Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention
Abstract
:1. Introduction
2. Results
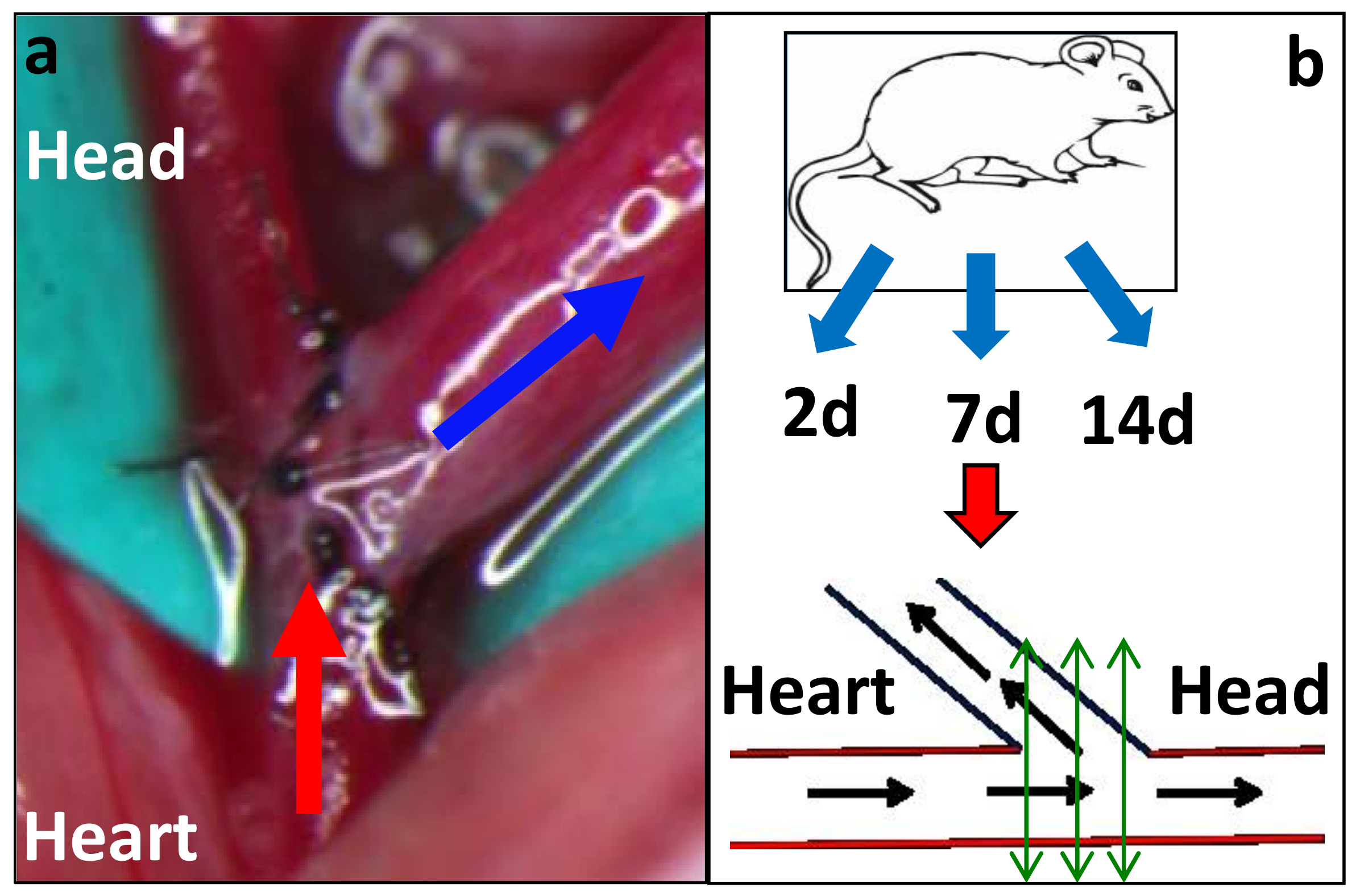
2.1. Mouse AVF Model Histology
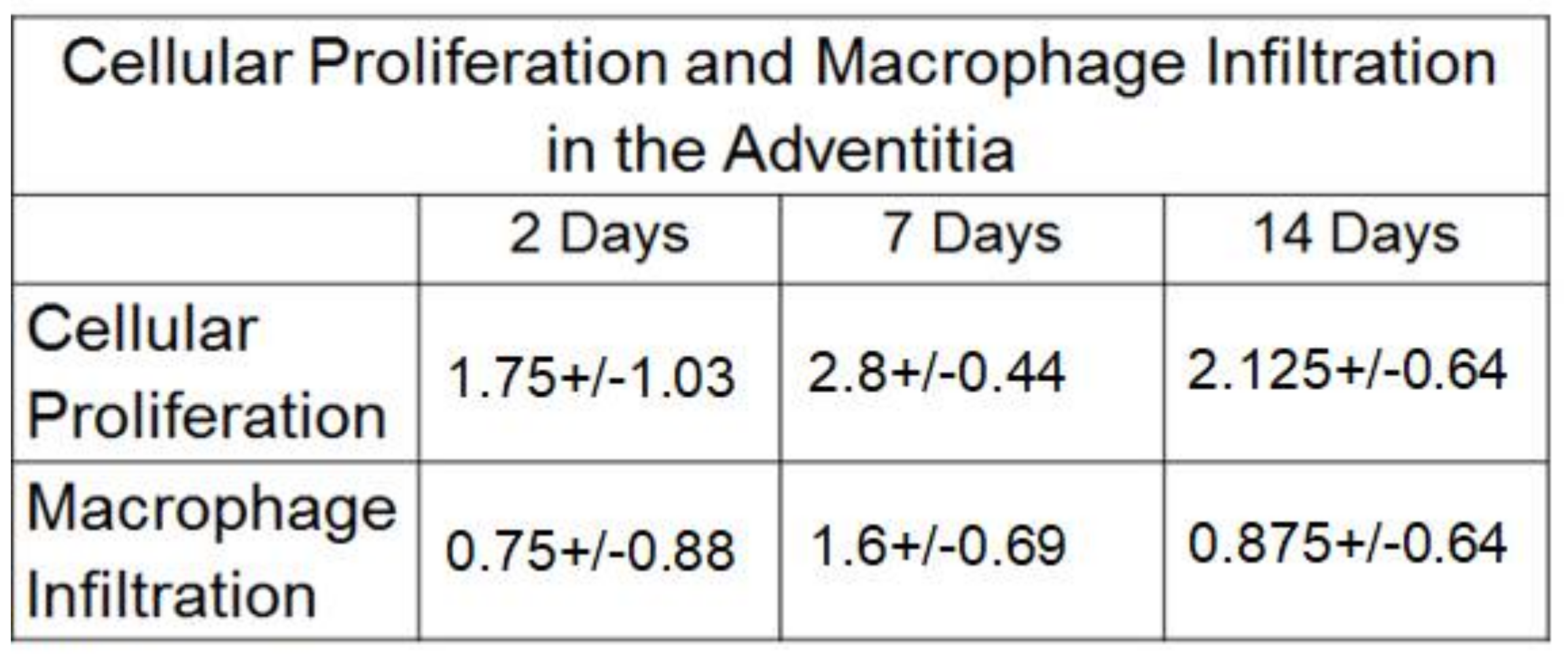
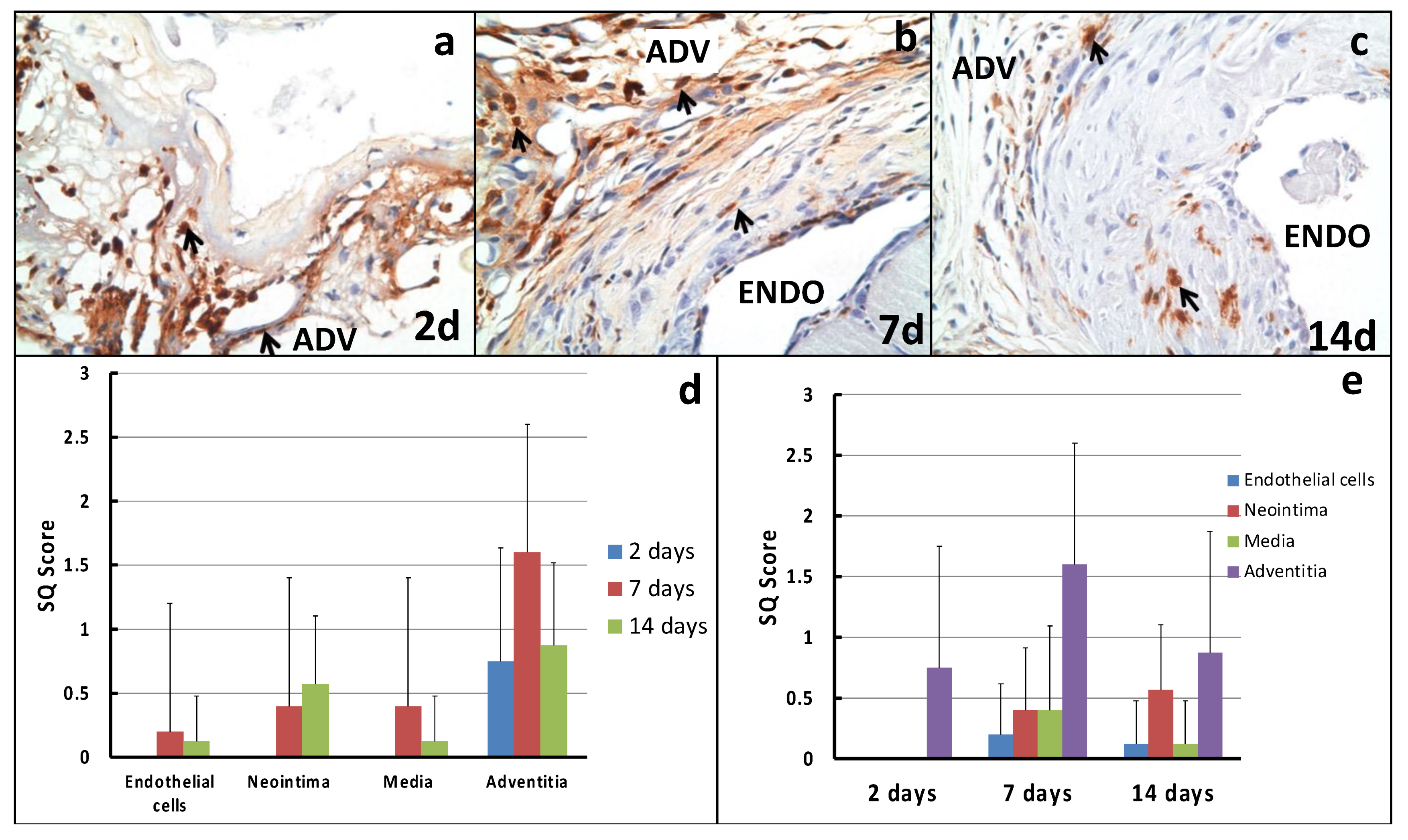
2.2. Cellular Proliferation
2.3. Macrophage Infiltration
3. Discussion
- (a)
- Non-peri-anastomotic stenotic lesions: We speculate that (a) needle stick injuries prior to AVF creation, (b) venous valves and (c) non laminar flow as a result of unique anatomical configurations could be responsible for non-peri-anastomotic clinical stenoses.
- (b)
- Forearm versus upper arm AVFs: In an earlier study by our group that described the cellular composition of neointimal hyperplasia in forearm and upper-arm AVFs, there was no real difference in the cellular phenotype, suggesting that the downstream venous response could be similar regardless of the type of vascular injury or the anatomical location of the same [23].
- (c)
- First time stenosis versus restenotic lesions: While the data on this are limited, we have previously not been able to demonstrate any significant histological changes between these two clinical settings.
4. Materials and Methods
4.1. Study Design
4.2. Arteriovenous Fistula Model Development
4.3. Specimen Collection and Processing
4.4. Processing of Samples for Histology
4.5. Immunohistochemistry
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, T.S.; Berceli, S.A.; Scali, S.T.; Neal, D.; Anderson, E.M.; Allon, M.; Cheung, A.K.; Dember, L.M.; Himmelfarb, J.; Roy-Chaudhury, P.; et al. Arteriovenous fistula maturation, functional patency, and intervention rates. JAMA Surg. 2021, 22, e214527. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.H.; Niklason, L.E.; Roy-Chaudhury, P. Challenges and novel therapies for vascular access in haemodialysis. Nat. Rev. Nephrol. 2020, 16, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Riella, M.C.; Roy-Chaudhury, P. Vascular access in haemodialysis: Strengthening the Achilles’ heel. Nat. Rev. Nephrol. 2013, 9, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Arend, L.; Zhang, J.; Krishnamoorthy, M.; Wang, Y.; Banerjee, R.; Samaha, A.; Munda, R. Neointimal hyperplasia in early arteriovenous fistula failure. Am. J. Kidney Dis. 2007, 50, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Roy-Chaudhury, P.; Kelly, B.S.; Melhem, M.; Zhang, J.; Li, J.; Desai, P.; Munda, R.; Heffelfinger, S.C. Vascular access in hemodialysis: Issues, management, and emerging concepts. Cardiol. Clin. 2005, 23, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chaudhury, P.; Sukhatme, V.P.; Cheung, A.K. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J. Am. Soc. Nephrol. 2006, 17, 1112–1127. [Google Scholar] [CrossRef]
- Wong, C.Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.J.; Hamming, J.F.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.; et al. A novel murine model of arteriovenous fistula failure: The surgical procedure in detail. J. Vis. Exp. 2016, 108, e53294. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; de Vries, M.R.; Wang, Y.; van der Vorst, J.R.; Vahrmeijer, A.L.; van Zonneveld, A.J.; Roy-Chaudhury, P.; Rabelink, T.J.; Quax, P.H.; Rotmans, J.I. Vascular remodeling and intimal hyperplasia in a novel murine model of arteriovenous fistula failure. J. Vasc. Surg. 2014, 59, 192–201.e191. [Google Scholar] [CrossRef] [Green Version]
- Roy-Chaudhury, P.; Khan, R.; Campos, B.; Wang, Y.; Kurian, M.; Lee, T.; Arend, L.; Munda, R. Pathogenetic role for early focal macrophage infiltration in a pig model of arteriovenous fistula (AVF) stenosis. J. Vasc. Access 2014, 15, 25–28. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Kruska, L. Future directions for vascular access for hemodialysis. Semin. Dial. 2015, 28, 107–113. [Google Scholar] [CrossRef]
- Havelka, G.E.; Kibbe, M.R. The vascular adventitia: Its role in the arterial injury response. Vasc. Endovasc. Surg. 2011, 45, 381–390. [Google Scholar] [CrossRef]
- Ji, J.; Xu, F.; Li, L.; Chen, R.; Wang, J.; Hu, W.C. Activation of adventitial fibroblasts in the early stage of the aortic transplant vasculopathy in rat. Transplantation 2010, 89, 945–953. [Google Scholar] [CrossRef]
- Singh, A.K.; Cai, C.; Kilari, S.; Zhao, C.; Simeon, M.L.; Takahashi, E.; Edelman, E.R.; Kong, H.J.; Macedo, T.; Singh, R.J.; et al. 1α, 25-dihydroxyvitamin D3 encapsulated in nanoparticles prevents venous neointimal hyperplasia and stenosis in porcine arteriovenous fistulas. J. Am. Soc. Nephrol. 2021, 32, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Kilari, S.; Zhao, C.; Simeon, M.L.; Misra, A.; Li, Y.; van Wijnen, A.J.; Mukhopadhyay, D.; Misra, S. Therapeutic effect of adipose derived mesenchymal stem cell transplantation in reducing restenosis in a murine angioplasty model. J. Am. Soc. Nephrol. 2020, 31, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Janardhanan, R.; Yang, B.; Kilari, S.; Leof, E.B.; Mukhopadhyay, D.; Misra, S. The role of repeat administration of adventitial delivery of lentivirus-shRNA-Vegf-A in arteriovenous fistula to prevent venous stenosis formation. J. Vasc. Interv. Radiol. 2016, 27, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Janardhanan, R.; Vohra, P.; Greene, E.L.; Bhattacharya, S.; Withers, S.; Roy, B.; Nieves Torres, E.C.; Mandrekar, J.; Leof, E.B.; et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 2014, 85, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.C.N.; Yang, B.; Roy, B.; Janardhanan, R.; Brahmbhatt, A.; Leof, E.; Mukhopadhyay, D.; Misra, S. Adventitial delivery of lentivirus-shRNA-ADAMTS-1 reduces venous stenosis formation in arteriovenous fistula. PLoS ONE 2014, 9, e94510. [Google Scholar] [CrossRef]
- Peden, E.K.; Lucas, J.F., 3rd; Browne, B.J.; Settle, S.M.; Scavo, V.A.; Bleyer, A.J.; Ozaki, C.K.; Teruya, T.H.; Wilson, S.E.; Mishler, R.E.; et al. PATENCY-2 trial of vonapanitase to promote radiocephalic fistula use for hemodialysis and secondary patency. J. Vasc. Access 2021. [Google Scholar] [CrossRef]
- Peden, E.K.; Leeser, D.B.; Dixon, B.S.; El-Khatib, M.T.; Roy-Chaudhury, P.; Lawson, J.H.; Menard, M.T.; Dember, L.M.; Glickman, M.H.; Gustafson, P.N.; et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula creation. J. Vasc. Access 2013, 14, 43–51. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Zepel, L.; Zhao, J.; Burke, S.; Lok, C.E.; Woodside, K.J.; Wasse, H.; Kawanishi, H.; Schaubel, D.E.; Zee, J.; et al. International Comparisons of Native Arteriovenous Fistula Patency and Time to Becoming Catheter-Free: Findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2021, 77, 245–254. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Zepel, L.; Fluck, R.; Lok, C.E.; Kawanishi, H.; Süleymanlar, G.; Wasse, H.; Tentori, F.; Zee, J.; Li, Y.; et al. International Differences in the Location and Use of Arteriovenous Accesses Created for Hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2018, 71, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, R.L.; Zepel, L.; Port, F.K.; Robinson, B.M. Trends in US Vascular Access Use, Patient Preferences, and Related Practices: An Update from the US DOPPS Practice Monitor with International Comparisons. Am. J. Kidney Dis. 2015, 65, 905–915. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Wang, Y.; Krishnamoorthy, M.; Zhang, J.; Banerjee, R.; Munda, R.; Heffelfinger, S.; Arend, L. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol. Dial. Transplant. 2009, 24, 2786–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hytonen, J.P.; Taavitsainen, J.; Laitinen, J.T.T.; Partanen, A.; Alitalo, K.; Leppänen, O.; Ylä-Herttuala, S. Local adventitial anti-angiogenic gene therapy reduces growth of vasa-vasorum and in-stent restenosis in WHHL rabbits. J. Mol. Cell Cardiol. 2018, 121, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarricone, A.; Ali, Z.; Rajamanickam, A.; Gujja, K.; Kapur, V.; Purushothaman, K.-R.; Purushothaman, M.; Vasquez, M.; Zalewski, A.; Parides, M.; et al. Histopathological Evidence of Adventitial or Medial Injury Is a Strong Predictor of Restenosis during Directional Atherectomy for Peripheral Artery Disease. J. Endovasc. Ther. 2015, 22, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Kilari, S.; Yang, B.; Sharma, A.; Wu, C.-C.; Vazquez-Padron, R.I.; Broadwater, J. Anti Human CX3CR1 VHH Molecule Attenuates Venous Neointimal Hyperplasia of Arteriovenous Fistula in Mouse Model. J. Am. Soc. Nephrol. 2021, 32, 1630–1648. [Google Scholar] [CrossRef]
- Cai, C.; Kilari, S.; Zhao, C.; Singh, A.K.; Simeon, M.L.; Misra, A.; Li, Y.; Takahashi, E.; Kumar, R.; Misra, S. Adventitial delivery of nanoparticles encapsulated with 1α, 25-dihydroxyvitamin D3 attenuates restenosis in a murine angioplasty model. Sci. Rep. 2021, 11, 4772. [Google Scholar] [CrossRef]
- Yamamoto, K.; Li, X.; Shu, C.; Miyata, T.; Dardik, A. Technical aspects of the mouse aortocaval fistula. J. Vis. Exp. 2013, 77, e50449. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Ishikawa, N.; Uchida, H.; Chasnoff, S.E.; Xie, X.; Mathew, S.; Hruska, K.A.; Choi, E.T. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J. Am. Soc. Nephrol. 2009, 20, 1236–1245. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, J.-S.; Wang, Y.; Cornea, V.; Roy-Chaudhury, P.; Campos, B. Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention. Int. J. Mol. Sci. 2021, 22, 12285. https://doi.org/10.3390/ijms222212285
Chan J-S, Wang Y, Cornea V, Roy-Chaudhury P, Campos B. Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention. International Journal of Molecular Sciences. 2021; 22(22):12285. https://doi.org/10.3390/ijms222212285
Chicago/Turabian StyleChan, Jenq-Shyong, Yang Wang, Virgilius Cornea, Prabir Roy-Chaudhury, and Begoña Campos. 2021. "Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention" International Journal of Molecular Sciences 22, no. 22: 12285. https://doi.org/10.3390/ijms222212285
APA StyleChan, J.-S., Wang, Y., Cornea, V., Roy-Chaudhury, P., & Campos, B. (2021). Early Adventitial Activation and Proliferation in a Mouse Model of Arteriovenous Stenosis: Opportunities for Intervention. International Journal of Molecular Sciences, 22(22), 12285. https://doi.org/10.3390/ijms222212285





