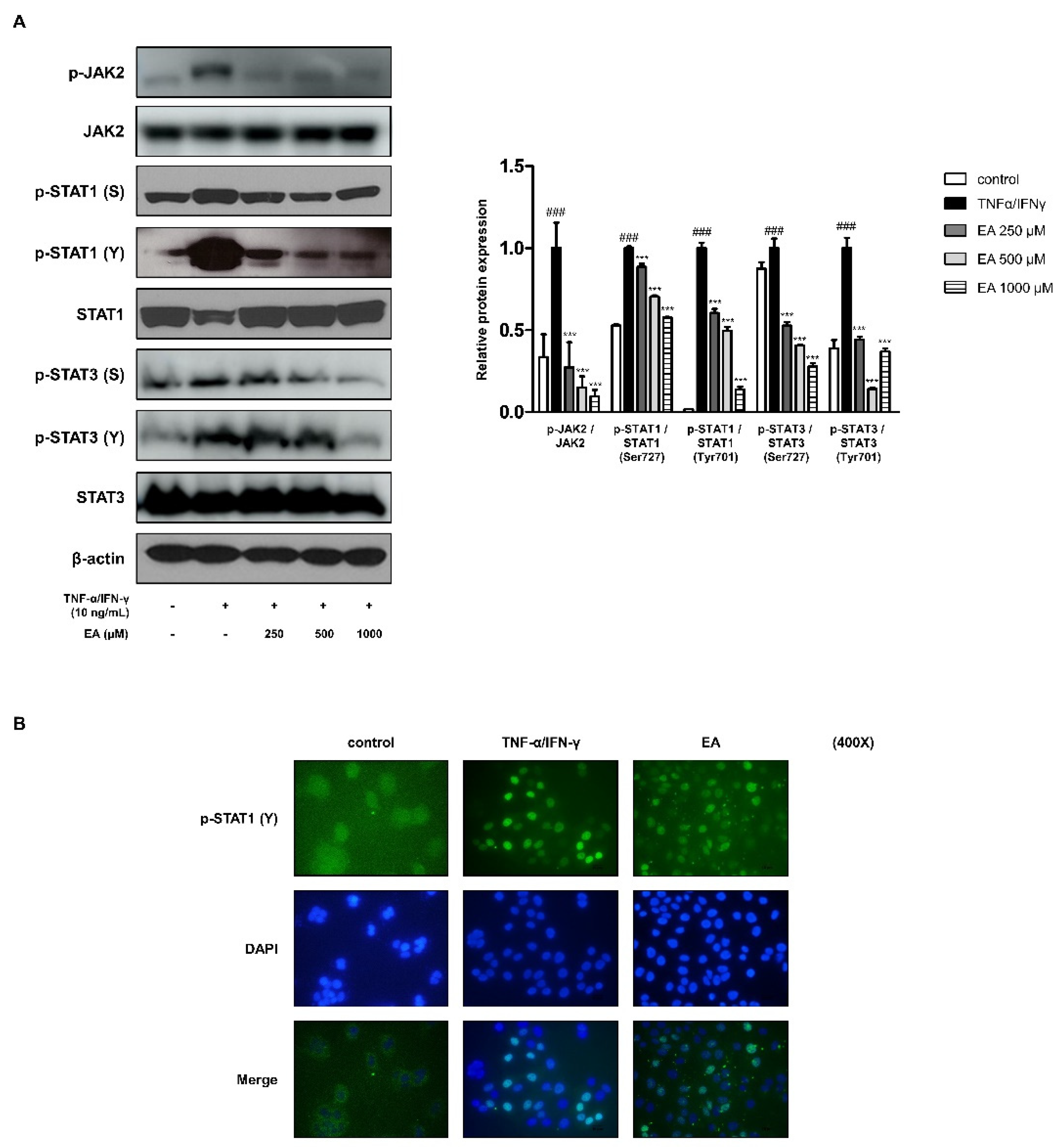
2.3. Effects of EA on the JAK/STAT Signaling Pathway in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
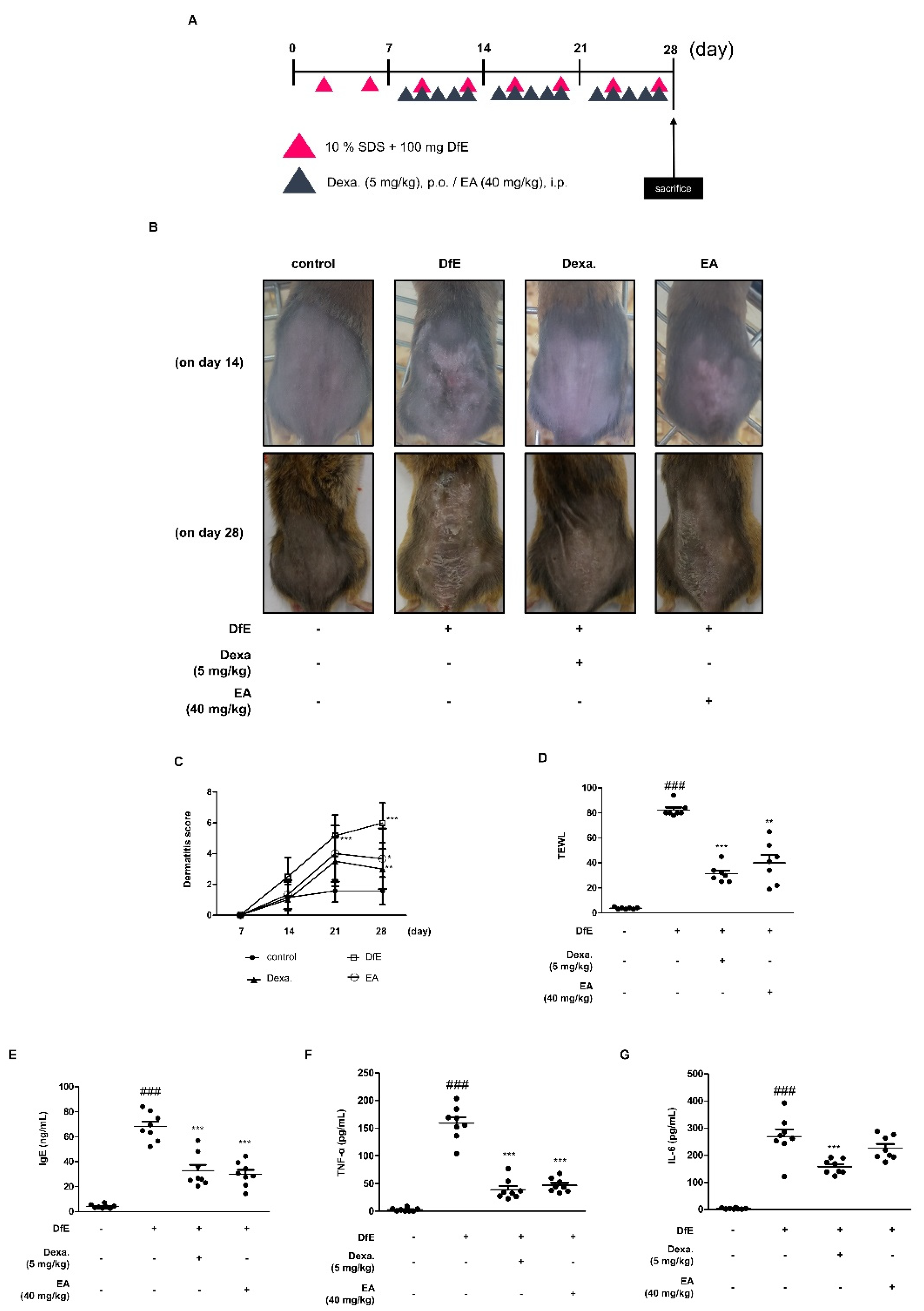
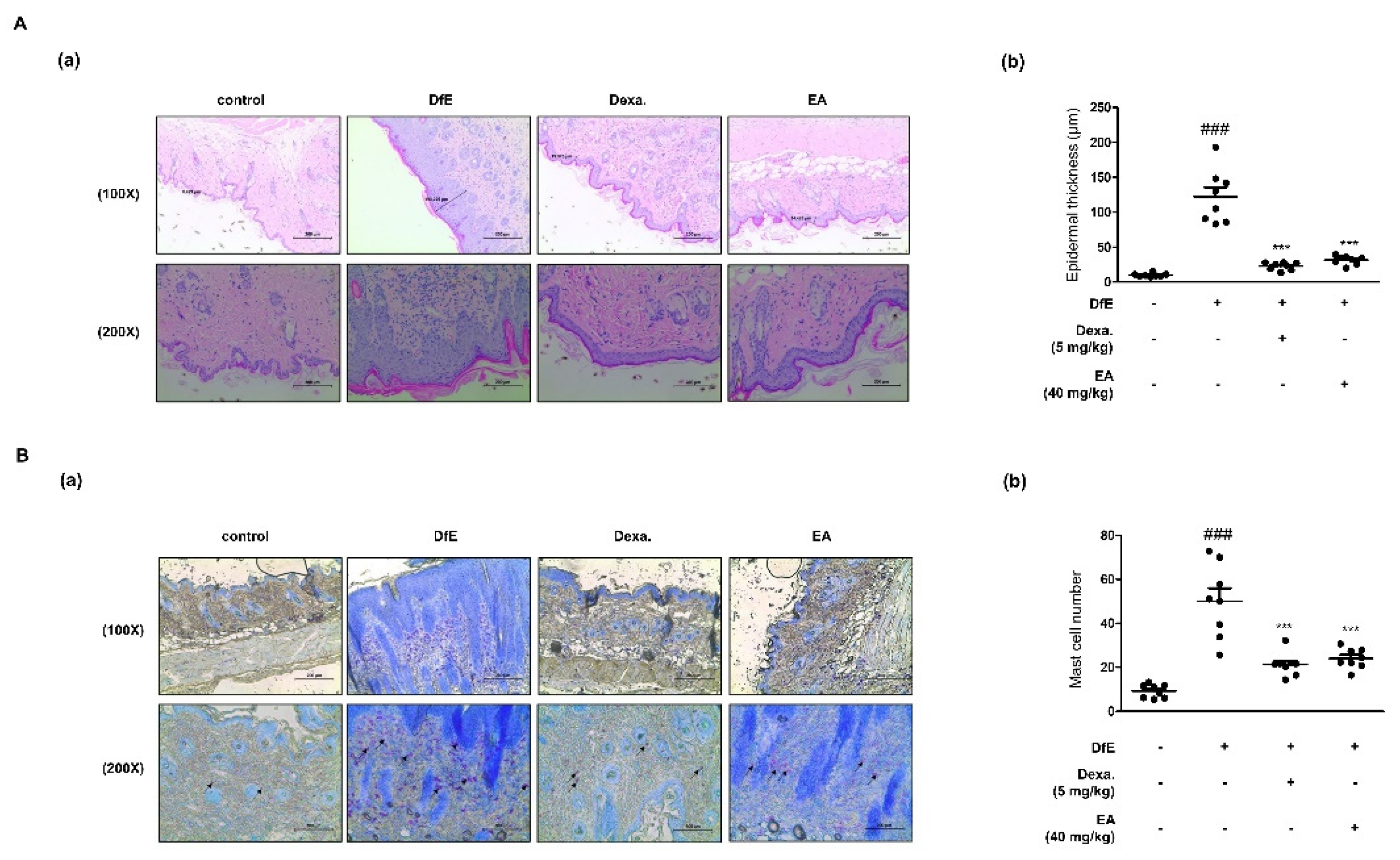
2.4. Effects of EA on Dermatophagoides Farinae Crude Extract (DfE)-Induced AD-Like Skin Lesions in NC/Nga Mice
2.5. Effects of EA on Skin Integrity and Mast Cell Infiltration in DfE-Induced AD-Like NC/Nga Mouse Skin Lesions
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of EA
4.3. Cell Culture and Treatment
4.4. Animals and Treatment
4.5. Evaluation of Dermatitis Severity
4.6. Transepidermal Water Loss
4.7. Histological Analysis of Skin Lesions
4.8. mRNA Extraction and Quantification
4.9. Western Blot Analysis
4.10. Cytokine Analysis
4.11. Immunofluorescence Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| EA | ellagic acid |
| DfE | Dermatophagoides farinae extract |
| MAPK | mitogen-activated protein kinase |
| STAT | signal transducer and activator of transcription |
References
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chun, S.Y.; Lee, M.G.; Kim, S.; Jang, T.J.; Nam, K.S. The prevention of TNF-alpha/IFN-gamma mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Silverberg, J.I. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol. Clin. 2017, 35, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26, 1045–1060. [Google Scholar] [CrossRef]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.P.; et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26, 1176–1193. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nistico, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Jung, M.R.; Lee, T.H.; Bang, M.H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-beta-D-glucopyanosylspinasterol via blocking NF-kappaB and STAT1 signaling pathways in TNF-alpha and IFN-gamma-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241. [Google Scholar] [CrossRef]
- Hassan, Z.; Luvsannyam, E.; Patel, D.; Nukala, S.; Puvvada, S.R.; Hamid, P. Review of Prominent Cytokines as Superior Therapeutic Targets for Moderate-to-Severe Atopic Dermatitis. Cureus 2020, 12, e9901. [Google Scholar] [CrossRef]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358.e1. [Google Scholar] [CrossRef] [PubMed]
- Wallmeyer, L.; Dietert, K.; Sochorova, M.; Gruber, A.D.; Kleuser, B.; Vavrova, K.; Hedtrich, S. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci. Rep. 2017, 7, 774. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Scarponi, C.; Sebastiani, S.; Cavani, A.; Federici, M.; Sozzani, S.; Girolomoni, G. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J. Leukoc. Biol. 2001, 70, 617–623. [Google Scholar] [PubMed]
- Nakahara, T.; Izuhara, K.; Onozuka, D.; Nunomura, S.; Tamagawa-Mineoka, R.; Masuda, K.; Ichiyama, S.; Saeki, H.; Kabata, Y.; Abe, R.; et al. Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: A study protocol. Medicine 2020, 99, e22043. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-kappaB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 473–479. [Google Scholar]
- Zeb, A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol. Cell. Biochem. 2018, 448, 27–41. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Sepand, M.R.; Seyednejad, S.A.; Omidi, A.; Akbariani, M.; Gholami, M.; Sabzevari, O. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IkBalpha/NFkB in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Marin, M.; Maria Giner, R.; Rios, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; Aparicio-Soto, M.; Sanchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Gad, A.M.; Fikry, E.M.; Eid, A.H. Ellagic acid attenuates testicular disruption in rheumatoid arthritis via targeting inflammatory signals, oxidative perturbations and apoptosis. Life Sci. 2019, 239, 117012. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Giner, R.M.; Marin, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C. Keratinocytes in allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, A.; Mastrofrancesco, A.; Flori, E.; Maresca, V.; Picardo, M.; Amerio, P.; Feliciani, C. Proinflammatory cytokine production in HaCaT cells treated by eosin: Implications for the topical treatment of psoriasis. Int. J. Immunopathol. Pharmacol. 2009, 22, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yoon, T.; Jang, J.Y.; Park, S.J.; Kim, H.K. Topical application of Rehmannia glutinosa extract inhibits mite allergen-induced atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011, 134, 37–44. [Google Scholar] [CrossRef]
- Kjellerup, R.B.; Kragballe, K.; Iversen, L.; Johansen, C. Pro-inflammatory cytokine release in keratinocytes is mediated through the MAPK signal-integrating kinases. Exp. Dermatol. 2008, 17, 498–504. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, Y.; Tan, G.; Han, Y.; Tang, Z.; Xu, W.; Zeng, F.; Guo, Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-alpha-induced ICAM-1 expression via NF-kappaB/MAPK in HaCaT cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 1335–1346. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Schabbauer, G.; Tencati, M.; Pedersen, B.; Pawlinski, R.; Mackman, N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Ohta, S.; Takagi, A.; Shiraishi, H.; Masuoka, M.; Ontsuka, K.; Suto, H.; Suzuki, S.; Yamamoto, K.; Ogawa, M.; et al. Periostin contributes to epidermal hyperplasia in psoriasis common to atopic dermatitis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2015, 64, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Welsch, K.; Holstein, J.; Laurence, A.; Ghoreschi, K. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur. J. Immunol. 2017, 47, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Clarysse, K.; Pfaff, C.M.; Marquardt, Y.; Huth, L.; Kortekaas Krohn, I.; Kluwig, D.; Luscher, B.; Gutermuth, J.; Baron, J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2019, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Haspel, R.L.; Darnell, J.E., Jr. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc. Natl. Acad. Sci. USA 1999, 96, 10188–10193. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhou, Y.; Ma, G.; Yang, L.; Wang, Y.; Shi, W. Cloning, bioinformatics analysis, and expression of the dust mite allergen Der f 5 of Dermatophagoides farinae. Braz. J. Med. Biol. Res. 2012, 45, 746–752. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Laudanska, H.; Reduta, T.; Szmitkowska, D. Evaluation of skin barrier function in allergic contact dermatitis and atopic dermatitis using method of the continuous TEWL measurement. Roczniki Akademii Medycznej w Bialymstoku 2003, 48, 123–127. [Google Scholar]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomas-Barberan, F.A.; Espin, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.P.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A.; et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food 2008, 11, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Alexis, A.F.; Beck, L.A.; Block, J.; Eichenfield, L.F.; Fonacier, L.; Guttman-Yassky, E.; Paller, A.S.; Pariser, D.; Silverberg, J.I.; et al. Expert Perspectives on Management of Moderate-to-Severe Atopic Dermatitis: A Multidisciplinary Consensus Addressing Current and Emerging Therapies. J. Allergy Clin. Immunol. Pract. 2017, 5, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, A.W.; Yin, E.S.; Antaya, R.J. Topical Corticosteroid Phobia in Atopic Dermatitis: A Systematic Review. JAMA Dermatol. 2017, 153, 1036–1042. [Google Scholar] [CrossRef]
- Shimada, Y.; Takehara, K.; Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, S.; Jagot, F.; Kyle, R.L.; Hyde, E.; White, R.F.; Prout, M.; Schmidt, A.J.; Yamane, H.; Lamiable, O.; Le Gros, G.; et al. Thymic stromal lymphopoietin drives the development of IL-13(+) Th2 cells. Proc. Natl. Acad. Sci. USA 2018, 115, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol. Res. 2012, 52, 211–223. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Arima, K.; Ohta, S.; Suzuki, S.; Inamitsu, M.; Yamamoto, K. Periostin in allergic inflammation. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2014, 63, 143–151. [Google Scholar] [CrossRef]
| Gene Name | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| TNF-α (h) | CGCTCCCCAAGAAGACAG | AGAGGCTGAGGAACAAGCAC |
| IL-6 (h) | ATTCCGGGAACGAAAGAGAA | TCTTCTCCTGGGGGTACTGG |
| TSLP (h) | CAGGCTATTCGGAAACTCAGA | GTAATTGTGACACTTGTTCCAGAC |
| RANTES (h) | CCGCGGCAGCCCTCGCTGTCATCC | CATCTCCAAAGAGTTGATGTACTCC |
| MDC (h) | AGGACAGAGCATGGATCGCCTACAGA | TAATGGCAGGGAGGTAGGGCTCCTGA |
| TARC (h) | CTTCTCTGCAGCACATCC | AAGACCTCTCAAGGCTTTG |
| GAPDH (h) | TGCACCACCACCTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. https://doi.org/10.3390/ijms22031277
Gil T-Y, Hong C-H, An H-J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. International Journal of Molecular Sciences. 2021; 22(3):1277. https://doi.org/10.3390/ijms22031277
Chicago/Turabian StyleGil, Tae-Young, Chul-Hee Hong, and Hyo-Jin An. 2021. "Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways" International Journal of Molecular Sciences 22, no. 3: 1277. https://doi.org/10.3390/ijms22031277
APA StyleGil, T.-Y., Hong, C.-H., & An, H.-J. (2021). Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. International Journal of Molecular Sciences, 22(3), 1277. https://doi.org/10.3390/ijms22031277






