The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway
Abstract
:1. Introduction
2. Results
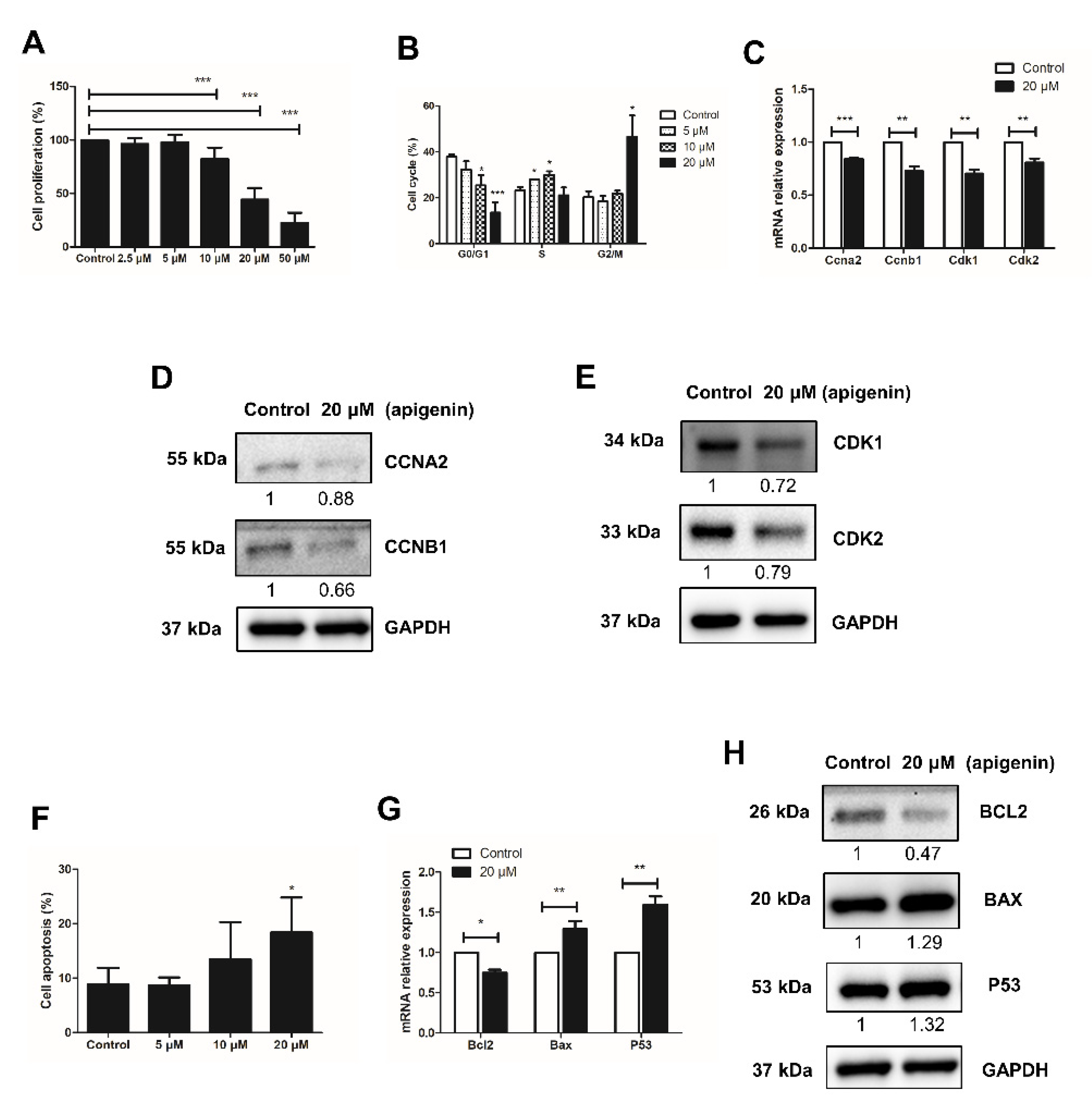
2.1. Apigenin Hampers the Proliferation of Spermatogonia
2.2. Apigenin Promotes Apoptosis of Spermatogonia
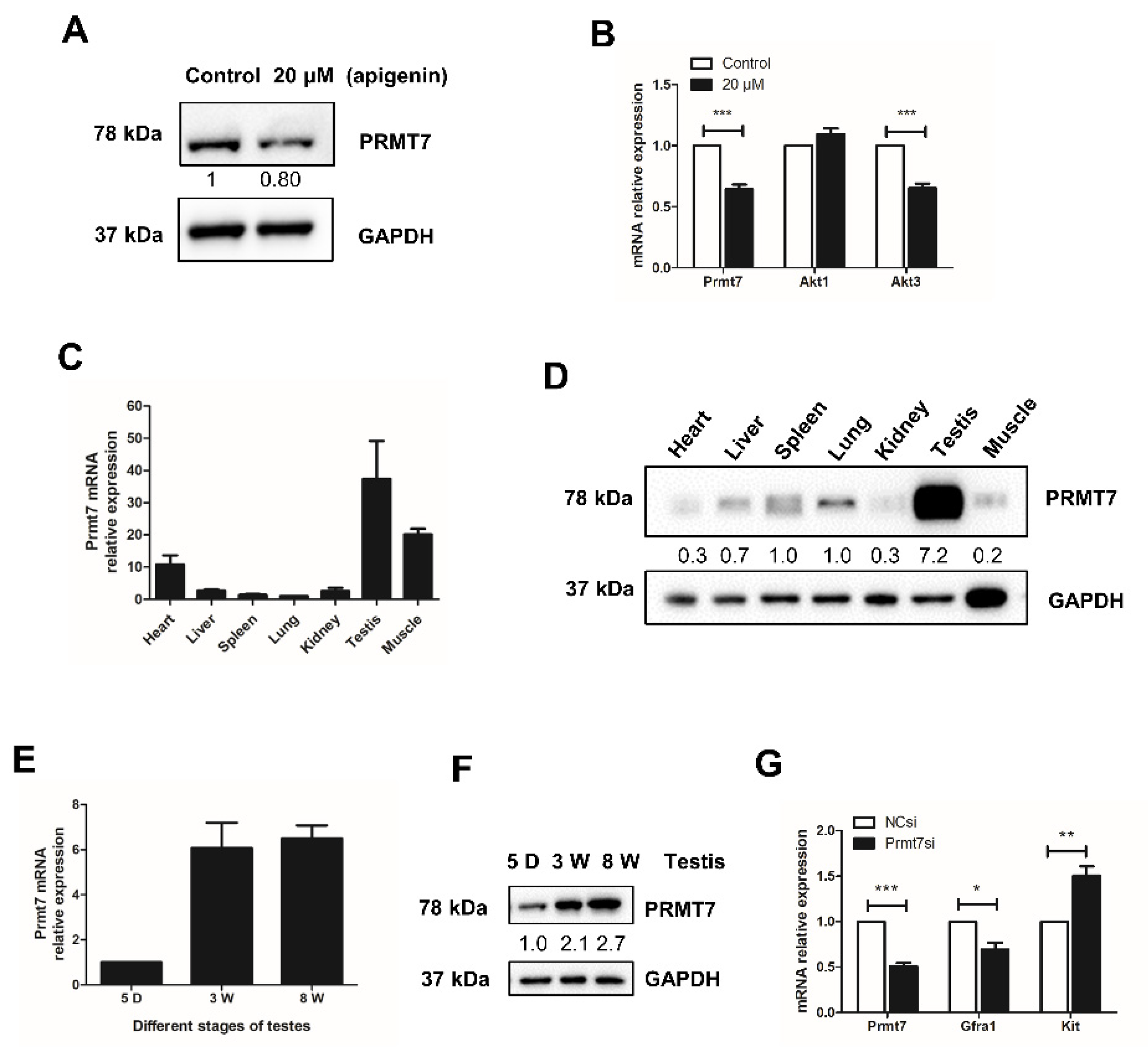
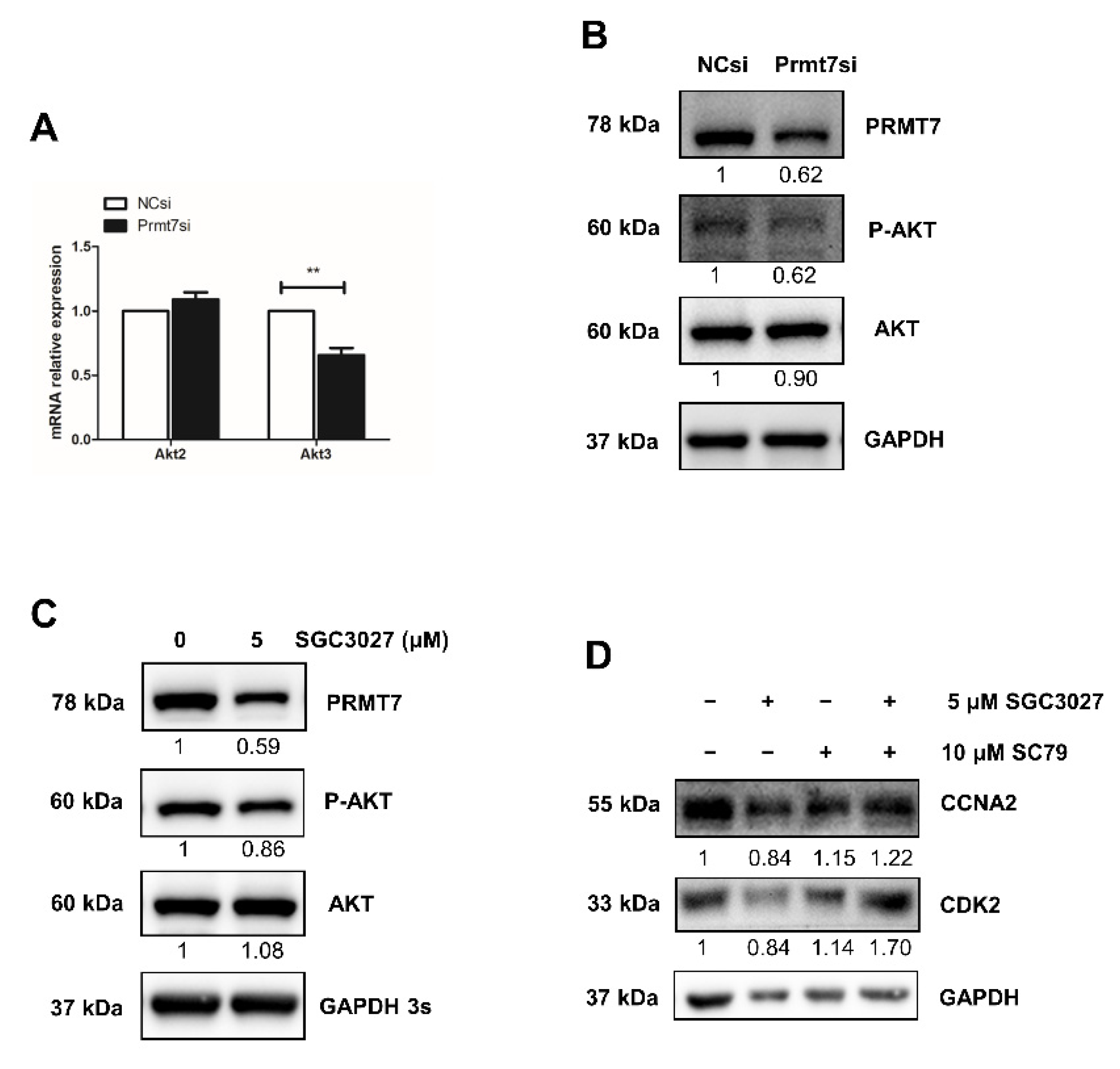
2.3. Apigenin Reduces the Expression of Prmt7 and Akt3 in Spermatogonia
2.4. Prmt7 Is Abundantly Expressed in Mouse Testis
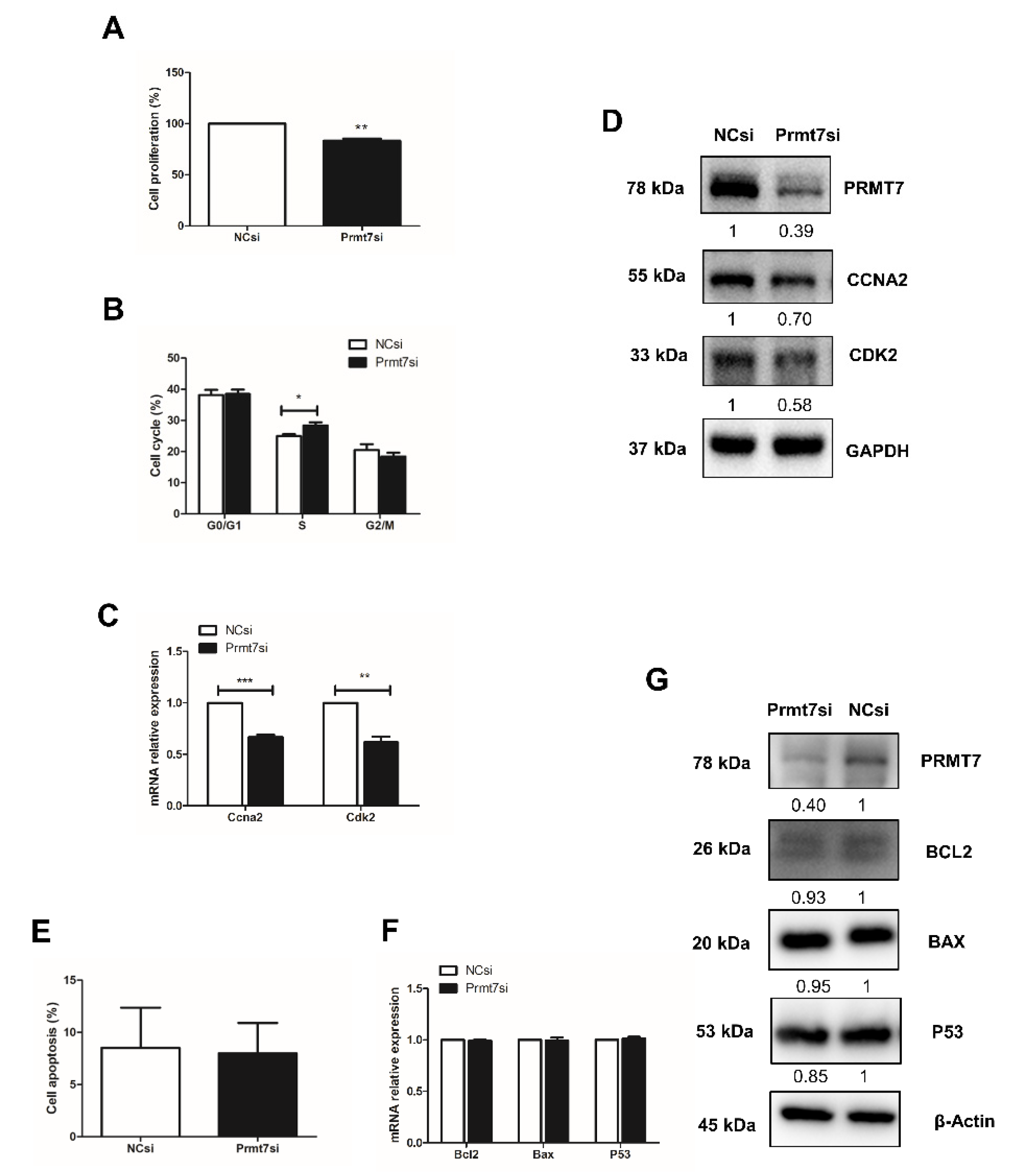
2.5. Prmt7 Knockdown Recapitulates the Inhibitory Effects of Apigenin on Spermatogonial Proliferation
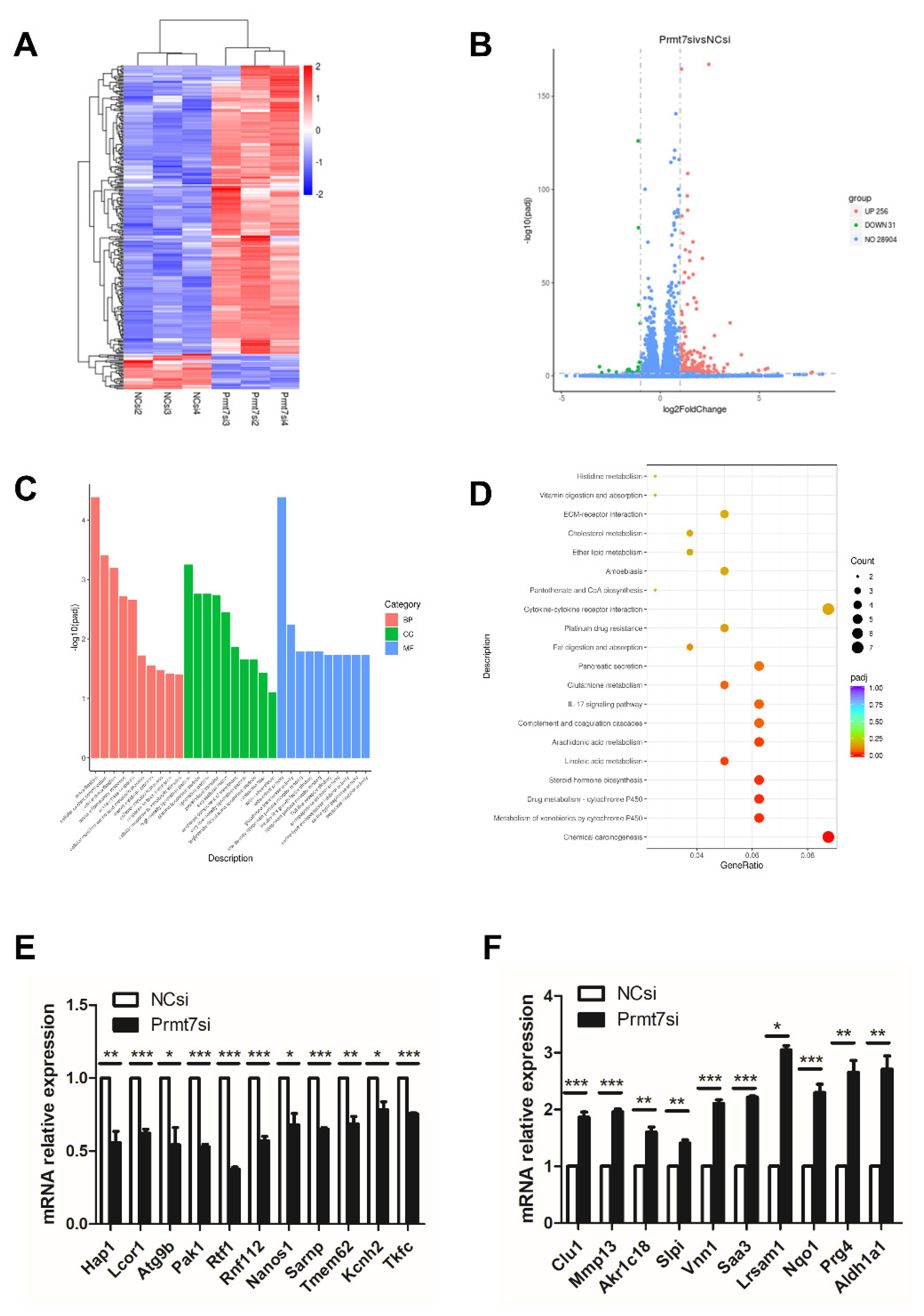
2.6. Downregulation of Prmt7 Expression Goes beyond This Gene and Perturbs Several Other Genes
2.7. The Possible Functional Pathway Underlying the Regulation of Spermatogonial Proliferation
3. Discussion
4. Materials and Methods
4.1. Animals and Chemicals
4.2. Cell Culture and Apigenin Treatment
4.3. Cell Proliferation Assay
4.4. siRNA Transfection
4.5. Cell Cycle Analysis by Flow Cytometry
4.6. Cell Apoptosis Analysis by Flow Cytometry
4.7. RNA Extraction and RT-qPCR
4.8. Western Blotting
4.9. Library Preparation for RNA Sequencing
4.10. RNA Sequencing Data Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Sengupta, P.; Borges, E., Jr.; Dutta, S.; Krajewska-Kulak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2018, 37, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Boulicault, M.; Perret, M.; Galka, J.; Borsa, A.; Gompers, A.; Reiches, M.; Richardson, S. The future of sperm: A biovariability framework for understanding global sperm count trends. Hum. Fertil. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Dang, Y.; Li, Z.; Wei, Q.; Zhang, R.; Xue, H.; Zhang, Y. Protective Effect of Apigenin on Acrylonitrile-Induced Inflammation and Apoptosis in Testicular Cells via the NF-κB Pathway in Rats. Inflammation 2018, 41, 1448–1459. [Google Scholar] [CrossRef]
- Dang, Y.; Li, Z.; Luo, B.; Pan, L.; Wei, Q.; Zhang, Y. Protective effects of apigenin against acrylonitrile-induced subchronic sperm injury in rats. Food Chem. Toxicol. 2017, 109, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Son, H.J.; Choi, Y.M.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin enhances skeletal muscle hypertrophy and myoblast differentiation by regulating Prmt7. Oncotarget 2017, 8, 78300–78311. [Google Scholar] [CrossRef] [Green Version]
- Fisk, J.C.; Sayegh, J.; Zurita-Lopez, C.; Menon, S.; Presnyak, V.; Clarke, S.G.; Read, L.K. A type III protein arginine methyltransferase from the protozoan parasite Trypanosoma brucei. J. Biol. Chem. 2009, 284, 11590–11600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, T.B.; Miranda, M.; Frankel, A.; Clarke, S. PRMT7 is a member of the protein arginine methyltransferase family with a distinct substrate specificity. J. Biol. Chem. 2004, 279, 22902–22907. [Google Scholar] [CrossRef] [Green Version]
- Blanc, R.S.; Vogel, G.; Chen, T.; Crist, C.; Richard, S. PRMT7 Preserves Satellite Cell Regenerative Capacity. Cell Rep. 2016, 14, 1528–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akawi, N.; McRae, J.; Ansari, M.; Balasubramanian, M.; Blyth, M.; Brady, A.F.; Clayton, S.; Cole, T.; Deshpande, C.; Fitzgerald, T.W.; et al. Discovery of four recessive developmental disorders using probabilistic genotype and phenotype matching among 4,125 families. Nat. Genet. 2015, 47, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Kernohan, K.D.; McBride, A.; Xi, Y.; Martin, N.; Schwartzentruber, J.; Dyment, D.A.; Majewski, J.; Blaser, S.; Boycott, K.M.; Chitayat, D. Loss of the arginine methyltranserase PRMT7 causes syndromic intellectual disability with microcephaly and brachydactyly. Clin. Genet. 2017, 91, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Agolini, E.; Dentici, M.L.; Bellacchio, E.; Alesi, V.; Radio, F.C.; Torella, A.; Musacchia, F.; Tartaglia, M.; Dallapiccola, B.; Nigro, V.; et al. Expanding the clinical and molecular spectrum of PRMT7 mutations: 3 additional patients and review. Clin. Genet. 2018, 93, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, R.; Yosha-Orpaz, N.; Yanoov-Sharav, M.; Kidron, D.; Gur, H.; Yosovich, K.; Lerman-Sagie, T.; Malinger, G.; Lev, D. Prenatal and postnatal presentation of PRMT7 related syndrome: Expanding the phenotypic manifestations. Am. J. Med. Genet. A 2019, 179, 78–84. [Google Scholar] [CrossRef]
- Valenzuela, I.; Segura-Puimedon, M.; Rodríguez-Santiago, B.; Fernández-Alvarez, P.; Vendrell, T.; Armengol, L.; Tizzano, E. Further delineation of the phenotype caused by loss of function mutations in PRMT7. Eur. J. Med. Genet. 2019, 62, 182–185. [Google Scholar] [CrossRef]
- Jelinic, P.; Stehle, J.C.; Shaw, P. The testis-specific factor CTCFL cooperates with the protein methyltransferase PRMT7 in H19 imprinting control region methylation. PLoS Biol. 2006, 4, e355. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Y.; Lin, L.; Dong, F.; Wu, H.; Bao, S.; Gao, F. PRMT7 is involved in regulation of germ cell proliferation during embryonic stage. Biochem. Biophys. Res. Commun. 2020, 533, 938–944. [Google Scholar] [CrossRef]
- Konishi, H.; Kuroda, S.; Tanaka, M.; Matsuzaki, H.; Ono, Y.; Kameyama, K.; Haga, T.; Kikkawa, U. Molecular cloning and characterization of a new member of the RAC protein kinase family: Association of the pleckstrin homology domain of three types of RAC protein kinase with protein kinase C subspecies and beta gamma subunits of G proteins. Biochem. Biophys. Res. Commun. 1995, 216, 526–534. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Chen, W.; Zhao, X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010, 103, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Strouch, M.J.; Milam, B.M.; Melstrom, L.G.; McGill, J.J.; Salabat, M.R.; Ujiki, M.B.; Ding, X.Z.; Bentrem, D.J. The flavonoid apigenin potentiates the growth inhibitory effects of gemcitabine and abrogates gemcitabine resistance in human pancreatic cancer cells. Pancreas 2009, 38, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Iizumi, Y.; Taniguchi, T.; Goi, W.; Miki, T.; Sakai, T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 2013, 8, e55922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Chunhua, L.; Donglan, L.; Xiuqiong, F.; Lihua, Z.; Qin, F.; Yawei, L.; Liang, Z.; Ge, W.; Linlin, J.; Ping, Z.; et al. Apigenin up-regulates transgelin and inhibits invasion and migration of colorectal cancer through decreased phosphorylation of AKT. J. Nutr. Biochem. 2013, 24, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wan, L.; Zou, H.; Pan, Z.; Zhou, W.; Lu, X. PRMT7 promotes the growth of renal cell carcinoma through modulating the β-catenin/C-MYC axis. Int. J. Biochem. Cell Biol. 2020, 120, 105686. [Google Scholar] [CrossRef]
- Baldwin, R.M.; Haghandish, N.; Daneshmand, M.; Amin, S.; Paris, G.; Falls, T.J.; Bell, J.C.; Islam, S.; Côté, J. Protein arginine methyltransferase 7 promotes breast cancer cell invasion through the induction of MMP9 expression. Oncotarget 2015, 6, 3013–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Jiang, J.; Hofmann, M.C.; Dym, M. Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol. Reprod. 2007, 77, 723–733. [Google Scholar] [CrossRef]
- Busada, J.T.; Chappell, V.A.; Niedenberger, B.A.; Kaye, E.P.; Keiper, B.D.; Hogarth, C.A.; Geyer, C.B. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev. Biol. 2015, 397, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakawa, T.; Yaman-Deveci, R.; Tomizawa, S.; Kamizato, Y.; Nakajima, K.; Sone, H.; Sato, Y.; Sharif, J.; Yamashita, A.; Takada-Horisawa, Y.; et al. An epigenetic switch is crucial for spermatogonia to exit the undifferentiated state toward a Kit-positive identity. Development 2013, 140, 3565–3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschopp, O.; Yang, Z.Z.; Brodbeck, D.; Dummler, B.A.; Hemmings-Mieszczak, M.; Watanabe, T.; Michaelis, T.; Frahm, J.; Hemmings, B.A. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 2005, 132, 2943–2954. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Huang, D.; Jiang, Z.; Luo, Y.; Norris, C.; Zhang, M.; Tian, X.; Tang, Y. Akt3 is responsible for the survival and proliferation of embryonic stem cells. Biol. Open 2017, 6, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Braun, R.E. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis. Development 2018, 145, dev151555. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, M.; Guo, J.; Liu, Z.; Zhou, R.; Guo, F.; Li, K.; Mu, Y. The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway. Int. J. Mol. Sci. 2021, 22, 12209. https://doi.org/10.3390/ijms222212209
Wang B, Zhang M, Guo J, Liu Z, Zhou R, Guo F, Li K, Mu Y. The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway. International Journal of Molecular Sciences. 2021; 22(22):12209. https://doi.org/10.3390/ijms222212209
Chicago/Turabian StyleWang, Bingyuan, Mingrui Zhang, Jiankang Guo, Zhiguo Liu, Rong Zhou, Fei Guo, Kui Li, and Yulian Mu. 2021. "The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway" International Journal of Molecular Sciences 22, no. 22: 12209. https://doi.org/10.3390/ijms222212209
APA StyleWang, B., Zhang, M., Guo, J., Liu, Z., Zhou, R., Guo, F., Li, K., & Mu, Y. (2021). The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway. International Journal of Molecular Sciences, 22(22), 12209. https://doi.org/10.3390/ijms222212209






