Mismatch between Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring of Tissue Respiration in Acute Brain Trauma: The Rationale for Implementing a Multimodal Monitoring Strategy
Abstract
1. Introduction
2. Tissue Respiration
2.1. Physiological Tissue Respiration
2.1.1. Oxygen Forms in the Blood and Oxygen Diffusion
2.1.2. Hemoglobin and Oxygen–Hemoglobin Dissociation Curve
2.1.3. Partial Oxygen Pressure Gradients in the Microcirculation and Interstitial Tissue
2.1.4. Roles of the Fåhraeus Effect and Glycocalyx Layer in Oxygen Diffusion in the Microcirculation
2.1.4.1. Reduction in Hematocrit Along the Microcirculation
2.1.4.2. Plasma Gap
2.1.5. Cerebral Blood Flow Autoregulation
2.1.6. Vascular Tone of the Microcirculation According to the Tissue Metabolic Status
2.2. Tissue Respiration in Traumatic Brain Injury
2.2.1. Reduction of Circulatory Oxygen Delivery Capacity
2.2.1.1. Reduction of Cerebral Blood Flow
2.2.1.2. Reduction of Hematocrit
2.2.1.3. Response to Therapeutic Hyperoxia
2.2.2. Metabolic Dysfunction
2.2.3. Microcirculatory Dysfunction
2.2.3.1. Anatomical Damage to the Vessels in the Microcirculation
2.2.3.2. Reduction of Vascular Density in the Microcirculation
2.2.4. Abnormalities in Cerebrovascular Regulation
2.2.5. Cerebral Vasospasm
2.2.6. Abnormalities in the Microcirculatory Reactivity to the Metabolic Status
2.2.7. Fåhraeus Effect and the Role of Endotheliopathy in Brain Trauma Microcirculation
2.2.8. Shifts in the Oxygen–Hemoglobin Dissociation Curve in the Tissue Microcirculation
2.2.8.1. Hydrogen Concentration
2.2.8.2. Carbon Dioxide Concentration
2.2.8.3. 2,3-Diphosphoglycerate Concentration
2.2.8.4. Chloride Concentration
2.2.8.5. Temperature
3. Intracranial Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring in Acute Traumatic Brain Injury
3.1. Inability to Infer the Whole Pathological Tissue Respiration Status by Analysis of Tissue Partial Oxygen Pressure Alone
3.1.1. The Interstitial Tissue Partial Oxygen Pressure Neuromonitoring Does Not Respond to All Pathological Abnormalities in Tissue Respiration
3.1.2. The Interstitial Tissue Partial Oxygen Pressure Is an Average of Different Pathogenetic Mechanisms Related to Brain Trauma
3.2. The Intracranial Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring Can Be Affected Differently by the Brain Trauma Pathogenesis
3.2.1. Metabolic Dysfunction
3.2.2. Reduction in Oxygen Diffusion and Oxygen‑Carrying Capacity
3.2.3. Right-Shift of the Oxygen–Hemoglobin Dissociation Curve
3.3. Different Volumes and Statuses Are Analyzed by the Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring
3.3.1. Different Tissue Statuses Across the Brain Can Influence the Values Reported
3.3.2. Changes in the Volumes Examined
3.4. Heterogeneity of Values within the Volume Analyzed by the Tissue Partial Oxygen Pressure and Near‑Infrared Spectroscopy Neuromonitoring
3.4.1. Tissue Partial Oxygen Pressure Neuromonitoring
3.4.2. Near-Infrared Spectroscopy Neuromonitoring
3.5. Barriers to Accurate Data Acquisition Using Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring
3.5.1. Tissue Partial Oxygen Pressure Neuromonitoring
3.5.2. Near-Infrared Spectroscopy Neuromonitoring
4. Future Application of Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring in Clinical Practice
4.1. Biosignatures
4.2. Multimodal Monitoring
4.2.1. Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring
4.2.1.1. Different Types of Near-Infrared Spectroscopy
- Absolute and Relative Values of Tissue Saturation
4.2.1.2. Contrast-Enhanced Near-Infrared Spectroscopy
4.2.1.3. Diffuse Optical Tomography
4.2.2. Computerized Tomography and Magnetic Resonance Imaging
4.2.3. Microdialysis
4.2.4. Mean Arterial Pressure and Intracranial Pressure Monitoring
4.2.5. Arterial Blood Gas Analysis
4.2.6. Blood Sampling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABG | Arterial blood gas analysis |
| 2,3-DPG | 2,3-Diphosphoglycerate |
| SD | Source-detector |
| CT | Computerized tomography |
| ICP | Intracranial pressure |
| CO2 | Carbon dioxide |
| ARDS | Acute respiratory distress syndrome |
| DOT | Diffuse optical tomography |
| CBF | Cerebral blood flow |
| CPP | Cerebral perfusion pressure |
| ECT | Extracranial tissue |
| Hb | Hemoglobin |
| HHb | Deoxyhemoglobin |
| ICG | Indocyanine green |
| FEM | Finite-element method |
| MAP | Mean arterial pressure |
| MRI | Magnetic resonance imaging |
| NIRS | Near-infrared spectroscopy |
| NO | Nitric oxide |
| BBB | Blood–brain barrier |
| O2 | Oxygen |
| O2Hb | Oxyhemoglobin |
| PaO2 | Arterial partial oxygen pressure |
| PbtO2 | Tissue partial oxygen pressure |
| SIADH | Syndrome of inappropriate antidiuretic hormone secretion |
References
- Ghajar, J. Traumatic brain injury. Lancet 2000, 356, 923–929. [Google Scholar] [CrossRef]
- Davies, D.J.; Su, Z.; Clancy, M.T.; Lucas, S.J.; Dehghani, H.; Logan, A.; Belli, A. Near-Infrared Spectroscopy in the Monitoring of Adult Traumatic Brain Injury: A Review. J. Neurotrauma 2015, 32, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Sehgal, S.; Tariq, R. Near-Infrared Spectroscopy: The New Must Have Tool in the Intensive Care Unit? Semin. Cardiothorac. Vasc. Anesth. 2016, 20, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Leal-Noval, S.R.; Cayuela, A.; Arellano-Orden, V.; Marin-Caballos, A.; Padilla, V.; Ferrandiz-Millon, C.; Corcia, Y.; Garcia-Alfaro, C.; Amaya-Villar, R.; Murillo-Cabezas, F. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med. 2010, 36, 1309–1317. [Google Scholar] [CrossRef]
- Davies, D.J.; Clancy, M.; Dehghani, H.; Lucas, S.J.E.; Forcione, M.; Yakoub, K.M.; Belli, A. Cerebral Oxygenation in Traumatic Brain Injury: Can a Non-Invasive Frequency Domain Near-Infrared Spectroscopy Device Detect Changes in Brain Tissue Oxygen Tension as Well as the Established Invasive Monitor? J. Neurotrauma 2019, 36, 1175–1183. [Google Scholar] [CrossRef]
- Rosenthal, G.; Furmanov, A.; Itshayek, E.; Shoshan, Y.; Singh, V. Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury. J. Neurotrauma 2014, 120, 901–907. [Google Scholar] [CrossRef]
- Büchner, K.; Meixensberger, J.; Dings, J.; Roosen, K. Near-infrared spectroscopy--not useful to monitor cerebral oxygenation after severe brain injury. Zent. Neurochir. 2000, 61, 69–73. [Google Scholar] [CrossRef]
- Weigl, W.; Milej, D.; Janusek, D.; Wojtkiewicz, S.; Sawosz, P.; Kacprzak, M.; Gerega, A.; Maniewski, R.; Liebert, A. Application of optical methods in the monitoring of traumatic brain injury: A review. J. Cereb. Blood Flow Metab. 2016, 36, 1825–1843. [Google Scholar] [CrossRef]
- Oddo, M.; Bösel, J. Monitoring of brain and systemic oxygenation in neurocritical care patients. Neurocrit. Care 2014, 21 (Suppl. 2), 103–120. [Google Scholar]
- Ganau, M.; Prisco, L. Comment on “neuromonitoring in traumatic brain injury”. Minerva Anestesiol. 2013, 79, 310–311. [Google Scholar]
- Bellelli, A.; Brunori, M. Control of Oxygen Affinity in Mammalian Hemoglobins: Implications for a System Biology Description of the Respiratory Properties of the Red Blood Cell. Curr. Protein Pept. Sci. 2020, 21, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Saunders/Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Valabrègue, R.; Aubert, A.; Burger, J.; Bittoun, J.; Costalat, R. Relation between Cerebral Blood Flow and Metabolism Explained by a Model of Oxygen Exchange. J. Cereb. Blood Flow Metab. 2003, 23, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; Macmillan: New York, NY, USA, 2004. [Google Scholar]
- Johnson, R.L.J.; Heigenhauser, G.J.F.; Hsia, C.C.W.; Jones, N.L.; Wagner, P.D. Determinants of Gas Exchange and Acid–Base Balance during Exercise. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2011; pp. 515–584. [Google Scholar]
- Tsai, A.G.; Johnson, P.C.; Intaglietta, M. Oxygen Gradients in the Microcirculation. Physiol. Revi. 2003, 83, 933–963. [Google Scholar] [CrossRef] [PubMed]
- Lübbers, D.W.; Baumgärtl, H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997, 51, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Duling, B.R.; Berne, R.M. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ. Res. 1970, 27, 669–678. [Google Scholar] [CrossRef] [PubMed]
- De Georgia, M.A. Brain Tissue Oxygen Monitoring in Neurocritical Care. J. Intensive Care Med. 2015, 30, 473–483. [Google Scholar] [CrossRef]
- Dings, J.; Meixensberger, J.; Jäger, A.; Roosen, K. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 1998, 43, 1082–1095. [Google Scholar] [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Popel, A.S.; Pittman, R.N. Role of Microvessels in Oxygen Supply to Tissue. News Physiol. Sci. 1994, 9, 119–123. [Google Scholar] [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Goldman, D.; Stephenson, A.H.; Dietrich, H.H.; Sprague, R.S. Erythrocytes: Oxygen sensors and modulators of vascular tone. Physiology 2009, 24, 107–116. [Google Scholar] [CrossRef]
- Tsai, A.; Johnson, P.; Intaglietta, M. Is the Distribution of Tissue pO2 Homogeneous? Antioxid. Redox Signal. 2007, 9, 979–984. [Google Scholar] [CrossRef]
- Tsai, A.; Cabrales, P.; Intaglietta, M. The Physics of Oxygen Delivery: Facts and Controversies. Antioxid. Redox Signal. 2009, 12, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Fåhraeus, R. The suspension stability of the blood. Physiol. Rev. 1929, 9, 241–274. [Google Scholar] [CrossRef]
- Goldsmith, H.L.; Cokelet, G.R.; Gaehtgens, P. Robin Fåhraeus: Evolution of his concepts in cardiovascular physiology. Am. J. Physiol. 1989, 257, H1005–H1015. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., III; Knudson, M.M.; Derugin, N.; Morabito, D.; Manley, G.T. Carbon Dioxide Reactivity and Pressure Autoregulation of Brain Tissue Oxygen. Neurosurgery 2001, 48, 377–384. [Google Scholar]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Ibaraki, M.; Shinohara, Y.; Nakamura, K.; Miura, S.; Kinoshita, F.; Kinoshita, T. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J. Cereb. Blood Flow Metab. 2010, 30, 1296–1305. [Google Scholar] [CrossRef]
- Tsai, A.; Vázquez, B.; Cabrales, P.; Kistler, E.; Tartakovsky, D.; Subramaniam, S.; Acharya, S.; Intaglietta, M. Replacing the Transfusion of 1–2 Units of Blood with Plasma Expanders that Increase Oxygen Delivery Capacity: Evidence from Experimental Studies. J. Funct. Biomater. 2014, 5, 232–245. [Google Scholar] [CrossRef]
- Chang, J.J.; Youn, T.S.; Benson, D.; Mattick, H.; Andrade, N.; Harper, C.R.; Moore, C.B.; Madden, C.J.; Diaz-Arrastia, R.R. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit. Care Med. 2009, 37, 283–290. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Poca, M.A.; Arribas, M.; Garnacho, A.; Rubio, E. Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: Are they clinically important? J. Neurosurg. 1999, 90, 16–26. [Google Scholar] [CrossRef]
- Vedantam, A.; Robertson, C.S.; Gopinath, S.P. Quantitative cerebral blood flow using xenon-enhanced CT after decompressive craniectomy in traumatic brain injury. J. Neurosurg. 2018, 129, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Stretti, F.; Gotti, M.; Pifferi, S.; Brandi, G.; Annoni, F.; Stocchetti, N. Body temperature affects cerebral hemodynamics in acutely brain injured patients: An observational transcranial color-coded duplex sonography study. Crit. Care 2014, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Doppenberg, E.M.; Zauner, A.; Bullock, R.; Ward, J.D.; Fatouros, P.P.; Young, H.F. Correlations between brain tissue oxygen tension, carbon dioxide tension, pH, and cerebral blood flow--a better way of monitoring the severely injured brain? Surg. Neurol. 1998, 49, 650–654. [Google Scholar] [CrossRef]
- Jaeger, M.; Soehle, M.; Schuhmann, M.U.; Winkler, D.; Meixensberger, J. Correlation of continuously monitored regional cerebral blood flow and brain tissue oxygen. Acta Neurochir. 2005, 147, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Marín-Caballos, A.J.; Murillo-Cabezas, F.; Cayuela-Domínguez, A.; Domínguez-Roldán, J.M.; Rincón-Ferrari, M.D.; Valencia-Anguita, J.; Flores-Cordero, J.M.; Muñoz-Sánchez, M.A. Cerebral perfusion pressure and risk of brain hypoxia in severe head injury: A prospective observational study. Crit. Care 2005, 9, R670–R676. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C.; Smith, W.S.; Sonne, D.C.; Morabito, D.; Manley, G.T. Relationship between Brain Tissue Oxygen Tension and CT Perfusion: Feasibility and Initial Results. Am. J. Neuroradiol. 2005, 26, 1095–1100. [Google Scholar]
- Lazaridis, C. Cerebral oxidative metabolism failure in traumatic brain injury: “Brain shock”. J. Crit. Care 2017, 37, 230–233. [Google Scholar] [CrossRef]
- Smith, M.J.; Stiefel, M.F.; Magge, S.; Frangos, S.; Bloom, S.; Gracias, V.; Le Roux, P.D. Packed red blood cell transfusion increases local cerebral oxygenation. Crit. Care Med. 2005, 33, 1104–1108. [Google Scholar] [CrossRef]
- Zimmerman, R.; Tsai, A.; Intaglietta, M.; Tartakovsky, D. A Mechanistic Analysis of Possible Blood Transfusion Failure to Increase Circulatory Oxygen Delivery in Anemic Patients. Ann. Biomed. Eng. 2019, 47, 1094–1105. [Google Scholar] [CrossRef]
- Naumann, D.N.; Hazeldine, J.; Bishop, J.; Midwinter, M.J.; Harrison, P.; Nash, G.; Hutchings, S.D. Impact of plasma viscosity on microcirculatory flow after traumatic haemorrhagic shock: A prospective observational study. Clin. Hemorheol. Microcirc. 2019, 71, 71–82. [Google Scholar] [CrossRef]
- Tolias, C.M.; Reinert, M.; Seiler, R.; Gilman, C.; Scharf, A.; Bullock, M.R. Normobaric hyperoxia--induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: A prospective historical cohort-matched study. J. Neurosurg. 2004, 101, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Nortje, J.; Coles, J.; Timofeev, I.; Fryer, T.; Aigbirhio, F.; Smielewski, P.; Outtrim, J.; Chatfield, D.; Pickard, J.; Hutchinson, P.; et al. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: Preliminary findings. Crit. Care Med. 2008, 36, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Stocchetti, N.; Longhi, L.; Balestreri, M.; Spagnoli, D.; Zanier, E.R.; Bellinzona, G. Brain oxygen tension, oxygen supply, and oxygen consumption during arterial hyperoxia in a model of progressive cerebral ischemia. J. Neurotrauma 2001, 18, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Merino, M.A.; Sánchez-Guerrero, A.; Arikan, F.; Vidal-Jorge, M.; Martínez-Valverde, T.; Rey, A.; Riveiro, M.; Poca, M.A. Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: A microdialysis study in patients with traumatic brain injuries. PLoS ONE 2014, 9, e102540. [Google Scholar] [CrossRef] [PubMed]
- Bergsneider, M.; Hovda, D.A.; Shalmon, E.; Kelly, D.F.; Vespa, P.M.; Martin, N.A.; Phelps, M.E.; McArthur, D.L.; Caron, M.J.; Kraus, J.F.; et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: A positron emission tomography study. J. Neurosurg. 1997, 86, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Coles, J.P.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Steiner, L.A.; Johnston, A.J.; Downey, S.P.; Williams, G.B.; Aigbirhio, F.; Hutchinson, P.J.; et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J. Cereb. Blood Flow Metab. 2004, 24, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.M.; Huang, S.C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000, 93, 815–820. [Google Scholar] [CrossRef]
- Vespa, P.M.; O’Phelan, K.; McArthur, D.; Miller, C.; Eliseo, M.; Hirt, D.; Glenn, T.; Hovda, D.A. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 2007, 35, 1153–1160. [Google Scholar] [CrossRef]
- Stein, N.R.; McArthur, D.L.; Etchepare, M.; Vespa, P.M. Early cerebral metabolic crisis after TBI influences outcome despite adequate hemodynamic resuscitation. Neurocrit. Care 2012, 17, 49–57. [Google Scholar] [CrossRef]
- Zygun, D.; Nortje, J.; Hutchinson, P.; Timofeev, I.; Menon, D.; Gupta, A. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury*. Crit. Care Med. 2009, 37, 1074–1078. [Google Scholar] [PubMed]
- Magnoni, S.; Ghisoni, L.; Locatelli, M.; Caimi, M.; Colombo, A.; Valeriani, V.; Stocchetti, N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: A microdialysis study. J. Neurosurg. 2003, 98, 952–958. [Google Scholar] [PubMed]
- Diringer, M.N.; Aiyagari, V.; Zazulia, A.R.; Videen, T.O.; Powers, W.J. Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J. Neurosurg. 2007, 106, 526–529. [Google Scholar] [PubMed]
- Glenn, T.C.; Kelly, D.F.; Boscardin, W.J.; McArthur, D.L.; Vespa, P.; Oertel, M.; Hovda, D.A.; Bergsneider, M.; Hillered, L.; Martin, N.A. Energy dysfunction as a predictor of outcome after moderate or severe head injury: Indices of oxygen, glucose, and lactate metabolism. J. Cereb. Blood Flow Metab. 2003, 23, 1239–1250. [Google Scholar] [PubMed]
- Timofeev, I.; Carpenter, K.; Nortje, J.; Al-Rawi, P.; O’Connell, M.; Czosnyka, M.; Smielewski, P.; Pickard, J.; Menon, D.; Kirkpatrick, P.; et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: A microdialysis study of 223 patients. Brain A J. Neurol. 2011, 134, 484–494. [Google Scholar]
- Rosenthal, G.; Hemphill, J.C., 3rd; Sorani, M.; Martin, C.; Morabito, D.; Obrist, W.D.; Manley, G.T. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit. Care Med. 2008, 36, 1917–1924. [Google Scholar]
- Menon, D.K.; Coles, J.P.; Gupta, A.K.; Fryer, T.D.; Smielewski, P.; Chatfield, D.A.; Aigbirhio, F.; Skepper, J.N.; Minhas, P.S.; Hutchinson, P.J.; et al. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004, 32, 1384–1390. [Google Scholar]
- Naumann, D.N.; Hazeldine, J.; Midwinter, M.J.; Hutchings, S.D.; Harrison, P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J. Trauma Acute Care Surg. 2018, 84, 81–88. [Google Scholar]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Purins, K.; Lewén, A.; Hillered, L.; Howells, T.; Enblad, P. Brain tissue oxygenation and cerebral metabolic patterns in focal and diffuse traumatic brain injury. Front. Neurol. 2014, 5, 64. [Google Scholar]
- Longhi, L.; Valeriani, V.; Rossi, S.; De Marchi, M.; Egidi, M.; Stocchetti, N. Effects of hyperoxia on brain tissue oxygen tension in cerebral focal lesions. Acta Neurochir. Suppl. 2002, 81, 315–317. [Google Scholar]
- Radolovich, D.K.; Czosnyka, M.; Timofeev, I.; Lavinio, A.; Kim, D.-J.; Jaeger, M.; Hutchinson, P.; Gupta, A.; Pickard, J.D.; Smielewski, P. Transient Changes in Brain Tissue Oxygen in Response to Modifications of Cerebral Perfusion Pressure: An Observational Study. Anesth. Analg. 2010, 110, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.W.; Lagopoulos, J.; Griffith, J.; Yip, K.; Yam, A.; Mudaliar, Y.; Mehdorn, H.M.; Dorsch, N.W. Cerebral vasomotor reactivity testing in head injury: The link between pressure and flow. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Carmona Suazo, J.A.; Maas, A.I.; van den Brink, W.A.; van Santbrink, H.; Steyerberg, E.W.; Avezaat, C.J. CO2 reactivity and brain oxygen pressure monitoring in severe head injury. Crit. Care Med. 2000, 28, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Feliciano, D.V.; Mattox, K.L. Trauma, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Clausen, T.; Khaldi, A.; Zauner, A.; Reinert, M.; Doppenberg, E.; Menzel, M.; Soukup, J.; Alves, O.L.; Bullock, M.R. Cerebral acid-base homeostasis after severe traumatic brain injury. J. Neurosurg. 2005, 103, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, I.; Nortje, J.; Al-Rawi, P.G.; Hutchinson, P.J.; Gupta, A.K. Extracellular brain pH with or without hypoxia is a marker of profound metabolic derangement and increased mortality after traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 422–427. [Google Scholar] [CrossRef]
- Jensen, F.B. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 2004, 182, 215–227. [Google Scholar] [CrossRef]
- Gupta, A.K.; Zygun, D.A.; Johnston, A.J.; Steiner, L.A.; Al-Rawi, P.G.; Chatfield, D.; Shepherd, E.; Kirkpatrick, P.J.; Hutchinson, P.J.; Menon, D.K. Extracellular Brain pH and Outcome following Severe Traumatic Brain Injury. J. Neurotrauma 2004, 21, 678–684. [Google Scholar] [CrossRef]
- Yokota, H.; Yamamoto, Y.; Naoe, Y.; Fuse, A.; Sato, H.; Unemoto, K.; Kurokawa, A. Measurements of cortical cellular pH by intracranial tonometer in severe head injury. Crit. Care Med. 2000, 28, 3275–3280. [Google Scholar] [CrossRef]
- Torres, R.B.; Terzi, R.G.G.; Falcão, A.L.E.; Hôer, N.F.; Dantas Filho, V.P. Hypophosphatemia in Severe Traumatic Brain Injury. RBTI 2007, 17, 116–120. [Google Scholar]
- Pas’ko, S.A.; Volosheniuk, T.G. Disordered phosphorus metabolism and its correction in the acute period of severe craniocerebral trauma. Zhurnal Voprosy Neirokhirurgii Imeni NN Burdenko 1990, 3, 14–16. [Google Scholar]
- Tsai, A.; Hofmann, A.; Cabrales, P.; Intaglietta, M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: The experimental evidence. Transfus. Apher. Sci. 2010, 43, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Posti, J.P.; Kamstrup-Nielsen, M.H.; Takala, R.S.K.; Lingsma, H.F.; Mattila, I.; Jäntti, S.; Katila, A.J.; Carpenter, K.L.H.; Ala-Seppälä, H.; et al. Human Serum Metabolites Associate with Severity and Patient Outcomes in Traumatic Brain Injury. EBioMedicine 2016, 12, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.A.M.; Bellomo, R.; Story, D.; Kellum, J. Bench-to-bedside review: Chloride in critical illness. Crit. Care 2010, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.-H.; Karmakar, M.K.; Contardi, L.H.; Ng, S.S.W.; Hewson, J.R. Excessive Use of Normal Saline in Managing Traumatized Patients in Shock: A Preventable Contributor to Acidosis. J. Trauma Acute Care Surg. 2001, 51, 173–177. [Google Scholar] [CrossRef]
- Yunos, N.M.; Kim, I.B.; Bellomo, R.; Bailey, M.; Ho, L.; Story, D.; Gutteridge, G.A.; Hart, G.K. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit. Care Med. 2011, 39, 2419–2424. [Google Scholar] [CrossRef]
- Roquilly, A.; Loutrel, O.; Cinotti, R.; Rosenczweig, E.; Flet, L.; Mahe, P.J.; Dumont, R.; Marie Chupin, A.; Peneau, C.; Lejus, C.; et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: A randomised double-blind pilot study. Crit. Care 2013, 17, R77. [Google Scholar] [CrossRef]
- Rossi, S.; Zanier, E.; Mauri, I.; Columbo, A.; Stocchetti, N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J. Neurol. Neurosurg. Psychiatry 2001, 71, 448–454. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. Hyperthermia and central nervous system injury. Prog. Brain Res. 2007, 162, 201–217. [Google Scholar]
- Diringer, M.N.; Reaven, N.L.; Funk, S.E.; Uman, G.C. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit. Care Med. 2004, 32, 1489–1495. [Google Scholar] [CrossRef]
- Busto, R.; Dietrich, W.D.; Globus, M.Y.; Valdés, I.; Scheinberg, P.; Ginsberg, M.D. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J. Cereb. Blood Flow Metab. 1987, 7, 729–738. [Google Scholar] [PubMed]
- Stocchetti, N.; Protti, A.; Lattuada, M.; Magnoni, S.; Longhi, L.; Ghisoni, L.; Egidi, M.; Zanier, E.R. Impact of pyrexia on neurochemistry and cerebral oxygenation after acute brain injury. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1135–1139. [Google Scholar] [PubMed]
- Oddo, M.; Frangos, S.; Maloney-Wilensky, E.; Andrew Kofke, W.; Le Roux, P.D.; Levine, J.M. Effect of shivering on brain tissue oxygenation during induced normothermia in patients with severe brain injury. Neurocrit. Care 2010, 12, 10–16. [Google Scholar] [PubMed]
- Nangunoori, R.; Maloney-Wilensky, E.; Stiefel, M.; Park, S.; Andrew Kofke, W.; Levine, J.M.; Yang, W.; Le Roux, P.D. Brain tissue oxygen-based therapy and outcome after severe traumatic brain injury: A systematic literature review. Neurocrit. Care 2012, 17, 131–138. [Google Scholar] [PubMed]
- van den Brink, W.A.; van Santbrink, H.; Steyerberg, E.W.; Avezaat, C.J.; Suazo, J.A.; Hogesteeger, C.; Jansen, W.J.; Kloos, L.M.; Vermeulen, J.; Maas, A.I. Brain oxygen tension in severe head injury. Neurosurgery 2000, 46, 868–876, discussion 876-8. [Google Scholar]
- Stiefel, M.F.; Spiotta, A.; Gracias, V.H.; Garuffe, A.M.; Guillamondegui, O.; Maloney-Wilensky, E.; Bloom, S.; Grady, M.S.; LeRoux, P.D. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 2005, 103, 805–811. [Google Scholar] [PubMed]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar]
- Narotam, P.K.; Morrison, J.F.; Nathoo, N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: Outcome analysis of a brain tissue oxygen-directed therapy. J. Neurosurg. 2009, 111, 672–682. [Google Scholar]
- Roh, D.; Park, S. Brain Multimodality Monitoring: Updated Perspectives. Curr. Neurol. Neurosci. Rep. 2016, 16, 56. [Google Scholar]
- Cunningham, A.S.; Salvador, R.; Coles, J.P.; Chatfield, D.A.; Bradley, P.G.; Johnston, A.J.; Steiner, L.A.; Fryer, T.D.; Aigbirhio, F.I.; Smielewski, P.; et al. Physiological thresholds for irreversible tissue damage in contusional regions following traumatic brain injury. Brain 2005, 128, 1931–1942. [Google Scholar]
- Eriksson, E.A.; Barletta, J.F.; Figueroa, B.E.; Bonnell, B.W.; Vanderkolk, W.E.; McAllen, K.J.; Ott, M.M. Cerebral perfusion pressure and intracranial pressure are not surrogates for brain tissue oxygenation in traumatic brain injury. Clin. Neurophysiol. 2012, 123, 1255–1260. [Google Scholar]
- Bouzat, P.; Marques-Vidal, P.; Zerlauth, J.B.; Sala, N.; Suys, T.; Schoettker, P.; Bloch, J.; Daniel, R.T.; Levivier, M.; Meuli, R.; et al. Accuracy of brain multimodal monitoring to detect cerebral hypoperfusion after traumatic brain injury*. Crit. Care Med. 2015, 43, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Sahuquillo, J.; Amoros, S.; Santos, A.; Poca, M.A.; Panzardo, H.; Dominguez, L.; Pedraza, S. Does an increase in cerebral perfusion pressure always mean a better oxygenated brain? A study in head-injured patients. Acta Neurochir. Suppl. 2000, 76, 457–462. [Google Scholar] [PubMed]
- Budohoski, K.P.; Zweifel, C.; Kasprowicz, M.; Sorrentino, E.; Diedler, J.; Brady, K.M.; Smielewski, P.; Menon, D.K.; Pickard, J.D.; Kirkpatrick, P.J.; et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br. J. Anaesth. 2012, 108, 89–99. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.D.; Igielman, F.; Elwell, C.; Cope, M.; Smith, M. Measuring cerebral oxygenation during normobaric hyperoxia: A comparison of tissue microprobes, near-infrared spectroscopy, and jugular venous oximetry in head injury. Anesth. Analg. 2003, 97, 851–856. [Google Scholar] [CrossRef]
- Goldberg, S.; Heitner, S.; Mimouni, F.; Joseph, L.; Bromiker, R.; Picard, E. The influence of reducing fever on blood oxygen saturation in children. Eur. J. Pediatrics 2018, 177, 95–99. [Google Scholar] [CrossRef]
- Bigio, I.J.; Fantini, S. Quantitative Biomedical Optics: Theory, Methods, and Applications; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Santora, R.J.; Moore, F.A. Monitoring trauma and intensive care unit resuscitation with tissue hemoglobin oxygen saturation. Crit. Care 2009, 13, S10. [Google Scholar] [CrossRef]
- Jaeger, M.; Soehle, M.; Meixensberger, J. Brain tissue oxygen (PtiO2): A clinical comparison of two monitoring devices. Acta Neurochir. Suppl. 2005, 95, 79–81. [Google Scholar]
- Sanchez, J.J.; Bidot, C.J.; O’Phelan, K.; Gajavelli, S.; Yokobori, S.; Olvey, S.; Jagid, J.; Garcia, J.A.; Nemeth, Z.; Bullock, R. Neuromonitoring with microdialysis in severe traumatic brain injury patients. Acta Neurochir. Suppl. 2013, 118, 223–227. [Google Scholar]
- Dunham, C.M.; Ransom, K.J.; Flowers, L.L.; Siegal, J.D.; Kohli, C.M. Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J. Trauma 2004, 56, 482–489. [Google Scholar] [CrossRef]
- Yoshitani, K.; Kawaguchi, M.; Miura, N.; Okuno, T.; Kanoda, T.; Ohnishi, Y.; Kuro, M. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near-infrared spectroscopy measurements. Anesthesiology 2007, 106, 458–462. [Google Scholar] [CrossRef]
- Láng, J.; Ganau, M.; Prisco, L.; Bozsik, K.; Banczerowski, P. Syndrome of trephined-underestimated and poorly understood complication after decompressive craniectomy. Ideggyogy Sz 2016, 69, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Ligarotti, G.K.I.; Ganau, L.; Prisco, L. Letter: Early Cranioplasty is Associated with Greater Neurological Improvement: A Systematic Review and Meta-Analysis. Neurosurgery 2018, 83, E87–E89. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.S.; Zager, E.L.; Narayan, R.K.; Handly, N.; Sharma, A.; Hanley, D.F.; Garza, H.; Maloney-Wilensky, E.; Plaum, J.M.; Koenig, C.H.; et al. Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. J. Neurotrauma 2010, 27, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.N.; Gopinath, S.P.; Robertson, C.S. Clinical application of near-infrared spectroscopy in patients with traumatic brain injury: A review of the progress of the field. Neurophotonics 2016, 3, 031409. [Google Scholar] [CrossRef] [PubMed]
- Stocchetti, N. Traumatic brain injury: Problems and opportunities. Lancet Neurol. 2014, 13, 14–16. [Google Scholar] [CrossRef]
- van den Brink, W.A.; Haitsma, I.K.; Avezaat, C.J.; Houtsmuller, A.B.; Kros, J.M.; Maas, A.I. Brain parenchyma/pO2 catheter interface: A histopathological study in the rat. J. Neurotrauma 1998, 15, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Haitsma, I.; Rosenthal, G.; Morabito, D.; Rollins, M.; Maas, A.I.; Manley, G.T. In vitro comparison of two generations of Licox and Neurotrend catheters. Acta Neurochir. Suppl. 2008, 102, 197–202. [Google Scholar] [PubMed]
- Ngwenya, L.B.; Burke, J.F.; Manley, G.T. Brain Tissue Oxygen Monitoring and the Intersection of Brain and Lung: A Comprehensive Review. Respir. Care 2016, 61, 1232–1244. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.S.; Kiening, K.L.; Bardt, T.F.; Schneider, G.H.; Unterberg, A.W.; Lanksch, W.R. Cerebral oxygenation in contusioned vs. nonlesioned brain tissue: Monitoring of PtiO2 with Licox and Paratrend. Acta Neurochir. Suppl. 1998, 71, 186–189. [Google Scholar]
- Ponce, L.L.; Pillai, S.; Cruz, J.; Li, X.; Julia, H.; Gopinath, S.; Robertson, C.S. Position of probe determines prognostic information of brain tissue PO2 in severe traumatic brain injury. Neurosurgery 2012, 70, 1492–1502. [Google Scholar] [CrossRef]
- Radolovich, D.; Czosnyka, M.; Timofeev, I.; Lavinio, A.; Hutchinson, P.; Gupta, A.; Pickard, J.; Smielewski, P. Reactivity of Brain Tissue Oxygen to Change in Cerebral Perfusion Pressure in Head Injured Patients. Neurocrit. Care 2009, 10, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, I.; Czosnyka, M.; Carpenter, K.L.H.; Nortje, J.; Kirkpatrick, P.J.; Al-Rawi, P.G.; Menon, D.K.; Pickard, J.D.; Gupta, A.K.; Hutchinson, P.J. Interaction between brain chemistry and physiology after traumatic brain injury: Impact of autoregulation and microdialysis catheter location. J. Neurotrauma 2011, 28, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Forcione, M.; Yakoub, K.M.; Chiarelli, A.M.; Perpetuini, D.; Merla, A.; Sun, R.; Sawosz, P.; Belli, A.; Davies, D.J. Dynamic contrast-enhanced near-infrared spectroscopy using indocyanine green on moderate and severe traumatic brain injury: A prospective observational study. Quant. Imaging Med. Surg. 2020, 10, 2085. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, A.; Skowno, J.; Lansdown, A.; Lupton, T.; Garner, A. Feasibility of cerebral near-infrared spectroscopy monitoring in the pre-hospital environment. Acta Anaesthesiol. Scand. 2012, 56, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.; Belli, A.; Davies, D.; Lucas, S.J.E.; Su, Z.J.; Dehghani, H. Comparison of Neurological NIRS signals during standing Valsalva maneuvers, pre and post vasopressor injection. In Diffuse Optical Imaging; Dehghani, V., Taroni, H.P., Eds.; SPIE-The International Society for Optical Engineering: Bellingham, WA, USA, 2015; Volume 9538. [Google Scholar]
- Sørensen, H.; Secher, N.H.; Siebenmann, C.; Nielsen, H.B.; Kohl-Bareis, M.; Lundby, C.; Rasmussen, P. Cutaneous Vasoconstriction Affects Near-infrared Spectroscopy Determined Cerebral Oxygen Saturation during Administration of Norepinephrine. Anesthesiology 2012, 117, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. Differential pathlength factor in continuous wave functional near-infrared spectroscopy: Reducing hemoglobin’s cross talk in high-density recordings. Neurophotonics 2019, 6, 035005. [Google Scholar] [CrossRef]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.G.; Rhodes, J.K.; Murray, G.D.; Eurotherm Trial, C. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef]
- Cooper, D.J.; Rosenfeld, J.V.; Murray, L.; Arabi, Y.M.; Davies, A.R.; D’Urso, P.; Kossmann, T.; Ponsford, J.; Seppelt, I.; Reilly, P.; et al. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011, 364, 1493–1502. [Google Scholar] [CrossRef]
- Robertson, C.S.; Ropper, A.H. Getting Warmer on Critical Care for Head Injury. N. Engl. J. Med. 2015, 373, 2469–2470. [Google Scholar] [CrossRef]
- Maas, A.; Menon, D.; Adelson, P.; Andelic, N.; Bell, M.; Belli, A.; Bragge, P.; Brazinova, A.; Buki, A.; Chesnut, R.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Ganau, M.; Syrmos, N.; Paris, M.; Ganau, L.; Ligarotti, G.K.I.; Moghaddamjou, A.; Chibbaro, S.; Soddu, A.; Ambu, R.; Prisco, L. Current and Future Applications of Biomedical Engineering for Proteomic Profiling: Predictive Biomarkers in Neuro-Traumatology. Medicines 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Hergenroeder, G.W.; Redell, J.B.; Moore, A.N.; Dash, P.K. Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 2008, 12, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Rusin, C.G.; Robertson, C.S. Secondary brain injury: Predicting and preventing insults. Neuropharmacology 2019, 145, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.A.; Densmore, M.; McKinnon, M.C.; Neufeld, R.W.J.; Frewen, P.A.; Théberge, J.; Jetly, R.; Richardson, J.D.; Lanius, R.A. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol. Med. 2019, 49, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Ghoreishi, S.F. Optimal Finite-Horizon Perturbation Policy for Inference of Gene Regulatory Networks. IEEE Intell. Syst. 2020. [Google Scholar] [CrossRef]
- Syrmos, N.; Ganau, M.; De Carlo, A.; Prisco, L.; Ganau, L.; Valadakis, V.; Grigoriou, K.; Iliadis, C.; Arvanitakis, D. Dealing with the Surgical and Medical Challenges of Penetrating Brain Injuries. Case Rep. Surg. 2013, 2013, 209750. [Google Scholar] [CrossRef]
- Froese, L.; Dian, J.; Batson, C.; Gomez, A.; Unger, B.; Zeiler, F.A. Cerebrovascular Response to Propofol, Fentanyl, and Midazolam in Moderate/Severe Traumatic Brain Injury: A Scoping Systematic Review of the Human and Animal Literature. Neurotrauma Rep. 2020, 1, 100–112. [Google Scholar] [CrossRef]
- Chen, H.I.; Malhotra, N.R.; Oddo, M.; Heuer, G.G.; Levine, J.M.; LeRoux, P.D. Barbiturate infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery 2008, 63, 880–886, discussion 886-7. [Google Scholar] [CrossRef]
- Hazeldine, J.; Naumann, D.N.; Toman, E.; Davies, D.; Bishop, J.R.B.; Su, Z.; Hampson, P.; Dinsdale, R.J.; Crombie, N.; Duggal, N.A.; et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med. 2017, 14, e1002338. [Google Scholar] [CrossRef]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care. Intensive Care Med. 2014, 40, 1189–1209. [Google Scholar] [CrossRef]
- Tajsic, T.; Gvozdanovic, A.; Timofeev, I.; Hutchinson, P. Multimodality Monitoring in Head Injury; Cambridge University Press: Cambridge, UK, 2020; pp. 132–145. [Google Scholar]
- Lazaridis, C.; Robertson, C.S. The Role of Multimodal Invasive Monitoring in Acute Traumatic Brain Injury. Neurosurg. Clin. 2016, 27, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Iqbal, M.; Ligarotti, G.K.I.; Syrmos, N. Breakthrough in the assessment of cerebral perfusion and vascular permeability after brain trauma through the adoption of dynamic indocyanin green-enhanced near-infrared spectroscopy. Quant. Imaging Med. Surg. 2020, 10, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, G.; Sanchez-Mejia, R.O.; Phan, N.; Hemphill, J.C., 3rd; Martin, C.; Manley, G.T. Incorporating a parenchymal thermal diffusion cerebral blood flow probe in bedside assessment of cerebral autoregulation and vasoreactivity in patients with severe traumatic brain injury. J. Neurosurg. 2011, 114, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hutchinson, P.J.; Fryer, T.; Al-Rawi, P.G.; Parry, D.A.; Minhas, P.S.; Kett-White, R.; Kirkpatrick, P.J.; Mathews, J.C.; Downey, S.; et al. Measurement of brain tissue oxygenation performed using positron emission tomography scanning to validate a novel monitoring method. J. Neurosurg. 2002, 96, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Forcione, M.; Chiarelli, A.M.; Davies, D.J.; Perpetuini, D.; Sawosz, P.; Merla, A.; Belli, A. Cerebral perfusion and blood–brain barrier assessment in brain trauma using contrast-enhanced near-infrared spectroscopy with indocyanine green: A review. J. Cereb. Blood Flow Metab. 2020. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Fabiani, M.; Gratton, G. Comparison of procedures for co-registering scalp-recording locations to anatomical magnetic resonance images. J. Biomed. Opt. 2015, 20, 016009. [Google Scholar] [CrossRef]
- Wheelock, M.D.; Culver, J.P.; Eggebrecht, A.T. High-density diffuse optical tomography for imaging human brain function. Rev. Sci. Instrum. 2019, 90, 051101. [Google Scholar] [CrossRef]
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. NeuroImage 2004, 23, S275–S288. [Google Scholar] [CrossRef]
- Weigl, W.; Milej, D.; Gerega, A.; Toczylowska, B.; Kacprzak, M.; Sawosz, P.; Botwicz, M.; Maniewski, R.; Mayzner-Zawadzka, E.; Liebert, A. Assessment of cerebral perfusion in post-traumatic brain injury patients with the use of ICG-bolus tracking method. NeuroImage 2014, 85, 555–565. [Google Scholar] [CrossRef]
- Larach, D.B.; Kofke, W.A.; Le Roux, P. Potential non-hypoxic/ischemic causes of increased cerebral interstitial fluid lactate/pyruvate ratio: A review of available literature. Neurocrit. Care 2011, 15, 609–622. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Jalloh, I.; Helmy, A.; Carpenter, K.L.; Rostami, E.; Bellander, B.M.; Boutelle, M.G.; Chen, J.W.; Claassen, J.; Dahyot-Fizelier, C.; et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med. 2015, 41, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.A.; Das, J.M. Cerebral Perfusion Pressure; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Régnier, M.A.; Raux, M.; Le Manach, Y.; Asencio, Y.; Gaillard, J.; Devilliers, C.; Langeron, O.; Riou, B. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology 2012, 117, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.S.; Buell, T.; Robertson, C.S. Systemic manifestations of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 205–218. [Google Scholar] [PubMed]
- Capatina, C.; Paluzzi, A.; Mitchell, R.; Karavitaki, N. Diabetes Insipidus after Traumatic Brain Injury. J. Clin. Med. 2015, 4, 1448–1462. [Google Scholar] [CrossRef]
- Jameson, J.L.; Kasper, D.L.; Longo, D.L.; Fauci, A.S.; Hauser, S.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine; Mcgraw-hill: New York, NY, USA, 2018. [Google Scholar]
- Bellelli, A.; Carey, J. Reversible Ligand Binding: Theory and Experiment; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Chen, D.; Bao, L.; Lu, S.-Q.; Xu, F. Serum Albumin and Prealbumin Predict the Poor Outcome of Traumatic Brain Injury. PLoS ONE 2014, 9, e93167. [Google Scholar] [CrossRef]
- Bernard, F.; Al-Tamimi, Y.Z.; Chatfield, D.; Lynch, A.G.; Matta, B.F.; Menon, D.K. Serum Albumin Level as a Predictor of Outcome in Traumatic Brain Injury: Potential for Treatment. J. Trauma Acute Care Surg. 2008, 64, 872–875. [Google Scholar] [CrossRef]

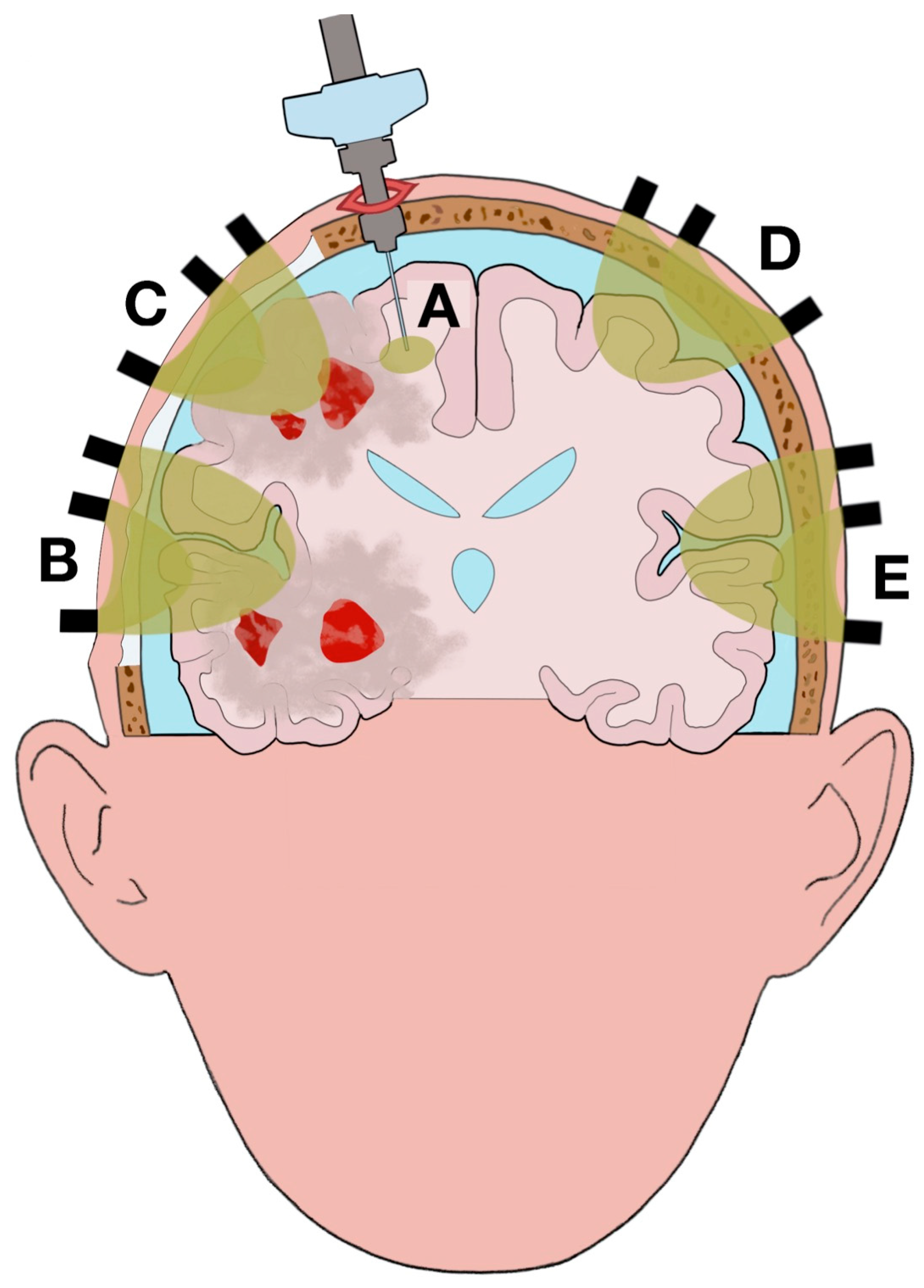
| Type | Time | Region Monitored | Parameters |
|---|---|---|---|
| PbtO2 1 monitor | Continuous | Brain | PbtO2 |
| NIRS 2 | Continuous | Brain | O2Hb 3, HHb 4 |
| Contrast-enhanced NIRS | Intermittent | Brain | ICG 5 |
| CT 6 head | Intermittent | Brain | Structural injuries |
| MRI 7 head | Intermittent | Brain | Structural injuries |
| Microdialysis | Intermittent | Brain | Lactate/pyruvate ratio |
| Arterial cannulation | Continuous | Systemic | MAP 8 |
| ICP 9 monitor | Continuous | Brain | ICP |
| ABG 10 | Intermittent | Systemic | Arterial pH, anion gap |
| Blood sample | Intermittent | Systemic | Hematocrit, electrolytes, plasma proteins, 2,3-DPG 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forcione, M.; Ganau, M.; Prisco, L.; Chiarelli, A.M.; Bellelli, A.; Belli, A.; Davies, D.J. Mismatch between Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring of Tissue Respiration in Acute Brain Trauma: The Rationale for Implementing a Multimodal Monitoring Strategy. Int. J. Mol. Sci. 2021, 22, 1122. https://doi.org/10.3390/ijms22031122
Forcione M, Ganau M, Prisco L, Chiarelli AM, Bellelli A, Belli A, Davies DJ. Mismatch between Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring of Tissue Respiration in Acute Brain Trauma: The Rationale for Implementing a Multimodal Monitoring Strategy. International Journal of Molecular Sciences. 2021; 22(3):1122. https://doi.org/10.3390/ijms22031122
Chicago/Turabian StyleForcione, Mario, Mario Ganau, Lara Prisco, Antonio Maria Chiarelli, Andrea Bellelli, Antonio Belli, and David James Davies. 2021. "Mismatch between Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring of Tissue Respiration in Acute Brain Trauma: The Rationale for Implementing a Multimodal Monitoring Strategy" International Journal of Molecular Sciences 22, no. 3: 1122. https://doi.org/10.3390/ijms22031122
APA StyleForcione, M., Ganau, M., Prisco, L., Chiarelli, A. M., Bellelli, A., Belli, A., & Davies, D. J. (2021). Mismatch between Tissue Partial Oxygen Pressure and Near-Infrared Spectroscopy Neuromonitoring of Tissue Respiration in Acute Brain Trauma: The Rationale for Implementing a Multimodal Monitoring Strategy. International Journal of Molecular Sciences, 22(3), 1122. https://doi.org/10.3390/ijms22031122







