Effects of Osthole on Inflammatory Gene Expression and Cytokine Secretion in Histamine-Induced Inflammation in the Caco-2 Cell Line
Abstract
:1. Introduction
2. Results
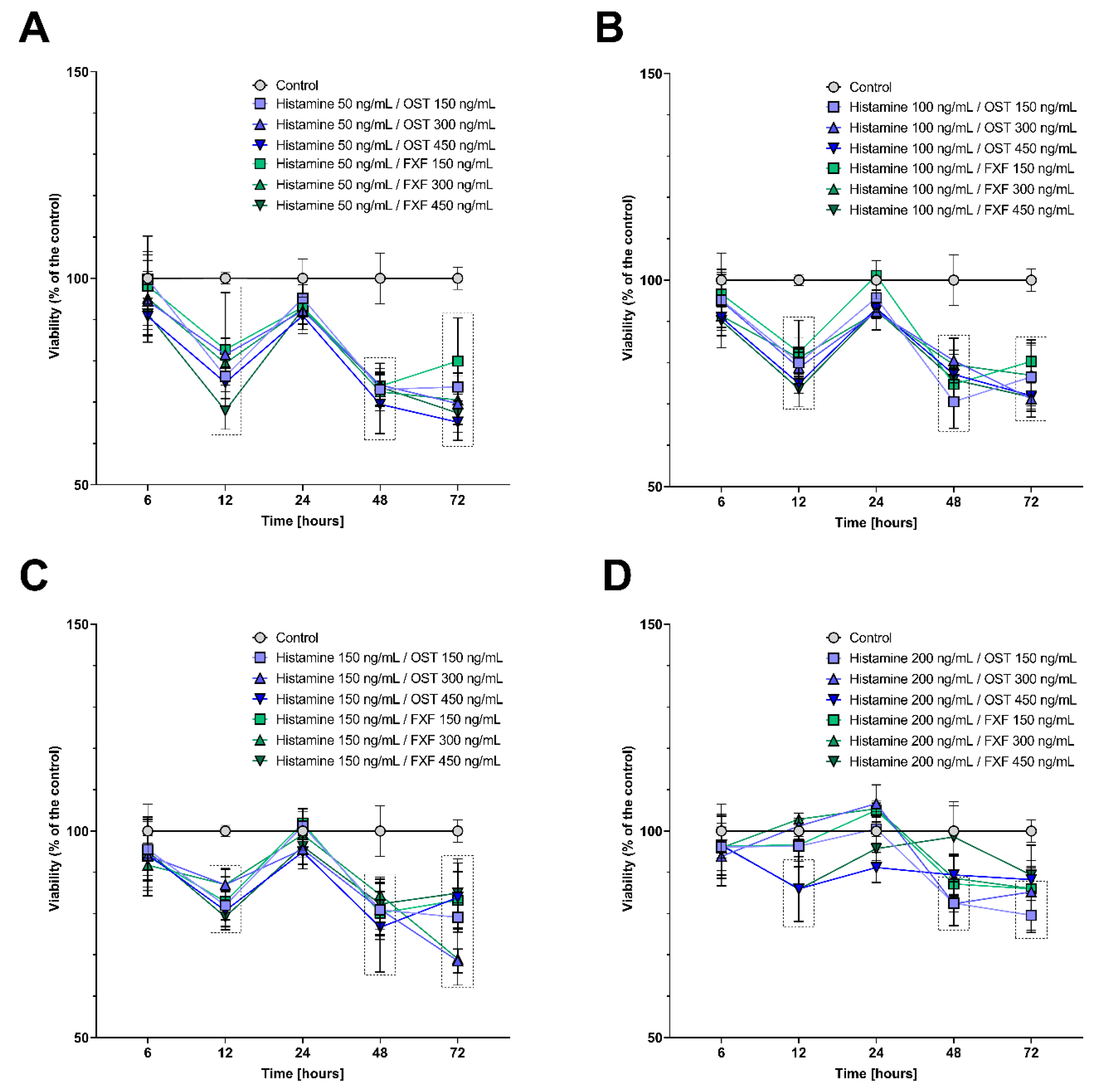
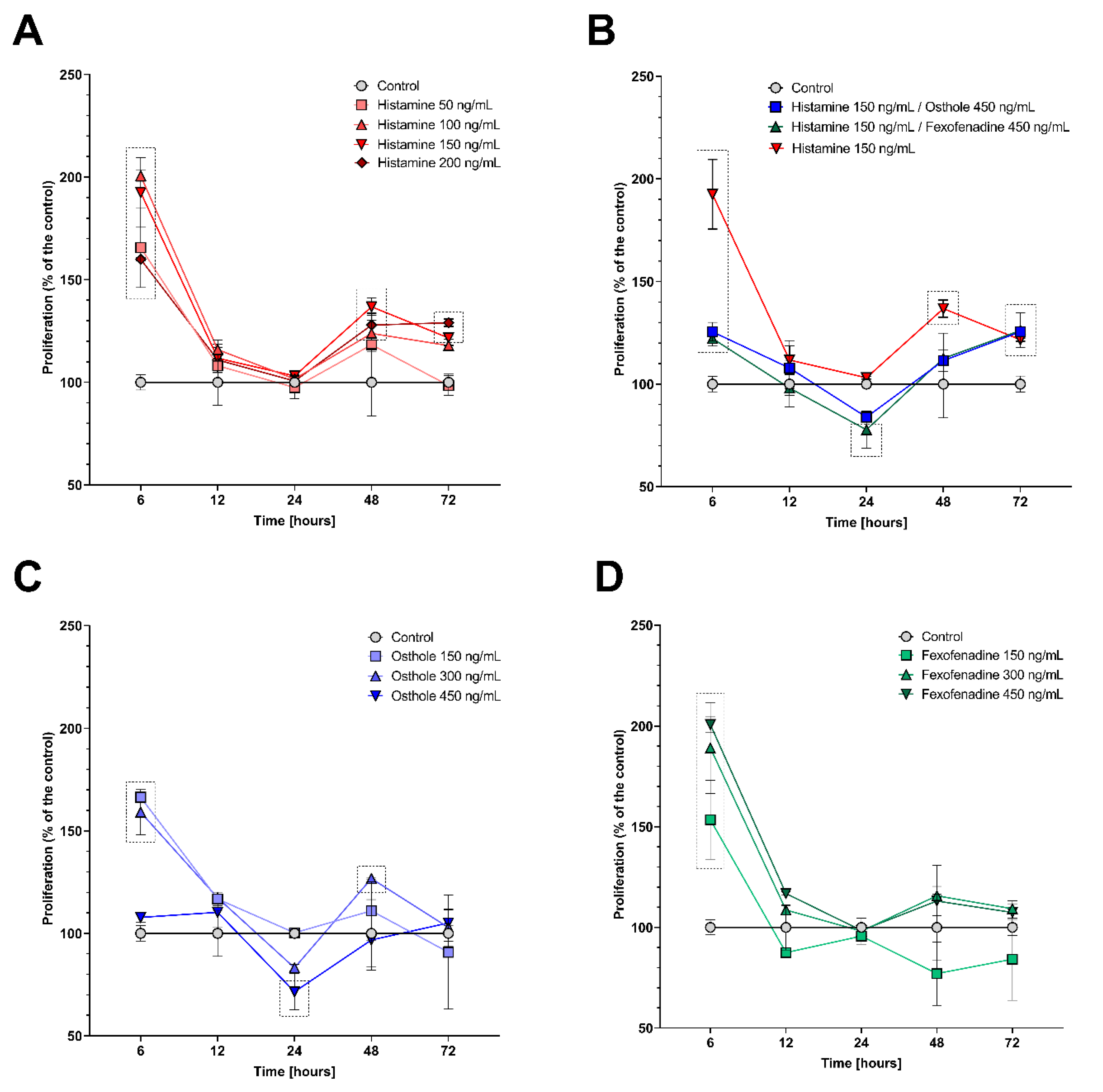
2.1. Histamine, Fexofenadine, and Osthole Do Not Affect Cell Viability and Proliferation in Time Point Chosen for Further Analyses
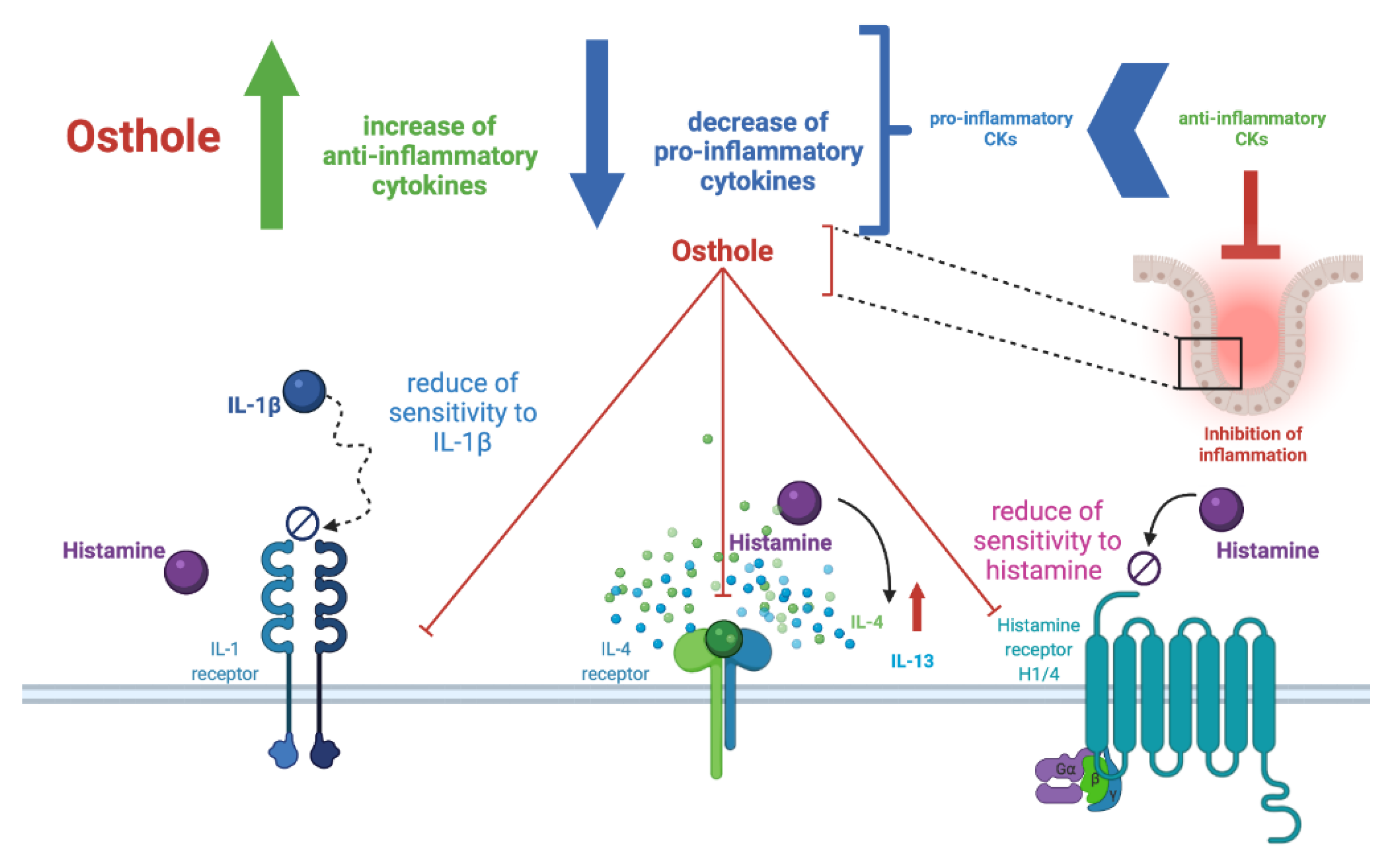
2.2. Osthole Decreases the Level of Pro-Inflammatory (IL-1β, IL-6, IL-8, TNF-α), but Increases the Level of Anti-Inflammatory CKs (IL-4, IL-10, IL-13) in the Histamine-Treated Caco-2 Cell Line
2.3. Osthole at the Lowest Concentration Decreases HRH1, HRH4, IL1R1, IL4R, NFκB, and COX-2 Expression
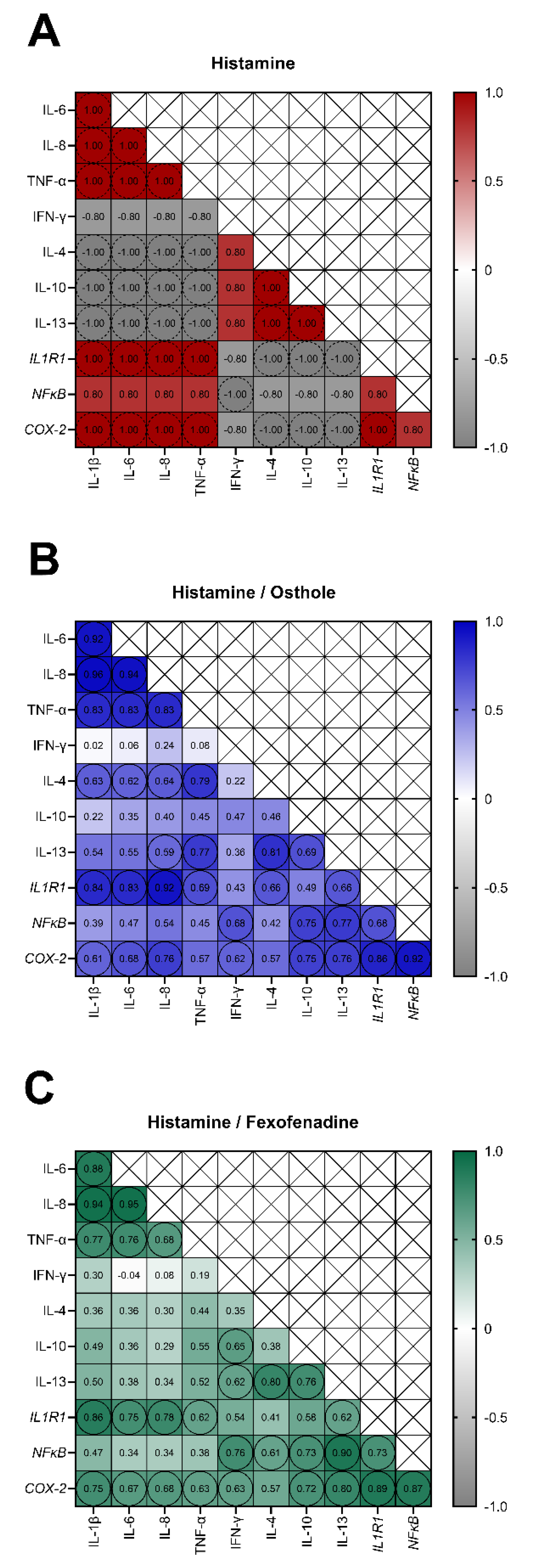
2.4. The Secretion of IL-1β, IL-6, IL-8, TNF-α, and the Expression of IL1R1 and COX-2 Are Interdependent in Osthole-Treated Caco-2 with Histamine-Induced Inflammation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cells Viability Analysis
4.4. Cells Proliferation Analysis
4.5. Incubation of Caco-2 Cells with Examined Substances
4.6. Post-Culture Media Collection and Total RNA Isolation
4.7. Reverse Transcription and Quantitative Real-Time PCR (qPCR)
4.8. Analysis of Cytokines Level
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTB | Actin beta |
| ASC | Apoptosis-associated speck-like protein containing CARD |
| ASD | Autism spectrum disorder |
| BrdU | Bromodeoxyuridine |
| CD | Crohn’s disease |
| CK | Cytokine |
| COX-2 | Cyclooxygenase 2 |
| DAMPs | Damage-associated molecular patterns |
| DEPC | Diethyl pyrocarbonate |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| FXF | Fexofenadine hydrochloride |
| HPA axis | Hypothalamic-pituitary-adrenal axis |
| HRH1 | Histamine receptor H1 |
| HRH4 | Histamine receptor H4 |
| IBD | Inflammatory bowel disease |
| IFN-γ | Interferon-gamma |
| IL1R1 | Interleukin 1 receptor type 1 |
| IL4R | Interleukin 4 receptor |
| LPS | Lipopolysaccharides |
| MTT | Methyl thiazolyl tetrazolium |
| NFκB | Nuclear factor-kappa B |
| NLR | Nod-like receptor |
| NLRP3 | NLR family pyrin domain containing protein 3 |
| PAMPs | Pathogen-associated molecular patterns |
| PBMCs | Peripheral blood mononuclear cells |
| PPAR | Peroxisome proliferator-activated receptor |
| TNF-α | Tumor necrosis factor-alpha |
| UC | Ulcerative colitis |
References
- Thoreson, R.; Cullen, J.J. Pathophysiology of inflammatory bowel disease: An overview. Surg. Clin. N. A. 2007, 87, 575–585. [Google Scholar] [CrossRef]
- Gentili, M.; Hidalgo-Garcia, L.; Vezza, T.; Ricci, E.; Migliorati, G.; Rodriguez-Nogales, A.; Riccardi, C.; Galvez, J.; Ronchetti, S. A recombinant glucocorticoid-induced leucine zipper protein ameliorates symptoms of dextran sulfate sodium-induced colitis by improving intestinal permeability. FASEB J. 2021, 35, e21950. [Google Scholar] [CrossRef]
- Cevallos, S.A.; Lee, J.Y.; Velazquez, E.M.; Foegeding, N.J.; Shelton, C.D.; Tiffany, C.R.; Parry, B.H.; Stull-Lane, A.R.; Olsan, E.E.; Savage, H.P.; et al. 5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli Expansion by Activating PPAR-γ Signaling in the Intestinal Epithelium. mBio 2021, 12, e03227-20. [Google Scholar] [CrossRef]
- Dalal, R.S.; Njie, C.; Marcus, J.; Gupta, S.; Allegretti, J.R. Predictors of Ustekinumab Failure in Crohn’s Disease After Dose Intensification. Inflamm. Bowel Dis. 2021, 27, 1294–1301. [Google Scholar] [CrossRef]
- Effenberger, M.; Reider, S.; Waschina, S.; Bronowski, C.; Enrich, B.; Adolph, T.E.; Koch, R.; Moschen, A.R.; Rosenstiel, P.; Aden, K.; et al. Microbial Butyrate Synthesis Indicates Therapeutic Efficacy of Azathioprine in IBD Patients. J. Crohns Colitis 2021, 15, 88–98. [Google Scholar] [CrossRef]
- Wei, X.; Zhuang, J.; Li, N.; Zheng, B.; Sun, H.; Cai, J.; Huang, X.; Zhang, G. NUDT15 genetic testing-guided 6-mercaptopurine dosing in children with ALL likely to be cost-saving in China. Int. J. Hematol. 2021, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, P.; Limdi, J.; Akbar, A.; Tucknott, S.; Kahol, D.N. Ulcerative colitis: Understanding the impact of ulcerative colitis on everyday life and exploring the unmet needs of patients. Curr. Med. Res. Opin. 2021, 37, 1901–1911. [Google Scholar] [CrossRef]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Spagnuolo, R.; Dastoli, S.; Silvestri, M.; Cosco, C.; Garieri, P.; Bennardo, L.; Nisticò, S.P. Anti-interleukin 12/23 in the treatment of erythema nodosum and Crohn disease: A case report. Dermatol. Ther. 2019, 32, e12811. [Google Scholar] [CrossRef]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Atreya, R.; Neurath, M.F.; Siegmund, B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front. Med. 2020, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, Y.; Shi, K.; Wang, H.; Lu, J.; Li, Y.; Ma, C. Osthole inhibits proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis. J. Biomed. Res. 2015, 29, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Che, Y.; Li, J.; Li, Z.; Wang, S.; Yan, Y.; Zou, K.; Zou, L. Osthole enhances antitumor activity and irradiation sensitivity of cervical cancer cells by suppressing ATM/NF-κB signaling. Oncol. Rep. 2018, 40, 737–747. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Zhang, Y. Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT. PLoS ONE 2018, 13, e0193449. [Google Scholar] [CrossRef] [PubMed]
- Abosharaf, H.A.; Diab, T.; Atlam, F.M.; Mohamed, T.M. Osthole extracted from a citrus fruit that affects apoptosis on A549 cell line by histone deacetylasese inhibition (HDACs). Biotechnol. Rep. 2020, 28, e00531. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. Cytokine production by PBMC and serum from allergic and non-allergic subjects following in vitro histamine stimulation to test fexofenadine and osthole anti-allergic properties. Eur. J. Pharmacol. 2016, 791, 763–772. [Google Scholar] [CrossRef]
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, P.; Chen, D.; et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef]
- Wu, S.J. Osthole Attenuates Inflammatory Responses and Regulates the Expression of Inflammatory Mediators in HepG2 Cells Grown in Differentiated Medium from 3T3-L1 Preadipocytes. J. Med. Food 2015, 18, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Kostyra, E.; Matysiewicz, M.; Cieślińska, A.; Jarmołowska, B. Impact of fexofenadine, osthole and histamine on peripheral blood mononuclear cell proliferation and cytokine secretion. Eur. J. Pharmacol. 2015, 761, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Kostyra, E.; Cieślińska, A.; Matysiewicz, M.; Fiedorowicz, E.; Sienkiewicz-Szłapka, E. Changes in gene expression induced by histamine, fexofenadine and osthole: Expression of histamine H. Immunobiology 2017, 222, 571–581. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B.; Piskorz-Ogórek, K.; Kostyra, E. Cytokines concentrations in serum samples from allergic children-Multiple analysis to define biomarkers for better diagnosis of allergic inflammatory process. Immunobiology 2018, 223, 648–657. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Piskorz-Ogórek, K.; Moszyńska, M.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. Serum cytokine levels in children with spectrum autism disorder: Differences in pro- and anti-inflammatory balance. J. Neuroimmunol. 2019, 337, 577066. [Google Scholar] [CrossRef]
- Kordulewska, N.K.; Kostyra, E.; Chwała, B.; Moszyńska, M.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. A novel concept of immunological and allergy interactions in autism spectrum disorders: Molecular, anti-inflammatory effect of osthole. Int. Immunopharmacol. 2019, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B.; Kostyra, E. High Expression of IL-1RI and EP2; Receptors in the IL-1β/COX-2 Pathway, and a New Alternative to Non-Steroidal Drugs-Osthole in Inhibition COX-2. Int. J. Mol. Sci. 2019, 20, 186. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Zhou, H.; Zhang, Y.; Yang, P.; Bai, H.; Zhang, T.; Hua, J.; Zhang, L.; Liu, Y.; Xie, X.; et al. Osthole ameliorates simulated microgravity-induced bone loss through down-regulation of miR-34c-5p. Acta Astronaut. 2021, 183, 141–152. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, L.; Yao, Y.; Li, S.; Tao, Z.; Yan, Y.; Yang, J. Osthole decreases beta amyloid levels through up-regulation of miR-107 in Alzheimer’s disease. Neuropharmacology 2016, 108, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.B.; Zhou, J.; Qu, Y.; Li, X.; Lu, C.T.; Xie, K.L.; Sun, X.L.; Fei, Z. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem. Int. 2010, 57, 206–215. [Google Scholar] [CrossRef]
- Huang, R.L.; Chen, C.C.; Huang, Y.L.; Hsieh, D.J.; Hu, C.P.; Chen, C.F.; Chang, C. Osthole increases glycosylation of hepatitis B surface antigen and suppresses the secretion of hepatitis B virus in vitro. Hepatology 1996, 24, 508–515. [Google Scholar] [CrossRef]
- Song, F.; Xie, M.L.; Zhu, L.J.; Zhang, K.P.; Xue, J.; Gu, Z.L. Experimental study of osthole on treatment of hyperlipidemic and alcoholic fatty liver in animals. World J. Gastroenterol. 2006, 12, 4359–4363. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, J.; Wang, H.; Zhang, Y.; Xie, M. Osthole improves alcohol-induced fatty liver in mice by reduction of hepatic oxidative stress. Phytother Res. 2011, 25, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Topa, J.; Tańska, M.; Cieślińska, A.; Fiedorowicz, E.; Savelkoul, H.F.J.; Jarmołowska, B. Modulatory Effects of Osthole on Lipopolysaccharides-Induced Inflammation in Caco-2 Cell Monolayer and Co-Cultures with THP-1 and THP-1-Derived Macrophages. Nutrients 2020, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Kitts, D.D. Antioxidant and anti-inflammatory activities of Maillard reaction products isolated from sugar-amino acid model systems. J. Agric. Food Chem. 2011, 59, 11294–11303. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Ehl, J.; Bretzel, R.G.; Kunz, C. Food derived carbonyl compounds affect basal and stimulated secretion of interleukin-6 and -8 in Caco-2 cells. Eur. J. Nutr. 2009, 48, 499–503. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.M.; Jing, H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar-amino acid maillard reaction products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef]
- Noda, T.; Iwakiri, R.; Fujimoto, K.; Rhoads, C.A.; Aw, T.Y. Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif. 2002, 35, 117–129. [Google Scholar] [CrossRef]
- Ramos, S.; Rodríguez-Ramiro, I.; Martín, M.A.; Goya, L.; Bravo, L. Dietary flavanols exert different effects on antioxidant defenses and apoptosis/proliferation in Caco-2 and SW480 colon cancer cells. Toxicol. In Vitro 2011, 25, 1771–1781. [Google Scholar] [CrossRef] [Green Version]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 cells as a model for intestinal absorption. Curr. Protoc. Toxicol. 2011, 47, 20–26. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Phytochemical and Over-The-Counter Drug Interactions: Involvement of Phase I and II Drug-Metabolizing Enzymes and Phase III Transporters. J. Med. Food 2021, 24, 786–805. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; He, S.H. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J. Gastroenterol. 2005, 11, 2851–2857. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Szabo, A.; Hsu, C.L.; Krier-Burris, R.A.; Schroeder, H.A.; Wang, M.Y.; Carter, R.G.; Velez, T.E.; Aguiniga, L.M.; Brown, J.B.; et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal. Immunol. 2018, 11, 861–870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, H.E.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Chen, T.J.; Wang, Y.P.; Chen, M.H. Inflammatory bowel disease is associated with higher dementia risk: A nationwide longitudinal study. Gut 2021, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.N.; Pavlov, V.A. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J. Neurochem. 2021, 158, 1359–1380. [Google Scholar] [CrossRef]
- Webster, J.C.; Oakley, R.H.; Jewell, C.M.; Cidlowski, J.A. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: A mechanism for the generation of glucocorticoid resistance. Proc. Natl. Acad. Sci. USA 2001, 98, 6865–6870. [Google Scholar] [CrossRef] [Green Version]
- Capuron, L.; Raison, C.L.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Miller, A.H. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am. J. Psychiatry 2003, 160, 1342–1345. [Google Scholar] [CrossRef]
- Simpson, K.; Jarvis, B. Fexofenadine: A review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs 2000, 59, 301–321. [Google Scholar] [CrossRef]
- Cianchi, F.; Cortesini, C.; Schiavone, N.; Perna, F.; Magnelli, L.; Fanti, E.; Bani, D.; Messerini, L.; Fabbroni, V.; Perigli, G.; et al. The role of cyclooxygenase-2 in mediating the effects of histamine on cell proliferation and vascular endothelial growth factor production in colorectal cancer. Clin. Cancer Res. 2005, 11, 6807–6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, S.A.; Wilkinson, L.J.; Robertson, J.F.; Hardcastle, J.D. Effect of histamine on the growth of human gastrointestinal tumours: Reversal by cimetidine. Gut 1993, 34, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Lügering, N.; Kucharzik, T.; Kraft, M.; Winde, G.; Sorg, C.; Stoll, R.; Domschke, W. Interleukin (IL)-13 and IL-4 are potent inhibitors of IL-8 secretion by human intestinal epithelial cells. Dig. Dis. Sci. 1999, 44, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Chun, J.; Kim, J.S. Fexofenadine regulates nuclear factor-κB signaling and endoplasmic reticulum stress in intestinal epithelial cells and ameliorates acute and chronic colitis in mice. J. Pharmacol. Exp. Ther. 2015, 352, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, R.; Chen, Y.; Hettinghouse, A.; Liu, C. Cytosolic Phospholipase A2 Is Required for Fexofenadine’s Therapeutic Effects against Inflammatory Bowel Disease in Mice. Int. J. Mol. Sci. 2021, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Dong, W.P.; Bi, S.H.; Pan, Z.G.; Yu, H.; Wang, X.W.; Ma, T.; Wang, J.; Zhang, W.D. Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. Int. J. Mol. Med. 2013, 32, 365–372. [Google Scholar] [CrossRef]
- Heller, N.M.; Matsukura, S.; Georas, S.N.; Boothby, M.R.; Rothman, P.B.; Stellato, C.; Schleimer, R.P. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004, 31, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Madsen, K.L.; Lewis, S.A.; Tavernini, M.M.; Hibbard, J.; Fedorak, R.N. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997, 113, 151–159. [Google Scholar] [CrossRef]
- Oshima, T.; Laroux, F.S.; Coe, L.L.; Morise, Z.; Kawachi, S.; Bauer, P.; Grisham, M.B.; Specian, R.D.; Carter, P.; Jennings, S.; et al. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 2001, 61, 130–143. [Google Scholar] [CrossRef]
- Mazzon, E.; Puzzolo, D.; Caputi, A.P.; Cuzzocrea, S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol. Med. 2002, 8, 353–366. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Albanesi, C.; Fluhr, J.; Gisondi, P.; Norgauer, J.; Girolomoni, G. H1 histamine receptor mediates inflammatory responses in human keratinocytes. J. Allergy Clin. Immunol. 2004, 114, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.A.; Schoonus, S.B.; Smit, M.J.; Timmerman, H.; Leurs, R. Histamine H(1)-receptor activation of nuclear factor-kappa B: Roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol. Pharmacol. 2001, 60, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Hong, C.Y.; Yu, C.L. Decreased serum IgE level, decreased IFN-gamma and IL-5 but increased IL-10 production, and suppressed cyclooxygenase 2 mRNA expression in patients with perennial allergic rhinitis after treatment with a new mixed formula of Chinese herbs. Int. Immunopharmacol. 2001, 1, 1173–1182. [Google Scholar] [CrossRef]
- Caioni, G.; Viscido, A.; d’Angelo, M.; Panella, G.; Castelli, V.; Merola, C.; Frieri, G.; Latella, G.; Cimini, A.; Benedetti, E. Inflammatory Bowel Disease: New Insights into the Interplay between Environmental Factors and PPARγ. Int. J. Mol. Sci. 2021, 22, 985. [Google Scholar] [CrossRef]
- Su, G.; Luo, Y.; Chen, D.; Yu, B.; He, J. NF-κB-dependent induction of porcine β-defensin 114 regulates intestinal epithelium homeostasis. Int. J. Biol. Macromol. 2021, 192, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Michalowski, C.B.; Beloqui, A. Oral Delivery of Biologics in Inflammatory Bowel Disease Treatment. Front. Bioeng. Biotechnol. 2021, 9, 675194. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zheng, Y.; Li, Y.; Xiong, Y.; Chen, D. Soluble ligands as drug targets for treatment of inflammatory bowel disease. Pharmacol. Ther. 2021, 226, 107859. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Malik, A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Malandro, M.; Stevens, B.R. Regulation of system y+ arginine transport capacity in differentiating human intestinal Caco-2 cells. Am. J. Physiol. 1995, 268, G578–G585. [Google Scholar] [CrossRef]
- Karin, M. How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastropietro, G.; Tiscornia, I.; Perelmuter, K.; Astrada, S.; Bollati-Fogolín, M. HT-29 and Caco-2 reporter cell lines for functional studies of nuclear factor kappa B activation. Mediat. Inflamm. 2015, 2015, 860534. [Google Scholar] [CrossRef] [Green Version]
- Duque, J.; Díaz-Muñoz, M.D.; Fresno, M.; Iñiguez, M.A. Up-regulation of cyclooxygenase-2 by interleukin-1beta in colon carcinoma cells. Cell Signal. 2006, 18, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Aspects Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid. Med. Cell Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay Between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Zahid, A.; Li, B.; Kombe, A.J.K.; Jin, T.; Tao, J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Garat, C.; Arend, W.P. Intracellular IL-1Ra type 1 inhibits IL-1-induced IL-6 and IL-8 production in Caco-2 intestinal epithelial cells through inhibition of p38 mitogen-activated protein kinase and NF-kappaB pathways. Cytokine 2003, 23, 31–40. [Google Scholar] [CrossRef]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordulewska, N.K.; Topa, J.; Rozmus, D.; Jarmołowska, B. Effects of Osthole on Inflammatory Gene Expression and Cytokine Secretion in Histamine-Induced Inflammation in the Caco-2 Cell Line. Int. J. Mol. Sci. 2021, 22, 13634. https://doi.org/10.3390/ijms222413634
Kordulewska NK, Topa J, Rozmus D, Jarmołowska B. Effects of Osthole on Inflammatory Gene Expression and Cytokine Secretion in Histamine-Induced Inflammation in the Caco-2 Cell Line. International Journal of Molecular Sciences. 2021; 22(24):13634. https://doi.org/10.3390/ijms222413634
Chicago/Turabian StyleKordulewska, Natalia K., Justyna Topa, Dominika Rozmus, and Beata Jarmołowska. 2021. "Effects of Osthole on Inflammatory Gene Expression and Cytokine Secretion in Histamine-Induced Inflammation in the Caco-2 Cell Line" International Journal of Molecular Sciences 22, no. 24: 13634. https://doi.org/10.3390/ijms222413634
APA StyleKordulewska, N. K., Topa, J., Rozmus, D., & Jarmołowska, B. (2021). Effects of Osthole on Inflammatory Gene Expression and Cytokine Secretion in Histamine-Induced Inflammation in the Caco-2 Cell Line. International Journal of Molecular Sciences, 22(24), 13634. https://doi.org/10.3390/ijms222413634






