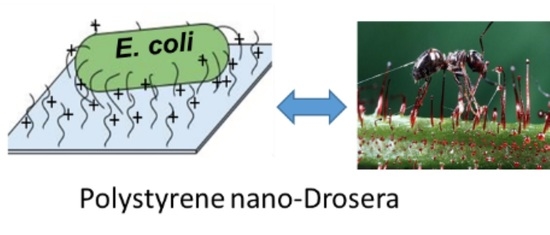
Soft Surface Nanostructure with Semi-Free Polyionic Components for Sustainable Antimicrobial Plastic
Abstract
:1. Introduction
2. Results and discussions
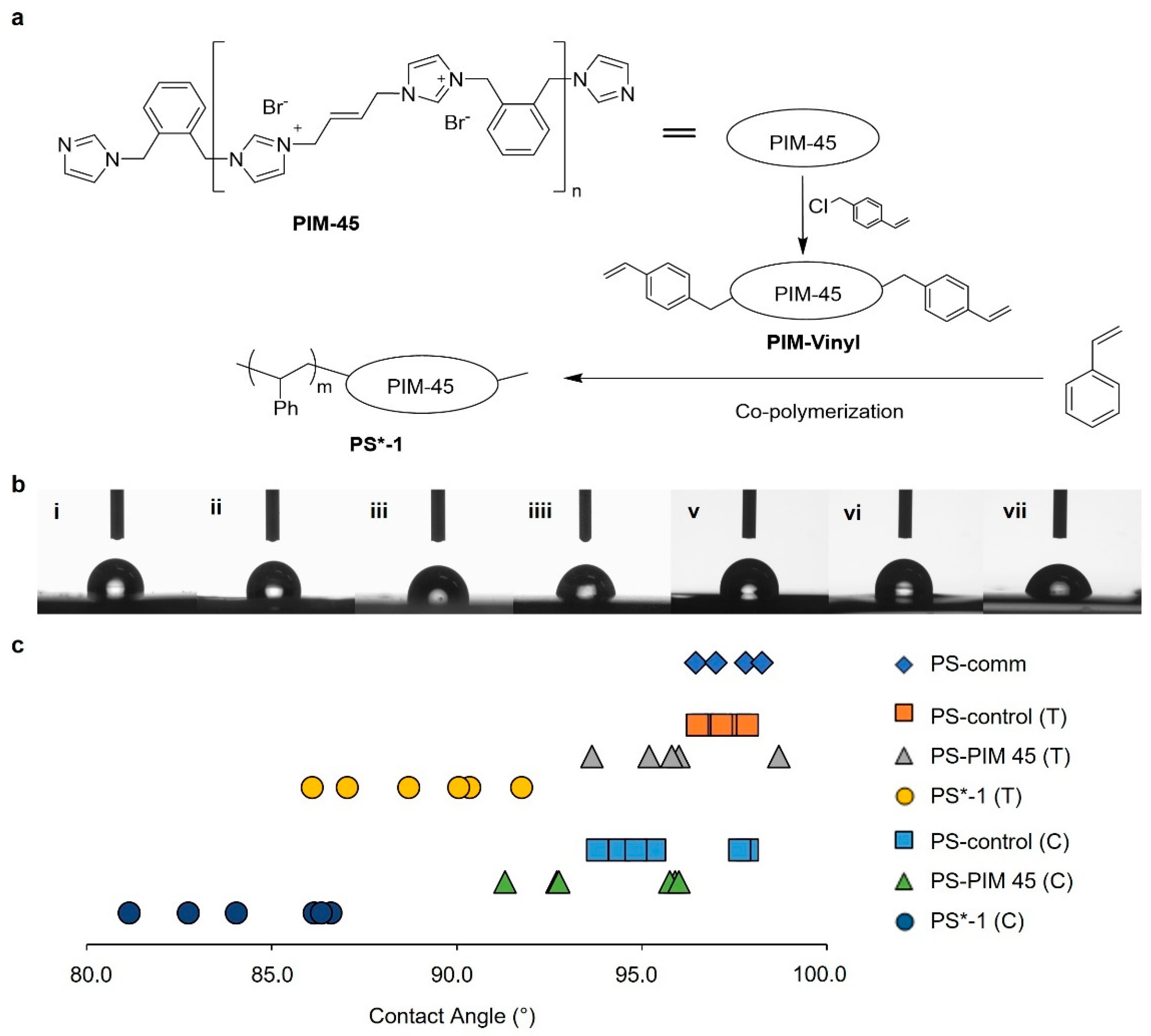
2.1. Synthesis and Characterization of Polystyrene Modified with Polyimidazoliums on Surface
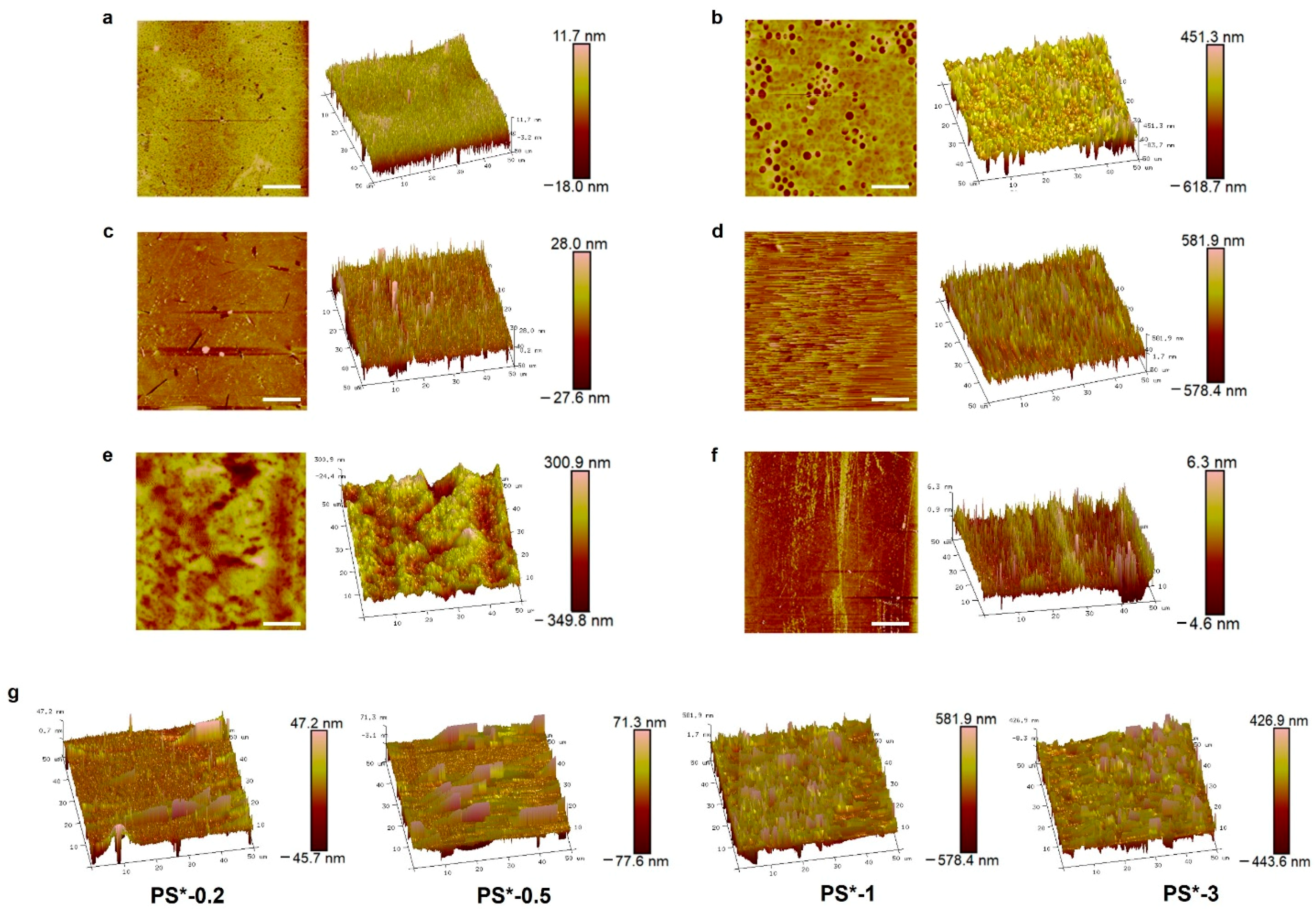
2.2. Surface Nanostructures of PS-Based Materials
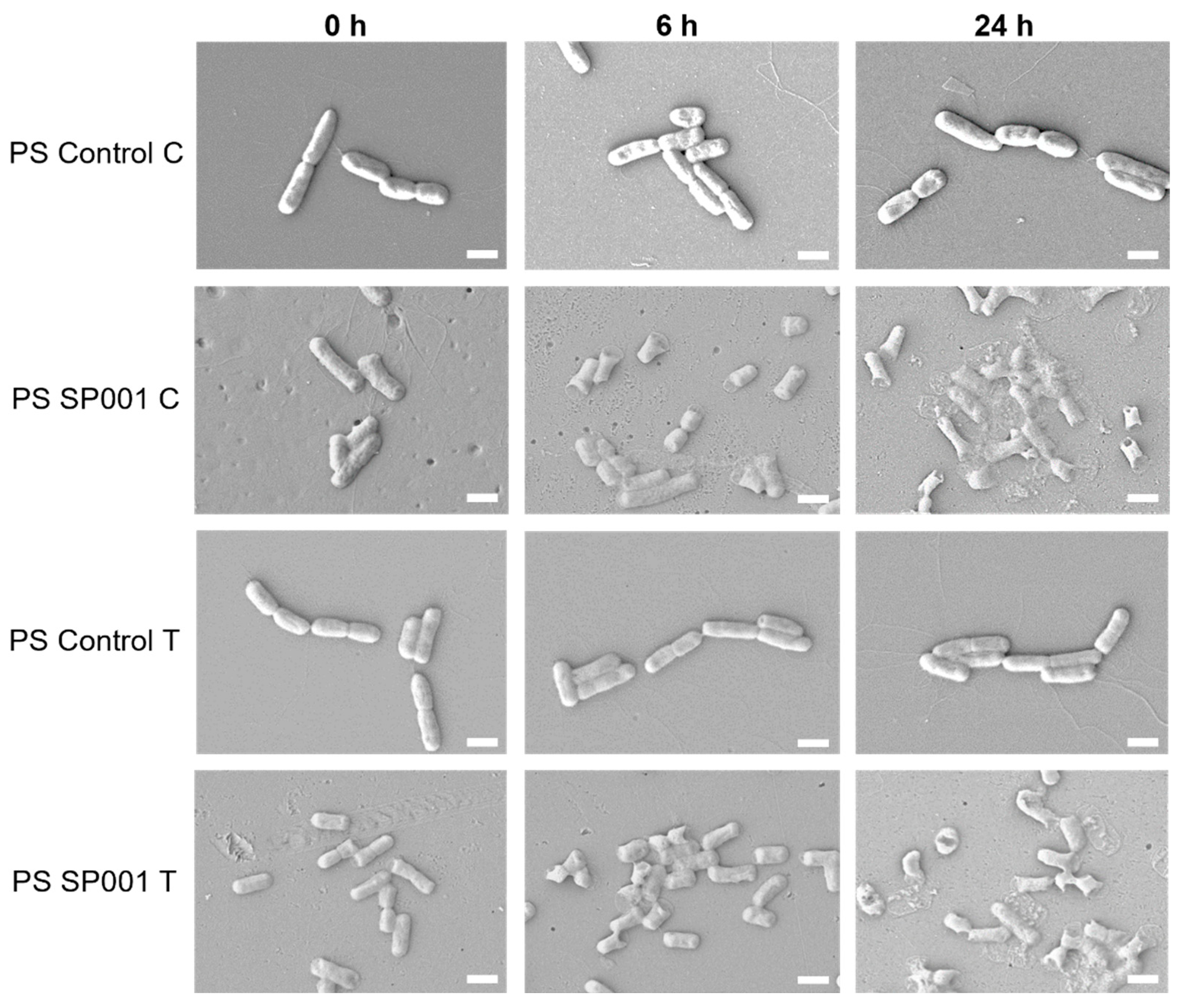
2.3. Antimicrobial Property Evaluation
2.4. Durability and Biocompatibility of PS-Based Antimicrobial Plastics
3. Materials and Methods
3.1. General Information
3.2. Compounds and Materials Synthesis
3.3. Water Contact Angle Measurement
3.4. Minimum Inhibitory Concentration (MIC)
3.5. Japanese Industrial Standard (JIS Z 2801/ISO 22196) Method
3.6. Time-Kill Kinetic Assay
3.7. GB 15979-2002 and Leaching Assay
3.8. Scanning Electron Microscopy (SEM) Imaging of Bacteria Morphology Changes
3.9. Surface Structure Analysis with SEM and Atomic Force Microscopy (AFM)
3.10. Accelerated Aging and Shelf Life Test
3.11. Cell Viability Assay
3.12. Hemolysis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banin, E.; Hughes, D.; Kuipers, O.P. Editorial: Bacterial pathogens, antibiotics and antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F. Versatile antibacterial materials: An emerging arsenal for combatting bacterial pathogens. Adv. Funct. Mater. 2018, 28, 1802140. [Google Scholar] [CrossRef]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Swartjes, J.J.T.M.; Sharma, P.K.; Kooten, T.G.; Mei, H.C.; Mahmoudi, M.; Busscher, H.J.; Rochford, E.T.J. Current developments in antimicrobial surface coatings for biomedical applications. Curr. Med. Chem. 2015, 22, 2116–2129. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.Y.; Sin, L.T.; Tee, T.; Bee, S.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Ogusona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Kim, J. Recent developments in biofouling control in membrane bioreactors for domestic wastewater treatment. Sep. Purif. Technol. 2018, 206, 297–315. [Google Scholar] [CrossRef]
- Wang, C.; Mu, C.; Lin, W.; Xiao, H. Functional-modified polyurethanes for rendering surfaces antimicrobial: An overview. Adv. Colloid Interface Sci. 2020, 283, 102235. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2017, 32, 698–725. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Rehim, M.H.A.; Youssef, A.M.; Ghanem, A. Polystyrene/hydrophobic TiO2 nanobelts as a novel packaging material. Polym. Bull. 2015, 72, 2353–2362. [Google Scholar] [CrossRef]
- Nassar, M.A.; Youssef, A.M. Mechanical and antibacterial properties of recycled carton paper coated by PS/Ag nanocomposites for packaging. Carbohydr. Polym. 2012, 89, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Kamel, S.; El-Samahy, M.A. Morphological and antibacterial properties of modified paper by PS nanocomposites for packaging applications. Carbohydr. Polym. 2013, 98, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.F.; Youssef, A.M.; Rehim, M.A. Hydrophobically modified graphene oxide as a barrier and antibacterial agent for polystyrene packaging. J. Mater. Sci. 2020, 55, 4685–4700. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Zhuang, Z.; Chen, X.; Ni, M.; Li, Y.; Cui, Y.; Zhan, P.; Yuan, C.; Ge, H.; et al. A New Strategy of Lithography Based on Phase Separation of Polymer Blends. Sci. Rep. 2015, 5, 15947. [Google Scholar] [CrossRef]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Liu, L.; Wu, H.; Riduan, S.N.; Ying, J.Y.; Zhang, Y. Short imidazolium chains effectively clear fungal biofilm in keratitis treatment. Biomaterials 2013, 34, 1018–1023. [Google Scholar] [CrossRef]
- Riduan, S.N.; Yuan, Y.; Zhou, F.; Leong, J.; Su, H.; Zhang, Y. Ultrafast killing and self-gelling antimicrobial imidazolium oligomers. Small 2016, 12, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, Y.; Riduan, S.N.; Gao, S.; Yang, Y.; Fan, W.; Zhang, Y. Main-chain imidazolium oligomer material as a selective biomimetic antimicrobial agent. Biomaterials 2012, 33, 8625–8631. [Google Scholar] [CrossRef] [PubMed]
- Revilla, R.I.; Guan, L.; Zhu, X.; Quan, B.; Yang, Y.; Wang, C. Electrowetting phenomenon on nanostructured surfaces studied by using atomic force microscopy. J. Phys. Chem. C 2012, 116, 14311–14317. [Google Scholar] [CrossRef]
- Sun, Y.; Akhremitchev, B.; Walker, G.C. Using the adhesive interaction between atomic force microscopy tips and polymer surfaces to measure the elastic modulus of compliant samples. Langmuir 2004, 20, 5837–5845. [Google Scholar] [PubMed]
- James, B.; Davis, C. Selecting suitable image dimensions for scanning probe microscopy. Surf. Interfaces 2017, 9, 132–142. [Google Scholar]
- Boussu, K.; Bruggen, B.; Volodin, A.; Snauwaert, J.; Haesendonck, C.V.; Vandecasteele, C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of AFM. J. Colloid Interface Sci. 2005, 286, 632–638. [Google Scholar] [CrossRef]
- Yuan, Y.; Liang, S.; Li, J.; Zhang, S.; Zhang, Y. Copolymers with both soft and rigid cationic rings as highly selective antimicrobials to combat antibiotic resistant microbes and biofilms. J. Mater. Chem. B 2019, 7, 5620–5625. [Google Scholar] [CrossRef]
- Yi, G.; Yuan, Y.; Li, X.; Zhang, Y. ZnO nanopillar coated surfaces with substrate-dependent superbactericidal property. Small 2018, 14, 1703159. [Google Scholar] [CrossRef]
- Yi, G.; Riduan, S.N.; Yuan, Y.; Zhang, Y. Microbicide surface nano-structures. Crit. Rev. Biotechnol. 2019, 39, 964–979. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Tzegkas, S.G.; Danezis, G.P. Nanomaterials in food packaging: State of the art and analysis. J. Food Sci. Technol. 2018, 55, 2862–2870. [Google Scholar] [CrossRef]
- Yuan, Y.; Lim, D.S.W.; Wu, H.; Lu, H.; Zheng, Y.; Wan, A.C.A.; Ying, J.Y.; Zhang, Y. pH-Degradable imidazolium oligomers as antimicrobial materials with tuneable loss of activity. Biomater. Sci. 2019, 7, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Teong, S.P.; Liu, S.; Chng, S.; Yang, Y.Y.; Zhang, Y. Iron-based nano-structured surfaces with antimicrobial properties. J. Mater. Chem. B 2020, 8, 10146. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices; ASTM F1980-16; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]

 ) indicates no colony observed on respective samples.
) indicates no colony observed on respective samples.
 ) indicates no colony observed on respective samples.
) indicates no colony observed on respective samples.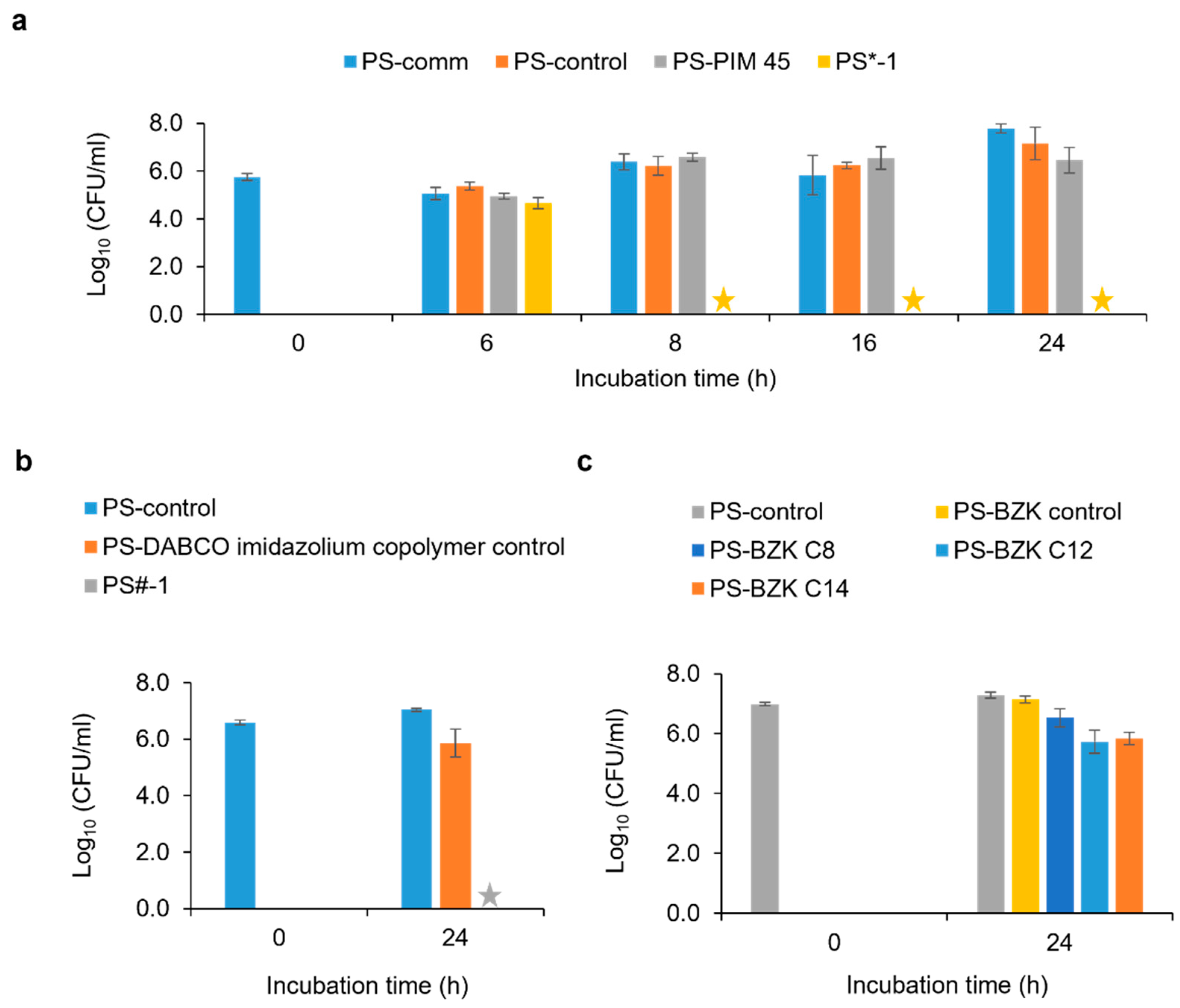
| Test Microbes | E. coli | S. aureus | C. albicans |
|---|---|---|---|
| 0 h 1 | 6.43 | 6.05 | 5.50 |
| 24 h 1 | |||
| PS-comm | 7.35 | 7.63 | 5.75 |
| PS-control (T) 2 | 7.16 | 7.37 | 5.83 |
| PS-PIM 45 (T) | 6.90 | 7.62 | 5.72 |
| PS*-1 (T) | 0.00 | 0.00 | 5.64 (0.00) 3 |
| 0 h 1 | 6.68 | 6.26 | 5.70 |
| 24 h 1 | |||
| PS-comm | 7.45 | 7.75 | 5.84 |
| PS-control (C) 2 | 7.28 | 7.76 | 5.87 |
| PS-PIM 45 (C) | 7.66 | 7.50 | 5.97 |
| PS*-1 (C) | 0.00 | 0.00 | 6.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.P.; Lim, D.S.W.; Armugam, A.; Yi, G.; Zhang, Y. Soft Surface Nanostructure with Semi-Free Polyionic Components for Sustainable Antimicrobial Plastic. Int. J. Mol. Sci. 2021, 22, 12315. https://doi.org/10.3390/ijms222212315
Chan SP, Lim DSW, Armugam A, Yi G, Zhang Y. Soft Surface Nanostructure with Semi-Free Polyionic Components for Sustainable Antimicrobial Plastic. International Journal of Molecular Sciences. 2021; 22(22):12315. https://doi.org/10.3390/ijms222212315
Chicago/Turabian StyleChan, Shook Pui, Diane S. W. Lim, Arunmozhiarasi Armugam, Guangshun Yi, and Yugen Zhang. 2021. "Soft Surface Nanostructure with Semi-Free Polyionic Components for Sustainable Antimicrobial Plastic" International Journal of Molecular Sciences 22, no. 22: 12315. https://doi.org/10.3390/ijms222212315
APA StyleChan, S. P., Lim, D. S. W., Armugam, A., Yi, G., & Zhang, Y. (2021). Soft Surface Nanostructure with Semi-Free Polyionic Components for Sustainable Antimicrobial Plastic. International Journal of Molecular Sciences, 22(22), 12315. https://doi.org/10.3390/ijms222212315