Diversified Biomineralization Roles of Pteria penguin Pearl Shell Lectins as Matrix Proteins
Abstract
1. Introduction
2. Results
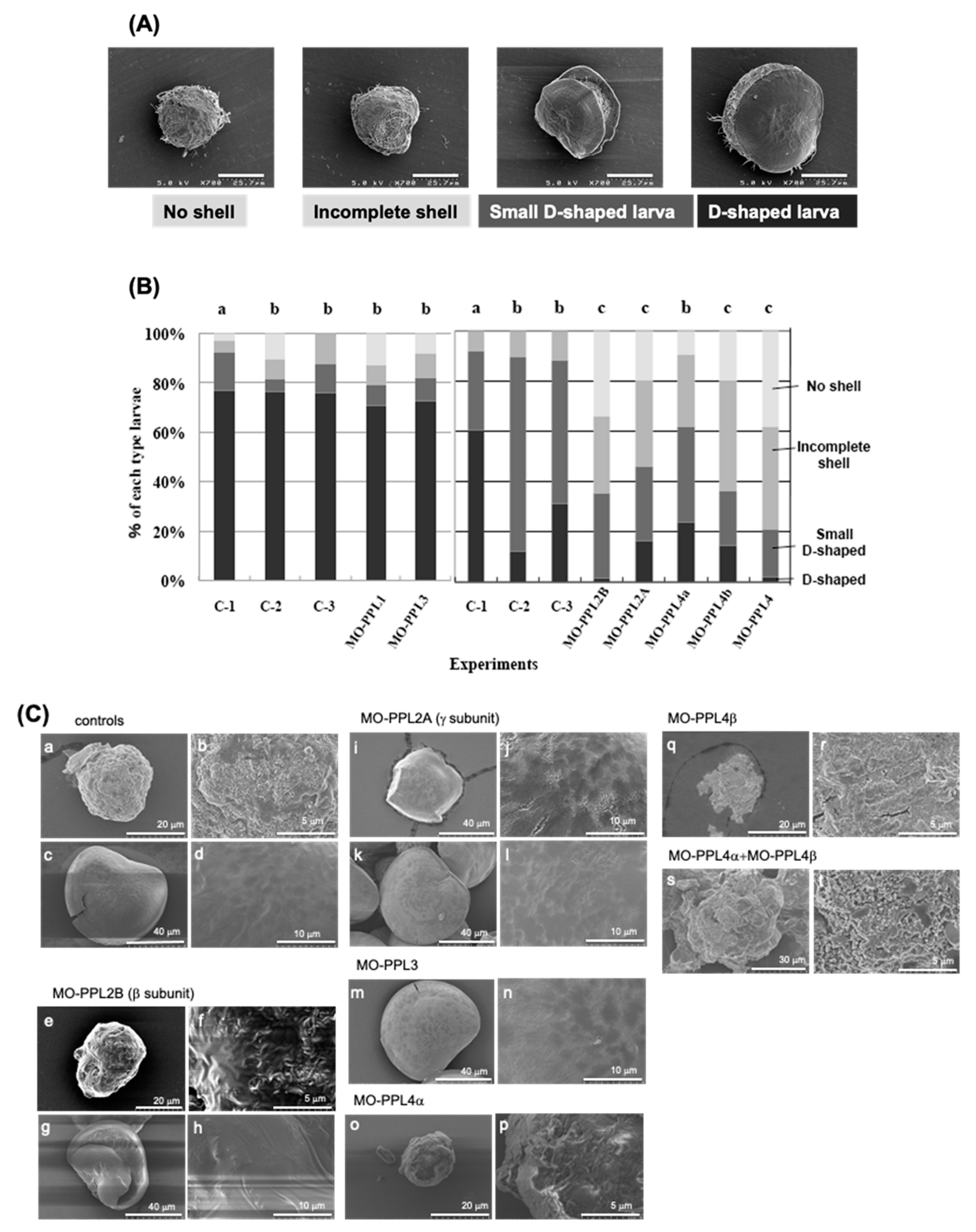
2.1. Knockdown Analysis of PPL3 and PPL4 in the Biomeneralization Processes
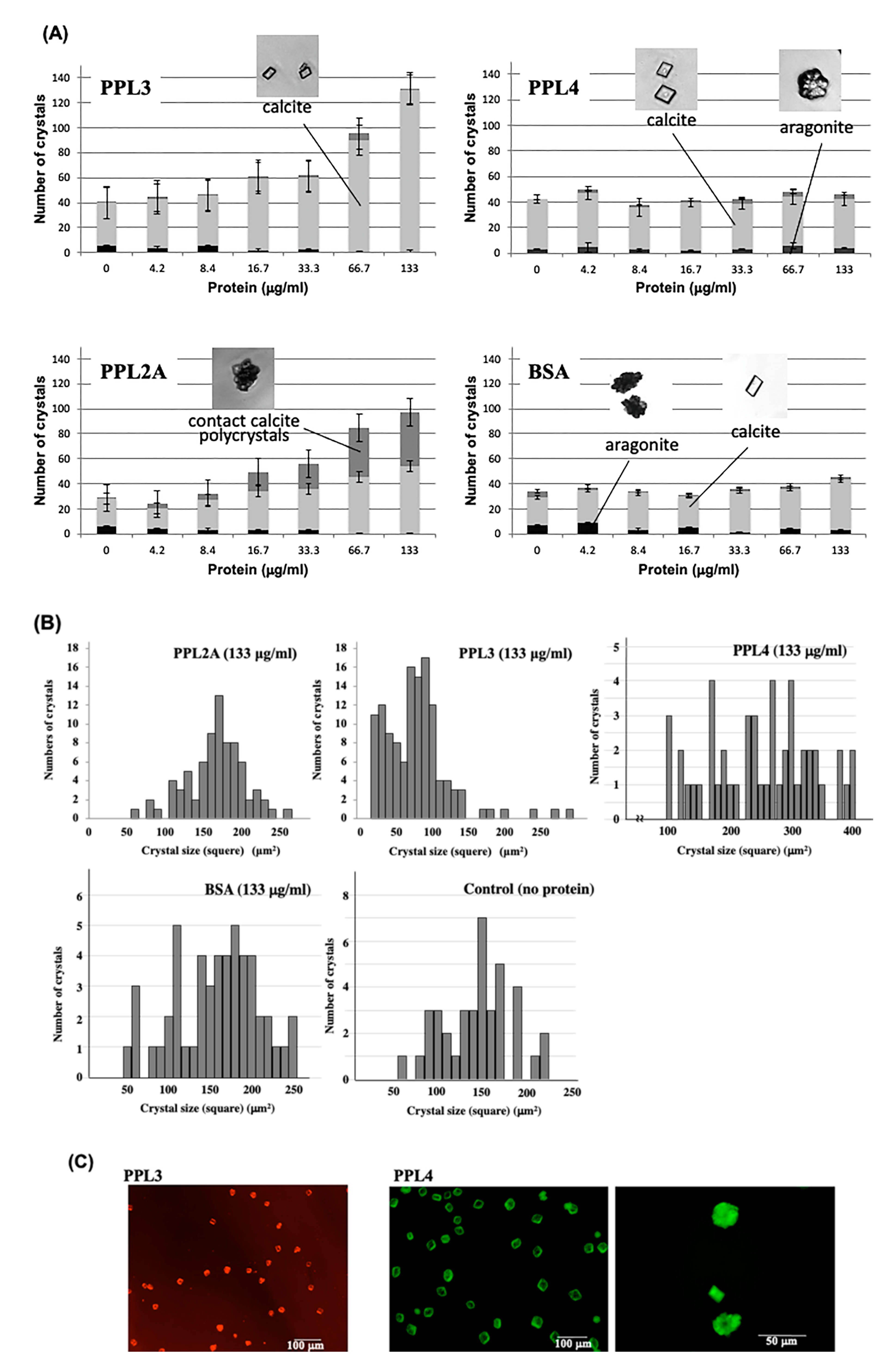
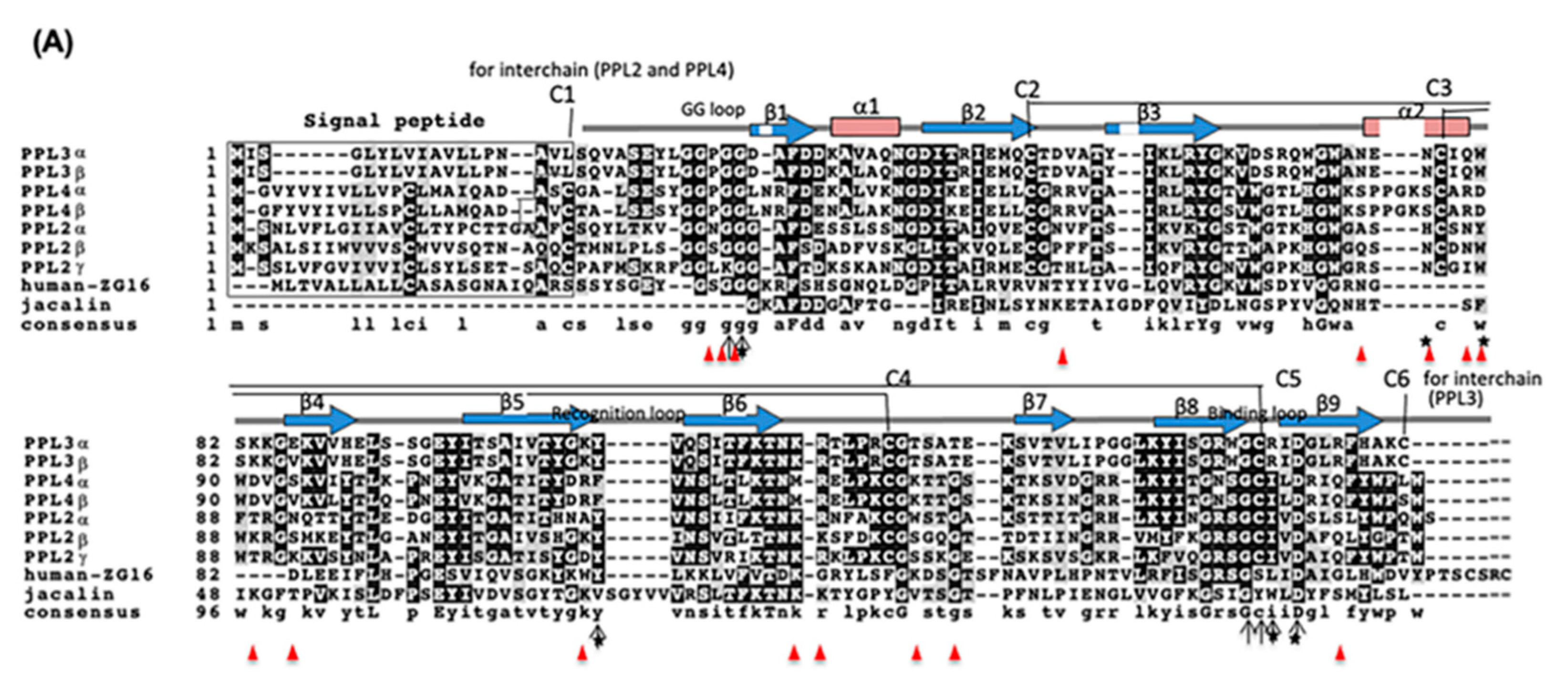
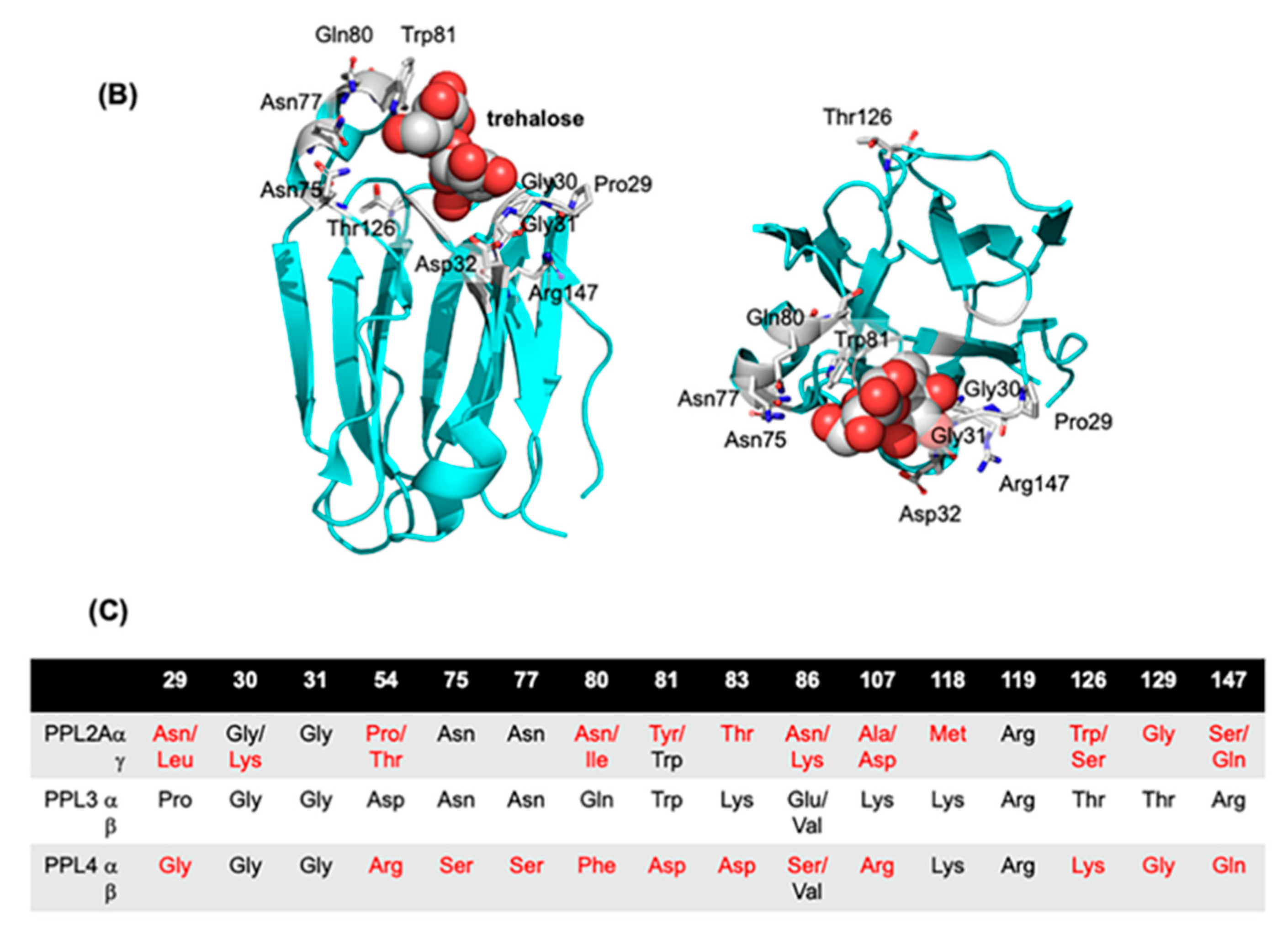
2.2. Molecular Properties of PPL3 and PPL4 on In Vitro Crystallization
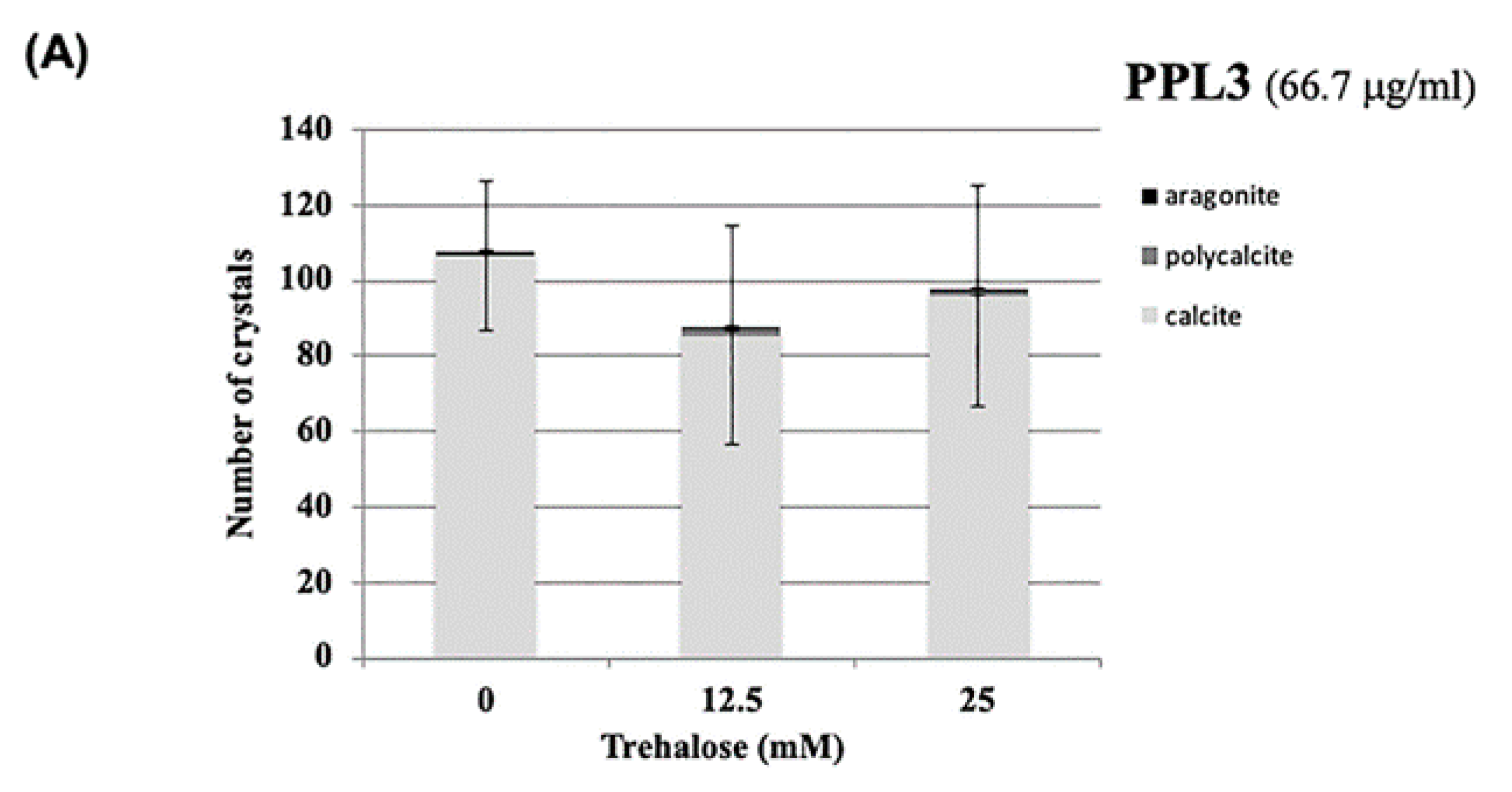
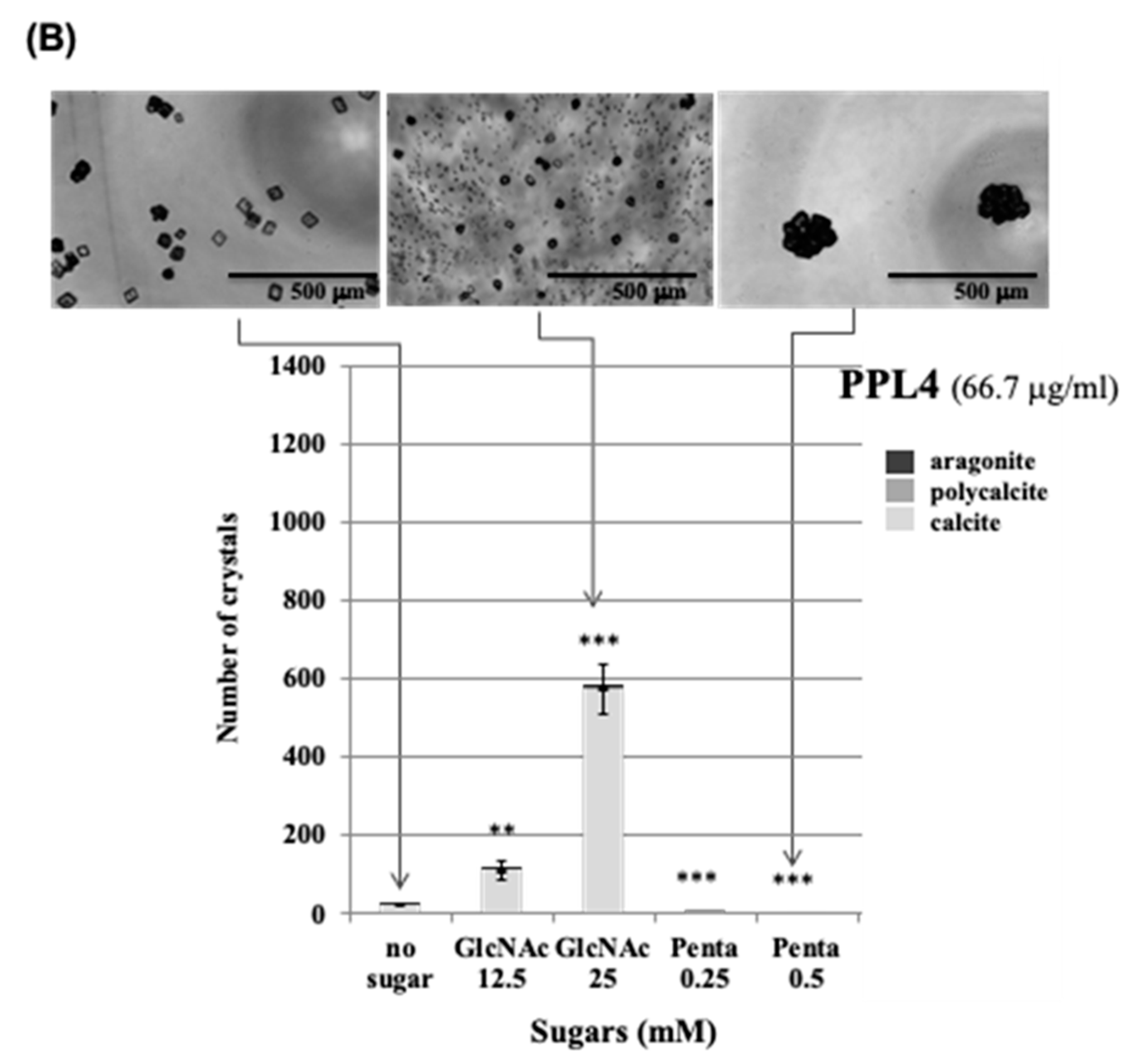
2.3. Effect of Carbohydrates on the Biomeneralization Processes of PPL3 and PPL4
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation of Lectins from Secretory Fluid of Mantle
4.3. Morpholino Design and Morphological Phenotype Analysis of Morphants of P. penguin D-Shaped Larva
4.4. In Vitro Crystallization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Alexa Fluor |
| BSA | bovine serum albumin |
| CRD | carbohydrate recognition domain |
| Fuc | fucose |
| GlcNAc | N-acetyl-D-glucosamine |
| HPLC | high performance liquid chromatography |
| JRL | jacalin related lectin |
| Man | mannose |
| MO | morpholino oligo |
| MS | mass spectrometry |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEM | scanning electron microscopy |
| Xyl | xylose |
| M3FX | Manαl~6(Manαl~3)(Xylβl~2)Manβl~4GlcNAcβ1~4(Fucαl~3)GlcNAc- |
References
- Huang, X.D.; Zhao, M.; Liu, W.G.; Guan, Y.Y.; Shi, Y.; Wang, Q.; Wu, S.Z.; He, M.X. Gigabase-scale transcriptome analysis on four species of pearl oysters. Mar. Biotechnol. 2013, 15, 253–264. [Google Scholar] [CrossRef]
- Li, H.; Liua, B.; Huang, G.; Fan, S.; Zhang, B.; Su, J.; Yu, D. Characterization of transcriptome and identification of biomineralization genes in winged pearl oyster (Pteria penguin) mantle tissue. Comp. Biochem. Physiol. Part D 2017, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Hoshino, W.; Shikanai, Y.; Sato, R.; Liu, K.; Sato, S.; Muramoto, K.; Osada, M.; Yoshimi, Y.; Ogawa, T. Novel matrix proteins of Pteria penguin pearl oyster shell nacre homologous to the Jacalin-related β-prism fold lectins. PLoS ONE 2014, 9, e112326. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Sato, R.; Naganuma, T.; Liu, K.; Lakudzala, A.E.; Muramoto, K.; Osada, M.; Yoshimi, K.; Hiemori, K.; Hirabayashi, J.; et al. Glycan Binding Profiling of Jacalin-Related Lectins from the Pteria penguin Pearl Shell. Int. J. Mol. Sci. 2019, 20, 4629. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Shionyu, M.; Ogawa, T.; Shirai, T. Structures of jacalin-related lectin PPL3 regulating pearl shell Biomineralization. Proteins 2018, 86, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sakuda, S.; Nagasawa, H. Identification of chitin in the prismatic layer of the shell and a chitin synthase gene from the Japanese pearl oyster, Pinctada fucata. Biosci. Biotechnol. Biochem. 2007, 71, 1735–1744. [Google Scholar] [CrossRef]
- Weiss, I.M.; Schönitzer, V.; Eichner, N.; Sumper, M. The chitin synthase involved in marine bivalve mollusk shell formation contains a myosin domain. FEBS Lett. 2006, 580, 1846–1852. [Google Scholar] [CrossRef]
- Weiss, I.M.; Lüke, F.; Eichner, N.; Guth, C.; Clausen-Schaumann, H. On the function of chitin synthase extracellular domains in biomineralization. J. Struct. Biol. 2013, 183, 216–225. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Furuhashi, T.; Schwarzinger, C.; Miksik, I.; Smrz, M.; Beran, A. Molluscan shell evolution with review of shell calcification hypothesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 351–371. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Falini, G.; Addadi, L.; Weiner, S. Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using Cryo-TEM. J. Struct. Biol. 2001, 135, 8–17. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W. X-ray diffraction study of the insoluble organic matrix of mollusk shells. FEBS Lett. 1980, 111, 311–316. [Google Scholar] [CrossRef]
- Weiss, I.M.; Renner, C.; Strigl, M.G.; Fritz, M. A simple and reliable method for the determination and localization of chitin in abalone nacre. Chem. Mater. 2002, 14, 3252–3259. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Shir, I.B.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within bivalve shells: Is chitin abundant? Acta Biomater. 2018, 80, 176–187. [Google Scholar] [CrossRef]
- Giuffrea, A.J.; Hamm, L.M.; Hana, N.; De Yoreo, J.J.; Dove, P.M. Polysaccharide chemistry regulates kinetics of calcite nucleation through competition of interfacial energies. Proc. Natl. Acad. Sci. USA 2013, 110, 9261–9266. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.S. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef]
- Suzuki, M.; Saruwatari, K.; Kogure, T.; Yamamoto, Y.; Nishimura, T.; Kato, T.; Nagasawa, H. An acidic matrix protein, Pif, is a key macromolecule for nacre formation. Science 2009, 325, 1388–1390. [Google Scholar] [CrossRef]
- Bahn, S.Y.; Jo, B.H.; Hwang, B.H.; Choi, Y.S.; Cha, H.J. Role of Pif97 in Nacre Biomineralization: In Vitro Characterization of Recombinant Pif97 as a Framework Protein for the Association of Organic−Inorganic Layers in Nacre. Cryst. Growth Des. 2015, 15, 3666–3673. [Google Scholar] [CrossRef]
- Montagnani, C.; Marie, B.; Marin, F.; Belliard, C.; Riquet, F.; Tayalé, A.; Zanella-Cléon, I.; Fleury, E.; Gueguen, Y.; Piquemal, D.; et al. Pmarg-Pearlin is a Matrix Protein Involved in Nacre Framework Formation in the Pearl Oyster Pinctada margaritifera. ChemBioChem 2011, 12, 2033–2043. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, J.; Pu, J.; Liu, X.; Li, J. Hichin, a chitin binding protein is essential for the self-assembly of organic frameworks and calcium carbonate during shell formation. Int. J. Biol. Macromol. 2019, 135, 745–751. [Google Scholar] [CrossRef]
- Arroyo-Loranca, R.G.; Hernandez-Saavedra, N.Y.; Hernandez-Adame, L.; Rivera-Perez, C. Ps19, a novel chitin binding protein from Pteria sterna capable to mineralize aragonite plates in vitro. PLoS ONE 2020, 15, e0230431. [Google Scholar] [CrossRef] [PubMed]
- Treccani, L.; Mann, K.; Heinemann, F.; Fritz, M. Perlwapin, an Abalone Nacre Protein with Three Four-Disulfide Core (Whey Acidic Protein) Domains, Inhibits the Growth of Calcium Carbonate Crystals. Biophys. J. 2006, 91, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Siedler, F.; Treccani, L.; Heinemann, F.; Fritz, M. Perlinhibin, a Cysteine-, Histidine-, and Arginine-Rich Miniprotein from Abalone (Haliotis laevigata) Nacre, Inhibits In Vitro Calcium Carbonate Crystallization. Biophys. J. 2007, 93, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Joubert, C.; Piquemal, D.; Marie, B.; Manchon, L.; Pierrat, F.; Zanella-Cléon, I.; Cochennec-Laureau, N.; Gueguen, Y.; Montagnani, C. Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: Focus on biomineralization. BMC Genom. 2010, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Wang, N.; Inoue, H.; Maeyama, K.; Okamoto, K.; Nagai, K.; Kondo, H.; Hirono, I.; Asakawa, S.; Watabe, S. Deep Sequencing of ESTs from Nacreous and Prismatic Layer Producing Tissues and a Screen for Novel Shell Formation-Related Genes in the Pearl Oyster. PLoS ONE 2011, 6, e21238. [Google Scholar] [CrossRef] [PubMed]
- Marie, B.; Joubert, C.; Tayaléa, A.; Zanella-Cléonc, I.; Belliarda, C.; Piquemald, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kawashima, T.; Koyanagi, R.; Gyoja, F.; Tanaka, M.; Ikuta, T.; Shoguchi, E.; Fujiwara, M.; Shinzato, C.; Hisata, K.; et al. Draft Genome of the Pearl Oyster Pinctada fucata: A Platform for Understanding Bivalve Biology. DNA Res. 2012, 19, 117–130. [Google Scholar] [CrossRef]
- Marie, B.; Arivalagan, J.; Mathéron, L.; Bolbach, G.; Berland, S.; Marie, S.; Marin, F. Deep conservation of bivalve nacre proteins highlighted by shell matrix proteomics of the Unionoida Elliptio complanata and Villosa lienosa. J. R. Soc. Interface 2017, 14, 20160846. [Google Scholar] [CrossRef]
- Mann, K.; Cerveau, N.; Gummich, M.; Fritz, M.; Mann, M.; Jackson, D.J. In-depth proteomic analyses of Haliotis laevigata (greenlip abalone) nacre and prismatic organic shell matrix. Proteome Sci. 2018, 16, 11. [Google Scholar] [CrossRef]
- Liao, Z.; Jiang, Y.-T.; Sun, Q.; Fan, M.-H.; Wang, J.-X.; Liang, H.-Y. Microstructure and in-depth proteomic analysis of Perna viridis shell. PLoS ONE 2019, 14, e0219699. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Wang, L.; Song, L. Recent Advances of Shell Matrix Proteins and Cellular Orchestration in Marine Molluscan Shell Biomineralization. Front. Mar. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, T.; Sato, R.; Naganuma, T.; Liu, K.; Sato, S.; Sakaue, S.; Osada, M.; Yoshimi, K.; Muramoto, K. Diversified Biomineralization Roles of Pteria penguin Pearl Shell Lectins as Matrix Proteins. Int. J. Mol. Sci. 2021, 22, 1081. https://doi.org/10.3390/ijms22031081
Ogawa T, Sato R, Naganuma T, Liu K, Sato S, Sakaue S, Osada M, Yoshimi K, Muramoto K. Diversified Biomineralization Roles of Pteria penguin Pearl Shell Lectins as Matrix Proteins. International Journal of Molecular Sciences. 2021; 22(3):1081. https://doi.org/10.3390/ijms22031081
Chicago/Turabian StyleOgawa, Tomohisa, Rie Sato, Takako Naganuma, Kayeu Liu, Saho Sato, Shizuka Sakaue, Makoto Osada, Kyosuke Yoshimi, and Koji Muramoto. 2021. "Diversified Biomineralization Roles of Pteria penguin Pearl Shell Lectins as Matrix Proteins" International Journal of Molecular Sciences 22, no. 3: 1081. https://doi.org/10.3390/ijms22031081
APA StyleOgawa, T., Sato, R., Naganuma, T., Liu, K., Sato, S., Sakaue, S., Osada, M., Yoshimi, K., & Muramoto, K. (2021). Diversified Biomineralization Roles of Pteria penguin Pearl Shell Lectins as Matrix Proteins. International Journal of Molecular Sciences, 22(3), 1081. https://doi.org/10.3390/ijms22031081





