Microglia Reduce Herpes Simplex Virus 1 Lethality of Mice with Decreased T Cell and Interferon Responses in Brains
Abstract
1. Introduction
2. Results
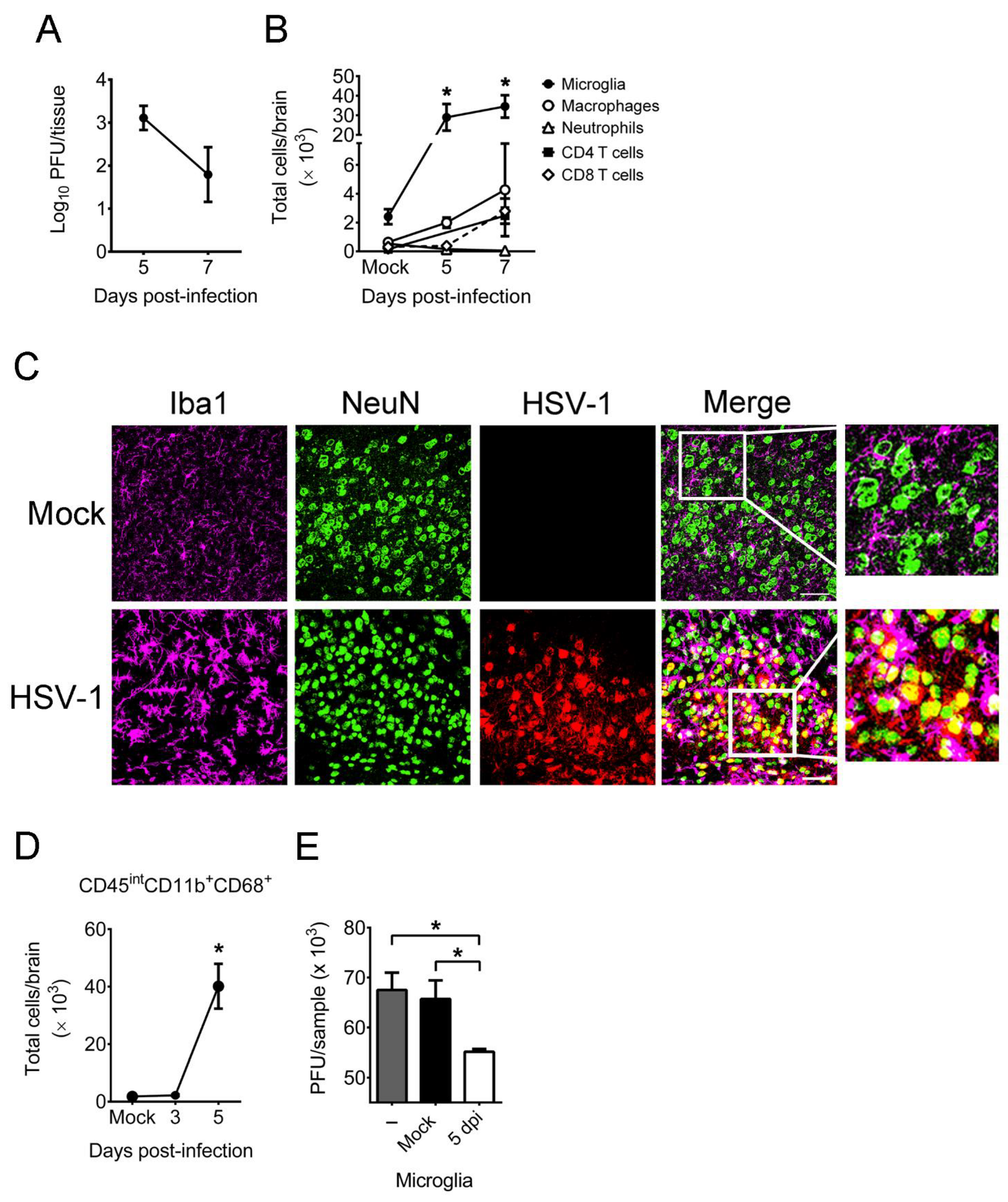
2.1. HSV-1 Infection of Mice Increases the Number, Activation, and Antiviral Activity of Brain Microglia
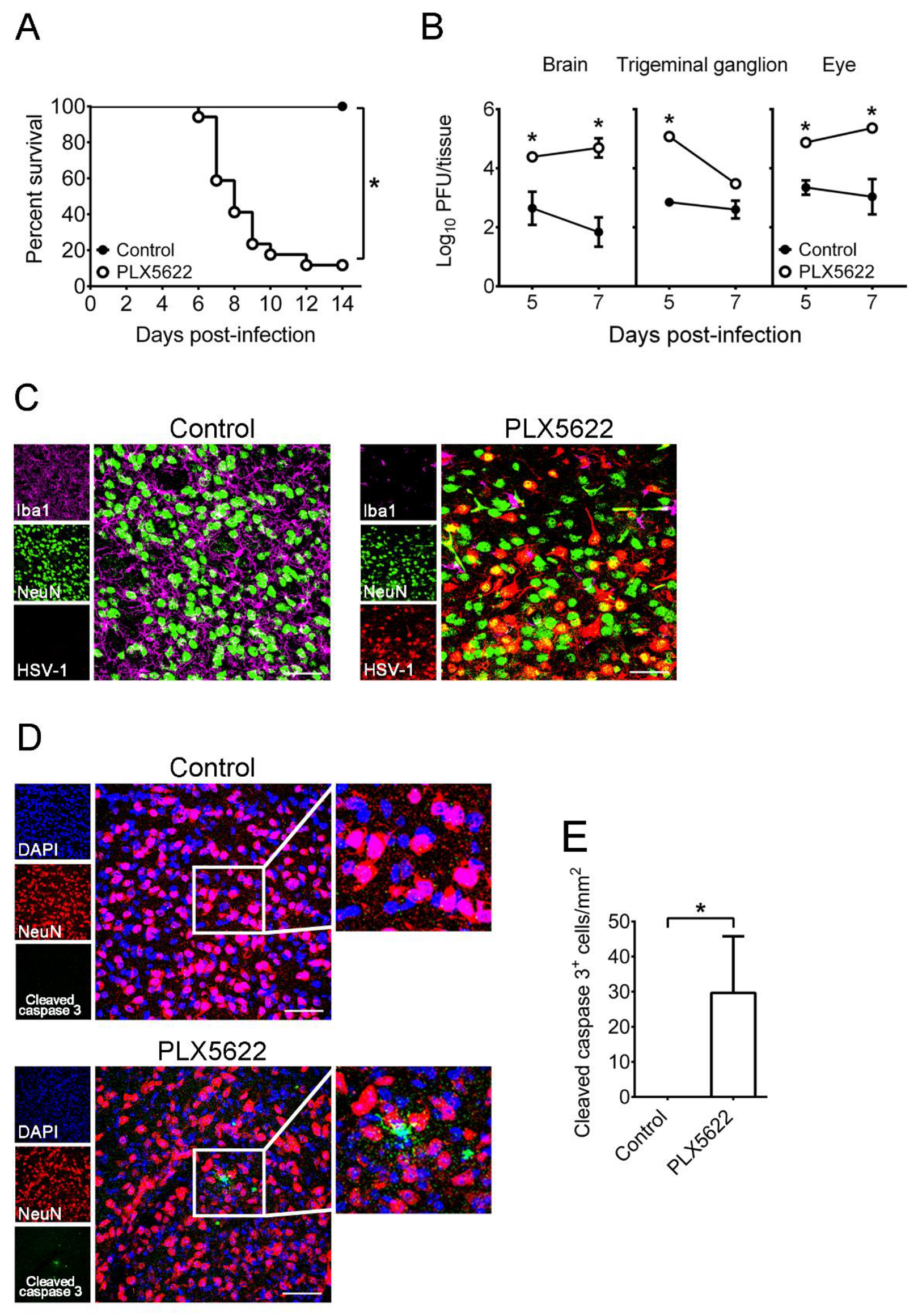
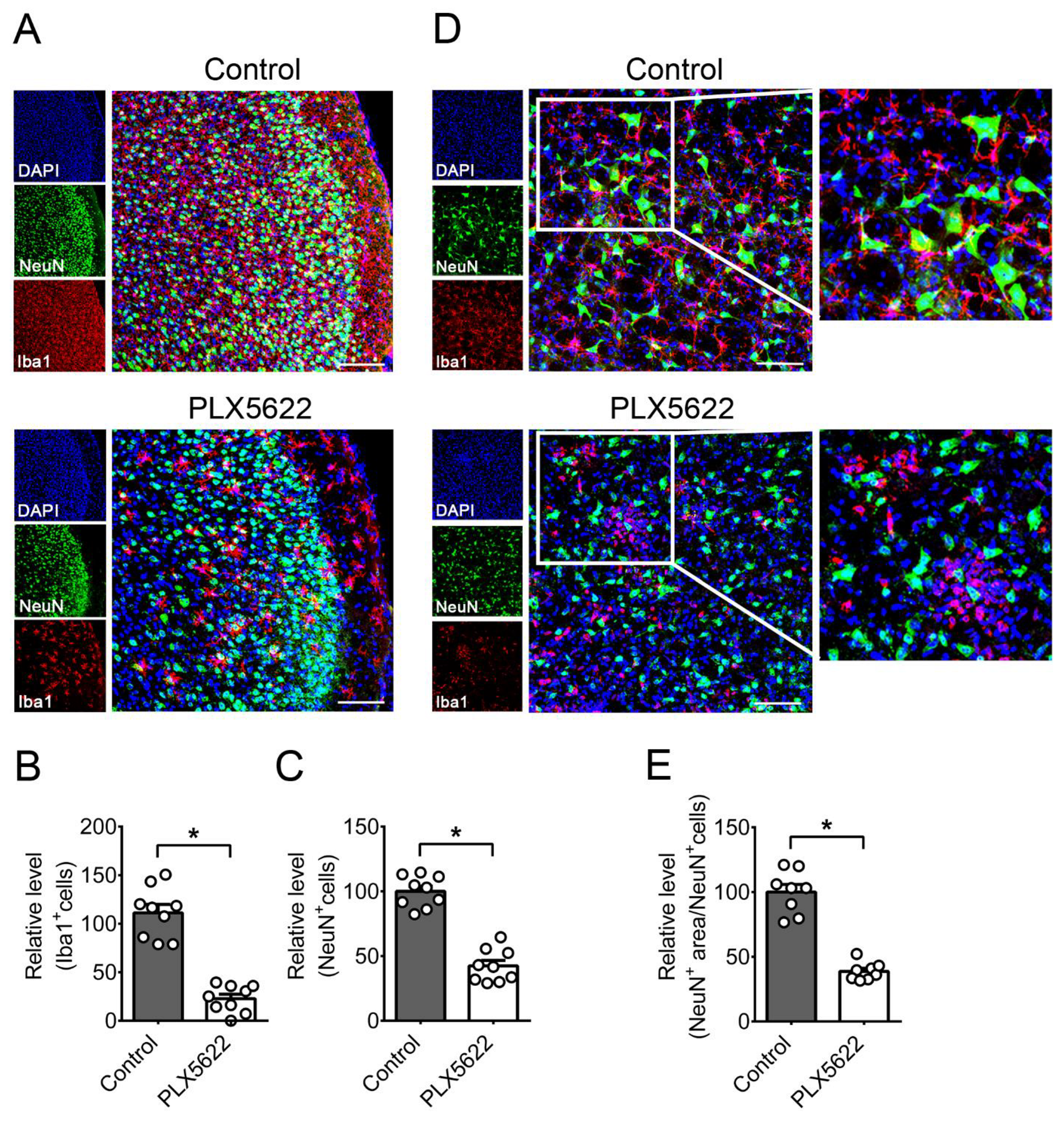
2.2. Depletion of Microglia by PLX5622 Increases HSV-1 Lethality, Tissue Viral Loads, and Brain Neuron Loss of Infected Mice
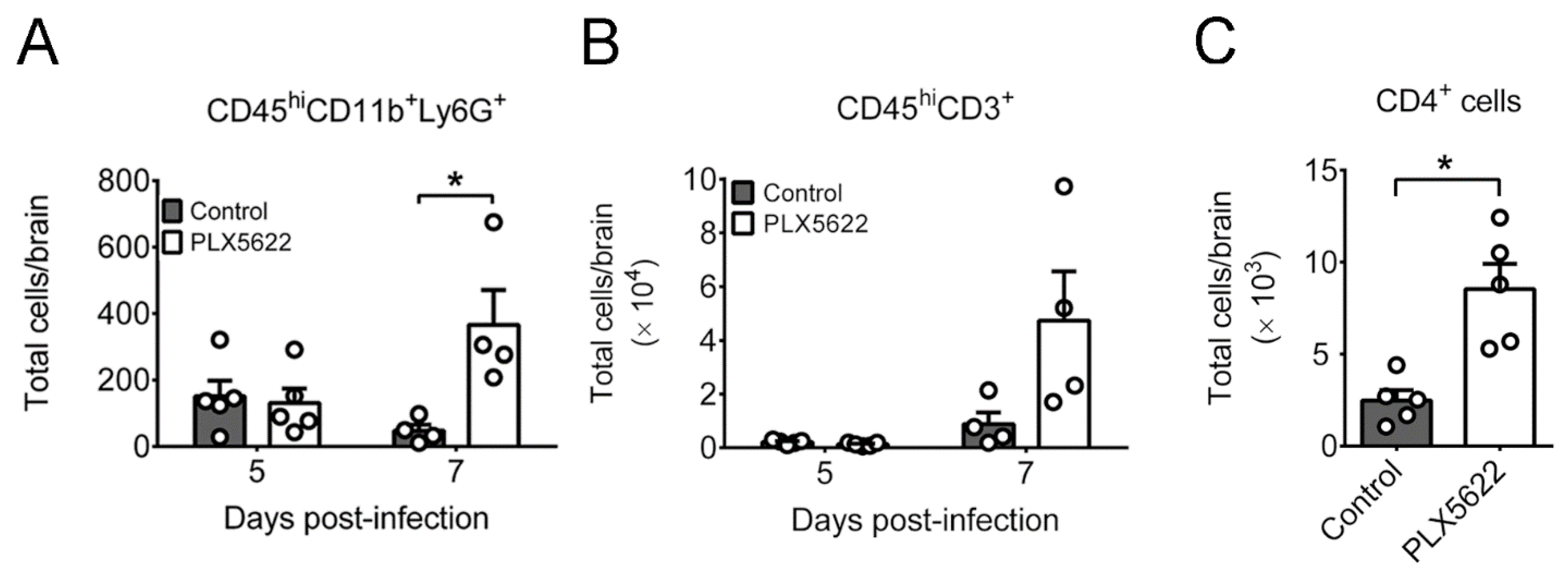
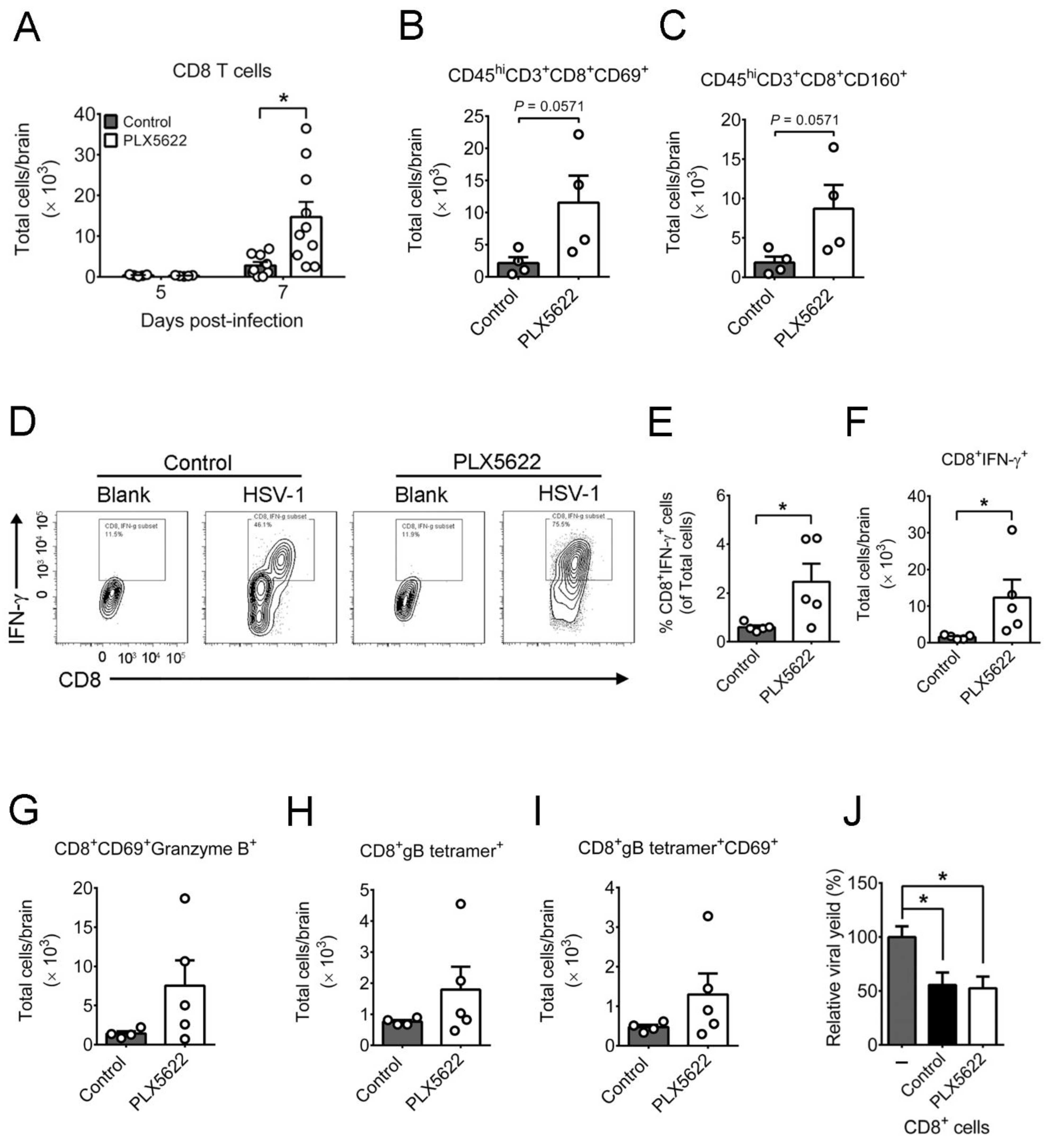
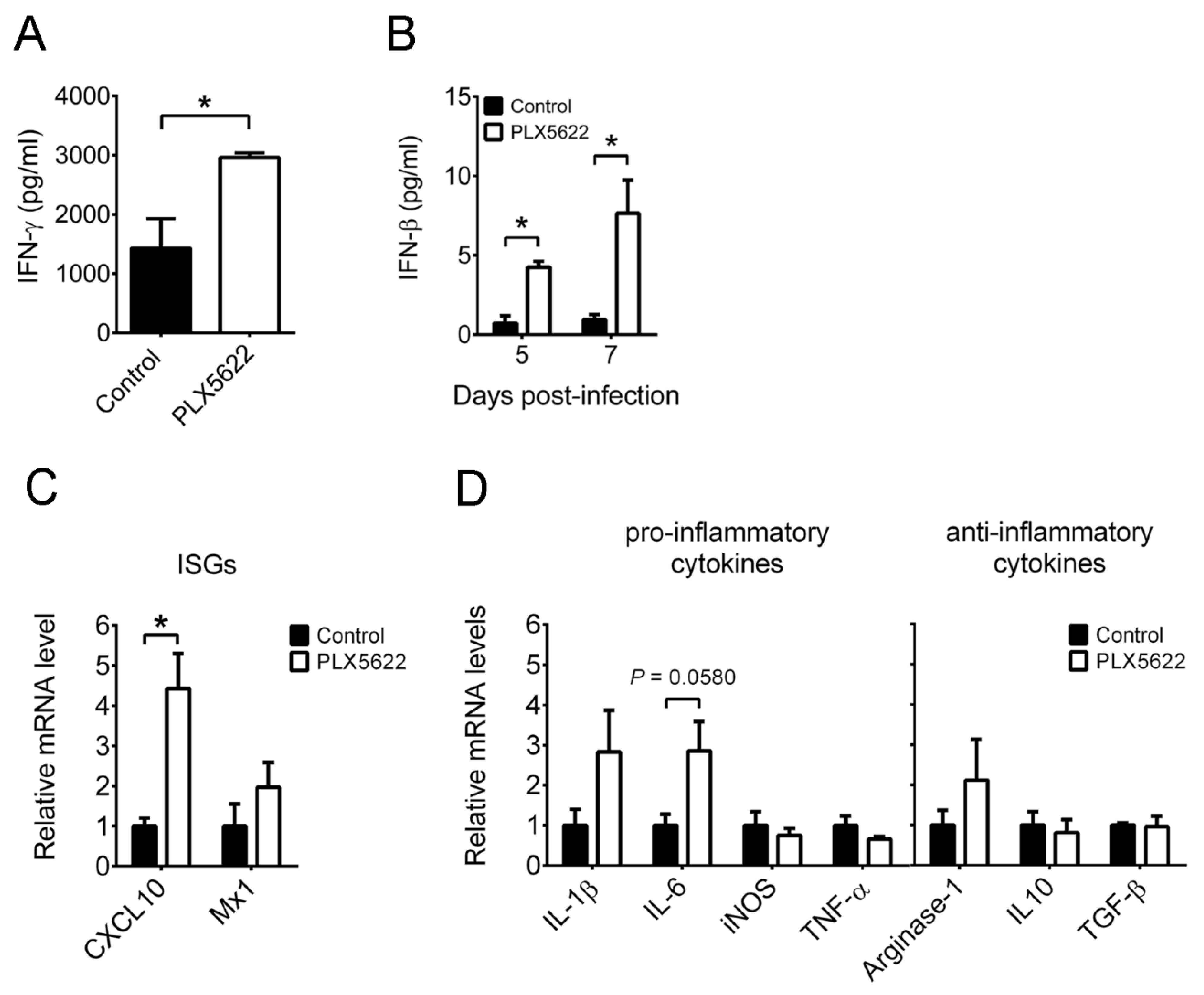
2.3. PLX5622 Decreases Microglia, but Increases the Levels of Neutrophils, CD4 T Cells, CD8 T Cells, IFN-β, and IFN-γ in Brains of Infected Mice
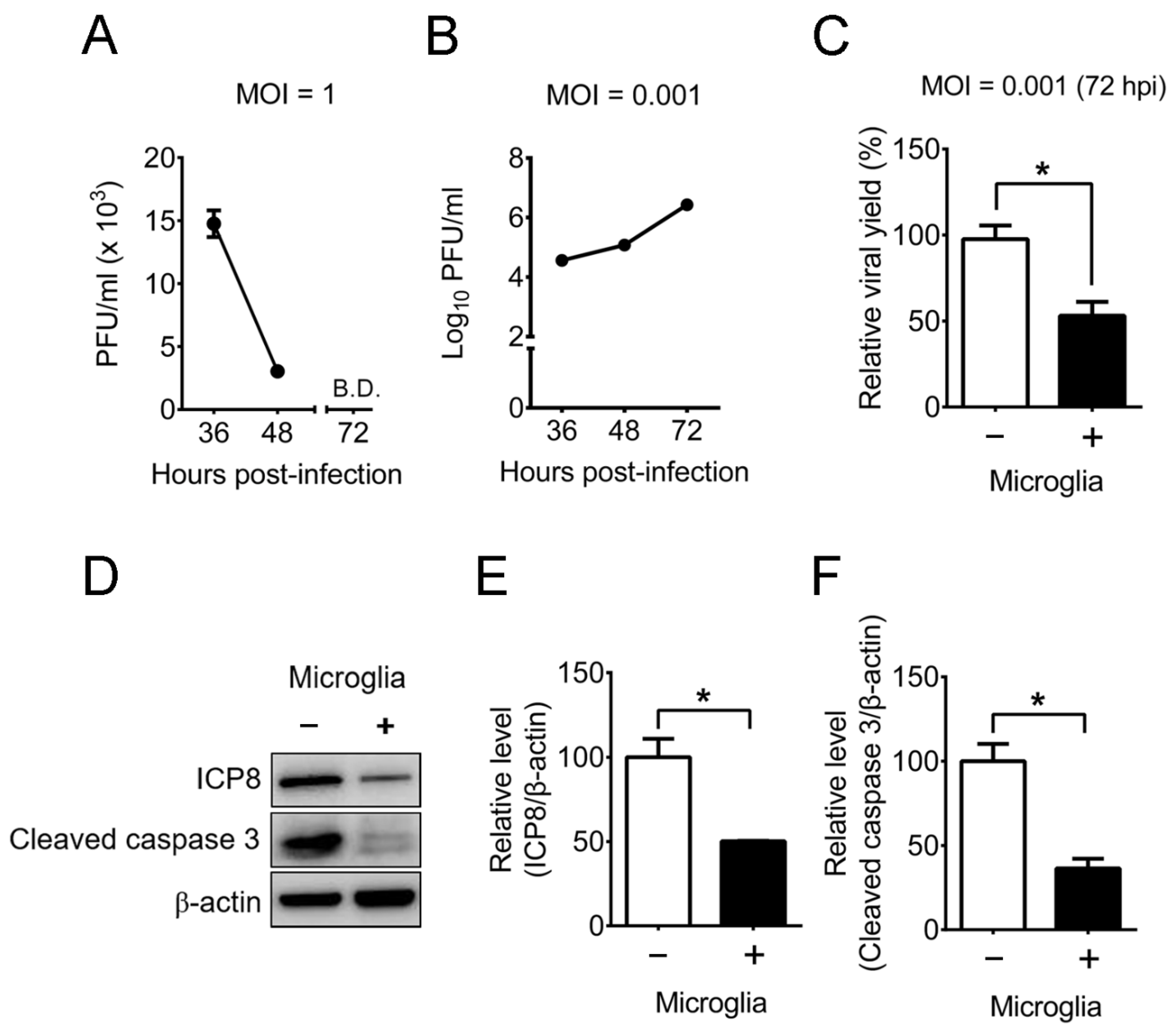
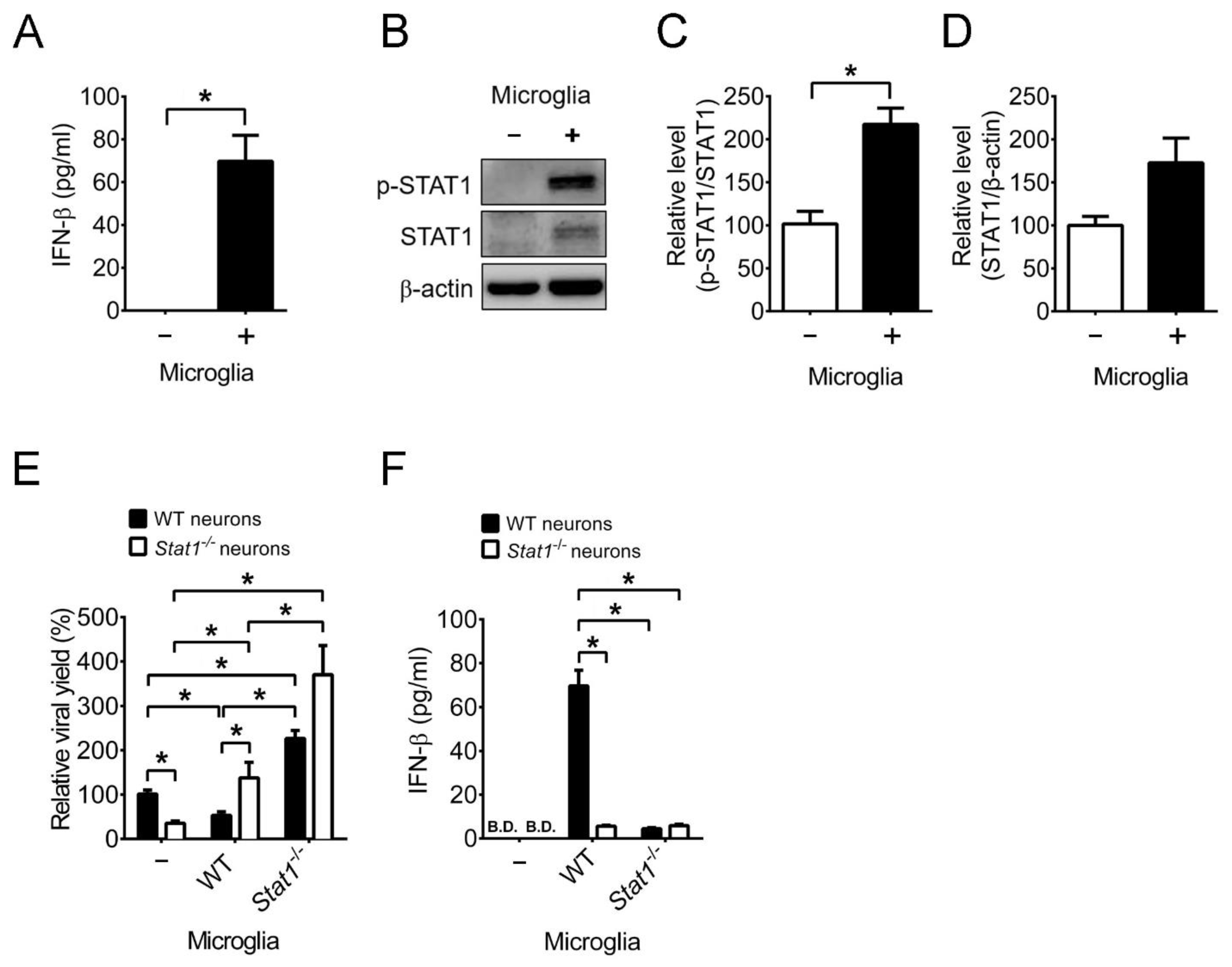
2.4. Mouse Primary Brain Microglia Inhibit HSV-1 Replication of Mouse Primary Brain Neurons with Increases of IFN-β Expression and STAT1 Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Cells, Virus, and Mice
4.2. Infection and Microglia Depletion of Mice
4.3. Flow Cytometry
4.4. Immunofluorescence Staining
4.5. Isolation of Microglia or CD8 T Cells from Mouse Brains
4.6. Quantification of Cytokines
4.7. RNA Isolation and Quantitative RT-PCR
4.8. Culture of Mouse Primary Cells
4.9. Western Blotting
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeBiasi, R.L.; Kleinschmidt-DeMasters, B.K.; Richardson-Burns, S.; Tyler, K.L. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J. Infect. Dis. 2002, 186, 1547–1557. [Google Scholar] [CrossRef]
- Fekete, R.; Cserép, C.; Lénárt, N.; Tóth, K.; Orsolits, B.; Martinecz, B.; Méhes, E.; Szabó, B.; Németh, V.; Gönci, B.; et al. Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. 2018, 136, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.S.; Lopušná, K.; Winther, H.; Sun, C.; Thomsen, M.K.; Nandakumar, R.; Mogensen, T.H.; Meyer, M.; Vægter, C.; Nyengaard, J.R.; et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016, 7, 13348. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.M.; Low, D.K.; Fan, Y.; Tan, J.J.; Lee, B.; Chan, J.K.; Rénia, L.; Ginhoux, F.; Ng, L. Zika virus infects human fetal brain microglia and induces inflammation. Clin. Infect. Dis. 2017, 64, 914–920. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, D.; Li, G. The role of microglia in viral encephalitis: A review. J. Neuroinflamm. 2019, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Alldred, M.J.; Gunnam, S.M.; Schiroli, C.; Lee, S.H.; Morgello, S.; Fischer, T. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018, 83, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Waltl, I.; Käufer, C.; Gerhauser, I.; Chhatbar, C.; Ghita, L.; Kalinke, U.; Löscher, W. Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage. Brain Behav. Immun. 2018, 74, 186–204. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Sariol, A.; Meyerholz, D.K.; Perlman, S. Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Investig. 2018, 128, 931–943. [Google Scholar] [CrossRef]
- Funk, K.; Klein, R.S. CSF1R antagonism limits local restimulation of antiviral CD8+ T cells during viral encephalitis. J. Neuroinflamm. 2019, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.M.S.; DePaula-Silva, A.B.; Doty, D.J.; Hanak, T.J.; Truong, A.; Libbey, J.E.; Fujinami, R.S. The CSF1R-microglia axis has protective host-specific roles during neurotropic picornavirus infection. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Clarke, P.; Tyler, K.L. Pharmacologic depletion of microglia increases viral load in the brain and enhances mortality in murine models of flavivirus-induced encephalitis. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Roizman, B.; Knipe, D.M.; Whitley, R.J. Herpes Simplex Viruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1823–1897. [Google Scholar]
- Whitley, R.J. Herpes simplex virus infections of the central nervous system. Encephalitis and neonatal herpes. Drugs 1991, 42, 406–427. [Google Scholar] [CrossRef]
- Pellett, P.E.; Roizman, B. Herpesviridae. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1802–1822. [Google Scholar]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef]
- Alsweed, A.; Alsuhaibani, M.; Casanova, J.-L.; Al-Hajjar, S. Approach to recurrent herpes simplex encephalitis in children. Int. J. Pediatr. Adolesc. Med. 2018, 5, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Zolini, G.P.; Lima, G.K.; Lucinda, N.; Silva, M.A.; Dias, M.F.; Pessoa, N.L.; Coura, B.P.; Cartelle, C.T.; Arantes, R.M.E.; Kroon, E.G.; et al. Defense against HSV-1 in a murine model is mediated by iNOS and orchestrated by the activation of TLR2 and TLR9 in trigeminal ganglia. J. Neuroinflamm. 2014, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Cantin, E.; Tanamachi, B.; Openshaw, H.; Mann, J.; Clarke, K. Gamma interferon (IFN-gamma) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-gamma ligand null-mutant mice. J. Virol. 1999, 73, 5196–5200. [Google Scholar] [CrossRef]
- Manickan, E.; Rouse, B.T. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol. 1995, 69, 8178–8179. [Google Scholar] [CrossRef]
- Parker, Z.M.; Pasieka, T.J.; Parker, G.A.; Leib, D.A. Immune- and nonimmune-compartment-specific interferon responses are critical determinants of herpes simplex virus-induced generalized infections and acute liver failure. J. Virol. 2016, 90, 10789–10799. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Bowen, G.N.; Zhou, S.; Cerny, A.; Zacharia, A.; Knipe, D.M.; Finberg, R.W.; Kurt-Jones, E.A. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 2011, 86, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Vidal, S.M. Insights into the pathogenesis of herpes simplex encephalitis from mouse models. Mamm. Genome 2018, 29, 425–445. [Google Scholar] [CrossRef]
- Uyar, O.; Laflamme, N.; Piret, J.; Venable, M.-C.; Carbonneau, J.; Zarrouk, K.; Rivest, S.; Boivin, G. An early microglial response is needed to efficiently control herpes simplex virus encephalitis. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Schwartz, G.S. Classification of herpes simplex virus keratitis. Cornea 1999, 18, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Armién, A.G.; Hu, S.; Little, M.R.; Robinson, N.; Lokensgard, J.R.; Low, W.C.; Cheeran, M.C.-J. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol. 2009, 20, 738–750. [Google Scholar] [CrossRef]
- Wu, H.-M.; Huang, C.-C.; Chen, S.-H.; Liang, Y.-C.; Tsai, J.-J.; Hsieh, C.-L.; Hsu, K.-S. Herpes simplex virus type 1 inoculation enhances hippocampal excitability and seizure susceptibility in mice. Eur. J. Neurosci. 2003, 18, 3294–3304. [Google Scholar] [CrossRef] [PubMed]
- Lokensgard, S.H.J.R.; Hu, S.; Sheng, W.; Vanoijen, M.; Cox, D.; Cheeran, M.; Peterson, P.K. Robust expression of TNFa, IL-1ß, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J. NeuroVirol. 2001, 7, 208–219. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Hu, S.; Rowen, T.N.; Palmquist, J.M.; Lokensgard, J.R. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 2005, 175, 4189–4193. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Xiong, S.; Sun, F.; Qin, G.; Hu, G.; Wang, J.; Zhao, L.; Liang, Y.-X.; Wu, T.; et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 2018, 21, 530–540. [Google Scholar] [CrossRef]
- Willis, E.; MacDonald, K.P.; Nguyen, Q.; Garrido, A.L.; Gillespie, E.R.; Harley, S.B.; Bartlett, P.F.; Schroder, W.A.; Yates, A.G.; Anthony, D.; et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 2020, 180, 833–846. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, Y.; Long, T.; He, W.; Qin, G.; Zhang, D.; Chen, L.; Zhou, J. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J. Neuroinflamm. 2019, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Okunuki, Y.; Mukai, R.; Nakao, T.; Tabor, S.J.; Butovsky, O.; Dana, R.; Ksander, B.R.; Connor, K.M. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9989–9998. [Google Scholar] [CrossRef]
- Tsuruta, F.; Okajima, T. Microglial dynamics during brain development. Neural Regen. Res. 2018, 13, 222–223. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Moseman, E.A.; Blanchard, A.C.; Nayak, D.; McGavern, D.B. T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Ramakrishna, C.; Brown, J.; Tyszka, J.M.; Hamamura, M.; Hinton, D.R.; Kovats, S.; Nalcioglu, O.; Weinberg, K.; Openshaw, H.; et al. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 2008, 82, 7078–7088. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Khanna, K.M.; Yee, M.B.; Baty, C.J.; Kinchington, P.R.; Hendricks, R.L. Noncytotoxic lytic granule–mediated CD8+ T Cell inhibition of HSV-1 reactivation from neuronal latency. Science 2008, 322, 268–271. [Google Scholar] [CrossRef]
- Fitzpatrick, L.; Makrigiannis, A.P.; Kaiser, M.; Hoskin, D.W. Anti-CD3-activated killer T cells: Interferon-γ and interleukin-10 cross-regulate granzyme B expression and the induction of major histocompatibility complex-unrestricted cytotoxicity. J. Interf. Cytokine Res. 1996, 16, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Chucair-Elliott, A.J.; Conrady, C.; Zheng, M.; Kroll, C.M.; Lane, T.E.; Carr, D.J.J. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 2014, 62, 1418–1434. [Google Scholar] [CrossRef]
- Mathur, V.; Burai, R.; Vest, R.T.; Bonanno, L.; Lehallier, B.; Zardeneta, M.E.; Mistry, K.N.; Do, D.; Marsh, S.; Abud, E.M.; et al. Activation of the STING-dependent type I interferon response reduces microglial reactivity and neuroinflammation. Neuron 2017, 96, 1290–1302. [Google Scholar] [CrossRef]
- Carr, D.J.; Al-Khatib, K.; James, C.M.; Silverman, R. Interferon-β suppresses herpes simplex virus type 1 replication in trigeminal ganglion cells through an RNase L-dependent pathway. J. Neuroimmunol. 2003, 141, 40–46. [Google Scholar] [CrossRef]
- Pasieka, T.J.; Collins, L.; O’Connor, M.A.; Chen, Y.; Parker, Z.M.; Berwin, B.L.; Piwnica-Worms, D.R.; Leib, D.A. Bioluminescent imaging reveals divergent viral pathogenesis in two strains of Stat1-deficient mice, and in αßγ interferon receptor-deficient mice. PLoS ONE 2011, 6, e24018. [Google Scholar] [CrossRef][Green Version]
- Rosato, P.C.; Leib, D.A. Neuronal interferon signaling is required for protection against herpes simplex virus replication and pathogenesis. PLoS Pathog. 2015, 11, e1005028. [Google Scholar] [CrossRef]
- Trinchieri, G. Type I interferon: Friend or foe? J. Exp. Med. 2010, 207, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Conti, J.; Zatta, V.; Russo, V.; Scarpa, M.; Kotsafti, A.; Porzionato, A.; De Caro, R.; Scarpa, M.; Fassan, M.; et al. Persistent herpes simplex virus type 1 infection of enteric neurons triggers CD8+ T cell response and gastrointestinal neuromuscular dysfunction. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Egan, K.P.; Wu, S.; Wigdahl, B.; Jennings, S.R. Immunological control of herpes simplex virus infections. J. NeuroVirol. 2013, 19, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Nishimura, T.; Kondo, H.; Ikeda, K.; Hayashi, Y.; McGeer, P.L. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res. 1994, 639, 171–174. [Google Scholar] [CrossRef]
- Sherr, C.J.; Rettenmier, C.W.; Sacca, R.; Roussel, M.F.; Look, A.T.; Stanley, E.R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF 1. Cell 1985, 41, 665–676. [Google Scholar] [CrossRef]
- Chitu, V.; Gokhan, Ş.; Nandi, S.; Mehler, M.F.; Stanley, E.R. Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci. 2016, 39, 378–393. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.; Rolland, D.C.; Polk, A.; Wolfe, A.; Dewar, H.; Chowdhury, P.; Özlem, Ö.; Dewar, R.; Brown, N.A.; Bailey, N.G.; et al. Colony-stimulating factor 1 receptor (CSF1R) activates AKT/mTOR signaling and promotes T-cell lymphoma viability. Clin. Cancer Res. 2019, 26, 690–703. [Google Scholar] [CrossRef]
- Green, K.N.; Hume, D.A. On the utility of CSF1R inhibitors. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Lei, F.; Cui, N.; Zhou, C.; Chodosh, J.; Vavvas, D.G.; Paschalis, E.I. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; Affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 23336–23338. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Roy, E.; Zheng, H. Protocol for primary microglial culture preparation. Bio-Protoc. 2016, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-H.; Wang, C.-Y.; Chen, P.-S.; Wang, J.-W.; Chuang, D.-M.; Yang, C.-S.; Tzeng, S.-F. Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4receptor expression in activated microglia. J. Neurosci. Res. 2013, 91, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.-W.; Ling, P.; Chen, S.-H.; Tung, Y.-Y.; Chen, S.-H. Factors affecting herpes simplex virus reactivation from the explanted mouse brain. J. Virol. 2012, 433, 116–123. [Google Scholar] [CrossRef]
- Korin, B.; Dubovik, T.; Rolls, A. Mass cytometry analysis of immune cells in the brain. Nat. Protoc. 2018, 13, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Assenmacher, M.; Schmitz, J.; Radbruch, A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: Expression of interleukin-10 in interferon-γ and in interleukin-4-expressing cells. Eur. J. Immunol. 1994, 24, 1097–1101. [Google Scholar] [CrossRef]
- Khanna, K.M.; Bonneau, R.H.; Kinchington, P.R.; Hendricks, R.L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunology 2003, 18, 593–603. [Google Scholar] [CrossRef]
- Shen, F.-H.; Wang, S.-W.; Yeh, T.-M.; Tung, Y.-Y.; Hsu, S.-M.; Chen, S.-H. Absence of CXCL10 aggravates herpes stromal keratitis with reduced primary neutrophil influx in mice. J. Virol. 2013, 87, 8502–8510. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Y.; Dang, C.; Han, B.; Han, R.; Ma, H.; Hao, J.; Wang, L. Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol. Med. 2020, 12, e11002. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Cahalan, S.; LaMonte, G.; Grubaugh, N.D.; Zeng, W.; Murthy, S.E.; Paytas, E.; Gamini, R.; Lukacs, V.; Whitwam, T.; et al. Common PIEZO1 allele in African populations causes RBC dehydration and attenuates plasmodium infection. Cell 2018, 173, 443–455.e12. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.-H.; Yeh, N.-H.; Huang, Y.-S. CPEB2 activates GRASP1 mRNA translation and promotes AMPA receptor surface expression, long-term potentiation, and memory. Cell Rep. 2017, 21, 1783–1794. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-S.; Wang, L.-C.; Tsai, H.-Y.; Lin, Y.-J.; Wu, H.-L.; Tzeng, S.-F.; Hsu, S.-M.; Chen, S.-H. Microglia Reduce Herpes Simplex Virus 1 Lethality of Mice with Decreased T Cell and Interferon Responses in Brains. Int. J. Mol. Sci. 2021, 22, 12457. https://doi.org/10.3390/ijms222212457
Tsai M-S, Wang L-C, Tsai H-Y, Lin Y-J, Wu H-L, Tzeng S-F, Hsu S-M, Chen S-H. Microglia Reduce Herpes Simplex Virus 1 Lethality of Mice with Decreased T Cell and Interferon Responses in Brains. International Journal of Molecular Sciences. 2021; 22(22):12457. https://doi.org/10.3390/ijms222212457
Chicago/Turabian StyleTsai, Meng-Shan, Li-Chiu Wang, Hsien-Yang Tsai, Yu-Jheng Lin, Hua-Lin Wu, Shun-Fen Tzeng, Sheng-Min Hsu, and Shun-Hua Chen. 2021. "Microglia Reduce Herpes Simplex Virus 1 Lethality of Mice with Decreased T Cell and Interferon Responses in Brains" International Journal of Molecular Sciences 22, no. 22: 12457. https://doi.org/10.3390/ijms222212457
APA StyleTsai, M.-S., Wang, L.-C., Tsai, H.-Y., Lin, Y.-J., Wu, H.-L., Tzeng, S.-F., Hsu, S.-M., & Chen, S.-H. (2021). Microglia Reduce Herpes Simplex Virus 1 Lethality of Mice with Decreased T Cell and Interferon Responses in Brains. International Journal of Molecular Sciences, 22(22), 12457. https://doi.org/10.3390/ijms222212457





