New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents
Abstract
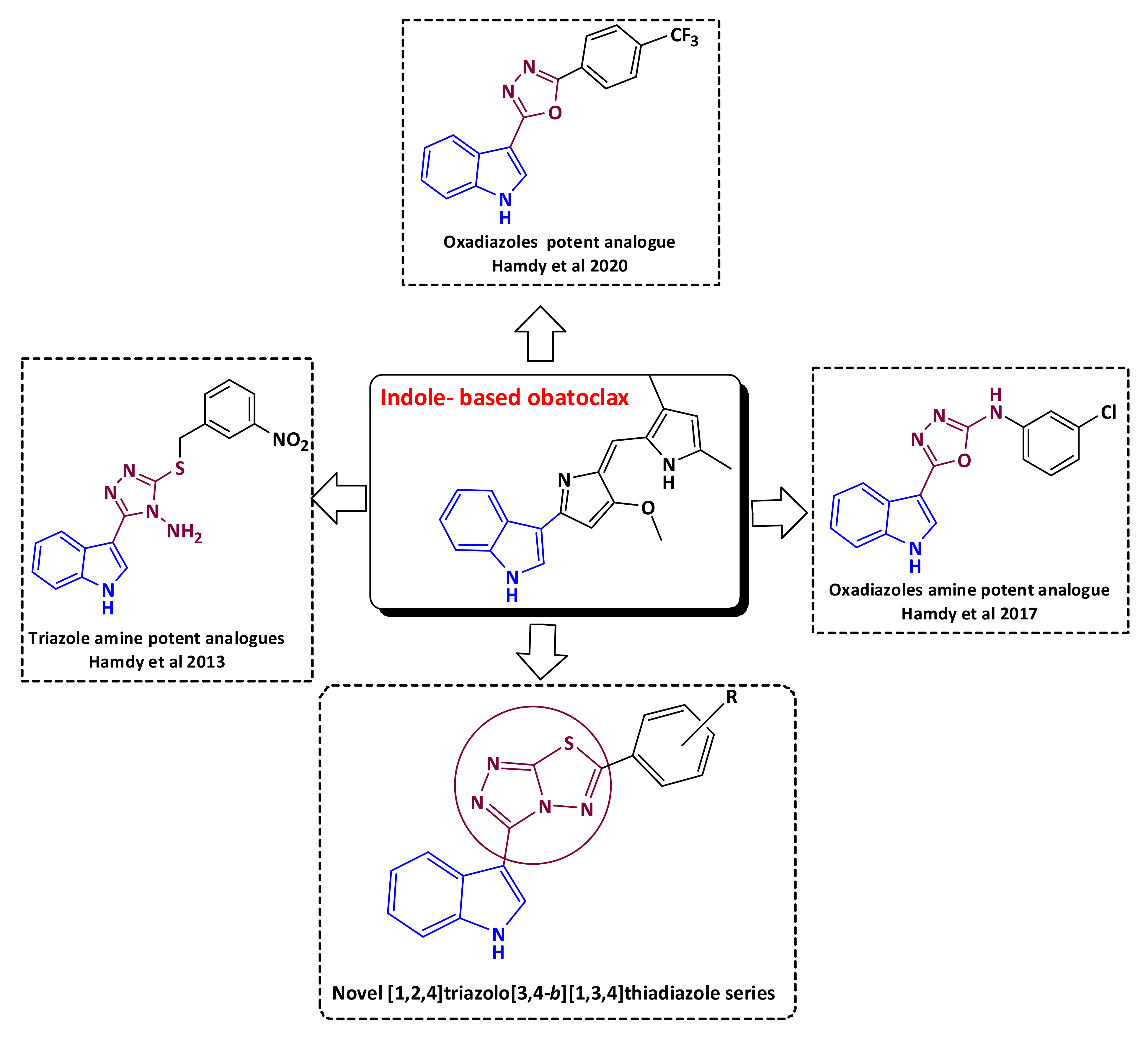
1. Introduction
2. Results
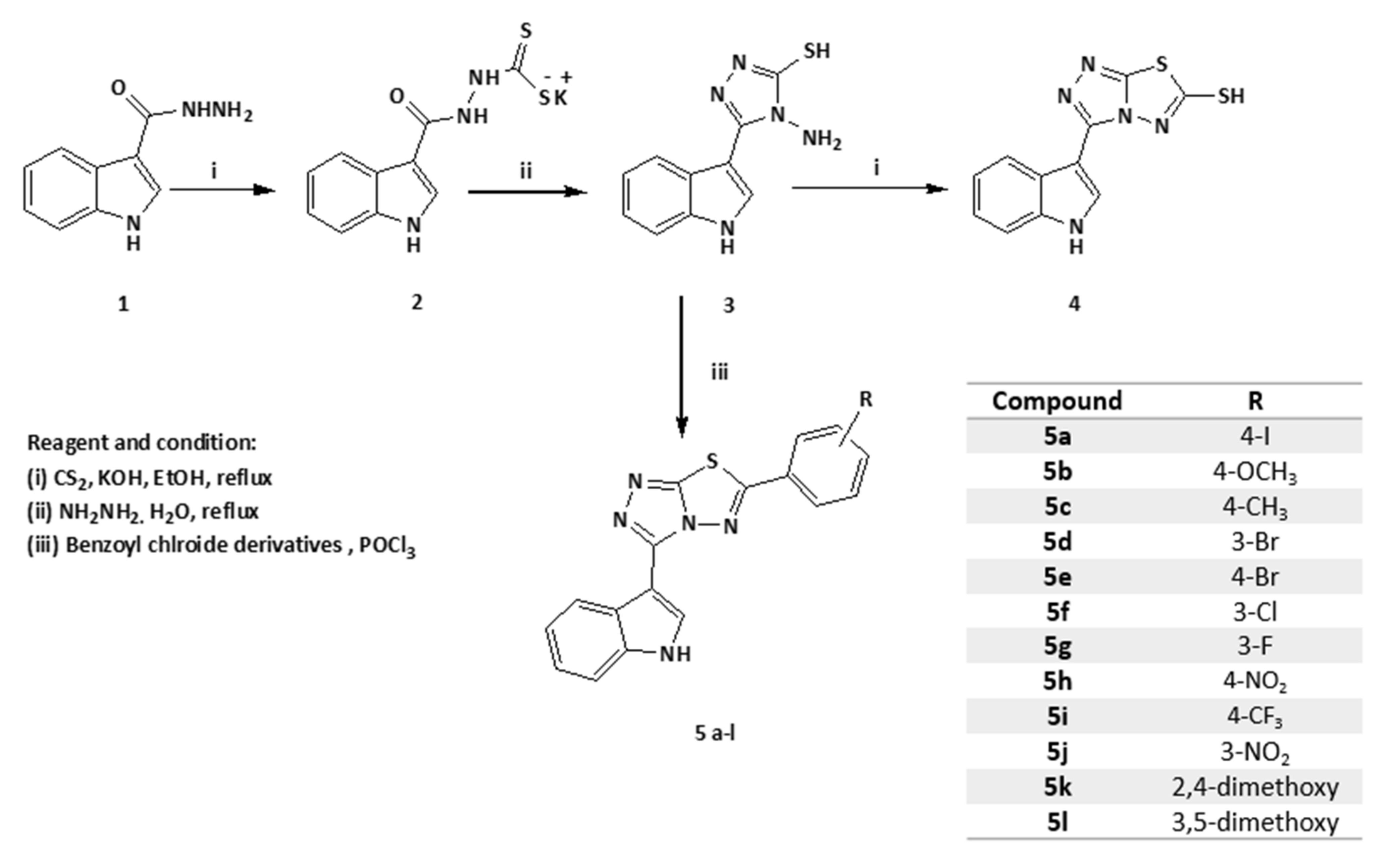
2.1. Synthesis of the Entitled Compounds 5a–l
2.2. Compound 5k and 5l Analogues Showed Potent and Selective Growth-Inhibitory Anticancer Activity on Bcl-2-Expressing Cell Lines
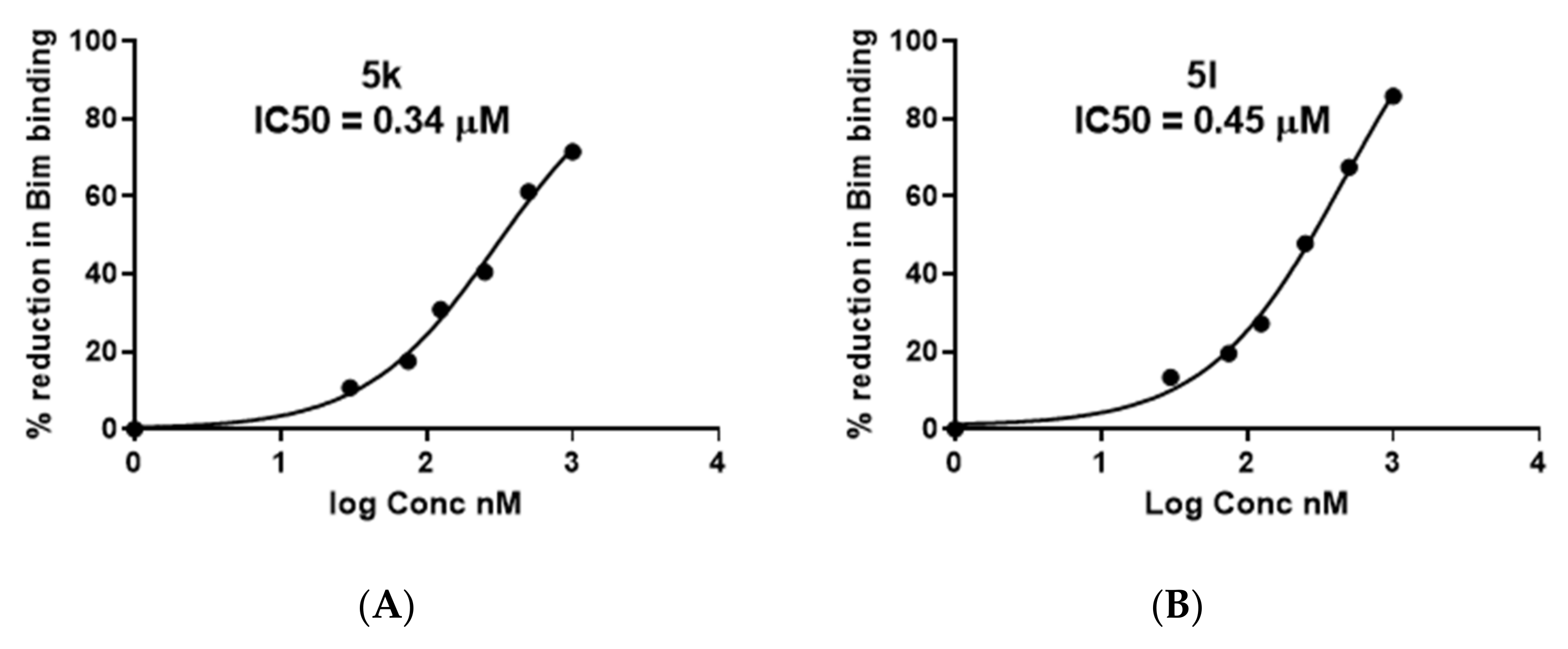
2.3. ELISA Assay Showed Potent Competitive Binding Activity of 5k and 5l
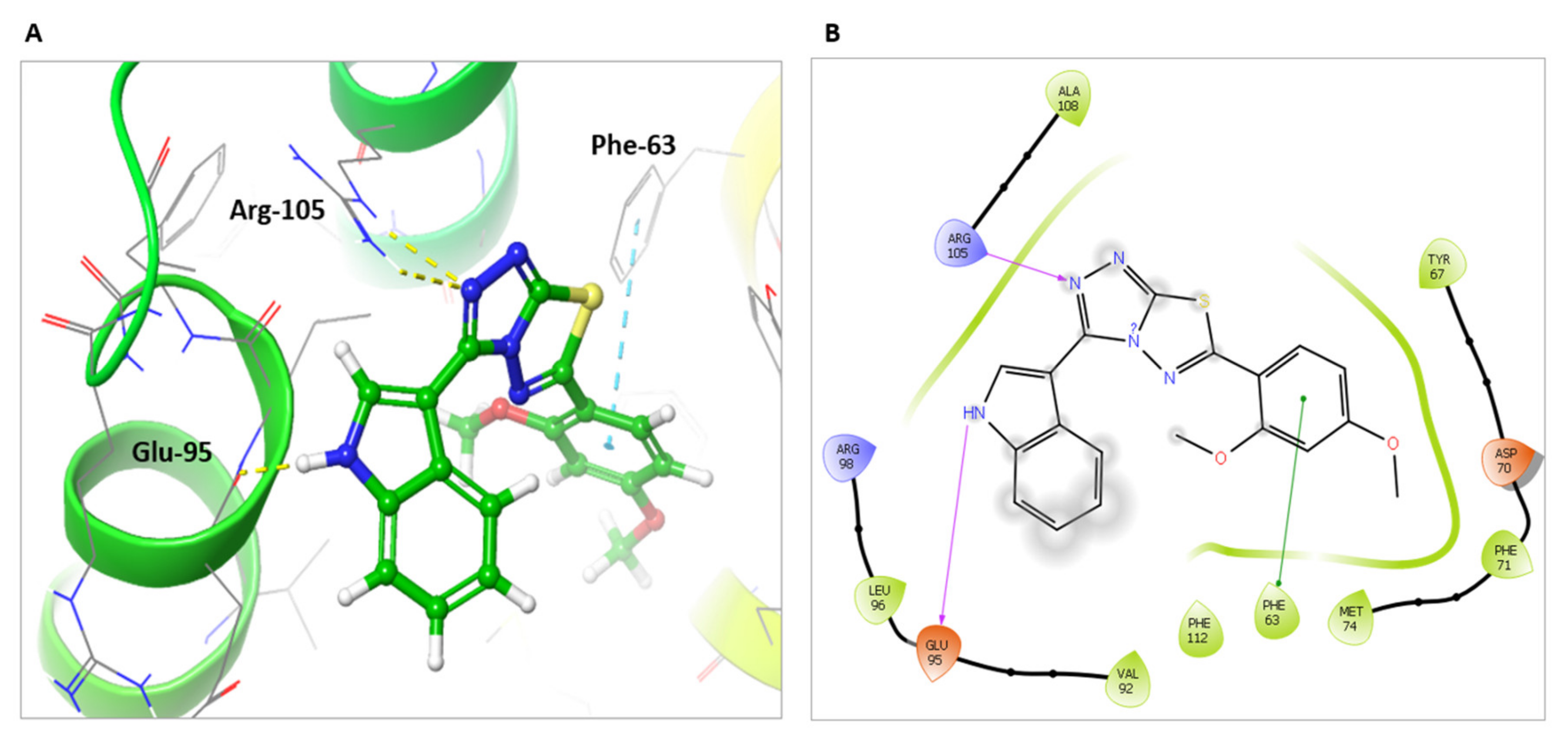
2.4. Molecular Docking to Rationalize the Potency and Selectivity of 5k against Bcl-2
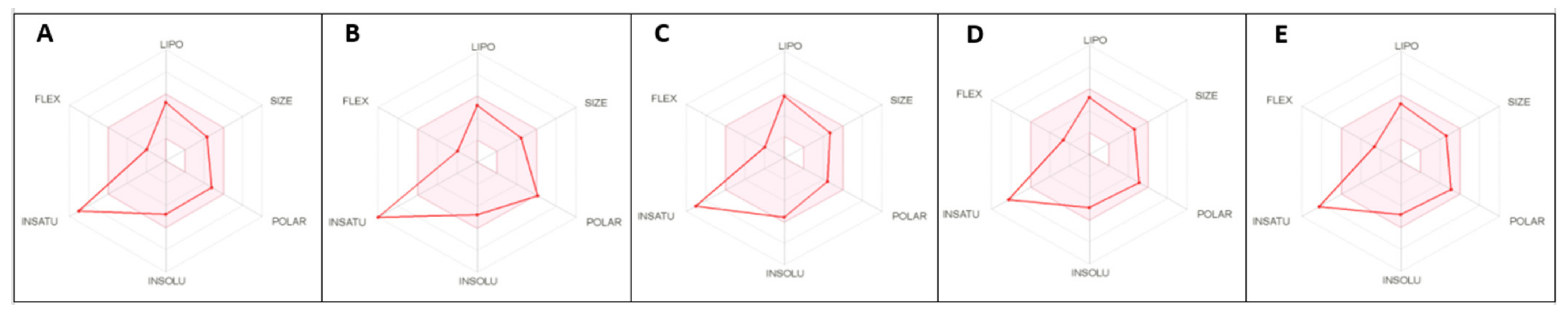
2.5. ADME Predictions Confirm the Drug-Like Properties of Compounds
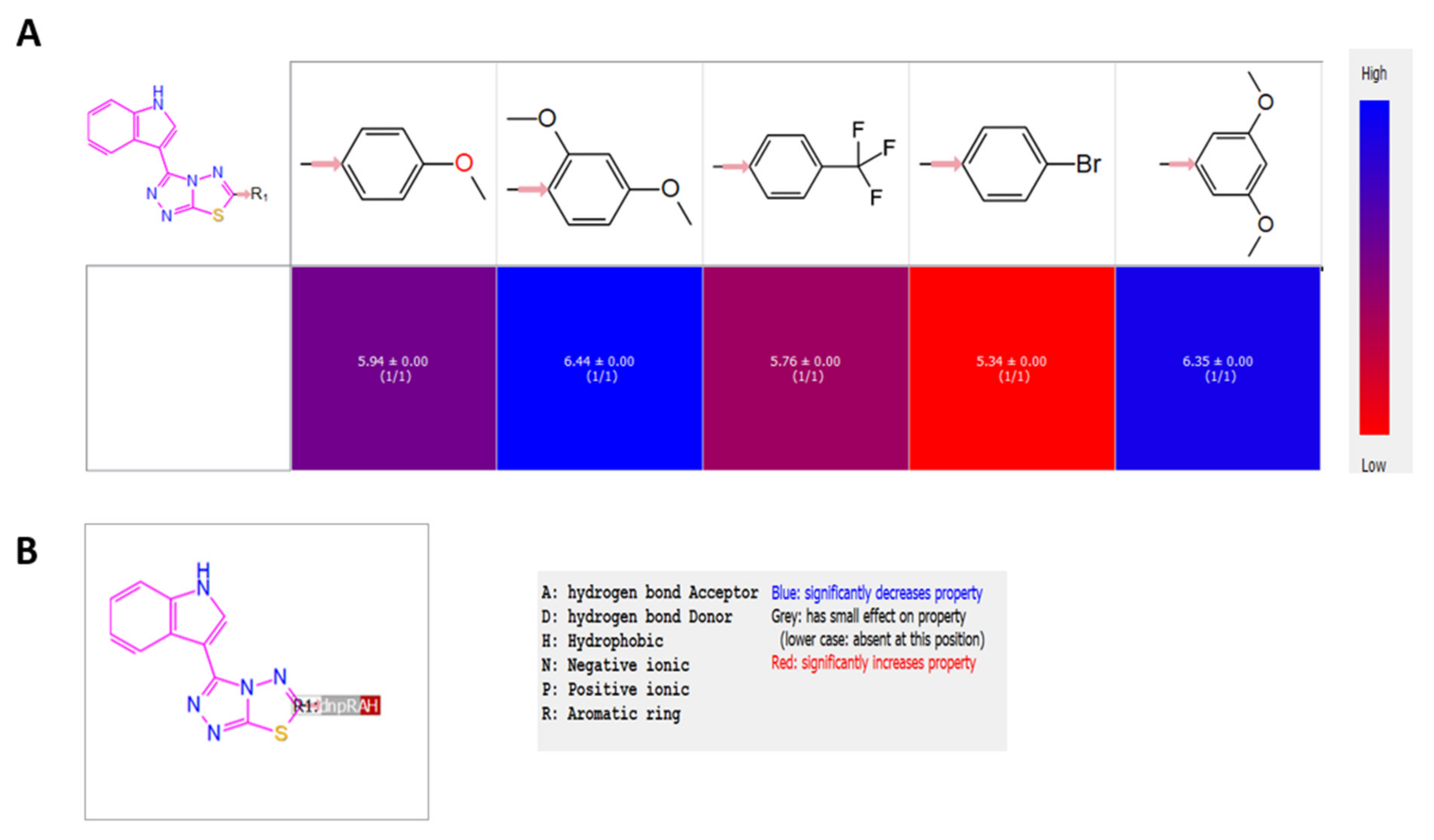
2.6. R-Group Analysis
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis of 4-Amino-5-(1H-indol-3-yl)-4H-[1,2,4]-triazole-3-thiol (3)
4.1.2. Synthesis of 3-(1H-Indol-3-yl)-[1,2,4]-triazolo[3,4-b]-[1,3,4]-thiadiazole-6-thiol (4)
4.1.3. General Preparation Procedure of 3-(6-Substituted phenyl-[1,2,4]-triazolo[3,4-b]-[1,3,4]-thiadiazol-3-yl)-1H-indol (5a–l)
4.2. Biology
4.2.1. Cell Viability—By MTT Assay
4.2.2. Cell Viability—CellTiter-Blue® Assay
4.2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Computational Design
Protein Preparation and Molecular Docking
4.4. Pharmacokinetics and Drug Likeness Filter and Target Prediction
4.5. R-Group Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar] [PubMed]
- Suvarna, V.; Singh, V.; Murahari, M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur. J. Pharmacol. 2019, 862, 172655. [Google Scholar] [CrossRef] [PubMed]
- Nachshon-Kedmi, M.; Yannai, S.; Haj, A.; Fares, F. Indole-3-carbinol and 3,3′-diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem. Toxicol. 2003, 41, 745–752. [Google Scholar] [CrossRef]
- Cai, B. p38 MAP Kinase Pathway Regulates Apoptosis through Phosphorylation and Up-Regulation of BimEL; University of Washington: Washington, DC, USA, 2008. [Google Scholar]
- Hamdy, R.; Ziedan, N.I.; Ali, S.; Bordoni, C.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 5-(1H-indol-3-yl)-N-aryl-1,3,4-oxadiazol-2-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 1037–1040. [Google Scholar] [CrossRef]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; El-Sadek, M.; Lashin, E.; Jones, A.T.; Westwell, A.D. Design, synthesis and evaluation of new bioactive oxadiazole derivatives as anticancer agents targeting bcl-2. Int. J. Mol. Sci. 2020, 21, 8980. [Google Scholar] [CrossRef]
- Ziedan, N.I.; Hamdy, R.; Cavaliere, A.; Kourti, M.; Prencipe, F.; Brancale, A.; Jones, A.T.; Westwell, A.D. Virtual screening, SAR, and discovery of 5-(indole-3-yl)-2-[(2-nitrophenyl) amino][1,3,4]-oxadiazole as a novel Bcl-2 inhibitor. Chem. Biol. Drug Des. 2017, 90, 147–155. [Google Scholar] [CrossRef]
- Hamdy, R.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Muhammad, Z.A.; El-Reedy, A.A. 5-(Thiophen-2-yl)-1,3,4-thiadiazole derivatives: Synthesis, molecular docking and in vitro cytotoxicity evaluation as potential anticancer agents. Drug Des. Dev. Ther. 2018, 12, 1511. [Google Scholar] [CrossRef]
- Metwally, K.A.; Yaseen, S.H.; Lashine, E.-S.M.; El-Fayomi, H.M.; El-Sadek, M.E. Non-carboxylic analogues of arylpropionic acids: Synthesis, anti-inflammatory activity and ulcerogenic potential. Eur. J. Med. Chem. 2007, 42, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; Jones, A.T.; Westwell, A.D. New quinoline-based heterocycles as anticancer agents targeting bcl-2. Molecules 2019, 24, 1274. [Google Scholar] [CrossRef]
- Voss, V.; Senft, C.; Lang, V.; Ronellenfitsch, M.W.; Steinbach, J.P.; Seifert, V.; Kögel, D. The pan-Bcl-2 inhibitor (−)-gossypol triggers autophagic cell death in malignant glioma. Mol. Cancer Res. 2010, 8, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Fayed, B.; Mostafa, A.; Shama, N.M.A.; Mahmoud, S.H.; Mehta, C.H.; Nayak, Y.; Soliman, S.S.M. Iterated Virtual Screening-Assisted Antiviral and Enzyme Inhibition Assays Reveal the Discovery of Novel Promising Anti-SARS-CoV-2 with Dual Activity. Int. J. Mol. Sci. 2021, 22, 9057. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Gaonkar, S.L.; Musad, E.A.; Dawsar, A.M.A. 1,3,4-Oxadiazole-containing hybrids as potential anticancer agents: Recent developments, mechanism of action and structure-activity relationships. J. Saudi Chem. Soc. 2021, 25, 101284. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef]
- Zhong, D.; Gu, C.; Shi, L.; Xun, T.; Li, X.; Liu, S.; Yu, L. Obatoclax Induces G1/G0-Phase Arrest via p38/p21waf1/Cip1 Signaling Pathway in Human Esophageal Cancer Cells. J. Cell. Biochem. 2014, 115, 1624–1635. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hu, S.; Tsui, K.-H.; Hwang, G.-S.; Chen, S.-T.; Tang, T.-K.; Cheng, H.-T.; Yu, J.-W.; Wang, H.-C.; Juang, H.-H. Anti-inflammatory effects of gossypol on human lymphocytic jurkat cells via regulation of MAPK signaling and cell cycle. Inflammation 2018, 41, 2265–2274. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.-M.; Hu, Z.-Y.; Zeng, Z.-L.; Yang, D.-J.; Jiang, W.-Q. Apogossypolone inhibits cell growth by inducing cell cycle arrest in U937 cells. Oncol. Rep. 2009, 22, 193–198. [Google Scholar]
- Takahashi, H.; Chen, M.C.; Pham, H.; Matsuo, Y.; Ishiguro, H.; Reber, H.A.; Takeyama, H.; Hines, O.J.; Eibl, G. Simultaneous knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax activation in pancreatic cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 2980–2987. [Google Scholar] [CrossRef]
- Zou, M.; Xia, S.; Zhuang, L.; Han, N.; Chu, Q.; Chao, T.; Peng, P.; Chen, Y.; Gui, Q.; Yu, S. Knockdown of the Bcl-2 gene increases sensitivity to EGFR tyrosine kinase inhibitors in the H1975 lung cancer cell line harboring T790M mutation. Int. J. Oncol. 2013, 42, 2094–2102. [Google Scholar] [CrossRef][Green Version]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef]
- Placzek, W.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, e40. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, srep42717. [Google Scholar] [CrossRef] [PubMed]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Al Joud, S.M.A.; El-Keblawy, A.A.; Soliman, S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
| Compound | IC50 1 | ||||
|---|---|---|---|---|---|
| R | MDA-MB-231 | HeLa | KG1A | Jurkat | |
| 4 | SH | 15 ± 0.60 | 18.20 ± 0.60 | 28.45 ±0.43 | 10.35 ± 0.14 |
| 5a | 4-I | 2.72 ±0.32 | 0.54 ± 0.02 | 1.66 ± 0.03 | 8.12 ± 0.16 |
| 5b | 4-OCH3 | 1.70 ± 0.61 | 2.70 ± 0.90 | 3.14 ± 0.19 | >100 |
| 5c | 4-CH3 | 16.28 ± 0.19 | 12.50 ± 0.23 | >100 | >100 |
| 5d | 3-Br | 4.9 ± 0.75 | 6.48 ± 0.67 | 20.15 ± 0.46 | 45.5 ± 0.40 |
| 5e | 4-Br | 8.29 ± 0.9 | 9.8 ± 0.3 | 4.9 ± 0.73 | >100 |
| 5f | 3-Cl | 12.5 ± 0.66 | 3.60 ± 0.52 | 10.23 ± 0.65 | 20.5 ± 0.52 |
| 5g | 3-F | 7.5 ± 0.98 | 1.99 ± 0.28 | 8.6 ± 0.22 | 65.08 ± 1.25 |
| 5h | 4-NO2 | 3.16 ± 0.32 | 22.13 ± 0.45 | 34.5 ± 0.30 | 58.09 ± 1.30 |
| 5i | 4-CF3 | 4.22 ± 0.38 | 2.7 ± 0.19 | 2.19 ± 0.72 | >100 |
| 5j | 3-NO2 | 3.6 ± 0.28 | 2.18 ± 0.73 | 6.82 ± 0.45 | 32.25 ± 1.2 |
| 5k | 2,4-dimethoxy | 0.7 ± 0.3 | 0.57 ± 0.08 | 0.31 ± 0.32 | >100 |
| 5l | 3,5-dimethoxy | 0.35 ±0.29 | 1.42 ± 0.17 | 1.15 ± 0.27 | >100 |
| Gossypol | 5.5 ± 0.35 | 4.43 ± 0.54 | 4.2 ± 0.35 | 18.1 ± 1.3 | |
| Compound | IC50 µM * |
|---|---|
| Gossypol | 0.6 ± 0.09 |
| 5b | 1.14 ± 0.09 |
| 5e | 4.6± 0.19 |
| 5i | 1.74 ± 0.15 |
| 5k | 0.36 ± 0.05 |
| 5l | 0.45 ± 0.03 |
| Compound | Mwt | HBA | HBD | TPSA | Ilogp | ESOL Class | Ali Log S | GI Absorption | BBB Permeation | Lipinski Violation | Bioavailability |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5b | 347 | 4 | 1 | 96.34 | 2.79 | Moderately soluble | −5.39 | High | No | 0 | 0.55 |
| 5e | 396.3 | 3 | 1 | 87.11 | 2.83 | Moderately soluble | −5.95 | High | No | 0 | 0.55 |
| 5i | 386.45 | 5 | 1 | 132.9 | 1.96 | Moderately soluble | −6.02 | Low | No | 0 | 0.55 |
| 5k | 377.42 | 5 | 1 | 105.6 | 3.05 | Moderately soluble | −5.56 | High | No | 0 | 0.55 |
| 5l | 377.42 | 5 | 1 | 105.6 | 2.94 | Moderately soluble | −5.56 | High | No | 0 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdy, R.; Jones, A.T.; El-Sadek, M.; Hamoda, A.M.; Shakartalla, S.B.; AL Shareef, Z.M.; Soliman, S.S.M.; Westwell, A.D. New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 12272. https://doi.org/10.3390/ijms222212272
Hamdy R, Jones AT, El-Sadek M, Hamoda AM, Shakartalla SB, AL Shareef ZM, Soliman SSM, Westwell AD. New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents. International Journal of Molecular Sciences. 2021; 22(22):12272. https://doi.org/10.3390/ijms222212272
Chicago/Turabian StyleHamdy, Rania, Arwyn T. Jones, Mohamed El-Sadek, Alshaimaa M. Hamoda, Sarra B. Shakartalla, Zainab M. AL Shareef, Sameh S. M. Soliman, and Andrew D. Westwell. 2021. "New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents" International Journal of Molecular Sciences 22, no. 22: 12272. https://doi.org/10.3390/ijms222212272
APA StyleHamdy, R., Jones, A. T., El-Sadek, M., Hamoda, A. M., Shakartalla, S. B., AL Shareef, Z. M., Soliman, S. S. M., & Westwell, A. D. (2021). New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents. International Journal of Molecular Sciences, 22(22), 12272. https://doi.org/10.3390/ijms222212272






