On Placental Toxicology Studies and Cerium Dioxide Nanoparticles
Abstract
:1. Background
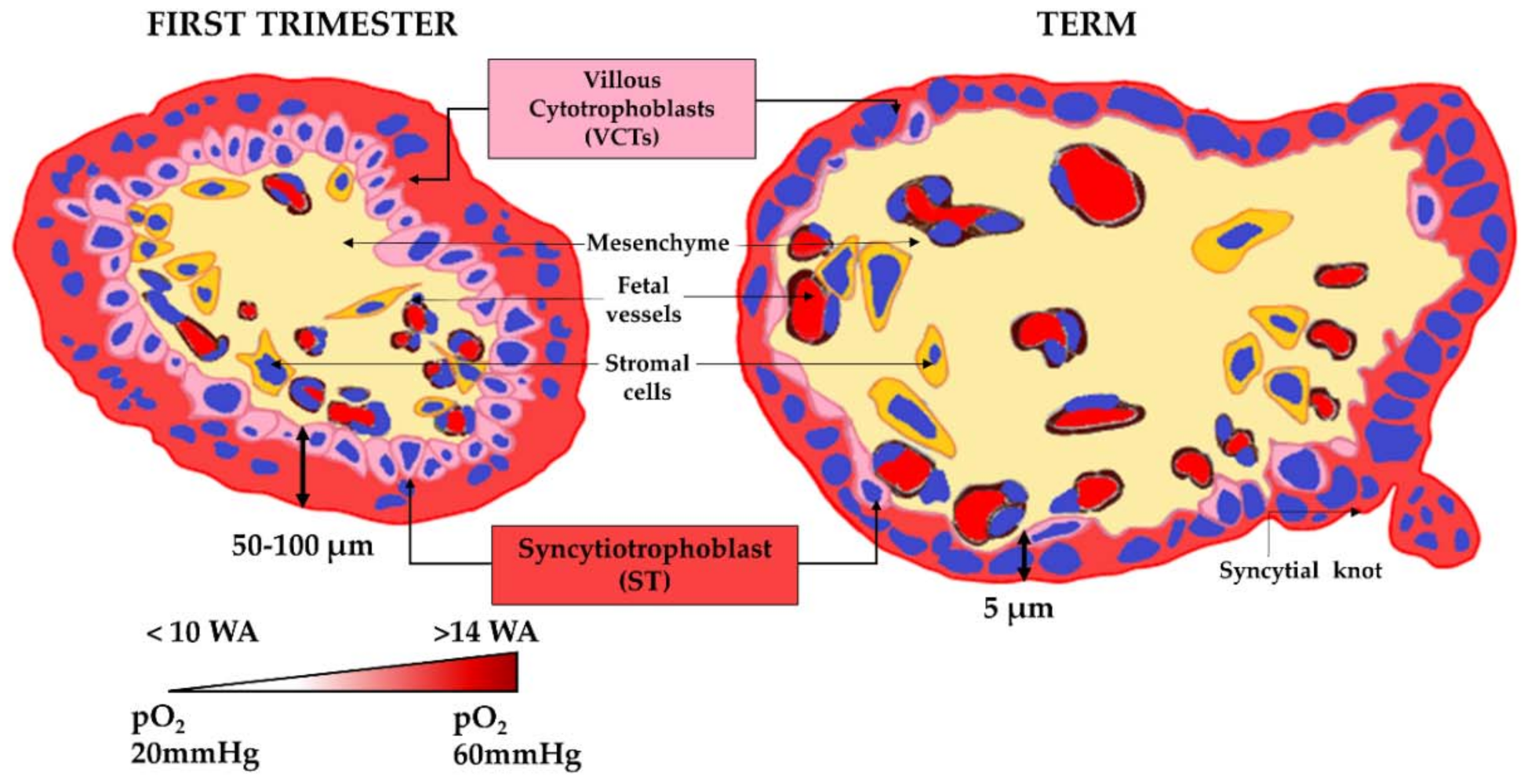
1.1. Human Placenta Ontogeny and Structure
- the villous cytotrophoblasts (VCTs), which differentiate into a syncytium called a syncytiotrophoblast (ST) by a cell-cell fusion process,
- the extravillous cytotrophoblasts (EVCTs), which invade the maternal decidua basalis up to the upper third of the myometrium, take part in the remodeling of the maternal spiral arteries and are responsible for the immune tolerance of the conceptus by expressing a non-classic human leucocyte antigen.
1.2. Human Placental Functions
1.3. The Placental Barrier
- an increase in the exchange surface area through the continuous ramifications of the villus tree to reach an area of 14 to 20 m2 at term;
- a decrease in the trophoblastic bilayer width, and therefore in the epithelial barrier between the maternal and fetal bloodstreams, dropping from 50 µm in the second month of pregnancy to 5 µm at the end of pregnancy;
- the arrival of maternal oxygenated blood in the intervillous chamber in contact with the ST between 10 and 14 WA after the removal of the trophoblastic plugs;
- an increase in the uterine blood flow up to 600 mL/min at the term of pregnancy.
- All these physiological changes must be considered when studying the effects of pollutants on the placental barrier and throughout placental ontogeny.
1.4. Impacts of Pollutants on the Human Placenta
2. Strategies to Study the Impact of Pollutants on the Placenta Barrier
2.1. Animal Models
- hemotrichorial placenta in rodents, composed of three trophoblast layers (one of VCT and two ST) instead of a trophoblast bilayer in humans (VCT and ST);
- the human placenta has several cotyledons on the maternal side of the placenta, unlike placenta in rodents;
- a labyrinthine organization (resulting from the fusion of villi around maternal blood gaps) in rodents;
- lack of hCG and of steroid hormone production by rodent placenta (e.g., steroids are secreted by the ovary during gestation);
- a more superficial invasion of maternal decidua in mice;
- the period of gestation (19–20 days for mice versus 270 days for humans).
2.2. Ex Vivo Placental Perfusion
2.3. Chorionic Villous Explant Cultures
2.4. Primary Culture of Trophoblasts (EVCT, VCT and ST)
2.5. Trophoblast Cell Lines
2.6. Co-Cultures and 2.5D Two-Chamber Models
2.7. Placenta-on-a-Chip Models
2.8. 3-D Models
3. Current Knowledge on Nanoceria
3.1. Introduction to Nanoparticles
3.2. Impact of Nanoparticles during Pregnancy
3.3. Nanoceria Properties
- As a pro-oxidant, in particular by the Fenton reaction, to kill cancer cells [103]
- As a carrier for targeted drug and gene delivery thanks to their coating ability and pH-dependent oxidation state, mainly in oncology therapies [104]
- As an antibacterial against Gram-positive and Gram-negative bacteria [105]
- In regenerative medicine and tissue engineering by enhancing long-term cell survival, enabling cell migration and proliferation and promoting stem cell differentiation [106].
3.4. Nanoceria and Human Health
3.5. CeO2 and Pregnancy
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evain-Brion, D. The 2 differentiation pathways of the human trophoblast. Gynecol. Obstet. Fertil. 2001, 29, 497–502. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Gundling, W.E.; Wildman, D.E. A review of inter- and intraspecific variation in the eutherian placenta. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massin, N.; Frendo, J.L.; Guibourdenche, J.; Luton, D.; Giovangrandi, Y.; Muller, F.; Vidaud, M.; Evain-Brion, D. Defect of syncytiotrophoblast formation and human chorionic gonadotropin expression in Down’s syndrome. Placenta 2001, 22 (Suppl. A), S93–S97. [Google Scholar] [CrossRef]
- Walker, O.S.; Ragos, R.; Gurm, H.; Lapierre, M.; May, L.L.; Raha, S. Delta-9-tetrahydrocannabinol disrupts mitochondrial function and attenuates syncytialization in human placental BeWo cells. Physiol. Rep. 2020, 8, e14476. [Google Scholar] [CrossRef]
- Costa, M.A.; Fonseca, B.M.; Marques, F.; Teixeira, N.A.; Correia-da-Silva, G. The psychoactive compound of Cannabis sativa, Δ(9)-tetrahydrocannabinol (THC) inhibits the human trophoblast cell turnover. Toxicology 2015, 334, 94–103. [Google Scholar] [CrossRef]
- Shoaito, H.; Petit, J.; Chissey, A.; Auzeil, N.; Guibourdenche, J.; Gil, S.; Laprévote, O.; Fournier, T.; Degrelle, S.A. The Role of Peroxisome Proliferator–Activated Receptor Gamma (PPARγ) in Mono(2-ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation. Environ. Health Perspect. 2019, 127, 27003. [Google Scholar] [CrossRef]
- Le Vee, M.; Kolasa, E.; Jouan, E.; Collet, N.; Fardel, O. Differentiation of human placental BeWo cells by the environmental contaminant benzo(a)pyrene. Chem. Biol. Interact. 2014, 210, 1–11. [Google Scholar] [CrossRef]
- Narciso, L.; Ietta, F.; Romagnoli, R.; Paulesu, L.; Mantovani, A.; Tait, S. Effects of Bisphenol A on endogenous retroviral envelopes expression and trophoblast fusion in BeWo cells. Reprod. Toxicol. 2019, 89, 35–44. [Google Scholar] [CrossRef]
- Loukeris, K.; Sela, R.; Baergen, R.N. Syncytial knots as a reflection of placental maturity: Reference values for 20 to 40 weeks’ gestational age. Pediatr. Dev. Pathol. 2010, 13, 305–309. [Google Scholar] [CrossRef]
- Heazell, A.E.; Moll, S.J.; Jones, C.J.; Baker, P.N.; Crocker, I.P. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta 2007, 28 (Suppl. A), S33–S40. [Google Scholar] [CrossRef]
- Evain-Brion, D.; Malassine, A. Human placenta as an endocrine organ. Growth Horm. IGF Res. 2003, 13 (Suppl. A), S34–S37. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S.; Tsantili-Kakoulidou, A. Current toxicological aspects on drug and chemical transport and metabolism across the human placental barrier. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1263–1275. [Google Scholar] [CrossRef]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef]
- Andres, R.L. The association of cigarette smoking with placenta previa and abruptio placentae. Semin. Perinatol. 1996, 20, 154–159. [Google Scholar] [CrossRef]
- Pineles, B.L.; Park, E.; Samet, J.M. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am. J. Epidemiol. 2014, 179, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Walker, N.; Filis, P.; O’Shaughnessy, P.J.; Bellingham, M.; Fowler, P.A. Nutrient transporter expression in both the placenta and fetal liver are affected by maternal smoking. Placenta 2019, 78, 10–17. [Google Scholar] [CrossRef]
- Mehendale, R.; Hibbard, J.; Fazleabas, A.; Leach, R. Placental angiogenesis markers sFlt-1 and PlGF: Response to cigarette smoke. Am. J. Obstet. Gynecol. 2007, 197, 363.e1–363.e5. [Google Scholar] [CrossRef]
- Zdravkovic, T.; Genbacev, O.; McMaster, M.T.; Fisher, S.J. The adverse effects of maternal smoking on the human placenta: A review. Placenta 2005, 26 (Suppl. A), S81–S86. [Google Scholar] [CrossRef]
- Rousseaux, S.; Seyve, E.; Chuffart, F.; Bourova-Flin, E.; Benmerad, M.; Charles, M.A.; Forhan, A.; Heude, B.; Siroux, V.; Slama, R.; et al. Immediate and durable effects of maternal tobacco consumption alter placental DNA methylation in enhancer and imprinted gene-containing regions. BMC Med. 2020, 18, 306. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fénichel, P.; Brucker-Davis, F.; Chevalier, N. The history of Distilbène® (Diethylstilbestrol) told to grandchildren--the transgenerational effect. Ann. Endocrinol. 2015, 76, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Kotrogianni, P.; Christopoulos-Timogiannakis, E.; Koutaki, D.; Daskalakis, G.; Papantoniou, N. Bisphenol A and adverse pregnancy outcomes: A systematic review of the literature. J. Matern.-Fetal Neonatal Med. 2018, 31, 3320–3327. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Lee, S.; Hong, Y.C.; Park, H.; Kim, Y.; Ha, M.; Ha, E. Combined effects of multiple prenatal exposure to pollutants on birth weight: The Mothers and Children’s Environmental Health (MOCEH) study. Environ. Res. 2020, 181, 108832. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.H.; Lee, I.; Kim, W.; Won, S.; Ku, J.L.; Moon, H.B.; Park, J.; Kim, S.; Choi, G.; et al. Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: A CHECK cohort study. Environ. Int. 2018, 119, 398–406. [Google Scholar] [CrossRef]
- Padula, A.M.; Yang, W.; Lurmann, F.W.; Balmes, J.; Hammond, S.K.; Shaw, G.M. Prenatal exposure to air pollution, maternal diabetes and preterm birth. Environ. Res. 2019, 170, 160–167. [Google Scholar] [CrossRef]
- Siddika, N.; Rantala, A.K.; Antikainen, H.; Balogun, H.; Amegah, A.K.; Ryti, N.R.I.; Kukkonen, J.; Sofiev, M.; Jaakkola, M.S.; Jaakkola, J.J.K. Synergistic effects of prenatal exposure to fine particulate matter (PM(2.5)) and ozone (O(3)) on the risk of preterm birth: A population-based cohort study. Environ. Res. 2019, 176, 108549. [Google Scholar] [CrossRef]
- van de Bor, M. Fetal toxicology. Handb. Clin. Neurol. 2019, 162, 31–55. [Google Scholar] [CrossRef]
- Suwannakul, B.; Sapbamrer, R.; Wiwattanadittakul, N.; Hongsibsong, S. Different Timing of Prenatal Organophosphate Pesticides Exposure Is Influenced Different Aspects of Infant Developmental Performance. Toxics 2021, 9, 99. [Google Scholar] [CrossRef]
- Montgomery, S.M.; Ekbom, A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ 2002, 324, 26–27. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.V.; Mack, W.J.; Yao, J.; Berhane, K.; Amadeus, M.; Lurmann, F.; Gilliland, F.; McConnell, R.; Hodis, H.N.; Künzli, N.; et al. Prenatal Air Pollution Exposure and Early Cardiovascular Phenotypes in Young Adults. PLoS ONE 2016, 11, e0150825. [Google Scholar] [CrossRef]
- Viluksela, M.; Pohjanvirta, R. Multigenerational and Transgenerational Effects of Dioxins. Int. J. Mol. Sci. 2019, 20, 2947. [Google Scholar] [CrossRef] [Green Version]
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the field forward! Nanomedicine 2016, 11, 941–957. [Google Scholar] [CrossRef]
- Buerki-Thurnherr, T.; von Mandach, U.; Wick, P. Knocking at the door of the unborn child: Engineered nanoparticles at the human placental barrier. Swiss Med. Wkly. 2012, 142, w13559. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.J.; Varberg, K.M.; Iqbal, K. Hemochorial placentation: Development, function, and adaptations. Biol. Reprod. 2018, 99, 196–211. [Google Scholar] [CrossRef] [Green Version]
- Soncin, F.; Natale, D.; Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: A comparative review. Cell Mol. Life Sci. 2015, 72, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Panigel, M.; Pascaud, M.; Brun, J.L. [Radioangiographic study of circulation in the villi and intervillous space of isolated human placental cotyledon kept viable by perfusion]. J. Physiol. 1967, 59, 277. [Google Scholar]
- Schneider, H.; Panigel, M.; Dancis, J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am. J. Obstet. Gynecol. 1972, 114, 822–828. [Google Scholar] [CrossRef]
- Myllynen, P.; Vähäkangas, K. Placental transfer and metabolism: An overview of the experimental models utilizing human placental tissue. Toxicol. In Vitro 2013, 27, 507–512. [Google Scholar] [CrossRef]
- Miller, R.K.; Genbacev, O.; Turner, M.A.; Aplin, J.D.; Caniggia, I.; Huppertz, B. Human placental explants in culture: Approaches and assessments. Placenta 2005, 26, 439–448. [Google Scholar] [CrossRef]
- Valero, L.; Alhareth, K.; Gil, S.; Simasotchi, C.; Roques, C.; Scherman, D.; Mignet, N.; Fournier, T.; Andrieux, K. Assessment of dually labelled PEGylated liposomes transplacental passage and placental penetration using a combination of two ex-vivo human models: The dually perfused placenta and the suspended villous explants. Int. J. Pharm. 2017, 532, 729–737. [Google Scholar] [CrossRef]
- Siman, C.M.; Sibley, C.P.; Jones, C.J.; Turner, M.A.; Greenwood, S.L. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1116–R1122. [Google Scholar] [CrossRef] [Green Version]
- Kliman, H.J.; Nestler, J.E.; Sermasi, E.; Sanger, J.M.; Strauss, J.F., 3rd. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 1986, 118, 1567–1582. [Google Scholar] [CrossRef] [Green Version]
- Pattillo, R.A.; Gey, G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968, 28, 1231–1236. [Google Scholar]
- Kohler, P.O.; Bridson, W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971, 32, 683–687. [Google Scholar] [CrossRef]
- Hochberg, A.; Rachmilewitz, J.; Eldar-Geva, T.; Salant, T.; Schneider, T.; de Groot, N. Differentiation of choriocarcinoma cell line (JAr). Cancer Res. 1992, 52, 3713–3717. [Google Scholar]
- Graham, C.H.; Hawley, T.S.; Hawley, R.C.; MacDougall, J.R.; Kerbel, R.S.; Khoo, N.; Lala, P.K. Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Exp. Cell Res. 1993, 206, 204–211. [Google Scholar] [CrossRef]
- Pavan, L.; Tarrade, A.; Hermouet, A.; Delouis, C.; Titeux, M.; Vidaud, M.; Thérond, P.; Evain-Brion, D.; Fournier, T. Human invasive trophoblasts transformed with simian virus 40 provide a new tool to study the role of PPARgamma in cell invasion process. Carcinogenesis 2003, 24, 1325–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 5892. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Gordon, L.; Wong, N.C.; Moffett, A.; Manuelpillai, U.; Craig, J.M.; Sharkey, A.; Saffery, R. Wide-ranging DNA methylation differences of primary trophoblast cell populations and derived cell lines: Implications and opportunities for understanding trophoblast function. Mol. Hum. Reprod. 2011, 17, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kallol, S.; Moser-Haessig, R.; Ontsouka, C.E.; Albrecht, C. Comparative expression patterns of selected membrane transporters in differentiated BeWo and human primary trophoblast cells. Placenta 2018, 72–73, 48–52. [Google Scholar] [CrossRef]
- Levkovitz, R.; Zaretsky, U.; Gordon, Z.; Jaffa, A.J.; Elad, D. In vitro simulation of placental transport: Part I. Biological model of the placental barrier. Placenta 2013, 34, 699–707. [Google Scholar] [CrossRef]
- Aengenheister, L.; Keevend, K.; Muoth, C.; Schönenberger, R.; Diener, L.; Wick, P.; Buerki-Thurnherr, T. An advanced human in vitro co-culture model for translocation studies across the placental barrier. Sci. Rep. 2018, 8, 5388. [Google Scholar] [CrossRef] [Green Version]
- Kreuder, A.E.; Bolaños-Rosales, A.; Palmer, C.; Thomas, A.; Geiger, M.A.; Lam, T.; Amler, A.K.; Markert, U.R.; Lauster, R.; Kloke, L. Inspired by the human placenta: A novel 3D bioprinted membrane system to create barrier models. Sci. Rep. 2020, 10, 15606. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Gilmore, C.; Sood, A.; Matsusaki, M.; Collett, G.; Tannetta, D.; Sargent, I.L.; McGarvey, J.; Halemani, N.D.; Hanley, J.; et al. In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium. Biomaterials 2019, 192, 140–148. [Google Scholar] [CrossRef]
- Tarrade, A.; Lai Kuen, R.; Malassiné, A.; Tricottet, V.; Blain, P.; Vidaud, M.; Evain-Brion, D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab. Investig. 2001, 81, 1199–1211. [Google Scholar] [CrossRef] [Green Version]
- Campagnolo, L.L.V.; Massimiani, M.; Magrini, A.; Pietroiusti, A. In vitro experimental models to study the efficiency of the placental barrier for environmental toxicants: Tumor cell lines versus trophoblast primary cells. Biomed. Prev. Issues 2018, 1, 210–212. [Google Scholar] [CrossRef]
- Blundell, C.; Tess, E.R.; Schanzer, A.S.; Coutifaris, C.; Su, E.J.; Parry, S.; Huh, D. A microphysiological model of the human placental barrier. Lab Chip 2016, 16, 3065–3073. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. In Vitro 2019, 54, 105–113. [Google Scholar] [CrossRef]
- Mandt, D.; Gruber, P.; Markovic, M.; Tromayer, M.; Rothbauer, M.; Kratz, S.R.A.; Ali, S.F.; Hoorick, J.V.; Holnthoner, W.; Mühleder, S.; et al. Fabrication of biomimetic placental barrier structures within a microfluidic device utilizing two-photon polymerization. Int. J. Bioprint. 2018, 4, 144. [Google Scholar] [CrossRef]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern.-Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef]
- Blundell, C.; Yi, Y.S.; Ma, L.; Tess, E.R.; Farrell, M.J.; Georgescu, A.; Aleksunes, L.M.; Huh, D. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Muoth, C.; Wichser, A.; Monopoli, M.; Correia, M.; Ehrlich, N.; Loeschner, K.; Gallud, A.; Kucki, M.; Diener, L.; Manser, P.; et al. A 3D co-culture microtissue model of the human placenta for nanotoxicity assessment. Nanoscale 2016, 8, 17322–17332. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, M.A.; Fernando, R.C.; Gardner, L.; Hollinshead, M.S.; Burton, G.J.; Moffett, A.; Turco, M.Y. Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta. Nat. Protoc. 2020, 15, 3441–3463. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Boland, S.; Hussain, S.; Baeza-Squiban, A. Carbon black and titanium dioxide nanoparticles induce distinct molecular mechanisms of toxicity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.T.; Feng, W.Y.; Wang, Y.; Wang, B.; Wang, M.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol. Sci. 2009, 107, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Garantziotis, S.; Rodrigues-Lima, F.; Dupret, J.M.; Baeza-Squiban, A.; Boland, S. Intracellular signal modulation by nanomaterials. Adv. Exp. Med. Biol. 2014, 811, 111–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, V.; Bhargava, A.; Bhanot, V.; Gupta, R.; Jain, N.; Panwar, J. Formation and Characterization of Protein Corona around Nanoparticles: A Review. J. Nanosci. Nanotechnol. 2018, 18, 6653–6670. [Google Scholar] [CrossRef] [PubMed]
- Puisney, C.; Baeza-Squiban, A.; Boland, S. Mechanisms of Uptake and Translocation of Nanomaterials in the Lung. Adv. Exp. Med. Biol. 2018, 1048, 21–36. [Google Scholar] [CrossRef]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Alstrup Jensen, K.; et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef]
- Ohlwein, S.; Kappeler, R.; Kutlar Joss, M.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [Green Version]
- Pereira, K.V.; Giacomeli, R.; Gomes de Gomes, M.; Haas, S.E. The challenge of using nanotherapy during pregnancy: Technological aspects and biomedical implications. Placenta 2020, 100, 75–80. [Google Scholar] [CrossRef]
- Aengenheister, L.; Favaro, R.R.; Morales-Prieto, D.M.; Furer, L.A.; Gruber, M.; Wadsack, C.; Markert, U.R.; Buerki-Thurnherr, T. Research on nanoparticles in human perfused placenta: State of the art and perspectives. Placenta 2021, 104, 199–207. [Google Scholar] [CrossRef]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.P.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2021, 751, 142235. [Google Scholar] [CrossRef]
- Guillard, A.; Gaultier, E.; Cartier, C.; Devoille, L.; Noireaux, J.; Chevalier, L.; Morin, M.; Grandin, F.; Lacroix, M.Z.; Coméra, C.; et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO(2) nanoparticles in an ex vivo placental perfusion model. Part. Fibre Toxicol. 2020, 17, 51. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef]
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnology 2019, 17, 84. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lee, K.B.; He, L.; Seiffert, J.; Subramaniam, P.; Yang, L.; Chen, S.; Maguire, P.; Mainelis, G.; Schwander, S.; et al. Effects of a nanoceria fuel additive on the physicochemical properties of diesel exhaust particles. Environ. Sci. Process. Impacts 2016, 18, 1333–1342. [Google Scholar] [CrossRef]
- Zhang, J.; Nazarenko, Y.; Zhang, L.; Calderon, L.; Lee, K.B.; Garfunkel, E.; Schwander, S.; Tetley, T.D.; Chung, K.F.; Porter, A.E.; et al. Impacts of a nanosized ceria additive on diesel engine emissions of particulate and gaseous pollutants. Environ. Sci. Technol. 2013, 47, 13077–13085. [Google Scholar] [CrossRef] [Green Version]
- Lung, S.; Cassee, F.R.; Gosens, I.; Campbell, A. Brain suppression of AP-1 by inhaled diesel exhaust and reversal by cerium oxide nanoparticles. Inhal. Toxicol. 2014, 26, 636–641. [Google Scholar] [CrossRef]
- Snaidr, S.M. Ceria/Zirconia Fibers for Use in Cigarettes. World Patent No. 2004023903 A2, 25 March 2004. [Google Scholar]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the Properties and Applications of Nanoceria: Is There Still Plenty of Room at the Bottom? Environ. Sci. Nano 2014, 46. [Google Scholar] [CrossRef] [Green Version]
- Böhlandt, A.; Schierl, R.; Diemer, J.; Koch, C.; Bolte, G.; Kiranoglu, M.; Fromme, H.; Nowak, D. High concentrations of cadmium, cerium and lanthanum in indoor air due to environmental tobacco smoke. Sci. Total Environ. 2012, 414, 738–741. [Google Scholar] [CrossRef]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L’Abbate, L.; Longo, V.; Colombo, P.; Frasca, D.; Balzan, M.; Cuttitta, G.; et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM(2.5). Environ. Res. 2018, 165, 71–80. [Google Scholar] [CrossRef]
- OECD. Current Developments/Activities on the Safety of Manufactured Nanomaterials. Safety of Manufactured Nanomaterials. 2010. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono (accessed on 22 April 2021).
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hoseini, S.J.; Hamzehlou, S.; Darroudi, M.; Verdi, J.; Hasanzadeh, L.; Kim, H.W.; Mozafari, M. Biomedical applications of nanoceria: New roles for an old player. Nanomedicine 2018, 13, 3051–3069. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic Applications of Cerium Oxide Nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383. [Google Scholar] [CrossRef]
- Carvajal, S.; Perramón, M.; Casals, G.; Oró, D.; Ribera, J.; Morales-Ruiz, M.; Casals, E.; Casado, P.; Melgar-Lesmes, P.; Fernández-Varo, G.; et al. Cerium Oxide Nanoparticles Protect against Oxidant Injury and Interfere with Oxidative Mediated Kinase Signaling in Human-Derived Hepatocytes. Int. J. Mol. Sci. 2019, 20, 5959. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, K.; Babu, K.N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine 2013, 9, 439–448. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Godugu, C. Nanoceria suppresses multiple low doses of streptozotocin-induced Type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction of apoptosis. Nanomedicine 2018, 13, 1905–1922. [Google Scholar] [CrossRef]
- Rocca, A.; Moscato, S.; Ronca, F.; Nitti, S.; Mattoli, V.; Giorgi, M.; Ciofani, G. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine 2015, 11, 1725–1734. [Google Scholar] [CrossRef]
- Genchi, G.G.; Degl’Innocenti, A.; Salgarella, A.R.; Pezzini, I.; Marino, A.; Menciassi, A.; Piccirillo, S.; Balsamo, M.; Ciofani, G. Modulation of gene expression in rat muscle cells following treatment with nanoceria in different gravity regimes. Nanomedicine 2018, 13, 2821–2833. [Google Scholar] [CrossRef]
- Pešić, M.; Podolski-Renić, A.; Stojković, S.; Matović, B.; Zmejkoski, D.; Kojić, V.; Bogdanović, G.; Pavićević, A.; Mojović, M.; Savić, A.; et al. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem. Biol. Interact. 2015, 232, 85–93. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Zeng, Y.P.; Hao, Y.H.; Huang, J.W.; Yang, Z.Y.; Li, R. Nanoceria-Mediated Drug Delivery for Targeted Photodynamic Therapy on Drug-Resistant Breast Cancer. ACS Appl. Mater. Interfaces 2016, 8, 31510–31523. [Google Scholar] [CrossRef]
- Babu, K.S.; Anandkumar, M.; Tsai, T.Y.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Cytotoxicity and antibacterial activity of gold-supported cerium oxide nanoparticles. Int. J. Nanomed. 2014, 9, 5515–5531. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Chigurupati, S.; Dowding, J.; Munusamy, P.; Baer, D.R.; McGinnis, J.F.; Mattson, M.P.; Self, W.; Seal, S. Therapeutic potential of nanoceria in regenerative medicine. MRS Bull. 2014, 39, 976–983. [Google Scholar] [CrossRef]
- Chen, B.H.; Stephen Inbaraj, B. Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles. Crit. Rev. Biotechnol. 2018, 38, 1003–1024. [Google Scholar] [CrossRef]
- Casals, E.; Zeng, M.; Parra-Robert, M.; Fernández-Varo, G.; Morales-Ruiz, M.; Jiménez, W.; Puntes, V.; Casals, G. Cerium Oxide Nanoparticles: Advances in Biodistribution, Toxicity, and Preclinical Exploration. Small 2020, 16, e1907322. [Google Scholar] [CrossRef]
- Geraets, L.; Oomen, A.G.; Schroeter, J.D.; Coleman, V.A.; Cassee, F.R. Tissue distribution of inhaled micro- and nano-sized cerium oxide particles in rats: Results from a 28-day exposure study. Toxicol Sci. 2012, 127, 463–473. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The Yin: An adverse health perspective of nanoceria: Uptake, distribution, accumulation, and mechanisms of its toxicity. Environ. Sci. Nano 2014, 1, 406–428. [Google Scholar] [CrossRef]
- Mauro, M.; Crosera, M.; Monai, M.; Montini, T.; Fornasiero, P.; Bovenzi, M.; Adami, G.; Turco, G.; Filon, F.L. Cerium Oxide Nanoparticles Absorption through Intact and Damaged Human Skin. Molecules 2019, 24, 3759. [Google Scholar] [CrossRef] [Green Version]
- Canoa, P.; Simón-Vázquez, R.; Popplewell, J.; González-Fernández, Á. A quantitative binding study of fibrinogen and human serum albumin to metal oxide nanoparticles by surface plasmon resonance. Biosens. Bioelectron. 2015, 74, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Mazzolini, J.; Weber, R.J.; Chen, H.S.; Khan, A.; Guggenheim, E.; Shaw, R.K.; Chipman, J.K.; Viant, M.R.; Rappoport, J.Z. Protein Corona Modulates Uptake and Toxicity of Nanoceria via Clathrin-Mediated Endocytosis. Biol. Bull. 2016, 231, 40–60. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Karakoti, A.; Seal, S.; Self, W.T. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol. BioSyst. 2010, 6, 1813–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Ly, A.; Das, S.; Sakthivel, T.S.; Barkam, S.; Seal, S. Cerium oxide nanoparticles at the nano-bio interface: Size-dependent cellular uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46, S956–S963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedder, M.; Boland, S.; Devineau, S.; Zerrad-Saadi, A.; Rogozarski, J.; Lai-Kuen, R.; Baya, I.; Guibourdenche, J.; Vibert, F.; Chissey, A.; et al. Uptake of Cerium Dioxide Nanoparticles and Impact on Viability, Differentiation and Functions of Primary Trophoblast Cells from Human Placenta. Nanomaterials 2020, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Höllriegl, V.; González-Estecha, M.; Trasobares, E.M.; Giussani, A.; Oeh, U.; Herraiz, M.A.; Michalke, B. Measurement of cerium in human breast milk and blood samples. J. Trace Elem. Med. Biol. 2010, 24, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Levack, V.M.; Hone, P.A.; Phipps, A.W.; Harrison, J.D. The placental transfer of cerium: Experimental studies and estimates of doses to the human fetus from 141Ce and 144Ce. Int. J. Radiat. Biol. 2002, 78, 227–235. [Google Scholar] [CrossRef]
- Wei, J.; Wang, C.; Yin, S.; Pi, X.; Jin, L.; Li, Z.; Liu, J.; Wang, L.; Yin, C.; Ren, A. Concentrations of rare earth elements in maternal serum during pregnancy and risk for fetal neural tube defects. Environ. Int. 2020, 137, 105542. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Zhang, L.; Bi, J.; Song, L.; Wang, L.; Liu, B.; Zhou, A.; Cao, Z.; Xiong, C.; et al. Prenatal exposure of rare earth elements cerium and ytterbium and neonatal thyroid stimulating hormone levels: Findings from a birth cohort study. Environ. Int. 2019, 133, 105222. [Google Scholar] [CrossRef]
- Zhong, H.; Geng, Y.; Chen, J.; Gao, R.; Yu, C.; Yang, Z.; Chen, X.; Mu, X.; Liu, X.; He, J. Maternal exposure to CeO(2)NPs during early pregnancy impairs pregnancy by inducing placental abnormalities. J. Hazard. Mater. 2020, 389, 121830. [Google Scholar] [CrossRef]
- Paul, E.; Franco-Montoya, M.L.; Paineau, E.; Angeletti, B.; Vibhushan, S.; Ridoux, A.; Tiendrebeogo, A.; Salome, M.; Hesse, B.; Vantelon, D.; et al. Pulmonary exposure to metallic nanomaterials during pregnancy irreversibly impairs lung development of the offspring. Nanotoxicology 2017, 11, 484–495. [Google Scholar] [CrossRef]
- Vafaei-Pour, Z.; Shokrzadeh, M.; Jahani, M.; Shaki, F. Embryo-Protective Effects of Cerium Oxide Nanoparticles against Gestational Diabetes in Mice. Iran. J. Pharm. Res. 2018, 17, 964–975. [Google Scholar]
- Lee, J.; Jeong, J.S.; Kim, S.Y.; Lee, S.J.; Shin, Y.J.; Im, W.J.; Kim, S.H.; Park, K.; Jeong, E.J.; Nam, S.Y.; et al. Safety assessment of cerium oxide nanoparticles: Combined repeated-dose toxicity with reproductive/developmental toxicity screening and biodistribution in rats. Nanotoxicology 2020, 14, 696–710. [Google Scholar] [CrossRef]
- Nemati, A.; Assadollahi, V.; Peluso, I.; Abbaszadeh, A.; Beigi-Boroujeni, M.; Khanipur, Z.; Gholami, M. A Stereological Study of the Toxic Effects of Cerium Oxide during Pregnancy on Kidney Tissues in Neonatal NMRI Mice. Oxid. Med. Cell. Longev. 2020, 2020, 9132724. [Google Scholar] [CrossRef]
- Nemati, A.; Farhadi, A.; Jalili, C.; Gholami, M. The Effect of Cerium Oxide During Pregnancy on the Development of the Testicular Tissue of Newborn NMRI Mice. Biol. Trace Elem. Res. 2020, 195, 196–204. [Google Scholar] [CrossRef]



| Models | Interests in Toxicology Studies | Advantages | Drawbacks |
|---|---|---|---|
| Animal models | • impact on pregnancy and outcomes • fetotoxicity studies | • in vivo • low cost • chronic exposure possible | • cautious extrapolation to animal model in view of the specificity of human placentation |
| Ex-vivo placental perfusion | • transplacental passage • placental kinetics and metabolism • placental accumulation of pollutants | • access to organized placental tissue (a whole cotyledon perfused) | • only possible in term placentas • do not allow chronic exposure • nonplacental pharmacokinetic factors |
| Chorionic villous explant cultures | • barrier permeability and tissular accumulation of pollutants • impact on cell viability • hormonal production | • physiological villi • near-physiological 3D microenvironment | • in vitro • fast ST necrosis • limited time exposures (less than 15 days) |
| Primary human trophoblast cultures | • impact on trophoblast viability • hormonal production • cellular internalization of pollutants | • recapitulate physiological differentiation to form the syncytium • isolation from term and first trimester placentas | • in vitro • limited period of culture due to cell necrosis • not adapted for chronic exposure |
| Cell line cultures | • impact on cell viability • cellular internalization of pollutants • cell signaling and hormonal production | • low cost • acquired resistance to apoptosis • possible adaptation to long term exposures | • in vitro • cancerous/immortalized cells’ properties distinct from physiological trophoblasts |
| 2D co-cultures and placenta-on-a-chip | • barrier permeability and bypassing • impact on cells’ viability • cell signaling and hormonal production | • near-physiological 3D microenvironment | • in vitro • cancerous/immortalized cells’ properties distinct from physiological trophoblasts |
| 3D models (organoids) | still under development | • recapitulate the human placenta villi • anatomically and functionally close to the villous placenta • long term culture possible (chronic exposure possible) | • in vitro • from first trimester placentas only • the polarity of the organoids (ST within the organoid cavity) needs to be reversed for toxicological studies. |
| Model | Sandwich Culture | Transwell Insert | Placenta-on-a-Chip System |
|---|---|---|---|
| Authors | Nishiguchi et al. 2019 [58] | Aengenheister et al., 2018 [56] | Blundell et al., 2018 [65] |
| http://creativecommons.org/licenses/by/4.0/, accessed on 10 November 2021 | |||
| Villous cytotrophoblasts | primary VCTs (third trimester) with collagen and laminin coating | BeWo b30 | BeWo b30 |
| Villous endothelial cells | human umbilical vein endothelial cells (HUVECs) with fibronectin and gelatin coating | microvascular human placental venous endothelial cell line (HPEC-A2) | human primary placental villous endothelial cells (HPVECs) |
| Villous mesenchymal fibroblasts | primary human villous mesenchymal fibroblasts (HVMFs) with fibronectin and gelatin coating | none | none |
| Technology | bottom-up approach using ECM (extracellular matrix) nanofilms | polycarbonate Transwell insert | upper and lower microchannels separated by a thin, semipermeable membrane |
| Description of the model |  |  |  |
| Model | 3D Spheroids | Organoids |
|---|---|---|
| Author | Muoth 2016 | Turco 2018 |
| Villous Cytotrophoblast | BeWo b30 and HTR-8/SVneo | Primary first trimester (8 to 11 WA) proliferative trophoblasts |
| Villous mesenchymal fibroblasts | Primary human villous mesenchymal fibroblasts (HVMF) | none |
| Technology | Scaffold-free hanging drop technology (GravityPLUS plates) | Isolation of first trimester proliferative trophoblasts seeded in drops of matrigel in a basal culture medium for the formation of organoids, including growth factors and inhibitors |
| Description of the model |  |  |
| Ce3+ → Ce4+ | |||
|---|---|---|---|
| Oxidation of Ce3+ | O2 + Ce3+ | → | O2•– + Ce4+ |
| •OH + Ce3+ | → | OH− + Ce4+ | |
| OH− + H+ →H2O | |||
| Superoxide Dismutase (SOD) mimetic activity | O2•– + 2H+ + Ce3+ | → | H2O2 + Ce4+ |
| Fenton-like reaction | H2O2 + Ce3+ | → | •OH + OH− + Ce4+ |
| Catalase (CAT) mimetic activity | H2O2 + 2H+ + 2Ce3+ | → | 2H2O + 2Ce4+ |
| Ce4+ → Ce3+ | |||
| Reduction of Ce4+ | H2O2 + Ce4+ | → | H+ + HO2 + Ce3+ |
| Superoxide Dismutase (SOD) mimetic activity | O2•– + Ce4+ | → | O2 + Ce3+ |
| Catalase (CAT) mimetic activity | H2O2 + 2Ce4+ | → | 2H+ + O2 + 2Ce3+ |
| Data Sources | Model Used | Nanoceria Effects | Type of Nanoceria | Dose and Time Exposure |
|---|---|---|---|---|
| Nedder et al. 2020 | Primary VCTs from human placentas at term of pregnancy | Internalization in both VCT and ST Dose and time dependent cytotoxicity Decrease in differentiation to form the ST Disrupted hormonal production Caspase activation | NM-212 (Joint Research Center nomenclature) polyhedral 28.4 ± 10.4 nm aggregate size 503 ± 55 nm | from 0.1 to 101 µg/cm2 until 72 h |
| Zhong et al. 2020 | BALB/c mice | Altered decidualization: disruption of decidual cell secretion of regulators of trophoblast invasion, altered uterine natural killer (uNK) cell recruitment and differentiation Decrease in birth weight Smaller litters because of failure in the fetus development | 3−5 nm | 5 mg/kg intravenous once a day at on D5, D6 and D7 |
| Paul et al. 2017 | C57BL6/J mice | Long-lasting impairment of lung development of the offspring Significant decrease in vascular endothelial growth factor (VEGF) mRNA and protein levels in amniotic fluid and pup lungs Significant decrease in fetal weight and placental efficiency | spherical shape 22.4 ± 0.2 nm aggregate size >1000 nm | intratracheal instillation of 300 µg (100 µg by week) on pregnant mice |
| Vafaei-Pour et al. 2018 | Swiss albino mice with diabetes induced by one dose of intraperitoneal injection of streptozotocin (60 mg/kg) | Reverse the elevation of oxidative stress markers induced by diabetes Diabetes-induced malformation in visceral and spinal of embryo partially restored | no data | 60 mg/kg for 16 days |
| Lee et al. 2020 | Sprague-Dawley rats | Cerium was not detected in either parental or pup tissues, not systemically absorbed in parental animals or their pups | polyhedral 14.2 ± 5.0 nm | 100, 300 and 1000 mg/kg orally administered during premating, mating, gestation and early lactation periods |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deval, G.; Boland, S.; Fournier, T.; Ferecatu, I. On Placental Toxicology Studies and Cerium Dioxide Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12266. https://doi.org/10.3390/ijms222212266
Deval G, Boland S, Fournier T, Ferecatu I. On Placental Toxicology Studies and Cerium Dioxide Nanoparticles. International Journal of Molecular Sciences. 2021; 22(22):12266. https://doi.org/10.3390/ijms222212266
Chicago/Turabian StyleDeval, Gaëlle, Sonja Boland, Thierry Fournier, and Ioana Ferecatu. 2021. "On Placental Toxicology Studies and Cerium Dioxide Nanoparticles" International Journal of Molecular Sciences 22, no. 22: 12266. https://doi.org/10.3390/ijms222212266
APA StyleDeval, G., Boland, S., Fournier, T., & Ferecatu, I. (2021). On Placental Toxicology Studies and Cerium Dioxide Nanoparticles. International Journal of Molecular Sciences, 22(22), 12266. https://doi.org/10.3390/ijms222212266








