Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals
Abstract
1. Biology of Spermatogenesis and Main Difficulties for Its Molecular Study
2. Most Common Approaches to the Study of Spermatogenesis
3. The Spermatogenic Process from a Flow Cytometric Perspective
4. FCM as an Analytical Tool of Spermatogenesis
4.1. Testis Postnatal Development
4.2. Rapid Spermatogenic Profiling for Infertility Research and Clinical Aims
5. FCM as a Preparative Tool in Spermatogenic Studies
5.1. Purification of Spermatogenic Cell Populations for Transcriptomic Studies
5.2. FACS Contributions in Single Cell-Based Approaches
5.3. FACS Contributions to Chromatin Conformation Studies
5.4. Purification of Spermatogenic Cells for In Vitro Culture Developments
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, R.A.; de Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, D.G.; Griswold, D.K.M. Questions about spermatogonia posed and answered since 2000. J. Androl. 2012, 33, 1085–1995. [Google Scholar] [CrossRef] [PubMed]
- Fraune, J.; Schramm, S.; Alsheimer, M.; Benavente, R. The mammalian synaptonemal complex: Protein components, assembly and role in meiotic recombination. Exp. Cell Res. 2012, 318, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Bolcun-Filas, E.; Ann Handel, M. Meiosis: The chromosomal foundation of reproduction. Biol. Reprod. 2018, 99, 112–126. [Google Scholar] [CrossRef]
- Cobb, J.; Handel, M.A. Dynamics of meiotic prophase I during spermatogenesis: From pairing to division. Semin. Cell Dev. Biol. 1998, 9, 445–450. [Google Scholar] [CrossRef]
- Handel, M.A.; Schimenti, J.C. Genetics of mammalian meiosis: Regulation, dynamics and impact on fertility. Nat. Rev. Genet. 2010, 11, 124–136. [Google Scholar] [CrossRef]
- Hann, M.C.; Lau, P.E.; Tempest, H.G. Meiotic recombination and male infertility: From basic science to clinical reality? Asian J. Androl. 2011, 13, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, A.; Benavente, R. Mutations in genes coding for synaptonemal complex proteins and their impact on human fertility. Cytogenet. Genome Res. 2016, 150, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, A.; Mackay, D.J.G.; Cheong, Y.; Verpoest, W. Genetic diagnosis of subfertility: The impact of meiosis and maternal effects. J. Med. Genet. 2019, 56, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 1972, 52, 198–236. [Google Scholar] [CrossRef] [PubMed]
- Gold, H.B.; Jung, Y.H.; Corces, V.G. Not just heads and tails: The complexity of the sperm epigenome. J. Biol. Chem. 2018, 293, 13815–13820. [Google Scholar] [CrossRef]
- Hao, S.L.; Ni, F.D.; Yang, W.X. The dynamics and regulation of chromatin remodeling during spermiogenesis. Gene 2019, 706, 201–210. [Google Scholar] [CrossRef]
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef]
- Skinner, M.; Griswold, M. Sertoli Cell Biology, 1st ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; p. 494. [Google Scholar]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015, 92, 37. [Google Scholar] [CrossRef]
- Handel, M.A.; Eppig, J.J.; Schimenti, J.C. Applying “Gold Standards” to in vitro-derived germ cells. Cell 2014, 157, 1257–1261. [Google Scholar] [CrossRef]
- Komeya, M.; Sato, T.; Ogawa, T. In vitro spermatogenesis: A century-long research journey, still half way around. Reprod. Med. Biol. 2018, 17, 407–420. [Google Scholar] [CrossRef]
- Bellvé, A.R.; Cavicchia, J.C.; Milletfe, C.F.; O’Brien, D.A.; Bhatnagar, Y.M.; Dym, M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977, 74, 68–85. [Google Scholar] [CrossRef]
- Malkov, M.; Fisher, Y.; Don, J. Developmental schedule of the postnatal rat testis determined by flow cytometry. Biol. Reprod. 1998, 59, 84–92. [Google Scholar] [CrossRef]
- Rodríguez-Casuriaga, R.; Geisinger, A.; Santiñaque, F.F.; López-Carro, B.; Folle, G.A. High-purity flow sorting of early meiocytes based on DNA analysis of guinea pig spermatogenic cells. Cytometry A 2011, 79, 625–634. [Google Scholar] [CrossRef]
- Thomas, K.H.; Wilkie, T.M.; Tomashefsky, P.; Bellvé, A.R.; Simon, M.I. Differential gene expression during spermatogenesis. Biol. Reprod. 1989, 41, 729–739. [Google Scholar] [CrossRef]
- Almstrup, K.; Nielsen, J.E.; Hansen, M.A.; Tanaka, M.; Skakkebaek, N.E.; Leffers, H. Analysis of cell-type-specific gene expression during mouse spermatogenesis. Biol. Reprod. 2004, 70, 1751–1761. [Google Scholar] [CrossRef]
- Maratou, K.; Forster, T.; Costa, Y.; Taggart, M.; Speed, R.M.; Ireland, J.; Teague, P.; Roy, D.; Cooke, H.J. Expression profiling of the developing testis in wild-type and Dazl knockout mice. Mol. Reprod. Dev. 2004, 67, 26–54. [Google Scholar] [CrossRef]
- Shima, J.E.; McLean, D.J.; McCarrey, J.R.; Griswold, M.D. The murine testicular transcriptome: Characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 2004, 71, 319–330. [Google Scholar] [CrossRef]
- Iguchi, N.; Tobias, J.W.; Hecht, N.B. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc. Natl. Acad. Sci. USA 2006, 103, 7712–7717. [Google Scholar] [CrossRef]
- Gong, W.; Pan, L.; Lin, Q.; Zou, Y.; Xin, C.; Yu, X.; Cui, P.; Hu, S.; Yu, J. Transcriptome profiling of the developing postnatal mouse testis using next-generation sequencing. Sci. China Life Sci. 2013, 56, 1–12. [Google Scholar] [CrossRef][Green Version]
- Laiho, A.; Kotaja, N.; Gyenesei, A.; Sironen, A. Transcriptome profiling of the murine testis during the first wave of spermatogenesis. PLoS ONE 2013, 8, e61558. [Google Scholar] [CrossRef]
- Meistrich, M.L. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977, 15, 15–54. [Google Scholar] [CrossRef]
- Lam, D.M.K.; Furrer, R.; Bruce, W.R. The separation, physical characterization, and differentiation kinetics of spermatogonial cells of the mouse. Proc. Natl. Acad. Sci. USA 1970, 65, 192–199. [Google Scholar] [CrossRef]
- Go, V.L.W.; Vernon, R.G.; Fritz, I.B. Studies on spermatogenesis in rats. I. Application of the sedimentation velocity technique to an investigation of spermatogenesis. Can. J. Biochem. 1971, 49, 753–760. [Google Scholar] [CrossRef]
- Romrell, L.J.; Bellvé, A.R.; Fawcet, D.W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev. Biol. 1976, 19, 119–131. [Google Scholar] [CrossRef]
- Geisinger, A.; Rodríguez-Casuriaga, R. Flow cytometry for gene expression studies in mammalian spermatogenesis. Cytogenet. Genome Res. 2010, 128, 46–56. [Google Scholar] [CrossRef]
- Bellvé, A.R. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993, 225, 84–113. [Google Scholar] [CrossRef]
- Wong, V.; Russell, L.D. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am. J. Anat. 1983, 167, 143–161. [Google Scholar] [CrossRef]
- Spano, M.; Evenson, D.P. Flow cytometric analysis for reproductive biology. Biol. Cell 1993, 78, 53–62. [Google Scholar] [CrossRef]
- Fallahi, M.; Getun, I.V.; Wu, Z.K.; Bois, P.R.J. A global expression switch marks pachytene initiation during mouse male meiosis. Genes 2010, 1, 469–483. [Google Scholar] [CrossRef]
- Getun, I.V.; Wu, Z.K.; Khalil, A.M.; Bois, P.R.J. Nucleosome occupancy landscape and dynamics at mouse recombination hotspots. EMBO Rep. 2010, 11, 555–560. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Yang, S.; Tian, R.; Wang, J.; Yuan, Q.; Dong, H.; He, Z.; Wang, S.; Li, Z. Dynamics of the transcriptome during human spermatogenesis: Predicting the potential key genes regulating male gametes generation. Sci. Rep. 2016, 6, 19069. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef]
- Gaysinskaya, V.; Miller, B.F.; De Luca, C.; van der Heijden, G.W.; Hansen, K.D.; Bortvin, A. Transient reduction of DNA methylation at the onset of meiosis in male mice. Epigenetics Chromatin 2018, 11, 15. [Google Scholar] [CrossRef]
- Ernst, C.; Eling, N.; Martinez-Jimenez, C.P.; Marioni, J.C.; Odom, D.T. Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat. Commun. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Jung, M.; Wells, D.; Rusch, J.; Ahmed, S.; Marchini, J.; Myers, S.; Conrad, D.F. Unified single-cell analysis of testis gene regulation and pathology in 5 mouse strains. eLife 2019, 8, e43966. [Google Scholar] [CrossRef]
- Vara, C.; Paytuvi-Gallart, A.; Cuartero, Y.; Le Dily, F.; Garcia, F.; Salva-Castro, J.; Gómez, H.L.; Julià, E.; Moutinho, C.; Aiese Cigliano, R.; et al. Three-dimensional genomic structure and cohesin occupancy correlate with transcriptional activity during spermatogenesis. Cell Rep. 2019, 28, 352–367. [Google Scholar] [CrossRef]
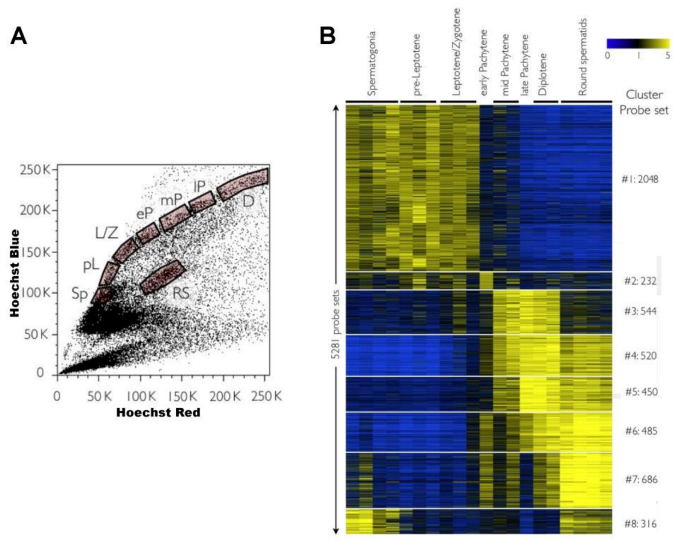
- Bastos, H.; Lassalle, B.; Chicheportiche, A.; Riou, L.; Testart, J.; Allemand, I.; Fouchet, P. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A 2005, 65A, 40–49. [Google Scholar] [CrossRef]
- Gaysinskaya, V.; Soh, I.Y.; van der Heijden, G.W.; Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytometry A 2014, 85, 556–565. [Google Scholar] [CrossRef]
- Lassalle, B.; Bastos, H.; Louis, J.P.; Riou, L.; Testart, J.; Dutrillaux, B.; Fouchet, B.; Allemand, I. ‘Side population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development 2004, 131, 479–487. [Google Scholar] [CrossRef]
- Falciatori, I.; Borsellino, G.; Haliassos, N.; Boitani, C.; Corallini, S.; Battistini, L.; Bernardi, G.; Stefanini, M.; Vicini, E. Identification and enrichment of spermatogonial stem cells displaying side-population phenotype in immature mouse testis. FASEB J. 2004, 18, 376–378. [Google Scholar] [CrossRef]
- Rodríguez-Casuriaga, R.; Santiñaque, F.F.; Folle, G.A.; Souza, E.; López-Carro, B.; Geisinger, A. Rapid preparation of rodent testicular cell suspensions and spermatogenic stages purification by flow cytometry using a novel blue laser-excitable vital dye. Methods X 2014, 1, 239–243. [Google Scholar] [CrossRef]
- Geisinger, A.; Rodríguez-Casuriaga, R. Flow cytometry for the isolation and characterization of rodent meiocytes. Methods Mol. Biol. 2017, 1471, 217–230. [Google Scholar] [CrossRef]
- Getun, I.V.; Torres, B.; Bois, P.R. Flow cytometry purification of mouse meiotic cells. J. Vis. Exp. 2011, 50, 2602. [Google Scholar] [CrossRef]
- Meistrich, M.L.; Lake, S.; Steinmetz, L.L.; Gledhill, B.L. Flow cytometry of DNA in mouse sperm and testis nuclei. Mutat. Res. 1978, 49, 383–396. [Google Scholar] [CrossRef]
- Janca, F.C.; Jost, L.K.; Evenson, D.P. Mouse testicular and sperm cell development characterized from birth to adulthood by dual parameter flow cytometry. Biol. Reprod. 1986, 34, 613–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petit, J.M.; Ratinaud, M.H.; Cordelli, E.; Spano, M.; Julien, R. Mouse testis cell sorting according to DNA and mitochondrial changes during spermatogenesis. Cytometry 1995, 19, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Suter, L.; Koch, E.; Bechter, R.; Bobadilla, M. Three-parameter flow cytometric analysis of rat spermatogenesis. Cytometry 1997, 27, 161–168. [Google Scholar] [CrossRef]
- Vigodner, M.; Lewin, L.M.; Shochat, L.; Mittelman, L.; Golan, R. Meiosis in the golden hamster: A confocal microscopy and flow cytometric analysis. Molec. Reprod. Dev. 2003, 64, 86–95. [Google Scholar] [CrossRef]
- Vigodner, M.; Lewy, H.; Lewin, L.M.; Shochat, L.; Golan, R. Evidence for biological rhythm in spermatogenesis in the pubertal hamster (Mesocricetus auratus): A flow cytometric study. Life Sci. 2004, 74, 1119–1126. [Google Scholar] [CrossRef]
- Neubauer, K.; Jewgenow, K.; Blottner, S.; Wildt, D.E.; Pukazhenthi, B.S. Quantity rather than quality in teratospermic males: A histomorphometric and flow cytometric evaluation of spermatogenesis in the domestic cat (Felis catus). Biol. Reprod. 2004, 71, 1517–1524. [Google Scholar] [CrossRef]
- Oskam, I.C.; Ropstad, E.; Andersen Berg, K.; Fredriksen, B.; Larsen, S.; Dahl, E.; Andresen, O. Testicular germ cell development in relation to 5alpha-androstenone levels in pubertal entire male pigs. Theriogenology 2008, 69, 967–976. [Google Scholar] [CrossRef]
- Aslam, H.; Schneiders, A.; Perret, M.; Weinbauer, G.F.; Hodges, J.K. Quantitative assessment of testicular germ cell production and kinematic and morphometric parameters of ejaculated spermatozoa in the grey lemur, Microebus murinus. Reproduction 2002, 123, 323–332. [Google Scholar] [CrossRef]
- Wistuba, J.; Schrod, A.; Greve, B.; Hodges, J.K.; Aslam, H.; Weinbauer, G.F.; Luetjens, M. Organization of seminiferous epithelium in primates: Relationship to spermatogenic efficiency, phylogeny, and mating system. Biol. Reprod. 2003, 69, 582–591. [Google Scholar] [CrossRef]
- Blanchard, Y.; Lavault, M.T.; Quernee, D.; Le Lannou, D.; Lobel, B.; Lescoat, D. Preparation of spermatogenic cell populations at specific stages of differentiation in the human. Mol. Reprod. Dev. 1991, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hittmair, A.; Rogatsch, H.; Offner, F.; Feichtinger, H.; Ofner, D.; Mikuz, G. Deoxyribonucleic acid flow cytometry and semiquantitative histology of spermatogenesis: A comparative study. Fertil. Steril. 1992, 58, 1040–1045. [Google Scholar] [CrossRef]
- Baksi, A.; Vasan, S.S.; Dighe, R.R. DNA Flow cytometric analysis of the human testicular tissues to investigate the status of spermatogenesis in azoospermic patients. Sci. Rep. 2018, 8, 11117. [Google Scholar] [CrossRef] [PubMed]
- Farah, O.I.; Cuiling, L.; Jiaojiao, W.; Huiping, Z. Use of fluorescent dyes for readily recognizing sperm damage. J. Reprod. Infertil. 2013, 14, 120–125. [Google Scholar] [PubMed]
- Evenson, D.; Jost, L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef]
- Domínguez-Fandos, D.; Camejo, M.I.; Ballescà, J.L.; Oliva, R. Human sperm DNA fragmentation: Correlation of TUNEL results as assessed by flow cytometry and optical microscopy. Cytometry A 2007, 71, 1011–1018. [Google Scholar] [CrossRef]
- Mitchell, L.A.; De Iuliis, G.N.; Aitken, R.J. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: Development of an improved methodology. Int. J. Androl. 2011, 34, 2–13. [Google Scholar] [CrossRef]
- Sharma, R.; Masaki, J.; Agarwal, A. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol. Biol. 2013, 927, 121–136. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A. A systematic review on sperm DNA fragmentation in male factor infertility: Laboratory assessment. Arab. J. Urol. 2018, 16, 65–76. [Google Scholar] [CrossRef]
- Skakkebaek, N.E. Abnormal morphology of germ cells in two infertile men. Acta Pathol. Microbiol. Scand. A 1972, 80, 374–378. [Google Scholar] [CrossRef]
- Cheng, L.; Lyu, B.; Roth, L.M. Perspectives on testicular germ cell neoplasms. Hum. Pathol. 2017, 59, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Karimi, N.; Wu, X.N.; Chen, S.R.; Liu, Y.X. Testicular germ cell tumor: A comprehensive review. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Stoop, H.; Sen, S.; Bokemeyer, C.; Oosterhuis, J.W.; Looijenga, L.H. Aneuploidy of human testicular germ cell tumors is associated with amplification of centrosomes. Oncogene 2003, 22, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Summersgill, B.; Yost, S.; Sultana, R.; Labreche, K.; Dudakia, D.; Renwick, A.; Seal, S.; Al-Saadi, R.; Broderick, P.; et al. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat. Commun. 2015, 6, 5973. [Google Scholar] [CrossRef]
- Boublikova, L.; Buchler, T.; Stary, J.; Abrahamova, J.; Trka, J. Molecular biology of testicular germ cell tumors: Unique features awaiting clinical application. Crit. Rev. Oncol. Hematol. 2014, 89, 366–385. [Google Scholar] [CrossRef]
- Litchfield, K.; Levy, M.; Huddart, R.A.; Shipley, J.; Turnbull, C. The genomic landscape of testicular germ cell tumours: From susceptibility to treatment. Nat. Rev. Urol. 2016, 13, 409–419. [Google Scholar] [CrossRef]
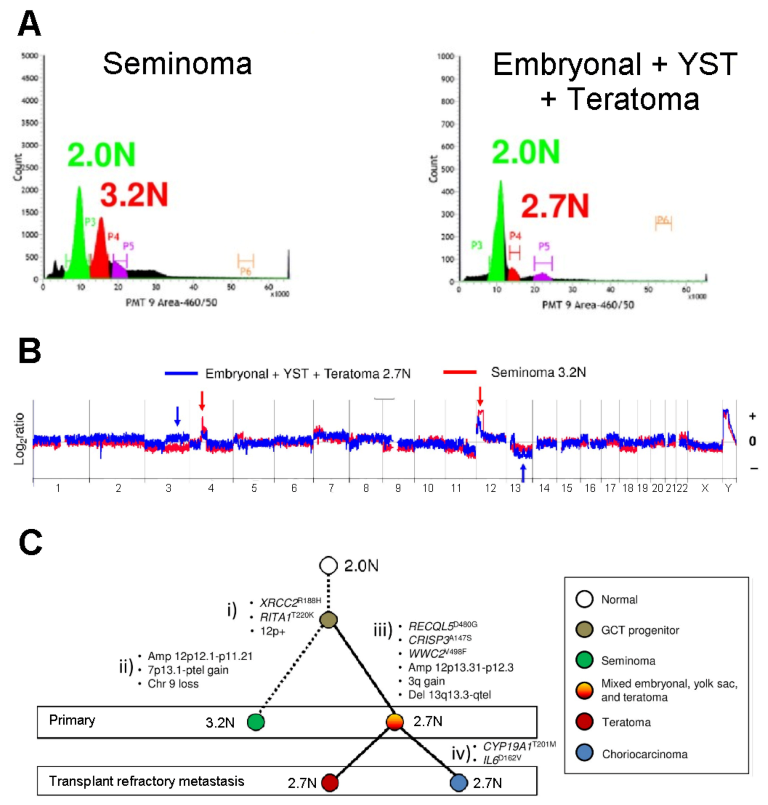
- Barrett, M.T.; Lenkiewicz, E.; Malasi, S.; Stanton, M.; Slack, J.; Andrews, P.; Pagliaro, L.; Bryce, A.H. Clonal analyses of refractory testicular germ cell tumors. PLoS ONE 2019, 14, e0213815. [Google Scholar] [CrossRef]
- Gaysinskaya, V.; Bortvin, A. Flow cytometry of murine spermatocytes. Curr. Protoc. Cytom. 2015, 72, 7.44.1-24. [Google Scholar] [CrossRef]
- Soper, S.F.; van der Heijden, G.W.; Hardiman, T.C.; Goodheart, M.; Martin, S.L.; de Boer, P.; Bortvin, A. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell 2008, 15, 285–297. [Google Scholar] [CrossRef]
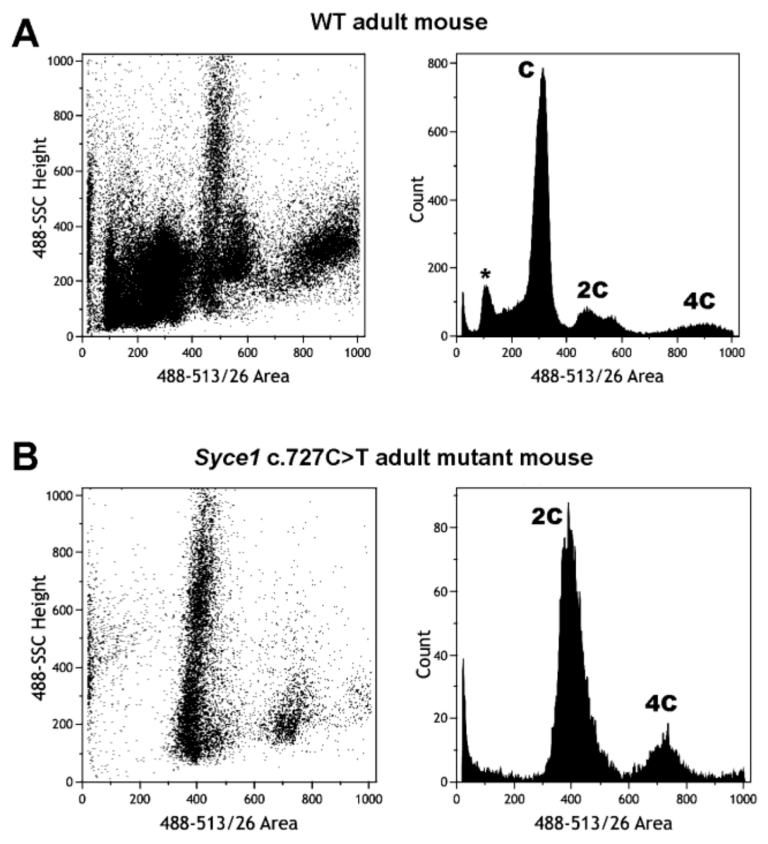
- Hernández-López, D.; Geisinger, A.; Trovero, M.F.; Santiñaque, F.F.; Brauer, M.; Folle, G.A.; Benavente, R.; Rodríguez-Casuriaga, R. Familial primary ovarian failure associated with a SYCE1 point mutation: Defective meiosis elucidated in humanized mice. Molec. Hum. Reprod. 2020, 26, 485–497. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H.; Xie, M.; Liu, M.; Guo, X.; Zheng, Y.; et al. Complete meiosis from embryonic stem Cell-Derived germ cells in vitro. Cell Stem Cell. 2016, 18, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Hamra, F.K.; Garbers, D.L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl. Acad. Sci. USA 2003, 100, 12201–12206. [Google Scholar] [CrossRef] [PubMed]
- Waldman Ben-Asher, H.; Shahar, I.; Yitzchak, A.; Mehr, R.; Don, J. Expression and chromosomal organization of mouse meiotic genes. Mol. Reprod. Dev. 2010, 77, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Ran, M.; Chen, B.; He, C.; Dong, L.; Peng, F. Genome-wide analysis of long non-coding RNAs and their role in postnatal porcine testis development. Genomics 2017, 109, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Dolci, S.; Sette, C.; Capolunghi, F.; Pellegrini, M.; Loiarro, M.; Di Agostino, S.; Paronetto, M.P.; Grimaldi, P.; Merico, D.; et al. Analysis of the gene expression profile of mouse male meiotic germ cells. Gene Expr. Patterns 2004, 4, 267–281. [Google Scholar] [CrossRef][Green Version]
- Pang, A.L.; Johnson, W.; Ravindranath, N.; Dym, M.; Rennert, O.M.; Chan, W.Y. Expression profiling of purified male germ cells: Stage-specific expression patterns related to meiosis and postmeiotic development. Physiol. Genom. 2006, 24, 75–85. [Google Scholar] [CrossRef]
- Chalmel, F.; Rolland, A.D.; Niederhauser-Wiederkehr, C.; Chung, S.S.; Demougin, P.; Gattiker, A.; Moore, J.; Patard, J.J.; Wolgemuth, D.J.; Jégou, B.; et al. The conserved transcriptome in human and rodent male gametogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 8346–8351. [Google Scholar] [CrossRef]
- Liang, M.; Li, W.; Tian, H.; Hu, T.; Wang, L.; Lin, Y.; Li, Y.; Huang, H.; Sun, F. Sequential expression of long noncoding RNA as mRNA gene expression in specific stages of mouse spermatogenesis. Sci. Rep. 2014, 4, 5966. [Google Scholar] [CrossRef]
- Soumillon, M.; Necsulea, A.; Weier, M.; Brawand, D.; Zhang, X.; Gu, H.; Barthès, P.; Kokkinaki, M.; Nef, S.; Gnirke, A.; et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 2013, 3, 2179–2190. [Google Scholar] [CrossRef]
- Chalmel, F.; Lardenois, A.; Evrard, B.; Rolland, A.D.; Sallou, O.; Dumargne, M.C.; Coiffec, I.; Collin, O.; Primig, M.; Jégou, B. High-resolution profiling of novel transcribed regions during rat spermatogenesis. Biol. Reprod. 2014, 91, 5. [Google Scholar] [CrossRef]
- Lin, X.; Han, M.; Cheng, L.; Chen, J.; Zhang, Z.; Shen, T.; Wang, M.; Wen, B.; Ni, T.; Han, C. Expression dynamics, relationships, and transcriptional regulations of diverse transcripts in mouse spermatogenic cells. RNA Biol. 2016, 13, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Wichman, L.; Somasundaram, S.; Breindel, C.; Valerio, D.M.; McCarrey, J.R.; Hodges, C.A.; Khalil, A.M. Dynamic expression of long noncoding RNAs reveals their potential roles in spermatogenesis and fertility. Biol. Reprod. 2017, 97, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, I.; Rodríguez-Casuriaga, R.; Santiñaque, F.F.; Farías, J.; Curti, G.; Capoano, C.A.; Folle, G.A.; Benavente, R.; Sotelo-Silveira, J.R.; Geisinger, A. Transcriptome analysis of highly purified mouse spermatogenic cell populations: Gene expression signatures switch from meiotic-to-postmeiotic-related processes at pachytene stage. BMC Genom. 2016, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Trovero, M.F.; Rodríguez-Casuriaga, R.; Romeo, C.; Santiñaque, F.F.; François, M.; Folle, G.A.; Benavente, R.; Sotelo-Silveira, J.R.; Geisinger, A. Revealing stage-specific expression patterns of long noncoding RNAs along mouse spermatogenesis. RNA Biol. 2020, 17, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Geisinger, A.; Rodríguez-Casuriaga, R.; Benavente, R. Transcriptomics of meiosis in the male mouse. Front. Cell Dev. Biol. under review.
- Zimmermann, C.; Stévant, I.; Borel, C.; Conne, B.; Pitetti, J.L.; Calvel, P.; Kaessmann, H.; Jégou, B.; Chalmel, F.; Nef, S. Research resource: The dynamic transcriptional profile of Sertoli cells during the progression of spermatogenesis. Mol. Endocrinol. 2015, 29, 627–642. [Google Scholar] [CrossRef]
- Gonen, N.; Lovell-Badge, R. The regulation of Sox9 expression in the gonad. Curr. Top. Dev. Biol. 2019, 134, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Zagore, L.L.; Sweet, T.J.; Hannigan, M.M.; Weyn-Vanhentenryck, S.M.; Jobava, R.; Hatzoglou, M.; Zhang, C.; Licatalosi, D.D. DAZL regulates germ cell survival through a network of PolyA-proximal mRNA interactions. Cell Rep. 2018, 25, 1225–1240. [Google Scholar] [CrossRef]
- Harichandan, A.; Sivasubramaniyan, K.; Hennenlotter, J.; Poths, S.; Bedke, J.; Kruck, S.; Stenzl, A.; Bühring, H.J. Molecular signatures of primary human spermatogonial progenitors and its neighboring peritubular stromal compartment. Stem Cells Dev. 2017, 26, 263–273. [Google Scholar] [CrossRef]
- Huang, X.Y.; Sha, J.H. Proteomics of spermatogenesis: From protein lists to understanding the regulation of male fertility and infertility. Asian J. Androl. 2011, 13, 18–23. [Google Scholar] [CrossRef]
- Suzuki, S.; Diaz, V.D.; Hermann, B.P. What has single-cell RNA-seq taught us about mammalian spermatogenesis? Biol. Reprod. 2019, 101, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Soraggi, S.; Riera, M.; Rajpert-De Meyts, E.; Schierup, M.H.; Almstrup, K. Evaluating genetic causes of azoospermia: What can we learn from a complex cellular structure and single-cell transcriptomics of the human testis? Hum. Genet. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.C.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep. 2018, 25, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 2018, 23, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-cell RNA sequencing of human, macaque, and mouse testes uncovers conserved and divergent features of mammalian spermatogenesis. Dev. Cell 2020, 54, 529–547. [Google Scholar] [CrossRef]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-Seq. Dev. Cell 2018, 46, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Lukassen, S.; Bosch, E.; Ekici, A.B.; Winterpacht, A. Characterization of germ cell differentiation in the male mouse through single-cell RNA sequencing. Sci. Rep. 2018, 8, 6521. [Google Scholar] [CrossRef]
- Grive, K.J.; Hu, Y.; Shu, E.; Grimson, A.; Elemento, O.; Grenier, J.K.; Cohen, P.E. Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLoS Genet. 2019, 15, e1007810. [Google Scholar] [CrossRef]
- La, H.M.; Mäkelä, J.A.; Chan, A.L.; Rossello, F.J.; Nefzger, C.M.; Legrand, J.M.D.; De Seram, M.; Polo, J.M.; Hobbs, R.M. Identification of dynamic undifferentiated cell states within the male germline. Nat. Commun. 2018, 9, 2819. [Google Scholar] [CrossRef]
- Song, H.W.; Bettegowda, A.; Lake, B.B.; Zhao, A.H.; Skarbrevik, D.; Babajanian, E.; Sukhwani, M.; Shum, E.Y.; Phan, M.H.; Plank, T.M.; et al. The homeobox transcription factor RHOX10 drives mouse spermatogonial stem cell establishment. Cell Rep. 2016, 17, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Ng, S.H.; Luk, A.C.; Suen, H.C.; Qian, Y.; Lee, A.W.T.; Tu, J.; Fung, J.C.L.; Tang, N.L.S.; Feng, B.; et al. Revealing cellular and molecular transitions in neonatal germ cell differentiation using single cell RNA sequencing. Development 2019, 146, dev174953. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Jensen, N.H.; Yokota, N.; Rossner, M.J.; Akiyama, H.; Shirahige, K.; Okada, Y. Single cell RNA-sequencing identified Dec2 as a suppressive factor for spermatogonial differentiation by inhibiting Sohlh1 expression. Sci. Rep. 2019, 9, 6063. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-cell RNA-seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell 2017, 20, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, J.; Zou, K. The progresses of spermatogonial stem cells sorting using Fluorescence-Activated Cell Sorting. Stem Cell Rev. Rep. 2020, 16, 94–102. [Google Scholar] [CrossRef]
- Ibtisham, F.; Honaramooz, A. Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef]
- Hogarth, C.A.; Evanoff, R.; Mitchell, D.; Kent, T.; Small, C.; Amory, J.K.; Griswold, M.D. Turning a spermatogenic wave into a tsunami: Synchronizing murine spermatogenesis using WIN 18,446. Biol. Reprod. 2013, 88, 40. [Google Scholar] [CrossRef]
- Romer, K.A.; de Rooij, D.G.; Kojima, M.L.; Page, D.C. Isolating mitotic and meiotic germ cells from male mice by developmental synchronization, staging, and sorting. Dev. Biol. 2018, 443, 19–34. [Google Scholar] [CrossRef]
- Russell, L.D.; Ettlin, R.A.; Sinha Hikim, A.P.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis; Cache River Press: St. Louis, MO, USA, 1990; pp. 49–53. [Google Scholar]
- Gelei, J. Weitere Studien über die Oogenese des Dendrocoelum lacteum. Die Konjugation der Chromosomen in der Literatur unde meine Befunde. Arch. Zellforsch. 1921, 16, 300–365. [Google Scholar]
- Scherthan, H. A bouquet makes ends meet. Nat. Rev. Mol. Cell. Biol. 2001, 2, 621–627. [Google Scholar] [CrossRef]
- Zickler, D.; Kleckner, N. A few of our favorite things: Pairing, the bouquet, crossover interference and evolution of meiosis. Semin. Cell. Dev. Biol. 2016, 54, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; McCarrey, J.R. Sex chromosome inactivation in the male. Epigenetics 2009, 4, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M. Meiotic silencing in mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 2002, 296, 2176–2178. [Google Scholar] [CrossRef]
- Rathke, C.; Baarends, W.M.; Awe, S.; Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta 2014, 1839, 155–168. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Low, D.H.; Yi, C.; Carrell, D.T.; Guccione, E.; Cairns, B.R. Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell 2014, 15, 239–253. [Google Scholar] [CrossRef]
- Lesch, B.J.; Silber, S.J.; McCarrey, J.R.; Page, D.C. Parallel evolution of male germline epigenetic poising and somatic development in animals. Nat. Genet. 2016, 48, 888–894. [Google Scholar] [CrossRef]
- Guo, J.; Grow, E.J.; Yi, C.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Murphy, P.J.; Wike, C.L.; Carrell, D.T.; Goriely, A.; et al. Chromatin and single-cell rna-seq profiling reveal dynamic signaling and metabolic transitions during human spermatogonial stem cell development. Cell Stem Cell. 2017, 21, 533–546. [Google Scholar] [CrossRef]
- Maezawa, S.; Yukawa, M.; Alavattam, K.G.; Barski, A.; Namekawa, S.H. Dynamic reorganization of open chromatin underlies diverse transcriptomes during spermatogenesis. Nucleic Acids Res. 2018, 46, 593–608. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, Y.; Du, Z.; Si, W.; Fan, S.; Qin, D.; Wang, M.; Duan, Y.; Lufan Li, L.; et al. Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol. Cell 2019, 73, 547–561. [Google Scholar] [CrossRef]
- Alavattam, K.G.; Maezawa, S.; Sakashita, A.; Khoury, H.; Barski, A.; Kaplan, N.; Namekawa, S.H. Attenuated chromatin compartmentalization in meiosis and its maturation in sperm development. Nat. Struct. Mol. Biol. 2019, 26, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Kang, R.; Rosenberg, S.C.; Qiu, Y.; Raviram, R.; Chee, S.; Hu, R.; Ren, B.; Cole, F.; Corbett, K.D. Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nat. Struct. Mol. Biol. 2019, 26, 164–174. [Google Scholar] [CrossRef]
- Lesch, B.J.; Dokshin, G.A.; Young, R.A.; McCarrey, J.R.; Page, D.C. A set of genes critical to development is epigenetically poised in mouse germ cells from fetal stages through completion of meiosis. Proc. Natl. Acad. Sci. USA. 2013, 110, 16061–16066. [Google Scholar] [CrossRef] [PubMed]
- Skene, P.J.; Henikoff, J.G.; Henikoff, S. Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat. Protoc. 2018, 13, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Cheng, S.F.; Sun, R.; Zhao, Y.; Shen, W. Reconstitution of gametogenesis in vitro: Meiosis is the biggest obstacle. J. Genet. Genom. 2014, 41, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K. In vitro reconstitution of germ cell development. Biol. Reprod. 2019, 101, 567–578. [Google Scholar] [CrossRef]
- Oliver, E.; Stukenborg, J.B. Rebuilding the human testis in vitro. Andrology 2020, 8, 825–834. [Google Scholar] [CrossRef]
- Richer, G.; Baert, Y.; Goossens, E. In-vitro spermatogenesis through testis modelling: Toward the generation of testicular organoids. Andrology 2020, 8, 879–891. [Google Scholar] [CrossRef]
- Pelzman, D.L.; Orwig, K.E.; Hwang, K. Progress in translational reproductive science: Testicular tissue transplantation and in vitro spermatogenesis. Fertil. Steril. 2020, 113, 500–509. [Google Scholar] [CrossRef]
- Shams, A.; Eslahi, N.; Movahedin, M.; Izadyar, F.; Asgari, H.; Koruji, M. Future of spermatogonial stem cell culture: Application of nanofiber scaffolds. Curr. Stem Cell Res. Ther. 2017, 12, 544–553. [Google Scholar] [CrossRef]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Fisher, A.; Kauffman, A.; Librach, L. Potential use of stem cells for fertility preservation. Andrology 2020, 8, 862–878. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
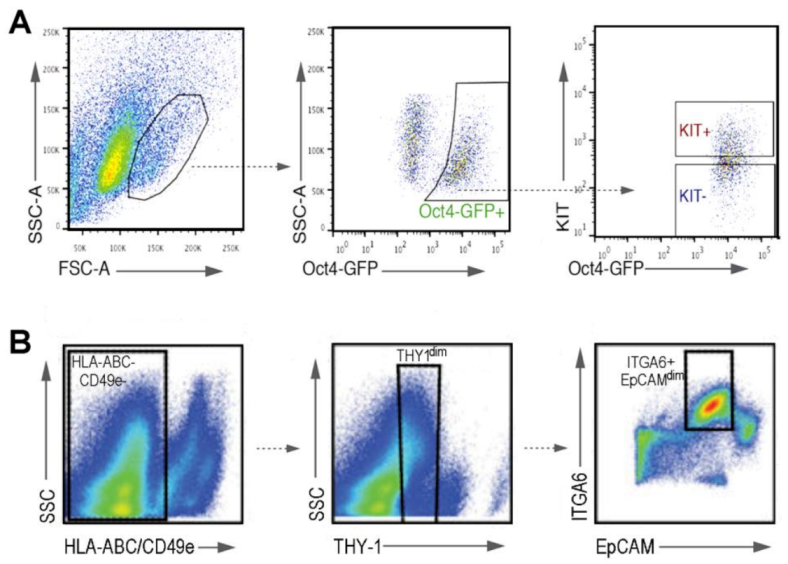
- Valli, H.; Sukhwani, M.; Dovey, S.L.; Peters, K.A.; Donohue, J.; Castro, C.A.; Chu, T.; Marshall, G.R.; Orwig, K.E. Fluorescence and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil. Steril. 2014, 102, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Wilkinson, M.F. A single-cell view of spermatogonial stem cells. Curr. Opin. Cell Biol. 2020, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.C. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 2003, 69, 701–707. [Google Scholar] [CrossRef]
- Chan, F.; Oatley, M.J.; Kaucher, A.V.; Yang, Q.-E.; Bieberich, C.J.; Shashikant, C.S.; Oatley, J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014, 28, 1351–1362. [Google Scholar] [CrossRef]
- Helsel, A.R.; Yang, Q.E.; Oatley, M.J.; Lord, T.; Sablitzky, F.; Oatley, J.M. ID4 levels dictate the stem cell state in mouse spermatogonia. Development 2017, 144, 624–634. [Google Scholar] [CrossRef]
- Law, N.C.; Oatley, M.J.; Oatley, J.M. Developmental kinetics and transcriptome dynamics of stem cell specification in the spermatogenic lineage. Nat. Commun. 2019, 10, 2787. [Google Scholar] [CrossRef]
- Nakagawa, T.; Sharma, M.; Nabeshima, Y.; Braun, R.E.; Yoshida, S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010, 328, 62–67. [Google Scholar] [CrossRef]
- Velte, E.K.; Niedenberger, B.A.; Serra, N.D.; Singh, A.; Roa-DeLaCruz, L.; Hermann, B.P.; Geyer, C.B. Differential RA responsiveness directs formation of functionally distinct spermatogonial populations at the initiation of spermatogenesis in the mouse. Development 2019, 146, dev173088. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Mutoji, K.N.; Velte, E.K.; Ko, D.; Oatley, J.M.; Geyer, C.B.; McCarrey, J.R. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol. Reprod. 2015, 92, 54. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Tan, K.; Song, H.W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The neonatal and adult human testis defined at the single-cell level. Cell Rep. 2019, 26, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, K.; Pei, C.B.; Zhang, S.H.; Li, X.; Zhou, Y.; Yan, B.; Wang, H.Y.; Ma, L.H. Relative safety of various spermatogenic stem cell purification methods for application in spermatogenic stem cell transplantation. Stem Cell Res. Ther. 2019, 10, 382. [Google Scholar] [CrossRef]
- Ziloochi Kashani, M.; Bagher, Z.; Asgari, H.R.; Najafi, M.; Koruji, M.; Mehraein, F. Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst. Biol. Reprod. Med. 2020, 66, 202–215. [Google Scholar] [CrossRef]

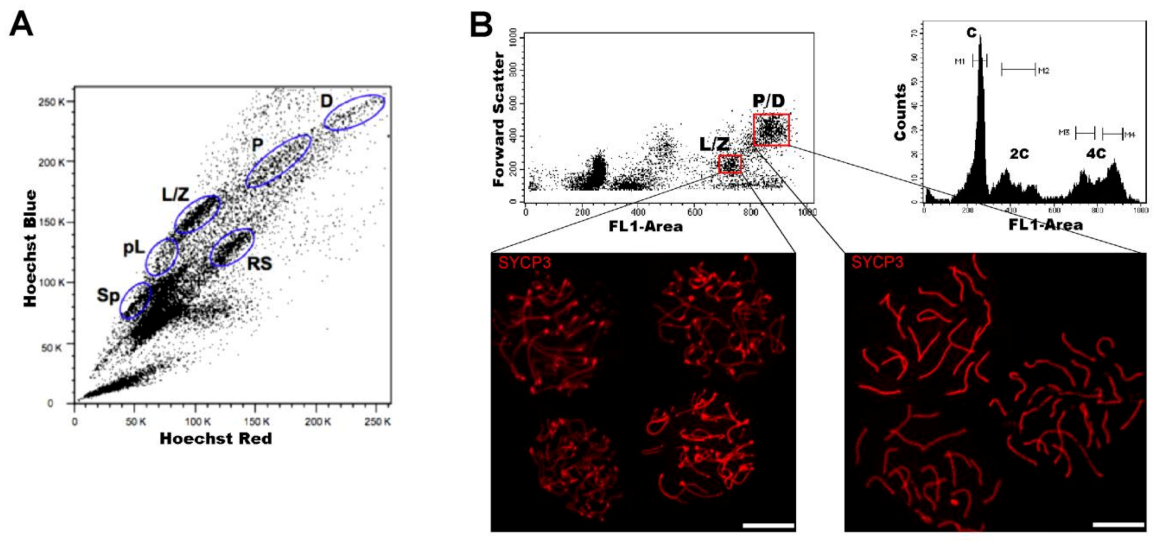
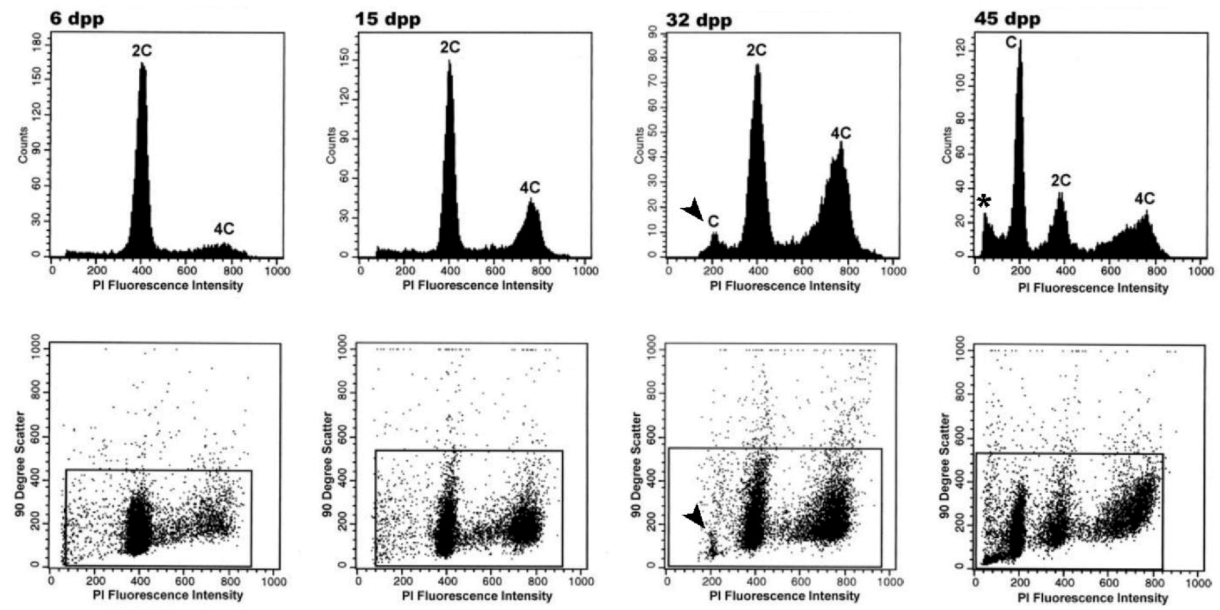
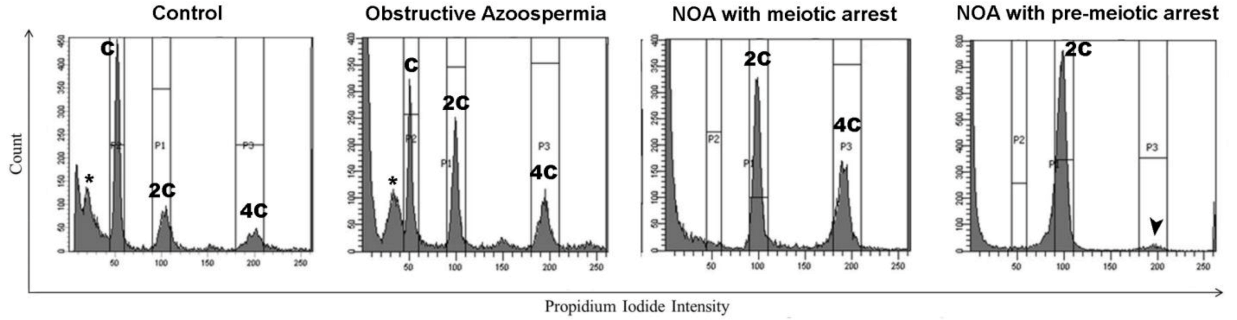
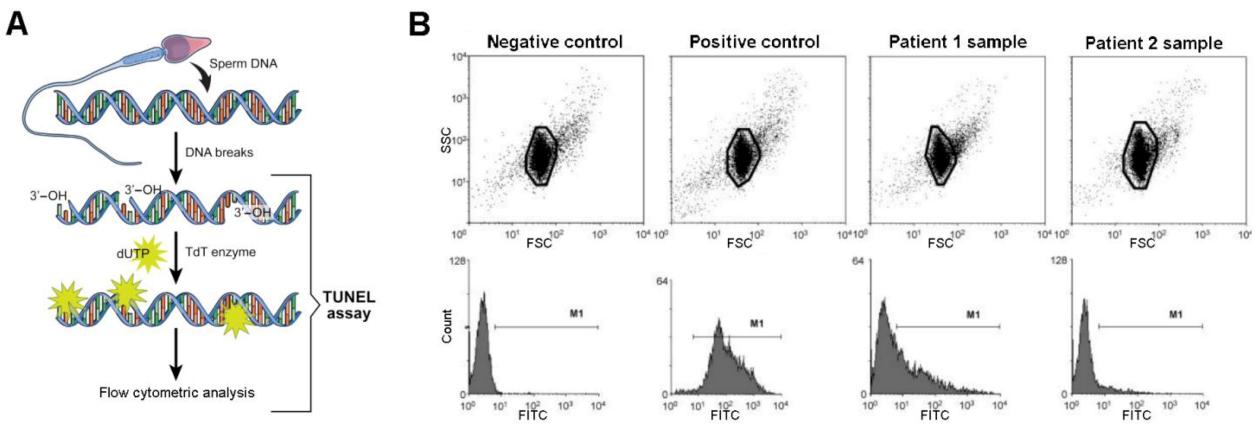
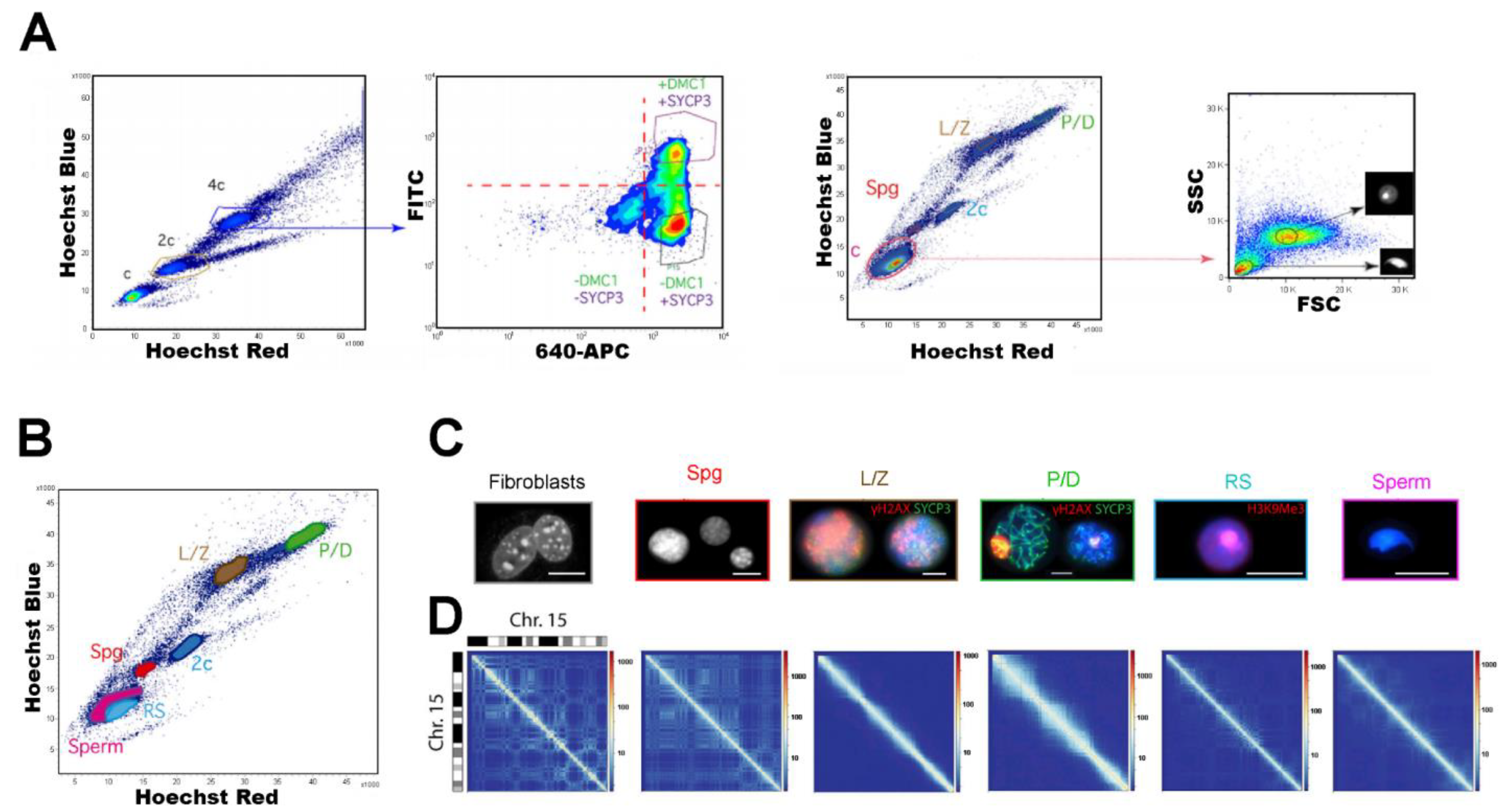
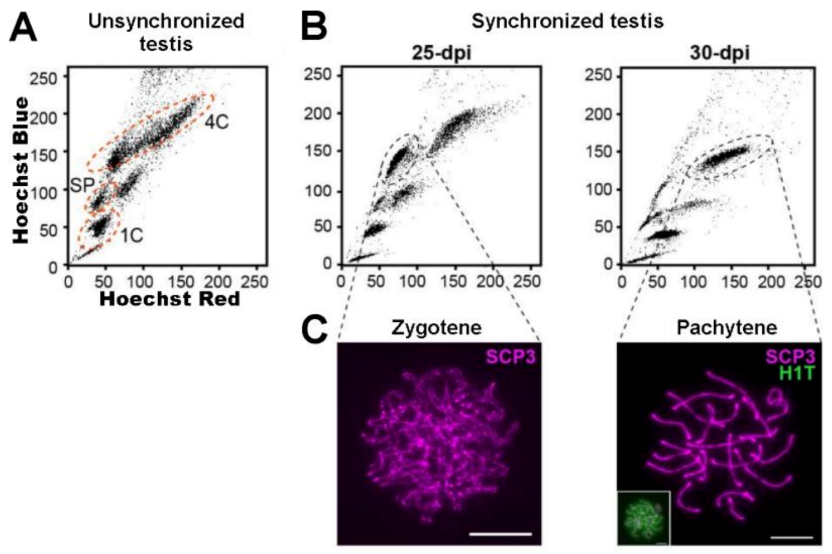
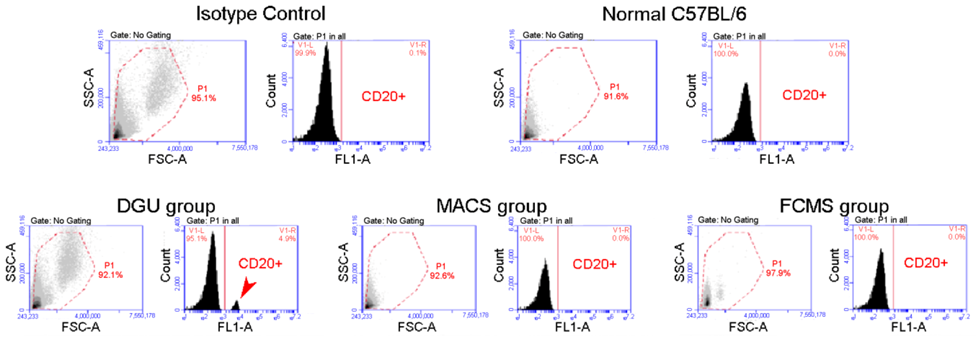
| Approximate Cellular Size (µm) | Cellular/Nuclear Shape | Inner Complexity | Chromatine Structure | DNA Content | |
|---|---|---|---|---|---|
| Type A spermatogonia | 12–14 | Ovoid nuclei | Very low | Homogenous euchromatin | 2C-4C * |
| Type B spermatogonia | 8–10 | Round nuclei | Low | Heterochromatin along nuclear periphery | 2C-4C * |
| PreLeptotene spermatocytes | 7.6–8.2 | Round nuclei | Low | Heterochromatin along nuclear periphery | 2C-4C * |
| Leptotene spermatocytes | 8–10 | Round nuclei | Low | Condensed chromosomes forming thin filaments | 4C |
| Zygotene spermatocytes | 10–12 | Round nuclei | Medium | Clumps of dense chromatin, mainly at the nuclear periphery | 4C |
| Pachytene spermatocytes | 12–18 | Large round nuclei; thin rim of cytoplasm; cell volume increases along the stage | High | Abundant clumps of dense chromatin; shorter and thicker chromosomes | 4C |
| Round spermatids | 10 | Round cells with round nuclei | Very low | Homogenous euchromatin; densely stained chromocenter in the middle | 1C |
| Elongating/elongated spermatids | 4–8 | Sickle-shaped nuclei | Low-Medium | Increasingly compacted chromatin | 1C |
| Leydig cells | 10–12 | Polyhedral cells with eccentrically located ovoid nuclei; abundant citoplasmic lipid droplets. | Very high | Prominent nucleoli; abundant peripheral heterochromatin | 2C |
| Sertoli cells | Height (from basal to apical surface): 90; Base: 30 | Columnar, Irregular, with apical and lateral invaginations; oval-shaped nuclei | High | Dark nucleolus; central condensation area | 2C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Casuriaga, R.; Geisinger, A. Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals. Int. J. Mol. Sci. 2021, 22, 1151. https://doi.org/10.3390/ijms22031151
Rodríguez-Casuriaga R, Geisinger A. Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals. International Journal of Molecular Sciences. 2021; 22(3):1151. https://doi.org/10.3390/ijms22031151
Chicago/Turabian StyleRodríguez-Casuriaga, Rosana, and Adriana Geisinger. 2021. "Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals" International Journal of Molecular Sciences 22, no. 3: 1151. https://doi.org/10.3390/ijms22031151
APA StyleRodríguez-Casuriaga, R., & Geisinger, A. (2021). Contributions of Flow Cytometry to the Molecular Study of Spermatogenesis in Mammals. International Journal of Molecular Sciences, 22(3), 1151. https://doi.org/10.3390/ijms22031151






