Elevated Cytokine Levels in Aqueous Humor Are Associated with Peripheral Anterior Synechiae after Penetrating Keratoplasty
Abstract
:1. Introduction
2. Results
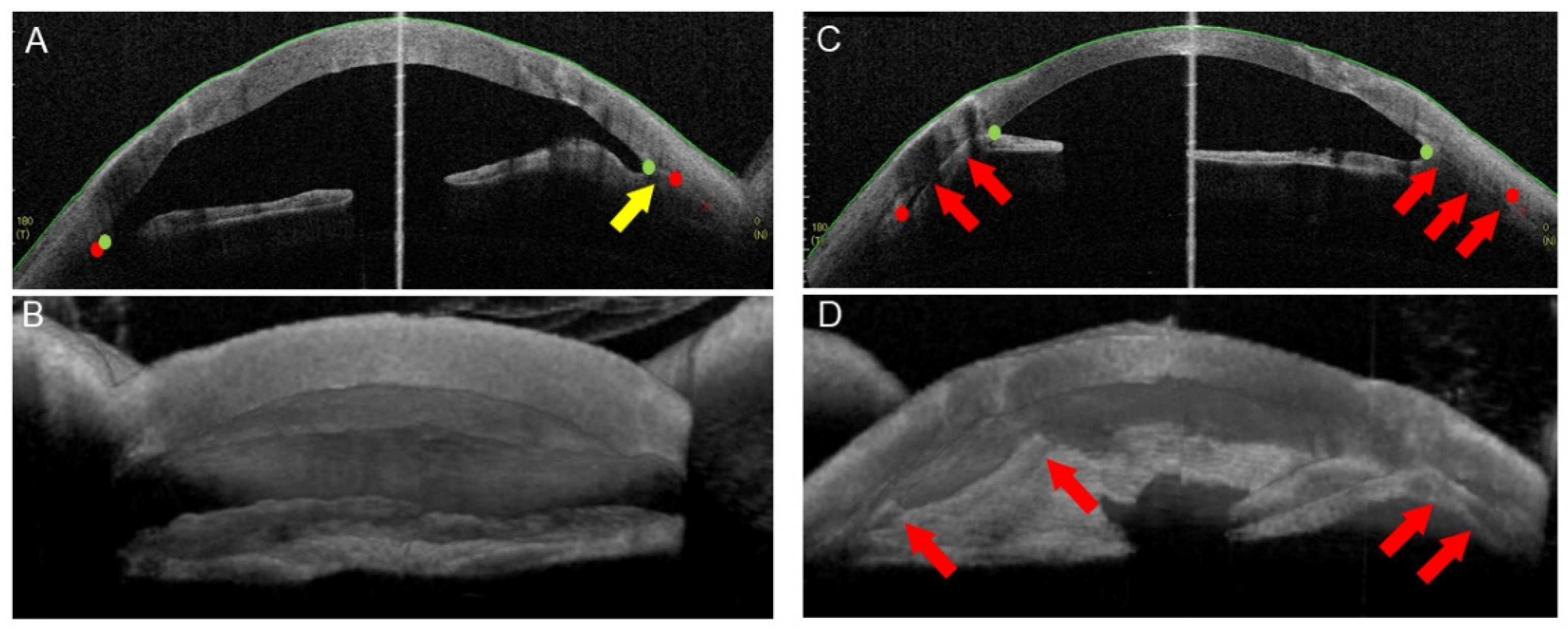
2.1. PAS after PKP
2.2. Influence of PAS on Intraocular Pressure after PKP
2.3. Association between PAS and Preoperative Cytokine Levels in the AqH
2.4. PAS and Preoperative Cytokine Levels in the AqH in a Mouse Model
3. Discussion
4. Materials and Methods
4.1. Participants and Surgical Technique
4.2. AS-OCT Imaging
4.3. Measurement of the ITC Area, Degree, and Maximum Height
4.4. Animal Experiments
4.5. Aqueous Humor Samples
4.6. Total Protein and Cytokine Level Measurements
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldberg, D.B.; Schanzlin, D.J.; Brown, S.I. Incidence of Increased Intraocular Pressure after Keratoplasty. Am. J. Ophthalmol. 1981, 92, 372–377. [Google Scholar] [CrossRef]
- Karesh, J.W.; Nirankari, V.S. Factors Associated with Glaucoma after Penetrating Keratoplasty. Am. J. Ophthalmol. 1983, 96, 160–164. [Google Scholar] [CrossRef]
- Chien, A.M.; Schmidt, C.M.; Cohen, E.J.; Rajpal, R.K.; Sperber, L.T.; Rapuano, C.J.; Moster, M.; Smith, M.; Laibson, P.R. Glaucoma in the Immediate Postoperative Period after Penetrating Keratoplasty. Am. J. Ophthalmol. 1993, 115, 711–714. [Google Scholar] [CrossRef]
- Foulks, G.N. Glaucoma Associated with Penetrating Keratoplasty. Ophthalmology 1987, 94, 871–874. [Google Scholar] [CrossRef]
- Ogawa, A.; Yamaguchi, T.; Mitamura, H.; Tomida, D.; Shimazaki-Den, S.; Murat, D.; Satake, Y.; Shimazaki, J. Aetiology-specific comparison of long-term outcome of deep anterior lamellar keratoplasty for corneal diseases. Br. J. Ophthalmol. 2015, 100, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, O.S.; Mehta, J.S.; Htoon, H.M.; Tan, D.T.; Wong, T.T. Incidence and Risk Factors of Elevated Intraocular Pressure Following Deep Anterior Lamellar Keratoplasty. Am. J. Ophthalmol. 2016, 170, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Musa, F.U.; Patil, S.; Rafiq, O.; Galloway, P.; Ball, J.; Morrell, A. Long-term risk of intraocular pressure elevation and glaucoma escalation after deep anterior lamellar keratoplasty. Clin. Exp. Ophthalmol. 2012, 40, 780–785. [Google Scholar] [CrossRef]
- Vajaranant, T.S.; Price, M.O.; Price, F.W.; Gao, W.; Wilensky, J.T.; Edward, D.P. Visual acuity and intraocular pressure after Descemet’s stripping endothelial keratoplasty in eyes with and without preexisting glaucoma. Ophthalmology 2009, 116, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.-K.B.; Klamann, M.K.J.; Torun, N.; Gonnermann, J.; Schroeter, J.; Joussen, A.M.; Rieck, P. Intraocular pressure elevation and post-DSEK glaucoma after Descemet‘s stripping endothelial keratoplasty. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 251, 1191–1198. [Google Scholar] [CrossRef]
- Ozeki, N.; Yuki, K.; Shiba, D.; Shimmura, S.; Murat, D.; Tsubota, K. Intraocular pressure elevation after Descemet’s stripping endothelial keratoplasty. Jpn. J. Ophthalmol. 2012, 56, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.-K.B.; Wolf, T.; Gundlach, E.; Klamann, M.K.J.; Gonnermann, J.; Bertelmann, E.; Joussen, A.M.; Torun, N. Intraocular pressure elevation and post-DMEK glaucoma following Descemet membrane endothelial keratoplasty. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Kaufmann, C.; Bachmann, L.M.; Tarantino-Scherrer, J.N.; Thiel, M.A.; Bochmann, F. Changes in intraocular pressure after descemet stripping automated endothelial keratoplasty: A retrospective analysis. Cornea 2015, 34, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Karadag, O.; Kugu, S.; Erdogan, G.; Kandemir, B.; Ozdil, S.E.; Dogan, O.K. Incidence of and Risk Factors for Increased Intraocular Pressure after Penetrating Keratoplasty. Cornea 2010, 29, 278–282. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Li, Y.; Francis, B.A.; Smith, R.E.; Gutmark, J.; Huang, D. Optical coherence tomography of the anterior segment in secondary glaucoma with corneal opacity after penetrating keratoplasty. Br. J. Ophthalmol. 2007, 91, 189–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, R.; Usui, T.; Tomidokoro, A.; Mishima, K.; Matagi, N.; Miyai, T.; Amano, S.; Araie, M. Noninvasive Observations of Peripheral Angle in Eyes after Penetrating Keratoplasty Using Anterior Segment Fourier-Domain Optical Coherence Tomography. Cornea 2012, 31, 259–263. [Google Scholar] [CrossRef]
- Teekhasaenee, C.; Ritch, R. Iridocorneal endothelial syndrome in Thai patients: Clinical variations. Arch. Ophthalmol. 2000, 118, 187–192. [Google Scholar] [CrossRef]
- Silva, L.; Najafi, A.; Suwan, Y.; Teekhasaenee, C.; Ritch, R. The iridocorneal endothelial syndrome. Surv. Ophthalmol. 2018, 63, 665–676. [Google Scholar] [CrossRef]
- Daniel, E.; Pistilli, M.; Kothari, S.; Khachatryan, N.; Kaçmaz, R.O.; Gangaputra, S.S.; Sen, H.N.; Suhler, E.B.; Thorne, J.E.; Foster, C.S.; et al. Risk of Ocular Hypertension in Adults with Noninfectious Uveitis. Ophthalmology 2017, 124, 1196–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, L.S.; Aung, T.; Husain, R.; Wu, Y.-J.; Gazzard, G.; Seah, S.K. Acute primary angle closure: Configuration of the drainage angle in the first year after laser peripheral iridotomy. Ophthalmology 2004, 111, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Parra, L.; Pardhan, S.; Buckley, R.J.; Parker, M.; Bourne, R.R.A. Diurnal Intraocular Pressure and the Relationship with Swept-Source OCT–Derived Anterior Chamber Dimensions in Angle Closure: The IMPACT Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2943–2949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazu, H.; Yamaguchi, T.; Aketa, N.; Higa, K.; Suzuki, T.; Yagi-Yaguchi, Y.; Satake, Y.; Abe, T.; Tsubota, K.; Shimazaki, J. Preoperative Aqueous Cytokine Levels are Associated with Endothelial Cell Loss after Descemet’s Stripping Automated Endothelial Keratoplasty. Investig. Ophthalmol. Vis. Sci. 2018, 59, 612–620. [Google Scholar] [CrossRef] [Green Version]
- Yagi-Yaguchi, Y.; Yamaguchi, T.; Higa, K.; Suzuki, T.; Yazu, H.; Aketa, N.; Satake, Y.; Tsubota, K.; Shimazaki, J. Preoperative Aqueous Cytokine Levels Are Associated with a Rapid Reduction in Endothelial Cells after Penetrating Keratoplasty. Am. J. Ophthalmol. 2017, 181, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Higa, K.; Yagi-Yaguchi, Y.; Ueda, K.; Noma, H.; Shibata, S.; Nagai, T.; Tomida, D.; Yasu-Mimura, R.; Ibrahim, O.; et al. Pathological processes in aqueous humor due to iris atrophy predispose to early corneal graft failure in humans and mice. Sci. Adv. 2020, 6, eaaz5195. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Yamaguchi, T.; Nishisako, S.; Matsumura, T.; Fukui, M.; Higa, K.; Inoue, T.; Shimazaki, J. Aqueous Cytokine Levels Are Associated with Progression of Peripheral Anterior Synechiae after Descemet Stripping Automated Endothelial Keratoplasty. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Irvine, A.R.; Kaufman, H.E. Intraolar pressure following penetrating keratoplasty. Am. J. Ophthalmol. 1969, 68, 835–844. [Google Scholar] [CrossRef]
- Maier, A.-K.B.; Gundlach, E.; Gonnermann, J.; Klamann, M.K.; Eulufi, C.; Joussen, A.M.; Bertelmann, E.; Rieck, P.; Torun, N. Anterior Segment Analysis and Intraocular Pressure Elevation after Penetrating Keratoplasty and Posterior Lamellar Endothelial Keratoplasty. Ophthalmic Res. 2014, 53, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Higa, K.; Suzuki, T.; Nakayama, N.; Yagi-Yaguchi, Y.; Dogru, M.; Satake, Y.; Shimazaki, J. Elevated Cytokine Levels in the Aqueous Humor of Eyes with Bullous Keratopathy and Low Endothelial Cell Density. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5954–5962. [Google Scholar] [CrossRef] [Green Version]
- Aketa, N.; Yamaguchi, T.; Suzuki, T.; Higa, K.; Yagi-Yaguchi, Y.; Satake, Y.; Tsubota, K.; Shimazaki, J. Iris Damage Is Associated with Elevated Cytokine Levels in Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO42–BIO51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanth, B.; Dubey, S.; Mathur, U. IOP Changes after DSEK. Ophthalmology 2010, 117, 1460–1461. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kawaji, T.; Tanihara, H. Monocyte chemotactic protein-1 level in the aqueous humour as a prognostic factor for the outcome of trabeculectomy. Clin. Exp. Ophthalmol. 2014, 42, 334–341. [Google Scholar] [CrossRef]
- Ohira, S.; Inoue, T.; Iwao, K.; Takahashi, E.; Tanihara, H. Factors Influencing Aqueous Proinflammatory Cytokines and Growth Factors in Uveitic Glaucoma. PLoS ONE 2016, 11, e0147080. [Google Scholar] [CrossRef]
- Kawai, M.; Inoue, T.; Inatani, M.; Tsuboi, N.; Shobayashi, K.; Matsukawa, A.; Yoshida, A.; Tanihara, H. Elevated Levels of Monocyte Chemoattractant Protein-1 in the Aqueous Humor after Phacoemulsification. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7951–7960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, Q.; Guo, F.; Chen, X.; Xie, L. Link between neurodegeneration and trabecular meshwork injury in glaucomatous patients. BMC Ophthalmol. 2017, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, E.R.; Midttun, O.; Ueland, P.M.; Schartum-Hansen, H.; Seifert, R.; Igland, J.; Nordrehaug, J.E.; Ebbing, M.; Svingen, G.; Bleie, O.; et al. Systemic markers of interferon-gamma-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriazopoulou, E.; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; Botsa, E.; et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017, 15, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spindler, J.; Zandi, S.; Pfister, I.B.; Gerhardt, C.; Garweg, J.G. Cytokine profiles in the aqueous humor and serum of patients with dry and treated wet age-related macular degeneration. PLoS ONE 2018, 13, e0203337. [Google Scholar] [CrossRef]
- Bonacini, M.; Soriano, A.; Cimino, L.; De Simone, L.; Bolletta, E.; Gozzi, F.; Muratore, F.; Nicastro, M.; Belloni, L.; Zerbini, A.; et al. Cytokine Profiling in Aqueous Humor Samples from Patients with Non-Infectious Uveitis Associated with Systemic Inflammatory Diseases. Front. Immunol. 2020, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heizmann, U.; Böhringer, D.; Kern, Y.; Reinhard, T. Distinct cytokine pattern in aqueous humor during immune reactions following penetrating keratoplasty. Mol. Vis. 2010, 16, 53–60. [Google Scholar] [PubMed]
- Chowdhury, U.R.; Madden, B.J.; Charlesworth, M.C.; Fautsch, M.P. Proteome Analysis of Human Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; Gabiati, L.; Laurenzo, M.; De-Giorgio, F.; Scorcia, V.; Grassi, S.; D’Aloja, E.; Fossarello, M. Repeatability and reproducibility of post-mortem central corneal thickness measurements using a portable optical coherence tomography system in humans: A prospective multicenter study. Sci. Rep. 2020, 10, 14508. [Google Scholar] [CrossRef]
- Locci, E.; Stocchero, M.; Noto, A.; Chighine, A.; Natali, L.; Napoli, P.; Caria, R.; De-Giogio, F.; Nioi, M.; d’Aloja, E. A1 HNMR metabolic approach for the estimation of the time since death using aqueous humor: An animal model. Metabolomics 2019, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Lass, J.; Benetz, B.; Verdier, D.; Szczotka-Flynn, L.B.; Ayala, A.R.; Liang, W.; Aldave, A.; Dunn, S.; McCall, T.; Mian, S.; et al. Corneal endothelial cell loss 3 years after successful Descemet stripping automated endothelial keratoplasty in the Cornea Preservation Time Study: A randomized clinical trial. JAMA Ophthalmol. 2017, 135, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Wall, R.V.; Gupta, V.; Klistorner, A.; Graham, S. DBA/2J mouse model for experimental glaucoma: Pitfalls and problems. Clin. Exp. Ophthalmol. 2017, 45, 911–922. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ohnuma, K.; Tomida, D.; Konomi, K.; Satake, Y.; Negishi, K.; Tsubota, K.; Shimazaki, J. The Contribution of the Posterior Surface to the Corneal Aberrations in Eyes after Keratoplasty. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6222–6229. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ITC | Preop | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| % Degree p-value * | 22.6 ± 34.2 | 19.3 ± 31.7 0.14 | 25.3 ± 37.0 0.14 | 29.8 ± 39.3 0.03 |
| Maximal height (mm) p-value * | 0.94 ± 1.56 | 0.76 ± 1.23 0.64 | 0.90 ± 1.41 0.86 | 1.22 ± 1.65 0.01 |
| Area (mm2) p-value * | 4.35 ± 8.29 | 3.06 ± 6.51 0.61 | 3.86 ± 7.16 0.55 | 6.51 ± 10.6 0.01 |
| ITC (+) | ITC (-) | Total | |
|---|---|---|---|
| IOP increase (+) | 10 | 1 | 11 |
| IOP increase (-) | 28 | 48 | 76 |
| Total | 38 | 49 | 87 |
| PAS (-) (n = 51) | PAS (+) (n = 36) | p-Value † | |
|---|---|---|---|
| Total protein | 0.88 ± 0.72 | 0.92 ± 0.47 | 0.322 |
| IL-1α | 52.0 ± 106 | 88.1 ±166 | 0.438 |
| IL-1β | 2.59 ± 2.89 | 21.4 ± 64.1 | 0.214 |
| IL-4 | 56.0 ± 75.0 | 79.8 ±118 | 0.084 |
| IL-6 | 505 ± 1290 | 1290 ±3072 | 0.017 |
| IL-8 | 26.6 ± 20.0 | 84.6 ± 150 | 0.008 |
| IL-10 | 2.91 ± 9.43 | 7.85 ± 17.6 | 0.028 |
| IL-12p70 | 7.59 ± 5.60 | 23.1 ± 36.2 | 0.014 |
| IL-13 | 6.92 ± 2.25 | 9.01 ± 6.58 | 0.302 |
| IL-17A | 10.3 ± 10.0 | 23.1 ± 42.8 | 0.401 |
| MIP-1α | 18.2 ± 33.0 | 16.5 ± 11.8 | 0.078 |
| MIP-1β | 177 ± 244 | 191 ± 287 | 0.816 |
| MCP-1 | 697 ± 317 | 904 ± 325 | 0.010 |
| TNF-α | 72.5 ± 65.4 | 69.0 ± 66.1 | 0.825 |
| IFN-α | 3.08 ± 1.89 | 2.83 ± 1.77 | 0.747 |
| IFN-γ | 99.6 ± 123 | 224 ± 489 | 0.097 |
| E-Selectin | 3890 ± 4990 | 4210 ± 3180 | 0.106 |
| P-Selectin | 5980 ± 5730 | 9700 ± 15,100 | 0.041 |
| sICAM-1 | 3680 ± 3790 | 7160 ± 12,500 | 0.089 |
| IP10 | 362 ± 966 | 394 ± 736 | 0.028 |
| GM-CSF | 7.11 ± 6.44 | 8.01 ± 8.00 | 0.871 |
| Preop PAS Area | PAS Area at 3 Months | PAS Area at 6 Months | PAS Area at 12 Months | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value * | r | p-Value * | r | p-Value * | r | p-Value * | |
| IL-1α | 0.006 | 0.964 | 0.073 | 0.591 | 0.049 | 0.742 | 0.116 | 0.414 |
| IL-1β | 0.165 | 0.291 | 0.140 | 0.394 | 0.017 | 0.927 | 0.056 | 0.750 |
| IL-4 | 0.138 | 0.262 | 0.237 | 0.064 | 0.364 | 0.010 | 0.303 | 0.025 |
| IL-6 | 0.385 | 0.001 | 0.312 | 0.014 | 0.387 | 0.007 | 0.396 | 0.003 |
| IL-8 | 0.269 | 0.026 | 0.358 | 0.004 | 0.300 | 0.034 | 0.263 | 0.053 |
| IL-10 | 0.341 | 0.010 | 0.173 | 0.207 | 0.235 | 0.144 | 0.251 | 0.100 |
| IL-12p70 | 0.270 | 0.076 | 0.406 | 0.012 | 0.491 | 0.005 | 0.329 | 0.054 |
| IL-13 | 0.218 | 0.239 | 0.163 | 0.428 | 0.052 | 0.815 | 0.168 | 0.403 |
| IL-17A | 0.190 | 0.229 | 0.286 | 0.087 | 0.191 | 0.320 | 0.171 | 0.342 |
| MIP-1α | 0.237 | 0.109 | 0.136 | 0.396 | 0.194 | 0.273 | 0.129 | 0.441 |
| MIP-1β | 0.105 | 0.388 | 0.078 | 0.542 | 0.001 | 0.993 | 0.033 | 0.809 |
| MCP-1 | 0.138 | 0.247 | 0.151 | 0.231 | 0.378 | 0.005 | 0.330 | 0.012 |
| TNF-α | 0.004 | 0.982 | 0.028 | 0.864 | −0.091 | 0.614 | 0.018 | 0.913 |
| IFN-α | −0.069 | 0.681 | −0.095 | 0.600 | −0.097 | 0.622 | −0.021 | 0.908 |
| IFN-γ | 0.211 | 0.134 | 0.271 | 0.069 | 0.395 | 0.015 | 0.378 | 0.015 |
| E-Selectin | 0.217 | 0.102 | 0.170 | 0.227 | 0.272 | 0.085 | 0.279 | 0.060 |
| P-Selectin | 0.071 | 0.553 | 0.100 | 0.428 | 0.282 | 0.041 | 0.171 | 0.198 |
| sICAM-1 | 0.174 | 0.141 | 0.213 | 0.085 | 0.270 | 0.049 | 0.261 | 0.046 |
| IP10 | 0.096 | 0.420 | 0.091 | 0.469 | 0.115 | 0.411 | 0.039 | 0.773 |
| GM-CSF | −0.016 | 0.962 | 0.189 | 0.601 | 0.155 | 0.670 | 0.212 | 0.556 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusano, Y.; Yamaguchi, T.; Nishisako, S.; Matsumura, T.; Fukui, M.; Higa, K.; Inoue, T.; Shimazaki, J. Elevated Cytokine Levels in Aqueous Humor Are Associated with Peripheral Anterior Synechiae after Penetrating Keratoplasty. Int. J. Mol. Sci. 2021, 22, 12268. https://doi.org/10.3390/ijms222212268
Kusano Y, Yamaguchi T, Nishisako S, Matsumura T, Fukui M, Higa K, Inoue T, Shimazaki J. Elevated Cytokine Levels in Aqueous Humor Are Associated with Peripheral Anterior Synechiae after Penetrating Keratoplasty. International Journal of Molecular Sciences. 2021; 22(22):12268. https://doi.org/10.3390/ijms222212268
Chicago/Turabian StyleKusano, Yuki, Takefumi Yamaguchi, Sota Nishisako, Takehiro Matsumura, Masaki Fukui, Kazunari Higa, Toshihiro Inoue, and Jun Shimazaki. 2021. "Elevated Cytokine Levels in Aqueous Humor Are Associated with Peripheral Anterior Synechiae after Penetrating Keratoplasty" International Journal of Molecular Sciences 22, no. 22: 12268. https://doi.org/10.3390/ijms222212268
APA StyleKusano, Y., Yamaguchi, T., Nishisako, S., Matsumura, T., Fukui, M., Higa, K., Inoue, T., & Shimazaki, J. (2021). Elevated Cytokine Levels in Aqueous Humor Are Associated with Peripheral Anterior Synechiae after Penetrating Keratoplasty. International Journal of Molecular Sciences, 22(22), 12268. https://doi.org/10.3390/ijms222212268







