USP14 Regulates Cancer Cell Growth in a Fatty Acid Synthase-Independent Manner
Abstract
:1. Introduction
2. Results
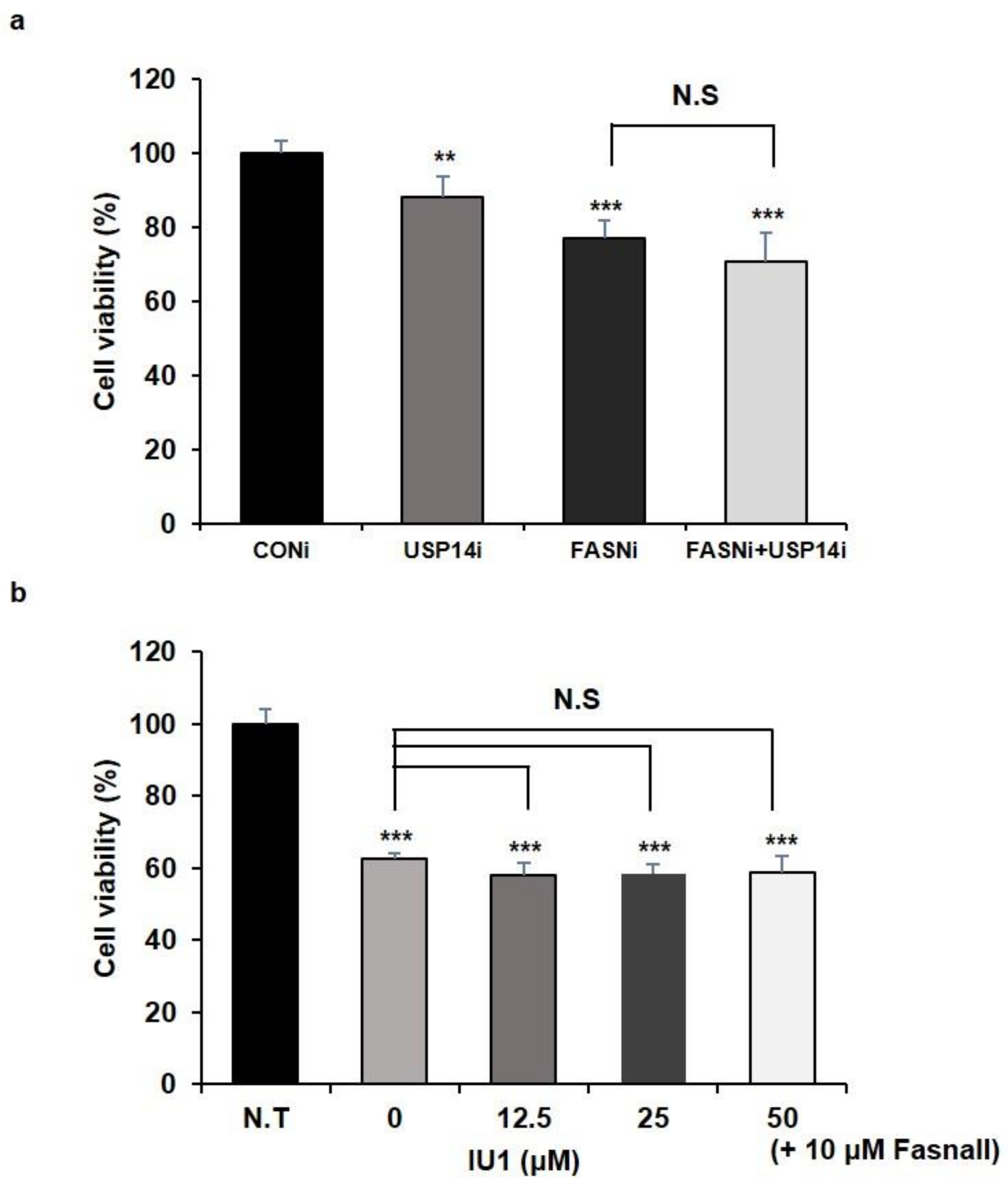
2.1. Inhibition of USP14 Has No Synergistic Effects on Cancer Cell Proliferation Reduced by FASN Inhibition
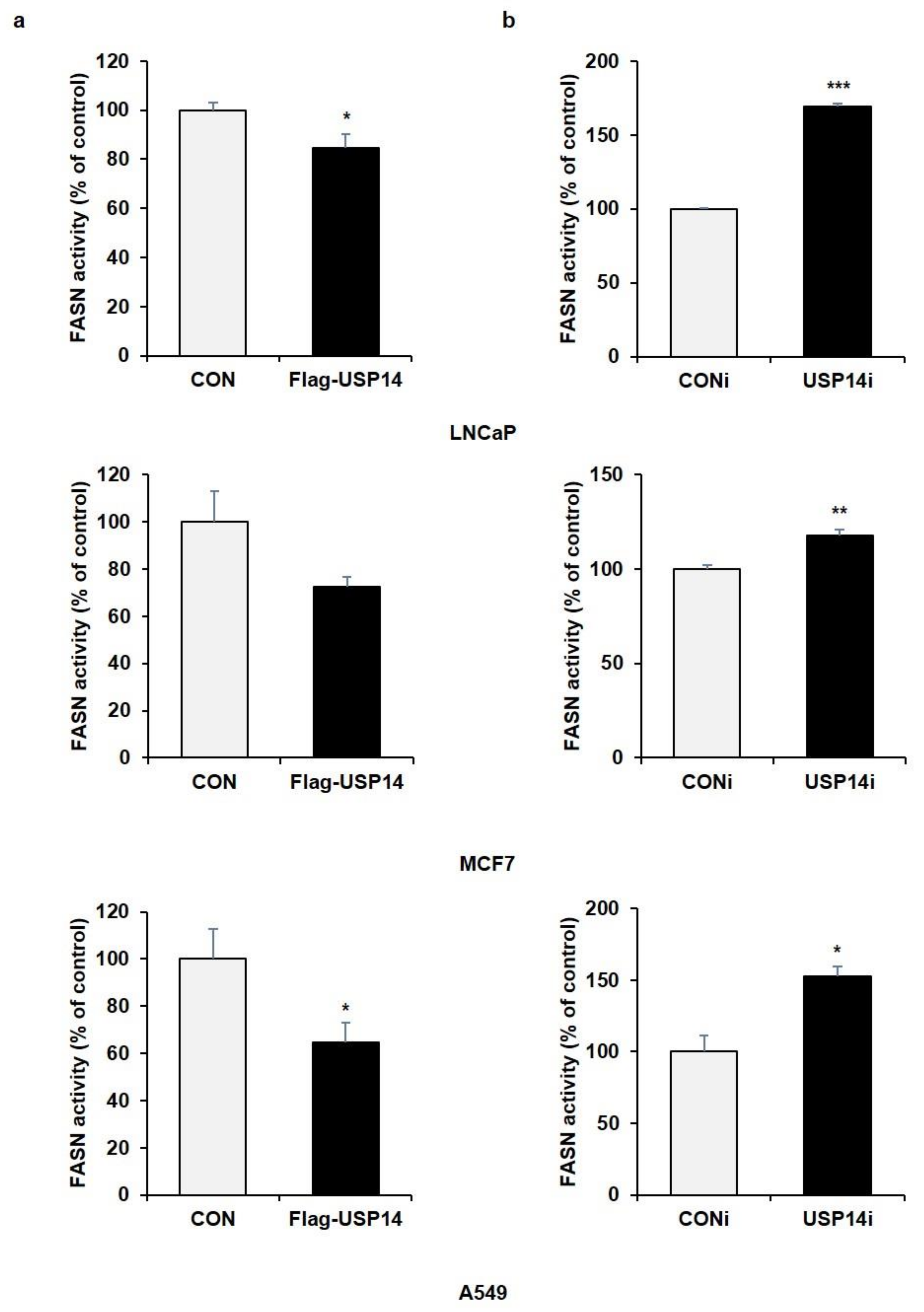
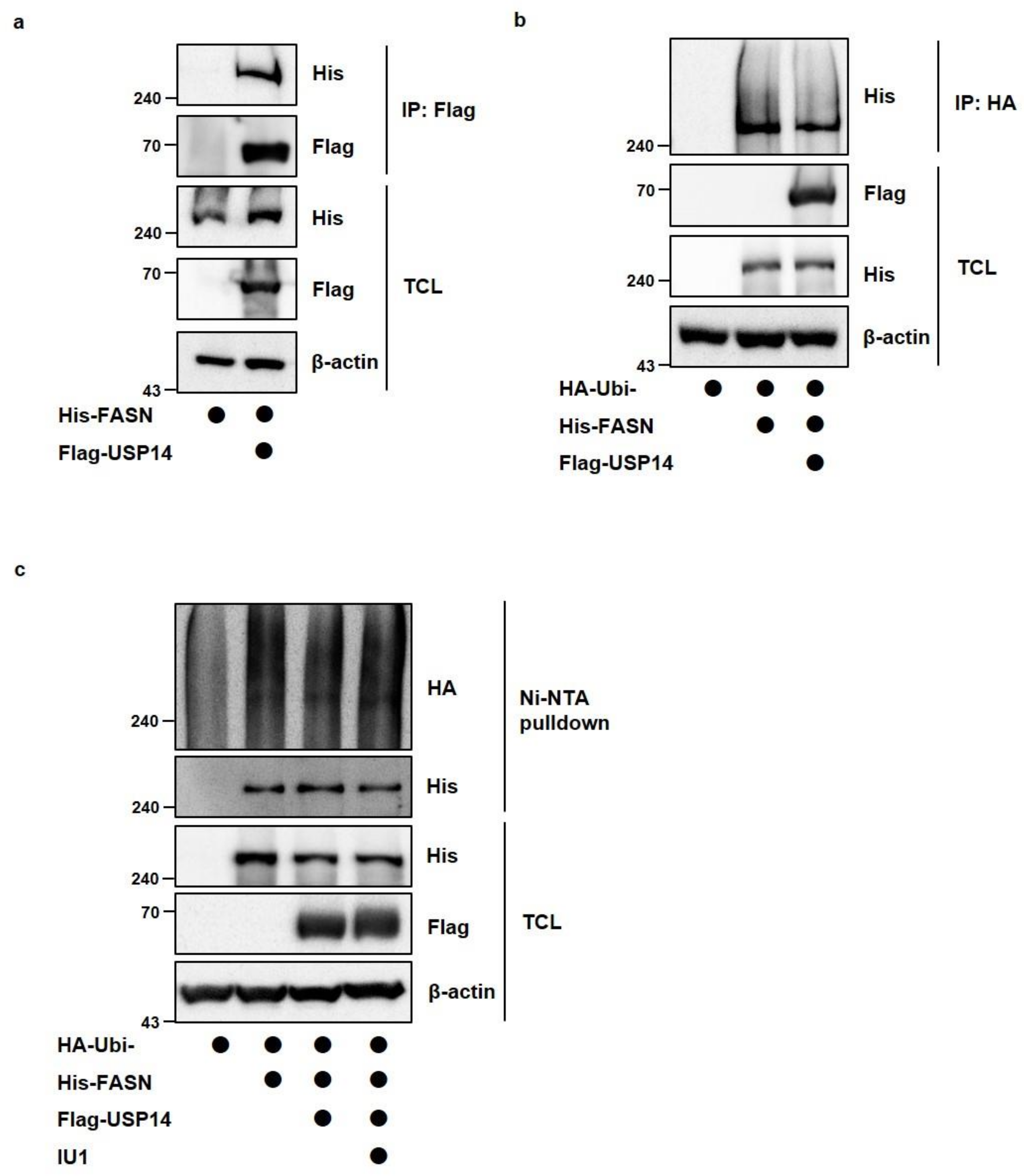
2.2. USP14 Negatively Regulates the Levels and Activity of FASN in Cancer Cells
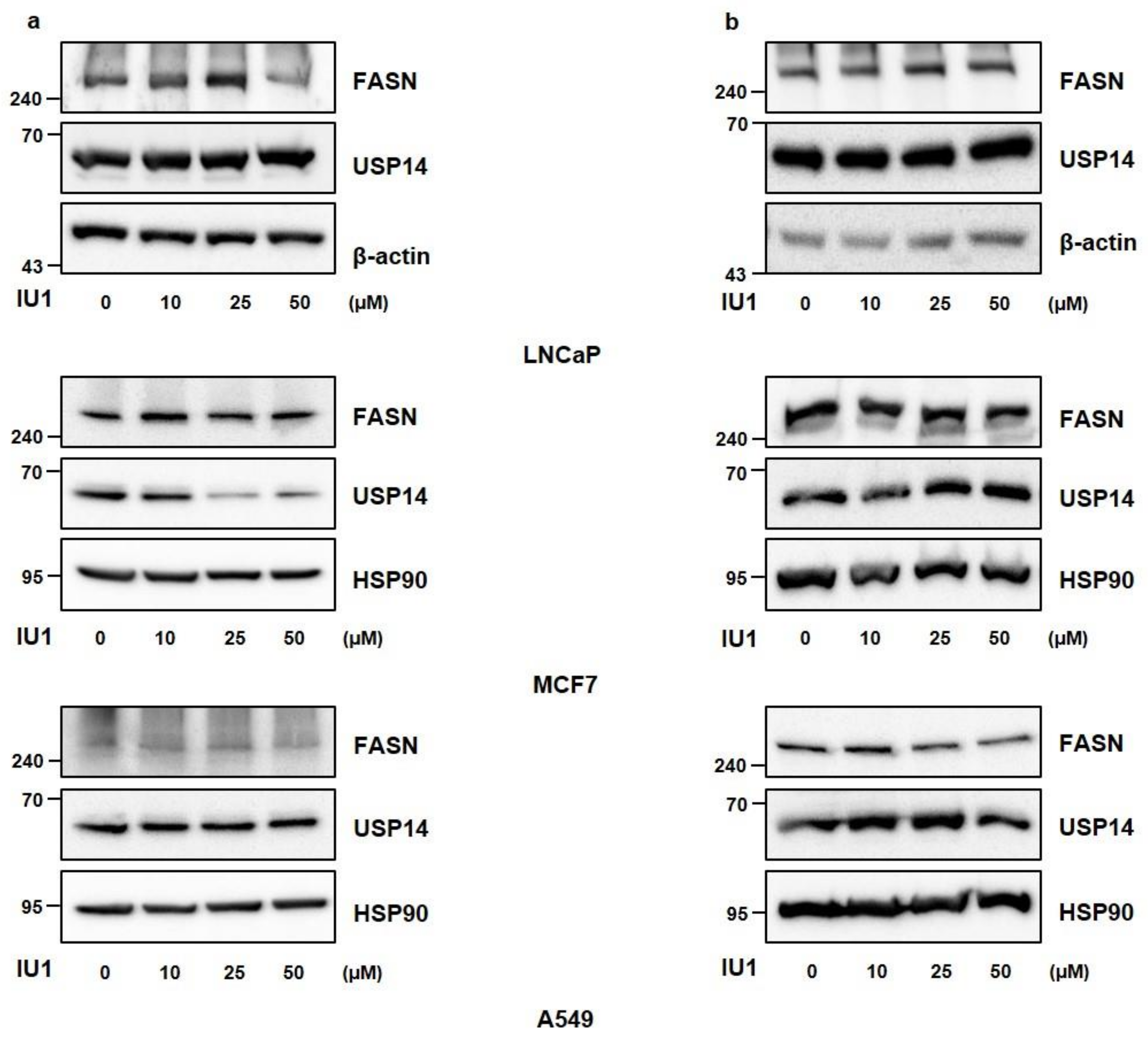
2.3. Inhibition of USP14 Does Not Significantly Affect FASN Protein Levels with IU1 in Cancer Cells
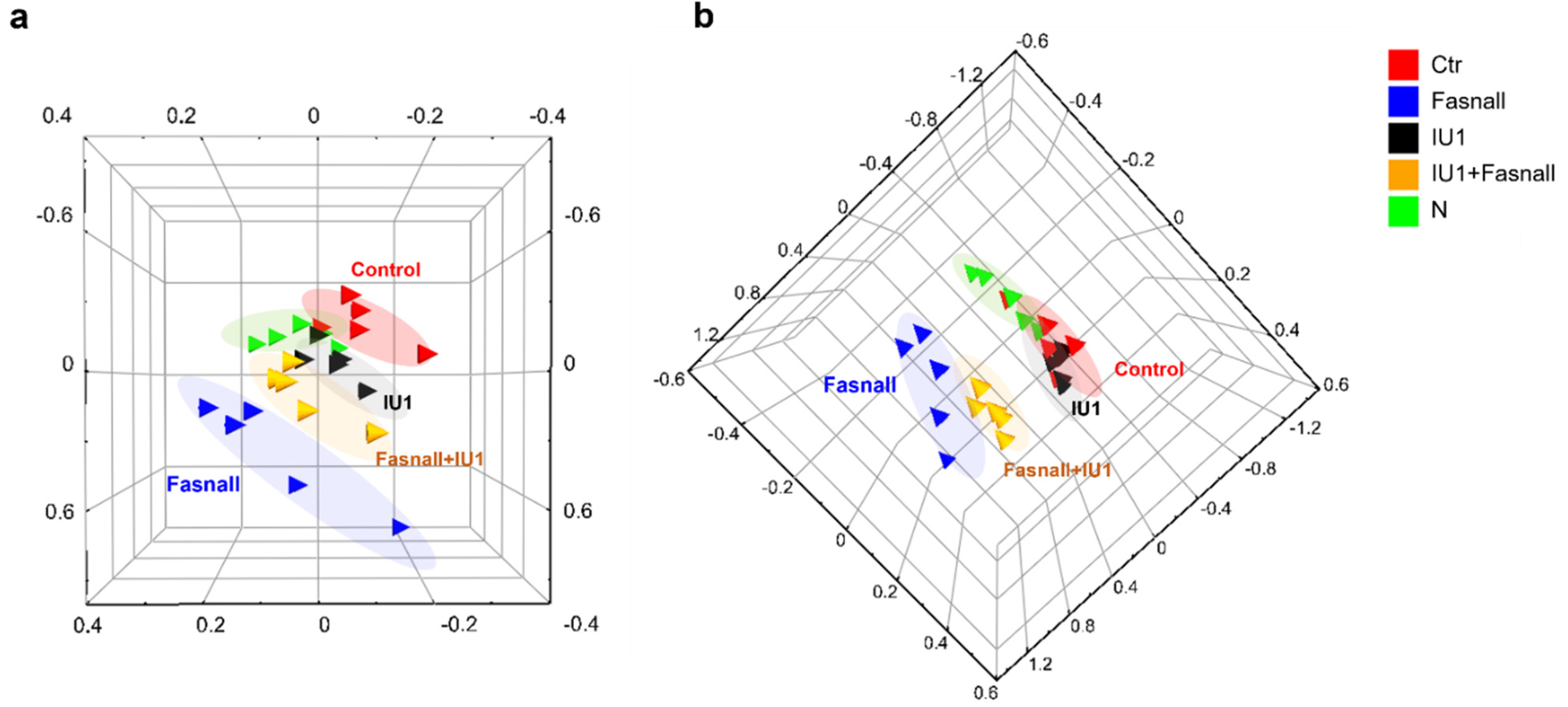
2.4. Metabolomic Profiling of IU1- and Fasnall-Treated LNCaP Cells
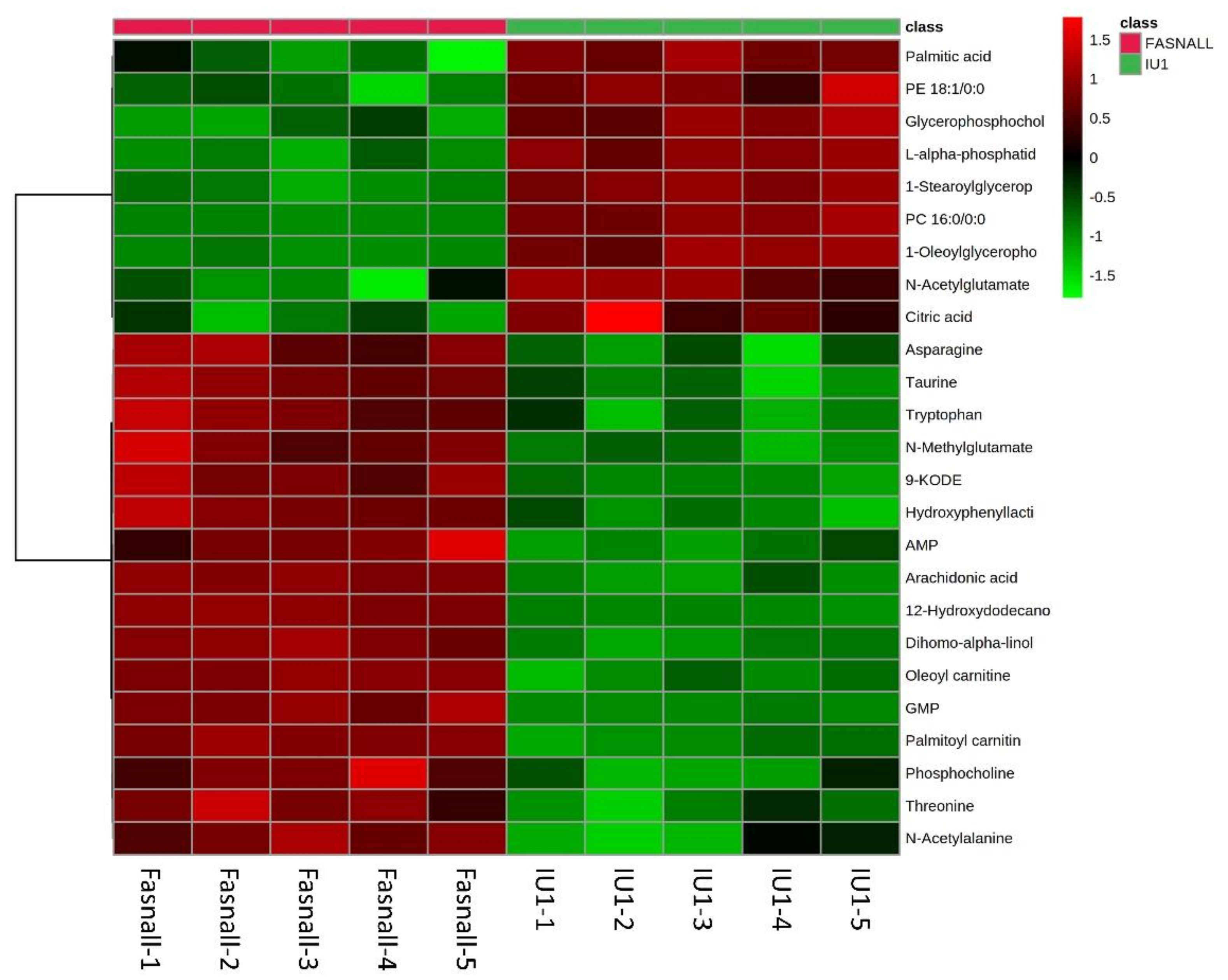
2.5. Altered Metabolite Identification in IU1- and Fasnall-Treated LNCaP Cells
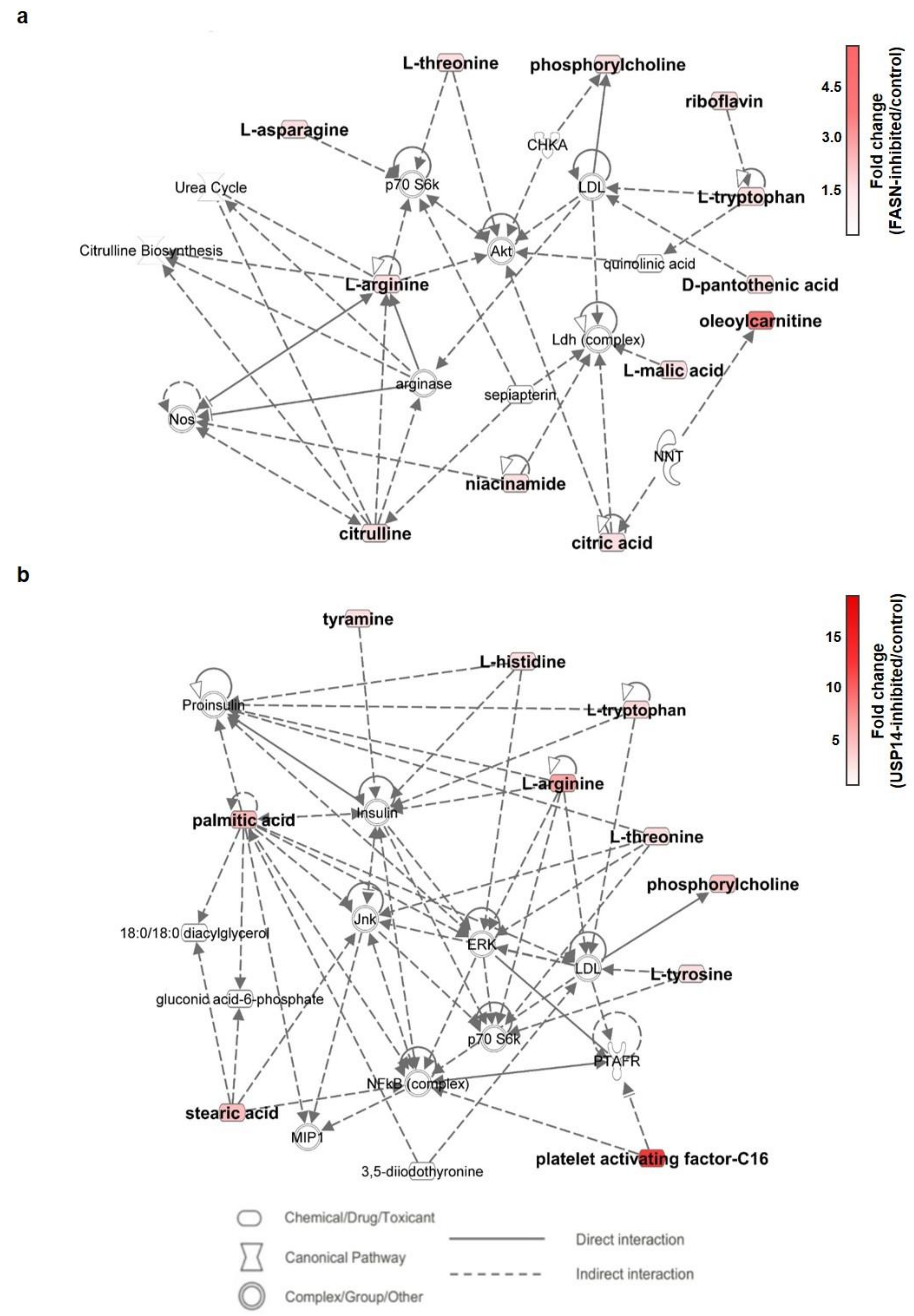
2.6. Metabolic Pathway Analysis of IU1- and Fasnall-Treated LNCaP Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Transfection
4.3. Plasmids and siRNAs
4.4. Western Blot Analysis
4.5. Immunoprecipitation
4.6. Ubiquitination Assay
4.7. Cell Viability Assay
4.8. Enzyme Activity Assay
4.9. Metabolite Extraction
4.10. Metabolomic Analysis
4.11. Data Preprocessing and Statistical Analysis for Identification of Metabolites
4.12. Ingenuity Pathway Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Metabolite | Fasnall | IU1 | Fasnall + IU1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p -Value | Fold Change | Trend | p -Value | Fold Change | Trend | p -Value | Fold Change | Trend | |
| Asparagine | <0.001 | 1.55 | up | NS | 1.18 | up | <0.0005 | 2.892 | up |
| Citric acid | <0.001 | 0.92 | down | NS | 1.36 | up | <0.005 | 0.55 | down |
| Citrulline | <0.05 | 1.04 | up | NS | 1.11 | up | <0.0005 | 0.61 | down |
| Glutamic acid | NS | 0.94 | down | NS | 0.94 | down | <0.005 | 0.616 | down |
| Glutathione | NS | 0.98 | down | NS | 0.93 | down | <0.0001 | 0.493 | down |
| Arginine | <0.01 | 1.52 | up | <0.01 | 4.7 | up | <0.05 | 4.014 | up |
| Leucine | NS | 2.82 | up | NS | 1.42 | up | <0.05 | 2.318 | up |
| Histidine | <0.0001 | 1.15 | up | <0.05 | 1.4 | up | <0.01 | 1.997 | up |
| Threonine | <0.0001 | 2.24 | 1.24 | <0.05 | 1.26 | up | <0.01 | 1.686 | up |
| Tryptophan | <0.01 | 2.51 | up | <0.01 | 1.96 | up | <0.0001 | 5.028 | up |
| Tyrosine | <0.05 | 1.42 | up | NS | 1.26 | up | <0.001 | 2.36 | up |
| N-Acetylaspartylglutamic acid | <0.001 | 1.14 | up | NS | 0.98 | down | NS | 0.959 | down |
| N-Acetyl-DL-alanine | <0.0001 | 1.23 | up | NS | 1.03 | up | <0.0001 | 1.651 | up |
| N-Acetyl-DL-aspartic acid | NS | 1.04 | up | <0.05 | 0.9 | down | <0.05 | 0.858 | down |
| N-Acetyl-DL-glutamic acid | NS | 0.98 | down | NS | 1.05 | up | NS | 0.474 | down |
| Nicotinamide | <0.001 | 0.94 | down | <0.05 | 0.71 | down | <0.001 | 0.475 | down |
| N-Methylglutamic acid | <0.05 | 2.28 | up | <0.05 | 1.78 | up | <0.0001 | 4.728 | up |
| Pantothenic acid | <0.05 | 1.12 | up | NS | 0.87 | down | <0.05 | 1.148 | up |
| Tyramine | <0.05 | 1.81 | up | <0.05 | 1.47 | up | <0.05 | 2.048 | up |
| Saccharopine | <0.001 | 1.15 | up | <0.05 | 1.29 | up | NS | 1.058 | up |
| 2-Furoglycine | <0.05 | 1.04 | up | NS | 1.02 | up | <0.05 | 0.801 | down |
| 12-Hydroxydodecanoic acid | <0.001 | 15.32 | up | NS | 0.74 | down | <0.0001 | 53.337 | up |
| 9-oxo-10E,12Z-octadecadienoic acid | NS | 1.42 | up | NS | 0.35 | down | NS | 5.502 | up |
| Arachidonic acid | <0.001 | 1.29 | up | NS | 0.44 | down | <0.05 | 7.322 | up |
| Adrenic acid | NS | 0.96 | down | <0.0001 | 0.56 | down | <0.05 | 1.996 | up |
| Arachidonic acid-(2-aminoethyl)-ester | NS | 1.41 | up | <0.05 | 2.08 | up | NS | 1.718 | up |
| Dihomo-alpha-linolenic acid | <0.001 | 1.16 | up | <0.005 | 0.43 | down | <0.05 | 3.825 | up |
| Dihomo-gamma-linolenic acid | NS | 2.65 | up | NS | 5.12 | up | NS | 3.153 | up |
| Palmitic acid | NS | 1.32 | up | <0.0001 | 3.63 | up | <0.001 | 2.044 | up |
| Stearic acid | <0.001 | 1.37 | up | <0.0001 | 3.43 | up | <0.05 | 2.046 | up |
| Oleoyl-L-carnitine | <0.0001 | 31.96 | 6.922 | NS | 1.56 | up | <0.0001 | 27.781 | up |
| Palmitoyl-L-carnitine | <0.001 | 9.85 | up | <0.05 | 2.89 | up | <0.0001 | 39.377 | up |
| Riboflavin | <0.05 | 1.23 | up | NS | 1.2 | up | <0.05 | 1.335 | up |
| 6-Hydroxyflavone | <0.001 | 1.36 | up | NS | 1.57 | up | <0.05 | 2.479 | up |
| Glycerophosphocholine | <0.01 | 0.83 | down | NS | 1.12 | up | <0.001 | 0.286 | down |
| 2-deoxy-2-thio Arachidonoyl PC | <0.05 | 1.01 | up | NS | 1.2 | up | NS | 1.268 | up |
| LysoPC(18:1/0:0) | <0.001 | 1.14 | up | <0.0001 | 3.74 | up | <0.001 | 1.821 | up |
| LysoPC(18:0/0:0) | <0.001 | 1.65 | up | <0.0001 | 7.87 | up | <0.05 | 3.069 | up |
| LysoPE(16:0/0:0) | <0.0001 | 1.46 | up | <0.0001 | 2.46 | up | <0.001 | 3.355 | up |
| LysoPE (22:6/0:0) | NS | 1.12 | up | NS | 1.04 | up | <0.05 | 1.325 | up |
| LysoPE alkenyl (18:1) | <0.001 | 1.19 | up | <0.05 | 1.34 | up | <0.05 | 1.431 | up |
| PC (18:1/2:0) | <0.001 | 1.2 | up | <0.0001 | 10.76 | up | <0.001 | 2.914 | up |
| PC (30:1) | <0.005 | 1.21 | up | NS | 1.13 | up | <0.05 | 1.357 | up |
| PC (36:5) | NS | 0.673 | down | NS | 0.56 | down | NS | 0.509 | down |
| PC (16:0/0:0) | <0.0001 | 1.16 | up | <0.0001 | 5.15 | up | <0.0001 | 2.119 | up |
| PE (18:1/0:0) | <0.0001 | 2.28 | up | <0.0001 | 2.95 | up | <0.05 | 1.649 | up |
| Malic acid | <0.05 | 1.04 | up | NS | 0.87 | down | NS | 0.892 | down |
| 5’-Deoxy-5’-methylthioadenosine | <0.05 | 0.97 | down | <0.05 | 1.33 | up | NS | 1.178 | up |
| Adenosine monophosphate (AMP) | <0.001 | 1.49 | up | <0.05 | 1.48 | up | <0.001 | 3.897 | up |
| Guanosine monophosphate (GMP) | <0.0001 | 1.49 | up | <0.05 | 0.64 | down | <0.001 | 2.445 | up |
| Uridine monophosphate (UMP) | <0.05 | 1.42 | up | NS | 1.1 | up | <0.05 | 2.365 | up |
| Taurine | <0.001 | 2.13 | up | NS | 1.17 | up | <0.0001 | 3.387 | up |
| Phosphocholine | <0.0001 | 2.134 | up | <0.01 | 3.1 | up | <0.05 | 4.275 | up |
| Hydroxyphenyllactic acid | <0.01 | 2.7 | up | <0.05 | 1.51 | up | <0.0001 | 5.856 | up |
| Cholic acid | <0.05 | 1.37 | up | NS | 1.21 | up | <0.0001 | 1.944 | up |
References
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Bueno, M.J.; Jimenez-Renard, V.; Samino, S.; Capellades, J.; Junza, A.; Lopez-Rodriguez, M.L.; Garcia-Carceles, J.; Lopez-Fabuel, I.; Bolanos, J.P.; Chandel, N.S.; et al. Essentiality of fatty acid synthase in the 2D to anchorage-independent growth transition in transforming cells. Nat. Commun. 2019, 10, 5011. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Cheng, Z.L.; He, R.Y.; Song, L.; Tian, M.X.; Zhou, L.S.; Groh, B.S.; Liu, W.R.; Ji, M.B.; Ding, C.; et al. Destabilization of Fatty Acid Synthase by Acetylation Inhibits De Novo Lipogenesis and Tumor Cell Growth. Cancer Res. 2016, 76, 6924–6936. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.E.; Jung, B.H.; Park, J.; Kang, S.; Lee, H. Deciphering Fatty Acid Synthase Inhibition-Triggered Metabolic Flexibility in Prostate Cancer Cells through Untargeted Metabolomics. Cells 2020, 9, 2447. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Soeters, M.R.; Soeters, P.B.; Schooneman, M.G.; Houten, S.M.; Romijn, J.A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1397–E1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Liu, N.; Hua, X.; Cai, J.; Xia, X.; Wang, X.; Huang, H.; Liu, J. Proteasome-associated deubiquitinase ubiquitin-specific protease 14 regulates prostate cancer proliferation by deubiquitinating and stabilizing androgen receptor. Cell Death Dis. 2017, 8, e2585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, H.; Li, J.; Fang, X.; Pan, H.; Yuan, X.; Zhang, P. Up-Regulated FASN Expression Promotes Transcoelomic Metastasis of Ovarian Cancer Cell through Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2014, 15, 11539–11554. [Google Scholar] [CrossRef]
- Hao, Q.; Li, T.; Zhang, X.; Gao, P.; Qiao, P.; Li, S.; Geng, Z. Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncol. Rep. 2014, 32, 2471–2476. [Google Scholar] [CrossRef] [Green Version]
- Van de Sande, T.; Roskams, T.; Lerut, E.; Joniau, S.; Van Poppel, H.; Verhoeven, G.; Swinnen, J.V. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J. Pathol. 2005, 206, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Alwarawrah, Y.; Hughes, P.; Loiselle, D.; Carlson, D.A.; Darr, D.B.; Jordan, J.L.; Xiong, J.; Hunter, L.M.; Dubois, L.G.; Thompson, J.W.; et al. Fasnall, a Selective FASN Inhibitor, Shows Potent Anti-tumor Activity in the MMTV-Neu Model of HER2(+) Breast Cancer. Cell Chem. Biol. 2016, 23, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, A.J.; Von Hoff, D.D.; Infante, J.R.; Patel, M.R.; Jones, S.F.; Burris, H.A.; Rubino, C.; McCulloch, W.; Zhukova-Harrill, V.; Kemble, G. First-in-human investigation of the oral first-in-class fatty acid synthase (FASN) inhibitor, TVB-2640. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Sagimet Biosciences. Available online: https://sagimet.com/programs/pipeline/ (accessed on 17 August 2020).
- Angeles, T.S.; Hudkins, R.L. Recent advances in targeting the fatty acid biosynthetic pathway using fatty acid synthase inhibitors. Expert Opin. Drug Discov. 2016, 11, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.M.; Jaworsky, D.E.; Frehywot, G.L.; Townsend, C.A.; Ronnett, G.V.; Lane, M.D.; Kuhajda, F.P. Reduced Food Intake and Body Weight in Mice Treated with Fatty Acid Synthase Inhibitors. Science 2000, 288, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Landree, L.E.; Hanlon, A.L.; Strong, D.W.; Rumbaugh, G.; Miller, I.M.; Thupari, J.N.; Connolly, E.C.; Huganir, R.L.; Richardson, C.; Witters, L.A.; et al. C75, a Fatty Acid Synthase Inhibitor, Modulates AMP-activated Protein Kinase to Alter Neuronal Energy Metabolism. J. Biol. Chem. 2004, 279, 3817–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Li, L.; Uttamchandani, M.; Yao, S.Q. In Situ Proteome Profiling of C75, a Covalent Bioactive Compound with Potential Anticancer Activities. Org. Lett. 2014, 16, 1414–1417. [Google Scholar] [CrossRef]
- Zhi, J.; Melia, A.T.; Eggers, H.; Joly, R.; Patel, I.H. Review of Limited Systemic Absorption of Orlistat, a Lipase Inhibitor, in Healthy Human Volunteers. J. Clin. Pharmacol. 1995, 35, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Graner, E.; Tang, D.; Rossi, S.; Baron, A.; Migita, T.; Weinstein, L.J.; Lechpammer, M.; Huesken, D.; Zimmermann, J.; Signoretti, S.; et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 2004, 5, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Deng, R.; Zhu, H.H.; Zhang, S.S.; Zhu, C.; Montminy, M.; Davis, R.; Feng, G.S. Modulation of Fatty Acid Synthase Degradation by Concerted Action of p38 MAP Kinase, E3 Ligase COP1, and SH2-Tyrosine Phosphatase Shp2. J. Biol. Chem. 2013, 288, 3823–3830. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Jiang, S.; Li, M.; Xiong, X.; Zhu, M.; Li, D.; Zhao, L.; Qian, L.; Zhai, L.; Li, J.; et al. Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN. Nat. Commun. 2018, 9, 4770. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Lee, B.H.; Hanna, J.; King, R.W.; Finley, D. Trimming of Ubiquitin Chains by Proteasome-associated Deubiquitinating Enzymes. Mol. Cell Proteom. 2011, 10, R110 003871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Xia, X.; Liu, N.; Cai, J.; Guo, Z.; Li, Y.; Jiang, L.; Dou, Q.P.; Tang, D.; Huang, H.; et al. Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene 2018, 37, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, X.; Ge, H.; Zheng, N.; Peng, F.; Fu, Y.; Tao, L.; Wang, Q. Inhibition of ubiquitinspecific protease 14 promotes connexin 32 internalization and counteracts cisplatin cytotoxicity in human ovarian cancer cells. Oncol. Rep. 2019, 42, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Wang, X.F.; Zhang, Y.J.; Luo, P.; Long, H.D.; Li, L.; Yang, H.Q.; Xie, R.T.; Jia, C.Y.; Lu, G.X.; et al. Inhibition of USP14 Deubiquitinating Activity as a Potential Therapy for Tumors with p53 Deficiency. Mol. Ther. Oncolytics 2020, 16, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Huang, C.; Liao, Y.; Liu, Y.; He, J.; Guo, Z.; Jiang, L.; Wang, X.; Liu, J.; Huang, H. Inhibition of USP14 enhances the sensitivity of breast cancer to enzalutamide. J. Exp. Clin. Cancer Res. 2019, 38, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Wang, J.; Yuan, X.; Yang, S.; Xu, X.; Li, K.; He, Y.; Wei, L.; Zhang, J.; Tian, Y. IU1 suppresses proliferation of cervical cancer cells through MDM2 degradation. Int. J. Biol. Sci. 2020, 16, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Kwak, M.; Lee, T.H.; Park, M.S.; Jeong, I.H.; Kim, M.J.; Jin, J.O.; Lee, P.C. USP14 Inhibition Regulates Tumorigenesis by Inducing Autophagy in Lung Cancer In Vitro. Int. J. Mol. Sci. 2019, 20, 5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, Y.; Furukawa, T.; Oyama, N.; Hasegawa, Y.; Kiyono, Y.; Nishii, R.; Waki, A.; Tsuji, A.B.; Sogawa, C.; Wakizaka, H.; et al. Fatty Acid Synthase is a Key Target in Multiple Essential Tumor Functions of Prostate Cancer: Uptake of Radiolabeled Acetate as a Predictor of the Targeted Therapy Outcome. PLoS ONE 2013, 8, e64570. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehic, M.; de Sa, V.K.; Hebestreit, S.; Heldin, C.H.; Heldin, P. The deubiquitinating enzymes USP4 and USP17 target hyaluronan synthase 2 and differentially affect its function. Oncogenesis 2017, 6, e348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Park, D.; Ryu, J.; Park, T. USP11 degrades KLF4 via its deubiquitinase activity in liver diseases. J. Cell Mol. Med. 2021, 25, 6976–6987. [Google Scholar] [CrossRef] [PubMed]
- Mialki, R.K.; Zhao, J.; Wei, J.; Mallampalli, D.F.; Zhao, Y. Overexpression of USP14 Protease Reduces I-kappaB Protein Levels and Increases Cytokine Release in Lung Epithelial Cells. J. Biol. Chem. 2013, 288, 15437–15441. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Shan, B.; Lee, B.H.; Zhu, K.; Zhang, T.; Sun, H.; Liu, M.; Shi, L.; Liang, W.; Qian, L.; et al. Phosphorylation and activation of ubiquitin-specific protease-14 by Akt regulates the ubiquitin-proteasome system. Elife 2015, 4, e10510. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, J.M.; Cho, J.Y.; Kim, T.E.; Lee, H.J.; Jung, B.H. Regulation of endogenic metabolites by rosuvastatin in hyperlipidemia patients: An integration of metabolomics and lipidomics. Chem. Phys. Lipids 2018, 214, 69–83. [Google Scholar] [CrossRef]
- Kong, X.; Yang, X.; Zhou, J.; Chen, S.; Li, X.; Jian, F.; Deng, P.; Li, W. Analysis of plasma metabolic biomarkers in the development of 4-nitroquinoline-1-oxide-induced oral carcinogenesis in rats. Oncol. Lett. 2015, 9, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.S.; Yoon, N.; Kong, M.; Jung, B.H.; Lee, H.; Park, J. USP14 Regulates Cancer Cell Growth in a Fatty Acid Synthase-Independent Manner. Int. J. Mol. Sci. 2021, 22, 13437. https://doi.org/10.3390/ijms222413437
Yang JS, Yoon N, Kong M, Jung BH, Lee H, Park J. USP14 Regulates Cancer Cell Growth in a Fatty Acid Synthase-Independent Manner. International Journal of Molecular Sciences. 2021; 22(24):13437. https://doi.org/10.3390/ijms222413437
Chicago/Turabian StyleYang, Ji Su, Naeun Yoon, Mingyu Kong, Byung Hwa Jung, Hyunbeom Lee, and Jinyoung Park. 2021. "USP14 Regulates Cancer Cell Growth in a Fatty Acid Synthase-Independent Manner" International Journal of Molecular Sciences 22, no. 24: 13437. https://doi.org/10.3390/ijms222413437
APA StyleYang, J. S., Yoon, N., Kong, M., Jung, B. H., Lee, H., & Park, J. (2021). USP14 Regulates Cancer Cell Growth in a Fatty Acid Synthase-Independent Manner. International Journal of Molecular Sciences, 22(24), 13437. https://doi.org/10.3390/ijms222413437





