KMU-1170, a Novel Multi-Protein Kinase Inhibitor, Suppresses Inflammatory Signal Transduction in THP-1 Cells and Human Osteoarthritic Fibroblast-Like Synoviocytes by Suppressing Activation of NF-κB and NLRP3 Inflammasome Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Effect of KMU-1170 on the Activity of Various Protein Kinases
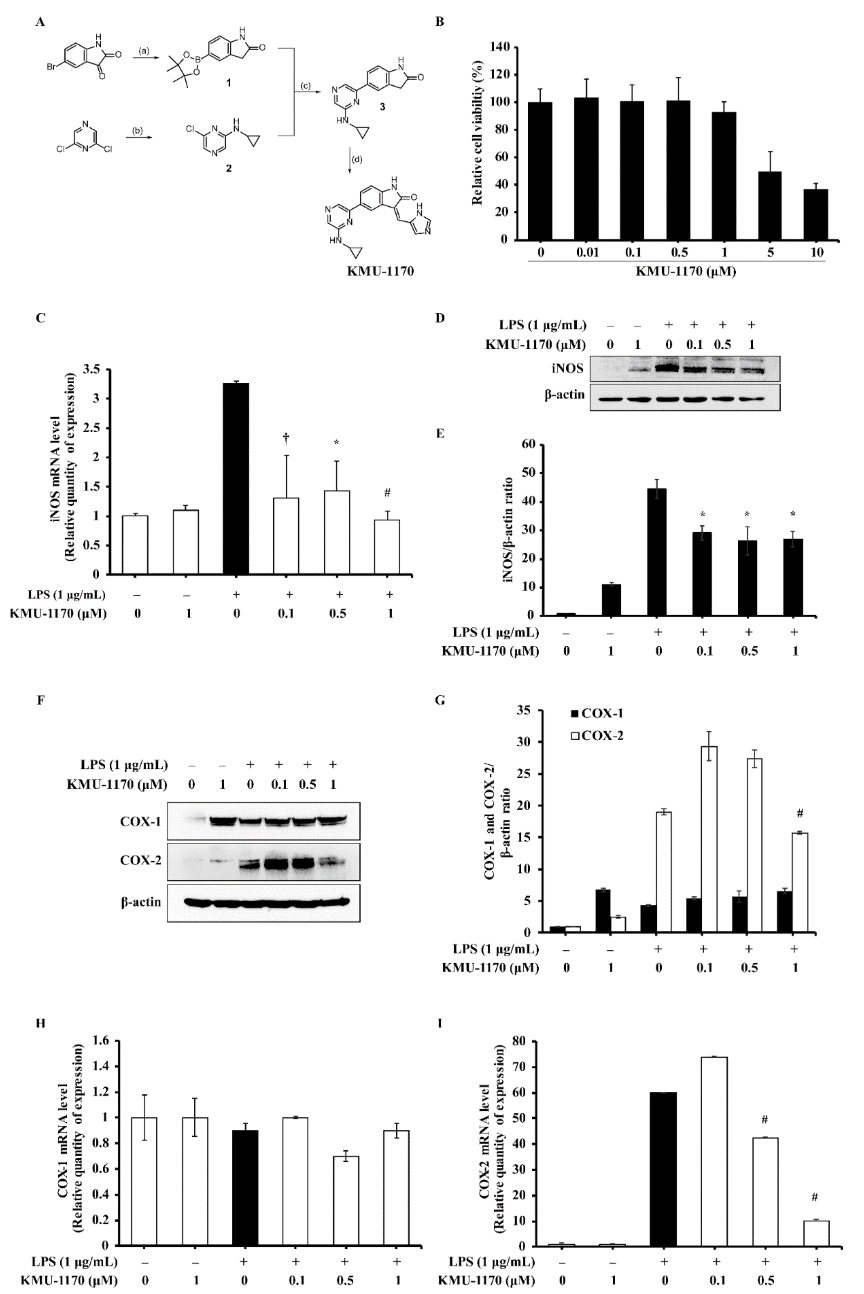
2.2. KMU-1170 Reduces LPS-Stimulated Upregulation of Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX-2) in THP-1 Cells
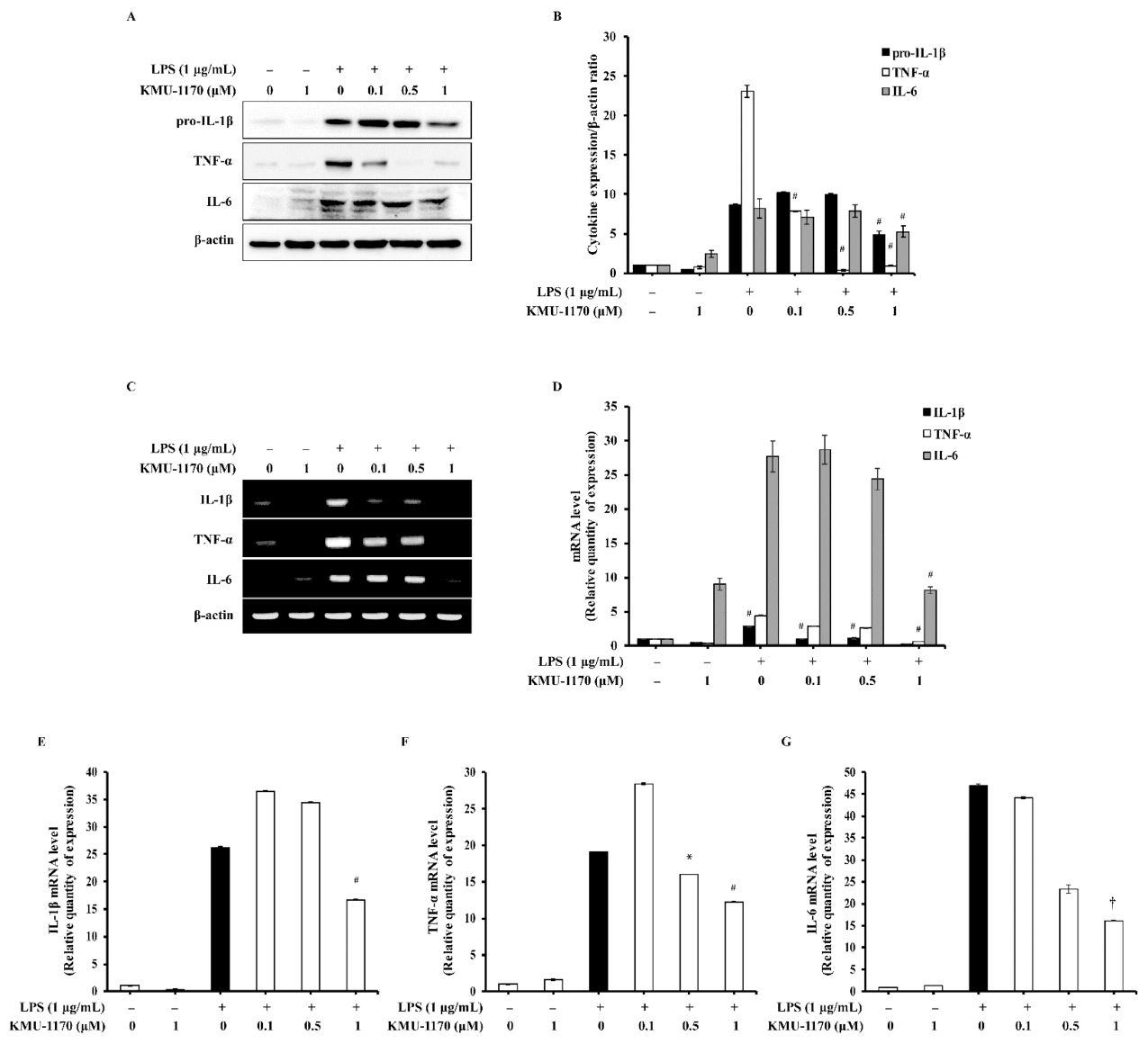
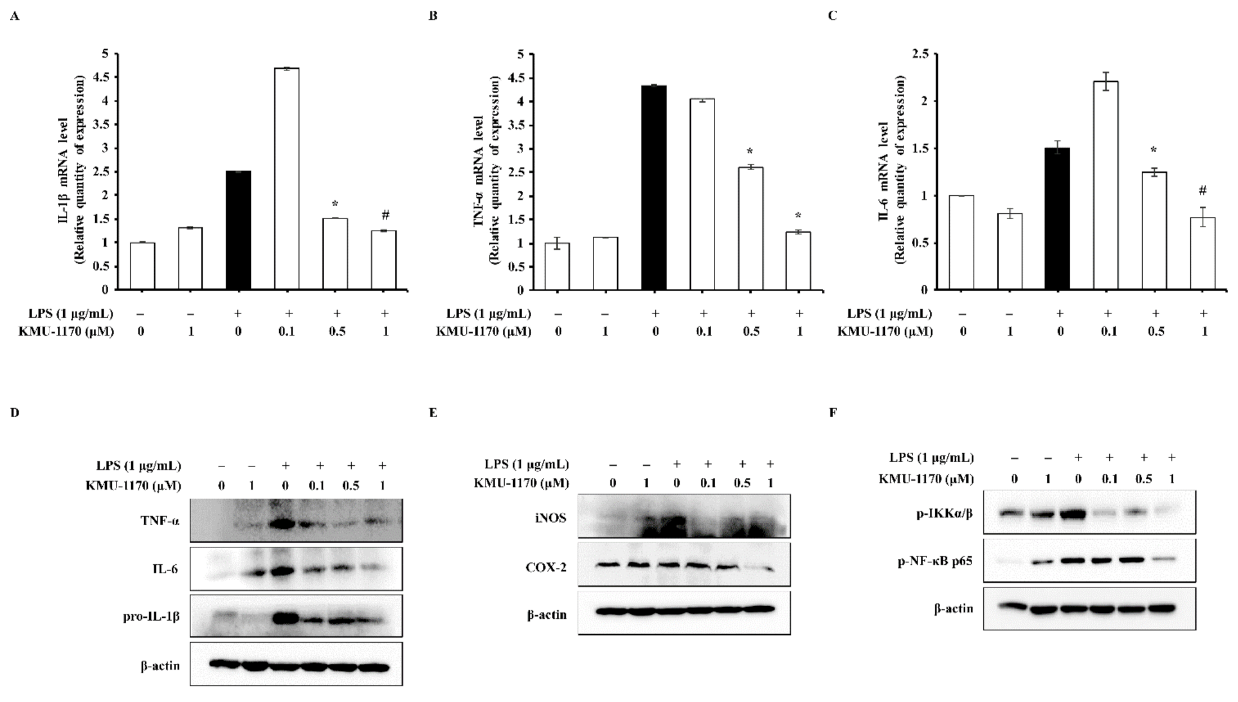
2.3. KMU-1170 Inhibits LPS-Induced Proinflammatory Cytokine Production in THP-1 Cells
2.4. KMU-1170 Suppresses LPS-Induced Phosphorylation of TAK1 and MAPKs in THP-1 Cells
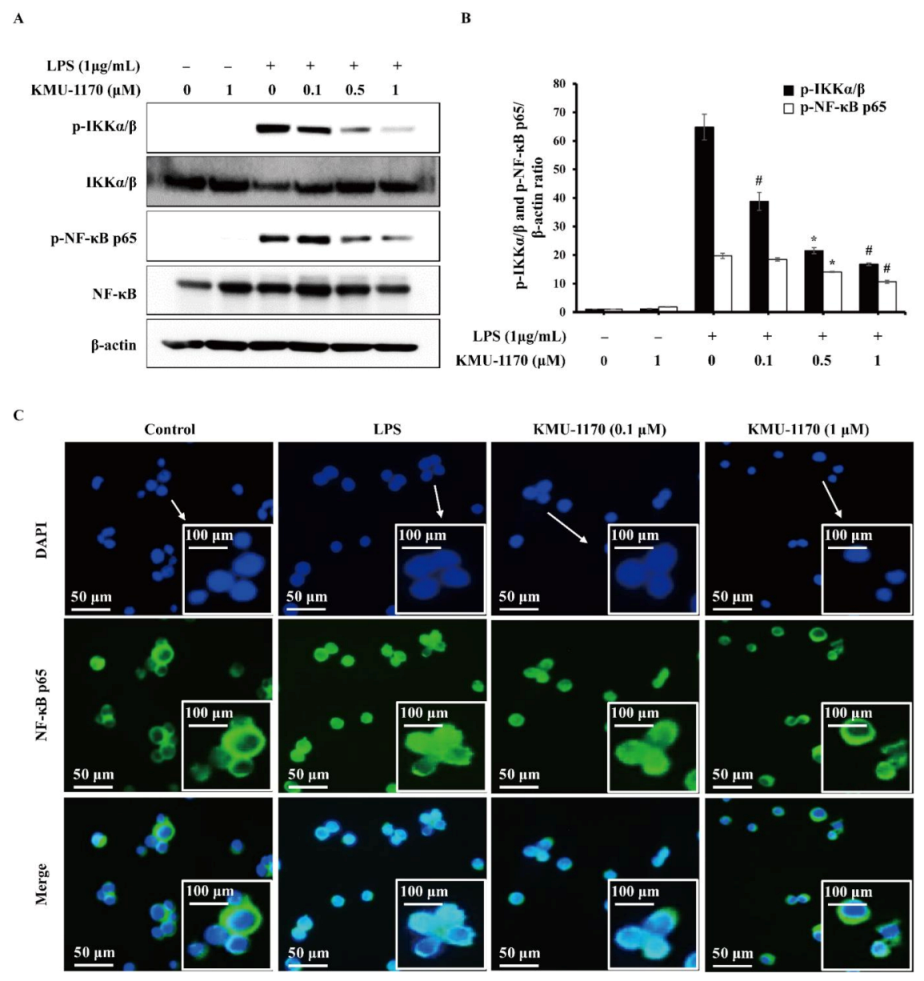
2.5. KMU-1170 Inhibits LPS-Induced Activation of NF-κB in THP-1 Cells
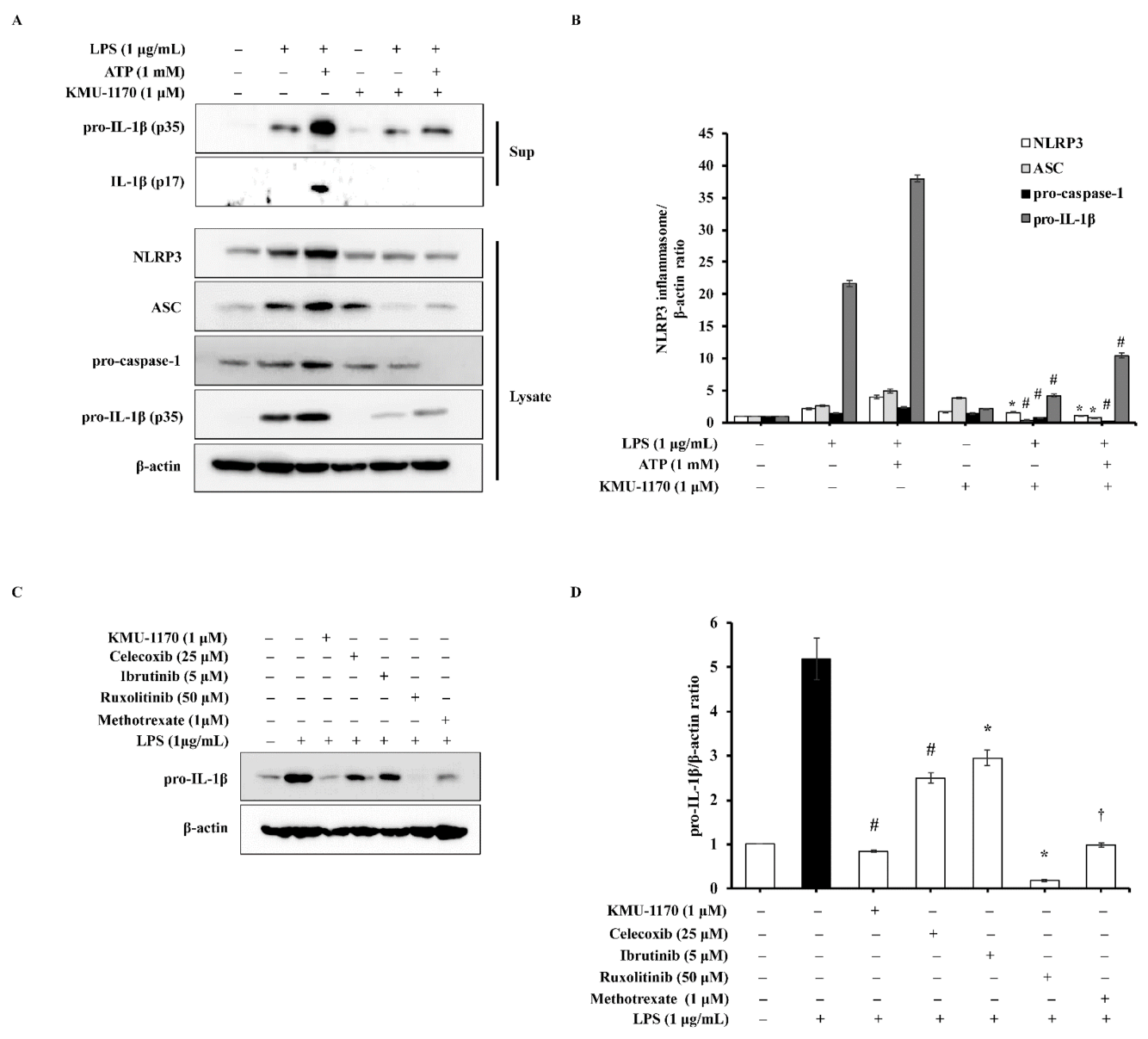
2.6. KMU-1170 Attenuates LPS-Induced Activation of NLRP3 in THP-1 Cells
2.7. KMU-1170 Inhibits LPS-Induced Inflammation in THP-1 Cells
2.8. KMU-1170 Suppresses LPS-Induced Inflammation in Osteoarthritic FLS
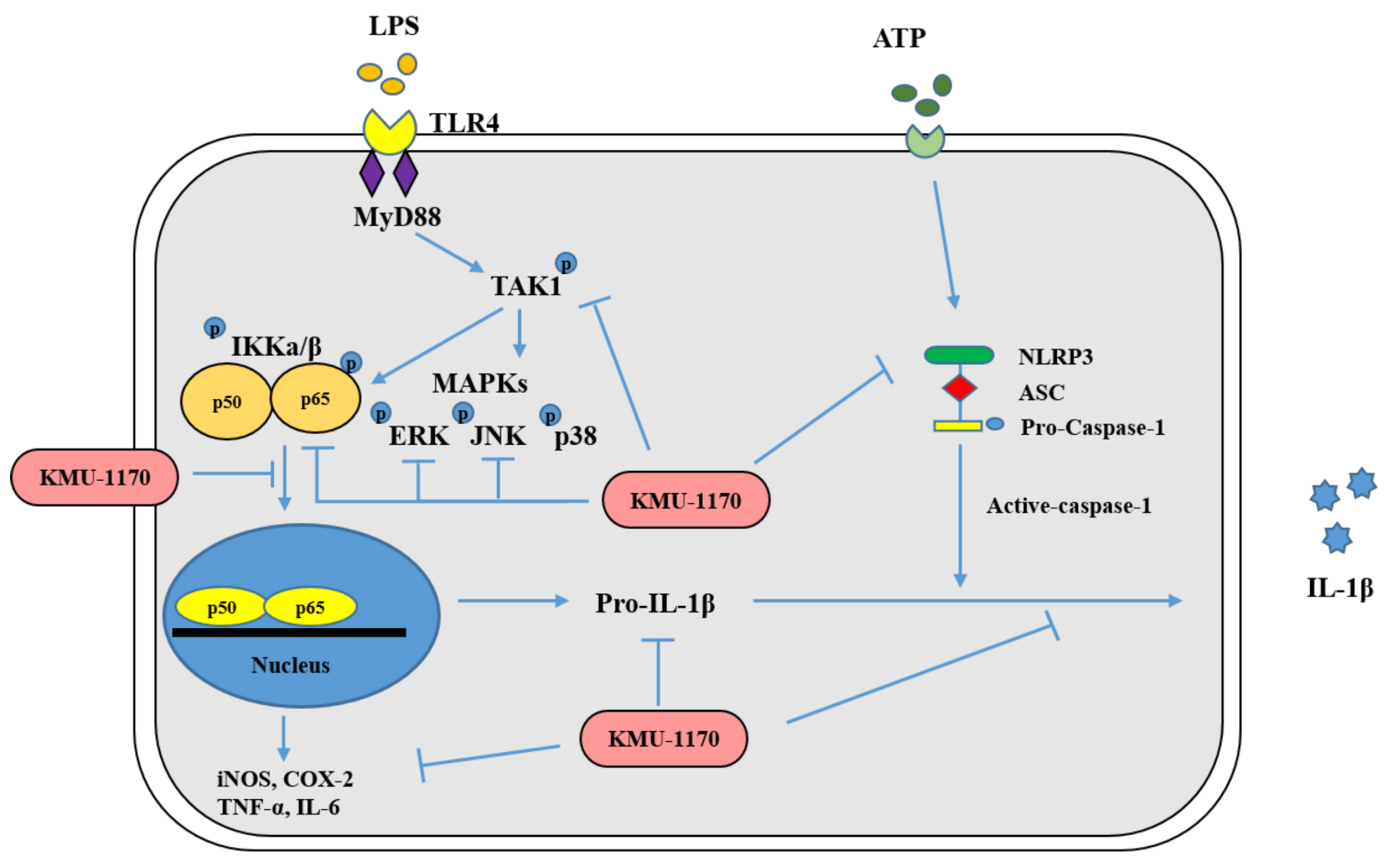
3. Discussion
4. Materials and Methods
4.1. Synthesis of KMU-1170
4.1.1. General Information
4.1.2. 5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)indolin-2-one (1)
4.1.3. 6-Chloro-N-cyclopropylpyrazin-2-amine (2)
4.1.4. 5-(6-(Cyclopropylamino)pyrazin-2-yl)indolin-2-one (3)
4.1.5. (Z)-3-((1H-Imidazol-5-yl)methylene)-5-(6-(cyclopropylamino)pyrazin-2-yl)indolin-2-one (KMU-1170)
4.2. Reagents
4.3. Cell Line and Culture
4.4. Isolation and Culture of Primary Human Osteoarthritic FLS
4.5. Kinase-Profiling Analysis
4.6. Cell Viability Assay
4.7. Western Blotting Analysis
4.8. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real Time Quantitative PCR (qPCR)
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FLS | fibroblast-like synoviocyte |
| LPS | lipopolysaccharide |
| iNOS | inducible nitric oxide synthase |
| COX | cyclooxygenase |
| TLR | toll-like receptor |
| IL | interleukin |
| TNF | tumor necrosis factor |
| NLRP3 | the NOD-, LRR- and pyrin domain-containing protein 3 |
| ASC | the adaptor molecule apoptosis-associated speck-like protein containing a CARD |
| JAK | Janus-associated kinase |
| FDA | the US Food and Drug Administration |
| BTK | Bruton’s tyrosine kinase |
| MAPK | mitogen-activated protein kinase |
| XTT | 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide |
| TAK1 | transforming growth factor-β-activated kinase 1 |
| IKK | inhibitor of NF-κB kinase |
| OA | osteoarthritis |
| HRP | horseradish peroxidase |
| FBS | fetal bovine serum |
| IRB | the Institutional Review Board |
| DMEM | Dulbecco’s modified Eagle medium |
| OD | optical density |
| TBS | Tris-buffered saline |
| RT-PCR | reverse transcription-polymerase chain reaction |
| qPCR | quantitative PCR |
| Ct | the threshold cycle number |
| SPSS | the Statistical Package for Social Science |
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Aga, M.; Watters, J.J.; Pfeiffer, Z.A.; Wiepz, G.J.; Sommer, J.A.; Bertics, P.J. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kappa B signaling pathways in murine RAW 264.7 macrophages. Am. J. Physiol. Cell Physiol. 2004, 286, C923–C930. [Google Scholar] [CrossRef] [PubMed]
- Taams, L.S. Inflammation and immune resolution. Clin. Exp. Immunol. 2018, 193, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Czerkies, M.; Kwiatkowska, K. Toll-like receptors and their contribution to innate immunity: Focus on TLR4 activation by lipopolysaccharide. Med. J. Cell Biol. 2014, 4, 1–23. [Google Scholar] [CrossRef]
- De Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Patterson, H.; Nibbs, R.; McInnes, I.; Siebert, S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 2014, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Kremer, J.; Cush, J.; Schulze-Koops, H.; Connell, C.A.; Bradley, J.D.; Gruben, D.; Wallenstein, G.V.; Zwillich, S.H.; Kanik, K.S.; et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 2012, 367, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Hommes, D.; van den Blink, B.; Plasse, T.; Bartelsman, J.; Xu, C.; Macpherson, B.; Tytgat, G.; Peppelenbosch, M.; Van Deventer, S. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology 2002, 122, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Robak, E. Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Expert Opin. Investig. Drugs 2012, 21, 921–947. [Google Scholar] [CrossRef]
- Lv, J.; Wu, J.; He, F.; Qu, Y.; Zhang, Q.; Yu, C. Development of Bruton’s Tyrosine Kinase Inhibitors for Rheumatoid Arthritis. Curr. Med. Chem. 2018, 25, 5847–5859. [Google Scholar] [CrossRef]
- Force, T.; Kuida, K.; Namchuk, M.; Parang, K.; Kyriakis, J.M. Inhibitors of protein kinase signaling pathways: Emerging therapies for cardiovascular disease. Circulation 2004, 109, 1196–1205. [Google Scholar] [CrossRef]
- Fabbro, D.; Cowan-Jacob, S.W.; Moebitz, H. Ten things you should know about protein kinases: IUPHAR Review 14. Br. J. Pharmacol. 2015, 172, 2675–2700. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. 2-Indolinone a versatile scaffold for treatment of cancer: A patent review (2008–2014). Expert Opin. Ther. Pat. 2016, 26, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Hoffmann, G.; Rieder, J. Inducible nitric oxide synthase—Time for reappraisal. Curr. Drug Targets Inflamm. Allergy 2002, 1, 89–108. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Aashaq, S.; Batool, A.; Andrabi, K.I. TAK1 mediates convergence of cellular signals for death and survival. Apoptosis Int. J. Program. Cell Death 2019, 24, 3–20. [Google Scholar] [CrossRef]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 control of cell death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef]
- Delaney, J.R.; Mlodzik, M. TGF-beta activated kinase-1: New insights into the diverse roles of TAK1 in development and immunity. Cell Cycle 2006, 5, 2852–2855. [Google Scholar] [CrossRef]
- Irie, T.; Muta, T.; Takeshige, K. TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett. 2000, 467, 160–164. [Google Scholar] [CrossRef]
- Brown, J.; Wang, H.; Hajishengallis, G.N.; Martin, M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 2011, 90, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Ouyang, C.; Lin, W.; Zhang, T.; Cao, X.; Xia, Z.; Wang, X. Phosphatase holoenzyme PP1/GADD34 negatively regulates TLR response by inhibiting TAK1 serine 412 phosphorylation. J. Immunol. 2014, 192, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Tsujimura, H.; Tamura, T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 2002, 70, 66–68. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Orlowsky, E.W.; Kraus, V.B. The role of innate immunity in osteoarthritis: When our first line of defense goes on the offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015, 67, 956–965. [Google Scholar] [CrossRef]

| KMU-1170 1 μM | |
|---|---|
| Kinase | Activity in % * |
| MAPK1 (h) | 2 |
| Yes (h) | 23 |
| Blk (h) | 20 |
| Fgr (h) | 29 |
| Lyn (h) | 13 |
| Lck (h) | 7 |
| TYK2 (h) | 6 |
| JAK3 (h) | 3 |
| Fyn (h) | 26 |
| Itk (h) | 45 |
| Syk (h) | 55 |
| JAK2 (h) | 41 |
| JNK1α1 (h) | 67 |
| Hck (h) | 30 |
| Pyk2 (h) | 67 |
| cSRC (h) | 36 |
| Bmx (h) | 26 |
| Txk (h) | −7 |
| Tec (h) activated | 41 |
| SAPK2a (h) | 108 |
| BTK (h) | 40 |
| ZAP-70 (h) | 108 |
| Primers | Sequences (5′ → 3′) | |
|---|---|---|
| iNOS | Forward | CTG TCT GGT TCC TAC GTC ACC |
| Reverse | CCC ACG TTA CAT GGG AGG ATA | |
| COX-1 | Forward | ACC TTG AAG GAG TCA GGC ATG AG |
| Reverse | TGT TCG GTG TCC AGT TCC AAT A | |
| COX-2 | Forward | ATC ACA GGC TTC CAT TGA CC |
| Reverse | TAT CAT CTA GTC CGG AGG GG | |
| IL-1β | Forward | CCT TGG GCC TCA AGG AAA A |
| Reverse | CTC CAG CTG TAG AGT GGG CTT A | |
| TNF-α | Forward | GGA GAA GGG TGA CCG ACT CA |
| Reverse | CTG CCC AGA CTC GGC AA | |
| IL-6 | Forward | ATG GCA CAG TAT CTG GAG GAG |
| Reverse | TAA GCT GGA CTC ACT CTC GGA | |
| β-actin | Forward | AAT CTG GCA CCA CAC CTT CTA |
| Reverse | ATA GCA CAG CCT GGA TAG CAA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, H.S.; Hong, V.S.; Kim, S.H.; Lee, J.; Kim, S. KMU-1170, a Novel Multi-Protein Kinase Inhibitor, Suppresses Inflammatory Signal Transduction in THP-1 Cells and Human Osteoarthritic Fibroblast-Like Synoviocytes by Suppressing Activation of NF-κB and NLRP3 Inflammasome Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 1194. https://doi.org/10.3390/ijms22031194
Baek HS, Hong VS, Kim SH, Lee J, Kim S. KMU-1170, a Novel Multi-Protein Kinase Inhibitor, Suppresses Inflammatory Signal Transduction in THP-1 Cells and Human Osteoarthritic Fibroblast-Like Synoviocytes by Suppressing Activation of NF-κB and NLRP3 Inflammasome Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(3):1194. https://doi.org/10.3390/ijms22031194
Chicago/Turabian StyleBaek, Hye Suk, Victor Sukbong Hong, Sang Hyon Kim, Jinho Lee, and Shin Kim. 2021. "KMU-1170, a Novel Multi-Protein Kinase Inhibitor, Suppresses Inflammatory Signal Transduction in THP-1 Cells and Human Osteoarthritic Fibroblast-Like Synoviocytes by Suppressing Activation of NF-κB and NLRP3 Inflammasome Signaling Pathway" International Journal of Molecular Sciences 22, no. 3: 1194. https://doi.org/10.3390/ijms22031194
APA StyleBaek, H. S., Hong, V. S., Kim, S. H., Lee, J., & Kim, S. (2021). KMU-1170, a Novel Multi-Protein Kinase Inhibitor, Suppresses Inflammatory Signal Transduction in THP-1 Cells and Human Osteoarthritic Fibroblast-Like Synoviocytes by Suppressing Activation of NF-κB and NLRP3 Inflammasome Signaling Pathway. International Journal of Molecular Sciences, 22(3), 1194. https://doi.org/10.3390/ijms22031194





