Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria
Abstract
1. Introduction
2. Results and Discussion
2.1. Quinolone Uptake
2.2. Inhibition of DNA Gyrase and Topoisomerase IV Assays
3. Materials and Methods
3.1. Quinolone Uptake
3.1.1. Bacterial Strains
3.1.2. Antibacterial Agents
3.1.3. Accumulation Assay
3.1.4. Preparation of Bioassay Plates
3.1.5. Bioassay Procedure
3.1.6. Standard Curve
3.2. Inhibition of DNA Gyrase and Topoisomerase IV Assays
3.2.1. Enzymatic Assay
3.2.2. Gel Analysis and IC50 Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarker, S.D.; Latif, Z.; Gray, A.I. Natural Product Isolation. In Methods in Biotechnology, 2nd ed.; Human Press: Totowa, NJ, USA, 2006; pp. 202–225. [Google Scholar]
- Wright, G.D. Something old, something new: Revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Rybenkov, V.V.; Zgurskaya, H.I.; Ganguly, C.; Leus, I.V.; Zhang, Z.; Moniruzzaman, M. The Whole Is Bigger than the Sum of Its Parts: Drug Transport in the Context of Two Membranes with Active Efflux. Chem. Rev. 2021, 121, 5597–5631. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V.; Jin, Y.-F.; Ricci, V.; Asuquo, A.E. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 1999, 43, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Six, D.A.; Krucker, T.; Leeds, J.A. Advances and challenges in bacterial compound accumulation assays for drug discovery. Curr. Opin. Chem. Biol. 2018, 44, 9–15. [Google Scholar] [CrossRef]
- Stone, M.R.L.; Łapińska, U.; Pagliara, S.; Masi, M.; Blanchfield, J.T.; Cooper, M.A.; Blaskovich, M.A.T. Fluorescent macrolide probes—Synthesis and use in evaluation of bacterial resistance. RSC Chem. Biol. 2020, 1, 395–404. [Google Scholar] [CrossRef]
- Cama, J.; Voliotis, M.; Metz, J.; Smith, A.; Iannucci, J.; Keyser, U.F.; Tsaneva-Atanasova, K.; Pagliara, S. Single-cell microfluidics facilitates the rapid quantification of antibiotic accumulation in Gram-negative bacteria. Lab Chip 2020, 20, 2765–2775. [Google Scholar] [CrossRef] [PubMed]
- Łapińska, U.; Voliotis, M.; Lee, K.K.; Campey, A.; Stone, R.; Phetsang, W.; Zhang, B.; Tsaneva-Atanasova, K.; Blaskovich, M.; Pagliara, S. Fast bacterial growth reduces antibiotic accumulation and efficacy. bioRxiv 2021. bioRxiv:2021.10.18.464851. [Google Scholar] [CrossRef]
- Zhou, Y.; Joubran, C.; Miller-Vedam, L.; Isabella, V.; Nayar, A.; Tentarelli, S.; Miller, A. Thinking Outside the “Bug”: A Unique Assay To Measure Intracellular Drug Penetration in Gram-Negative Bacteria. Anal. Chem. 2015, 87, 3579–3584. [Google Scholar] [CrossRef]
- Mortimer, P.G.S.; Piddock, L. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 1991, 28, 639–653. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Michot, J.-M.; Van Eldere, J.; Tulkens, P. Quinolones in 2005: An update. Clin. Microbiol. Infect. 2005, 11, 256–280. [Google Scholar] [CrossRef]
- Han, J.H.; Bilker, W.B.; Nachamkin, I.; Tolomeo, P.; Mao, X.; Fishman, N.O.; Lautenbach, E. Impact of antibiotic use during hospitalization on the development of gastrointestinal colonization with Escherichia coli with reduced fluoroquinolone susceptibility. Infect. Control. Hosp. Epidemiol. 2013, 34, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Marco, F.; Martínez, J.A.; Pisos, E.; Almela, M.; Dimova, V.P.; Álamo-Junquera, D.; Ortega, M.; Lopez, J.; Mensa, J. Influence of Vancomycin Minimum Inhibitory Concentration on the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2008, 46, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Maina, E.K.; Kiiyukia, C.; Wamae, C.N.; Waiyaki, P.G.; Kariuki, S. Characterization of methicillin-resistant Staphylococcus aureus from skin and soft tissue infections in patients in Nairobi, Kenya. Int. J. Infect. Dis. 2013, 17, e115–e119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhagwat, S.S.; McGhee, P.; Kosowska-Shick, K.; Patel, M.V.; Appelbaum, P.C. In Vitro Activity of the Quinolone WCK 771 against Recent U.S. Hospital and Community-Acquired Staphylococcus aureus Pathogens with Various Resistance Types. Antimicrob. Agents Chemother. 2009, 53, 811–813. [Google Scholar] [CrossRef]
- Taher, M.K.; Alami, A.; Gravel, C.A.; Tsui, D.; Bjerre, L.M.; Momoli, F.; Mattison, D.; Krewski, D. Systemic quinolones and risk of retinal detachment I: Analysis of data from the US FDA adverse event reporting system. Expert Opin. Drug Saf. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.A.; Albareda, N.; Rosen, T.; Torrelo, A.; Grimalt, R.; Rosenberg, N.; Zsolt, I.; Masramon, X. Topical Antibacterial Agent for Treatment of Adult and Pediatric Patients With Impetigo: Pooled Analysis of Phase 3 Clinical Trials. J. Drugs Dermatol. 2018, 17, 1051–1057. [Google Scholar]
- Canton, R.; Morrissey, I.; Vila, J.; Tato, M.; García-Castillo, M.; López, Y.; Gargallo-Viola, D.; Zsolt, I. Comparative in vitro antibacterial activity of ozenoxacin against Gram-positive clinical isolates. Future Microbiol. 2018, 13, 3–19. [Google Scholar] [CrossRef]
- Morrissey, I.; Cantón, R.; Vila, J.; Gargallo-Viola, D.; Zsolt, I.; Garcia-Castillo, M.; López, Y. Microbiological profile of ozenoxacin. Future Microbiol. 2019, 14, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Lopez, Y.; Tato, M.; Espinal, P.; Garcia-Alonso, F.; Gargallo-Viola, D.; Canton, R.; Vila, J. In vitro selection of mutants resistant to ozenoxacin compared with levofloxacin and ciprofloxacin in Gram-positive cocci. J. Antimicrob. Chemother. 2014, 70, 57–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López, Y.; Tato, M.; Gargallo-Viola, D.; Cantón, R.; Vila, J.; Zsolt, I. Comparative activity of ozenoxacin and other quinolones in Staphylococcus aureus strains overexpressing the efflux pump-encoding genes mepA and norA. Int. J. Antimicrob. Agents 2020, 56, 106082. [Google Scholar] [CrossRef] [PubMed]
- Khodursky, A.B.; Peter, B.J.; Schmid, M.B.; DeRisi, J.; Botstein, D.; Brown, P.O.; Cozzarelli, N.R. Analysis of topoisomerase function in bacterial replication fork movement: Use of DNA microarrays. Proc. Natl. Acad. Sci. USA 2000, 97, 9419–9424. [Google Scholar] [CrossRef]
- Vila, J.; Fàbrega, A.; Roca, I.; Hernández, A.; Martínez, J.L. Efflux pumps as an important mechanism for quinolone resistance. Adv. Enzymol. Relat. Areas Mol. Biol. 2011, 77, 167–235. [Google Scholar] [CrossRef]
- Hooper, D.C. Structural determinants of quinolone potency and bacterial resistance. In next generation quinolones for a new therapeutic millennium. Clin. Microbiol. Infect. 1999, 5, 23–24. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, L.; Pascual, A.; Jacoby, G.A. Quinolone resistance from a transferable plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.M.; Velasco, C.; Briales, A.; García, I.; Conejo, M.C.; Pascual, A. Qnr-like pentapeptide repeat proteins in Gram-positive bacteria. J. Antimicrob. Chemother. 2008, 61, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, J.M.; Briales, A.; Velasco, C.; Conejo, M.C.; Martínez-Martínez, L.; Pascual, A. Mutational analysis of quinolone resistance in the plasmid-encoded pentapeptide repeat proteins QnrA, QnrB and QnrS. J. Antimicrob. Chemother. 2009, 63, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Mitsuyama, J.; Hayashi, K. In vitro and in vivo antibacterial activity of T-3912, a novel non-fluorinated topical quinolone. J. Antimicrob. Chemother. 2002, 49, 455–465. [Google Scholar] [CrossRef]
- Vila, J.; Hebert, A.A.; Torrelo, A.; Lopez, Y.; Tato, M.; García-Castillo, M.; Cantón, R. Ozenoxacin: A review of preclinical and clinical efficacy. Expert Rev. Anti-Infect. Ther. 2019, 17, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Cazedey, E.C.L.; Salgado, H.R.N. Development and Validation of a Microbiological Agar Assay for Determination of Orbifloxacin in Pharmaceutical Preparations. Pharmaceutics 2011, 3, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.; Jin, Y.F. Antimicrobial activity and accumulation of moxifloxacin in quinolone-susceptible bacteria. J. Antimicrob. Chemother. 1999, 43 (Suppl. 2), 39–42. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V.; Jin, Y.F.; Griggs, D. Effect of hydrophobicity and molecular mass on the accumulation of fluoroquinolones by Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 47, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.V.; Johnson, M.M. Accumulation of 10 Fluoroquinolones by Wild-Type or Efflux Mutant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2002, 46, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Cama, J.; Schaich, M.; Al Nahas, K.; Hernández-Ainsa, S.; Pagliara, S.; Keyser, U. Direct Optofluidic Measurement of the Lipid Permeability of Fluoroquinolones. Sci. Rep. 2016, 6, 32824. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of fluoroquinolone resistance. Drug Resist. Updat. 1999, 2, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef]
- Cheng, J.; Thanassi, J.A.; Thoma, C.L.; Bradbury, B.J.; Deshpande, M.; Pucci, M.J. Dual Targeting of DNA Gyrase and Topoisomerase IV: Target Interactions of Heteroaryl Isothiazolones in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 2445–2453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopez, Y.; Tato, M.; Espinal, P.; Garcia-Alonso, F.; Gargallo-Viola, D.; Cantón, R.; Vila, J. In Vitro Activity of Ozenoxacin against Quinolone-Susceptible and Quinolone-Resistant Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2013, 57, 6389–6392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-F.; Zhang, S.; Pan, B.; Liu, X.; Feng, L.-S. 4-Quinolone derivatives and their activities against Gram positive pathogens. Eur. J. Med. Chem. 2018, 143, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.S.; Mundkur, L.A.; Gupte, S.V.; Patel, M.V.; Khorakiwala, H.F. The Anti-Methicillin-Resistant Staphylococcus aureus Quinolone WCK 771 Has Potent Activity against Sequentially Selected Mutants, Has a Narrow Mutant Selection Window against Quinolone-Resistant Staphylococcus aureus, and Preferentially Targets DNA Gyrase. Antimicrob. Agents Chemother. 2006, 50, 3568–3579. [Google Scholar] [CrossRef] [PubMed]
- Liebetrau, A.; Rodloff, A.C.; Dubreuil, L. In Vitro Activities of a New Des-Fluoro (6) Quinolone, Garenoxacin, against Clinical Anaerobic Bacteria. Antimicrob. Agents Chemother. 2003, 47, 3667–3671. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Critchley, I.A.; Karlowsky, J.A.; Blosser-Middleton, R.S.; Schmitz, F.-J.; Thornsberry, C.; Sahm, D.F. In Vitro Activities of Novel Nonfluorinated Quinolones PGE 9262932 and PGE 9509924 against Clinical Isolates of Staphylococcus aureus and Streptococcus pneumoniae with Defined Mutations in DNA Gyrase and Topoisomerase IV. Antimicrob. Agents Chemother. 2002, 46, 1651–1657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-H.; Liu, C.-Y.; Lu, J.-J.; King, C.-H.R.; Hsueh, P.-R. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J. Antimicrob. Chemother. 2009, 64, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J. Modifications of quinolones and fluoroquinolones: Hybrid compounds and dual-action molecules. Chem. Mon. 2018, 149, 1199–1245. [Google Scholar] [CrossRef]
- Takenouchi, T.; Tabata, F.; Iwata, Y.; Hanzawa, H.; Sugawara, M.; Ohya, S. Hydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 1996, 40, 1835–1842. [Google Scholar] [CrossRef]
- Giraud, E.; Cloeckaert, A.; Kerboeuf, D.; Chaslus-Dancla, E. Evidence for Active Efflux as the Primary Mechanism of Resistance to Ciprofloxacin in Salmonella enterica Serovar Typhimurium. Antimicrob. Agents Chemother. 2000, 44, 1223–1228. [Google Scholar] [CrossRef]
- Alt, S.; Mitchenall, L.A.; Maxwell, A.; Heide, L. Inhibition of DNA gyrase and DNA topoisomerase IV of Staphylococcus aureus and Escherichia coli by aminocoumarin antibiotics. J. Antimicrob. Chemother. 2011, 66, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
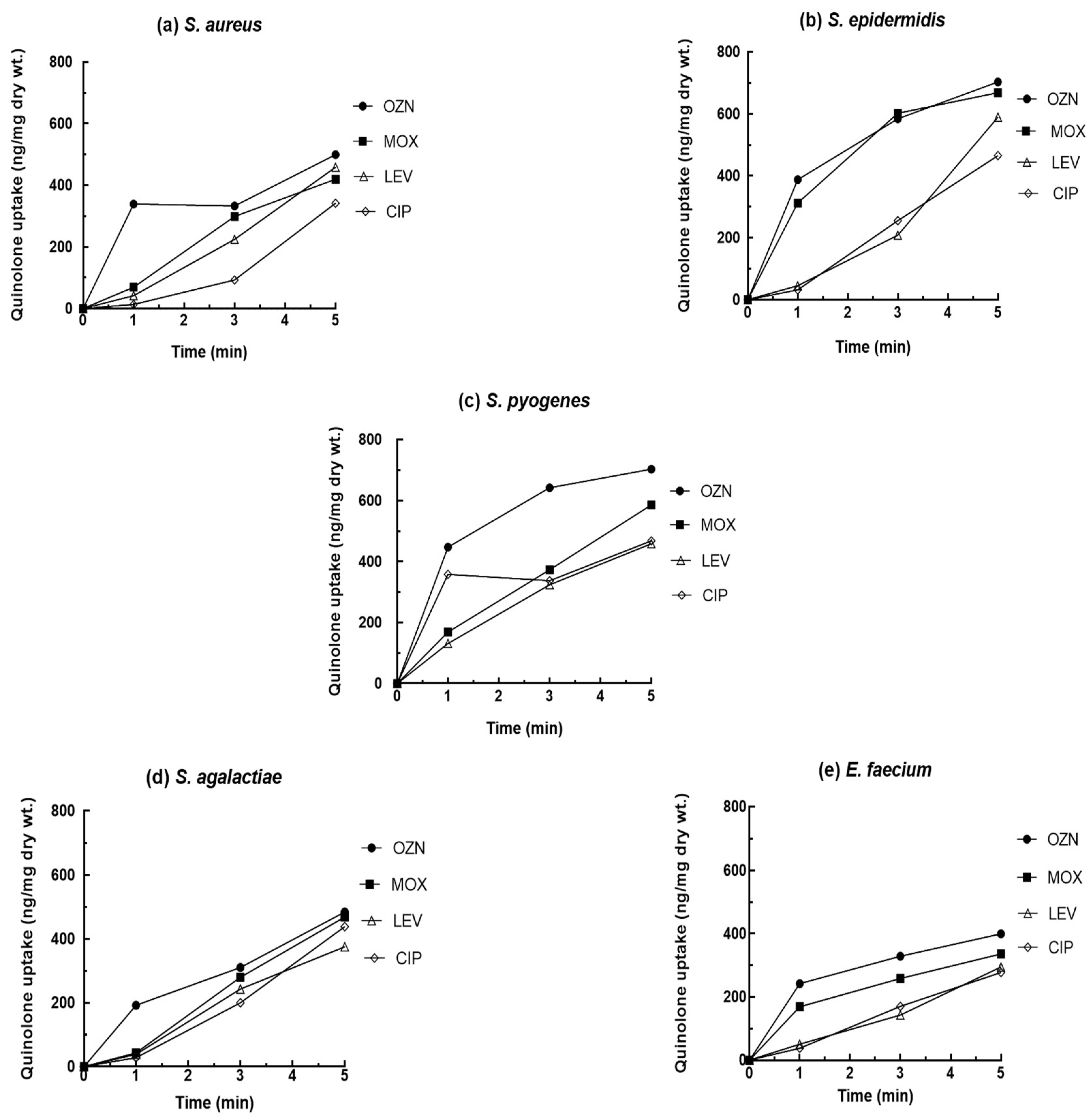
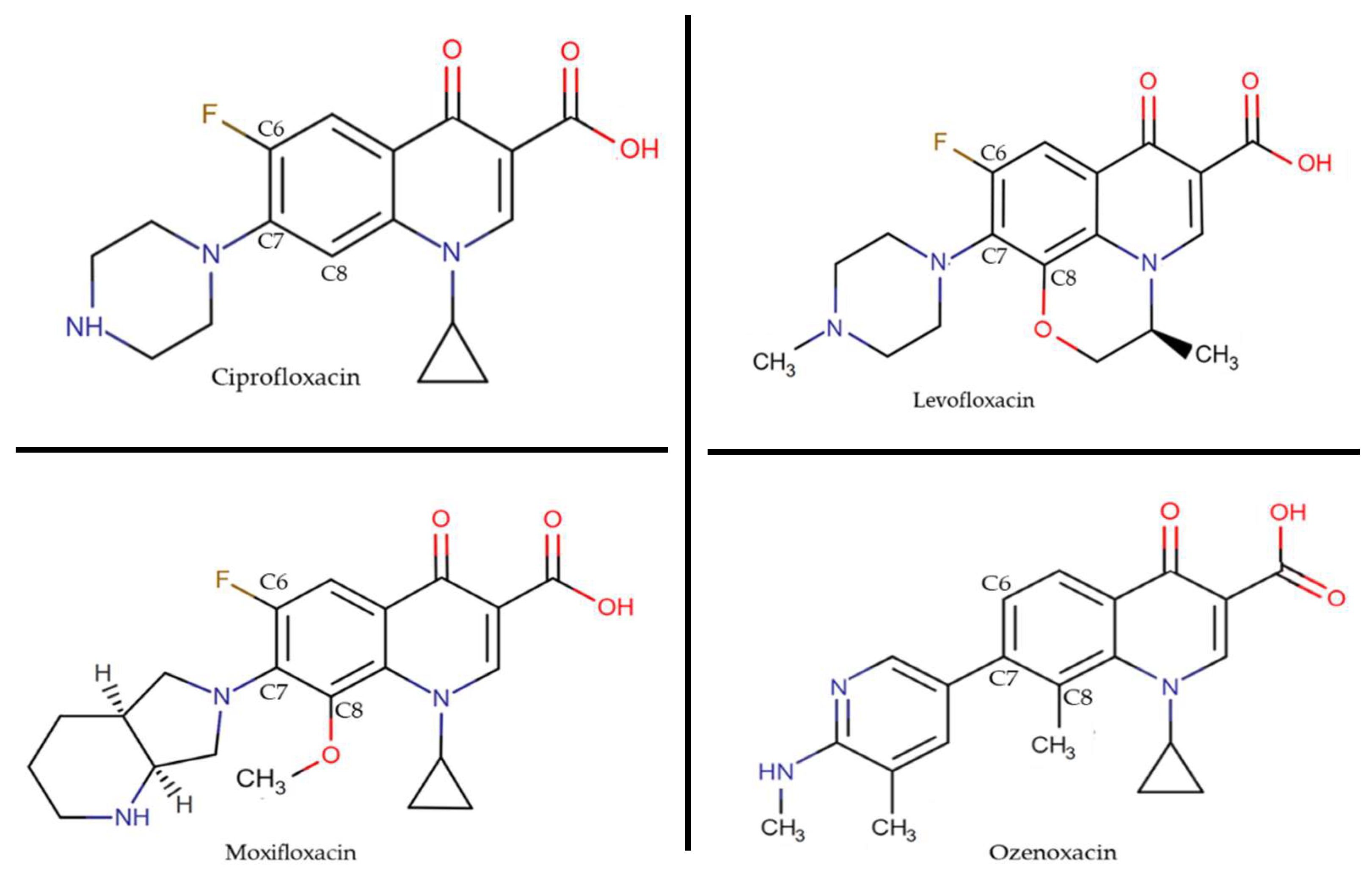
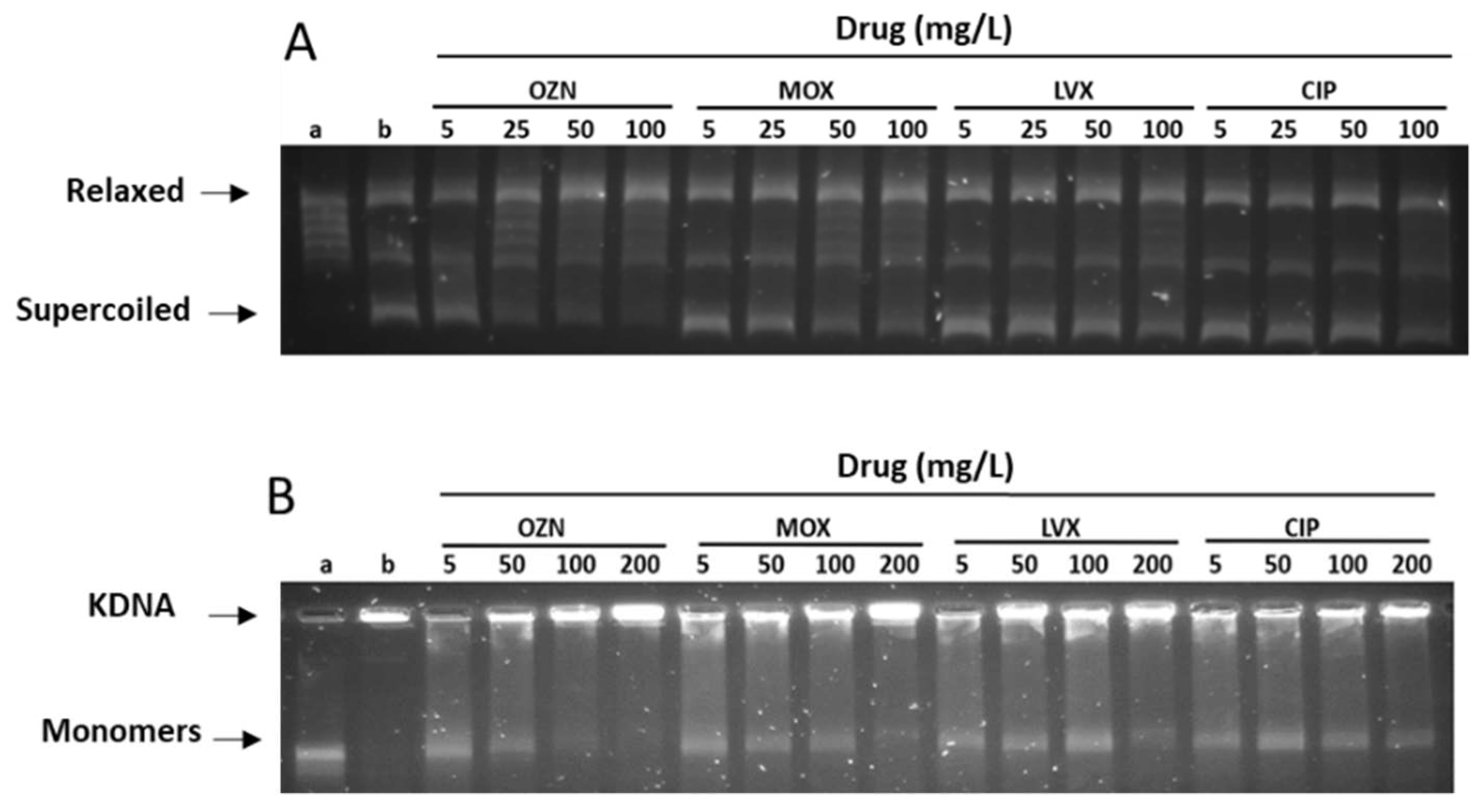
| Antibiotic | S. aureus IC50 (mg/L) | |
|---|---|---|
| DNA Gyrase | Topoisomerase IV | |
| Ozenoxacin | 10 | 0.5 |
| Moxifloxacin | 56 | 0.95 |
| Levofloxacin | >100 | 0.45 |
| Ciprofloxacin | 98 | 1.3 |
| Strains | Minimum Inhibitory Concentration (MIC) (mg/L) | Mutation in QRDR | |||
|---|---|---|---|---|---|
| OZN −R/+R 1 | MOX −R/+R | LVX −R/+R | CIP −R/+R | ||
| S. aureus (4-149) | 0.0039/0.0039 | 0.25/0.25 | 0.25/0.25 | 0.38/0.38 | WM 2 |
| S. epidermidis (7602) | 0.03/0.008 | 0.06/0.06 | 0.5/0.25 | 1/0.5 | WM |
| S. pyogenes (165) | 0.12/0.12 | 1/0.06 | 16/0.06 | 4/2 | WM |
| S. agalactiae (146) | 0.06/0.03 | 0.25/0.25 | 2/1 | 0.25/0.25 | WM |
| E. faecium (897) | 0.25/0.25 | 0.5/0.5 | 2/2 | 1/1 | Ser80Ile (parC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, Y.; Muñoz, L.; Gargallo-Viola, D.; Cantón, R.; Vila, J.; Zsolt, I. Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria. Int. J. Mol. Sci. 2021, 22, 13363. https://doi.org/10.3390/ijms222413363
López Y, Muñoz L, Gargallo-Viola D, Cantón R, Vila J, Zsolt I. Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria. International Journal of Molecular Sciences. 2021; 22(24):13363. https://doi.org/10.3390/ijms222413363
Chicago/Turabian StyleLópez, Yuly, Laura Muñoz, Domingo Gargallo-Viola, Rafael Cantón, Jordi Vila, and Ilonka Zsolt. 2021. "Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria" International Journal of Molecular Sciences 22, no. 24: 13363. https://doi.org/10.3390/ijms222413363
APA StyleLópez, Y., Muñoz, L., Gargallo-Viola, D., Cantón, R., Vila, J., & Zsolt, I. (2021). Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria. International Journal of Molecular Sciences, 22(24), 13363. https://doi.org/10.3390/ijms222413363







