Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering
Abstract
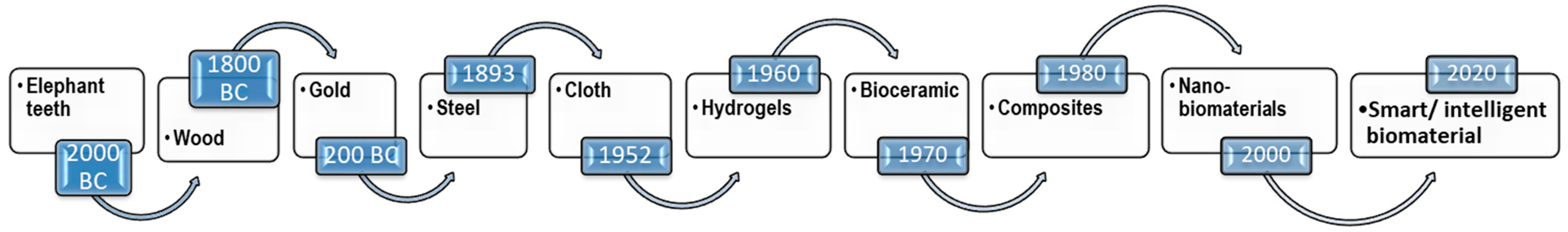
1. Introduction
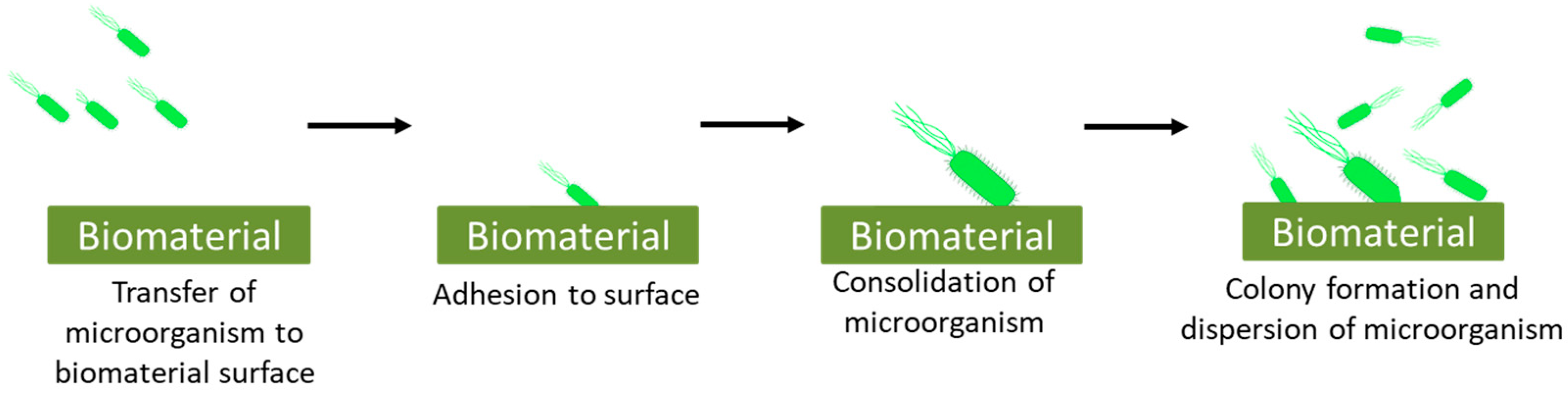
2. Microbial Interaction with Biomaterial: Biocontaminations in Biomaterials
- Transfer of microorganism:
- Adhesion:
- Consolidation:
- Colony formation:
Bacterial Infections
3. Host Tissue Reaction: Cellular Responses and Immune Response
3.1. Cellular Response of Surface Modified Biomaterial
3.2. Immune Response: Natural Instinctive Immune Reaction Following Implantation of Biomaterial
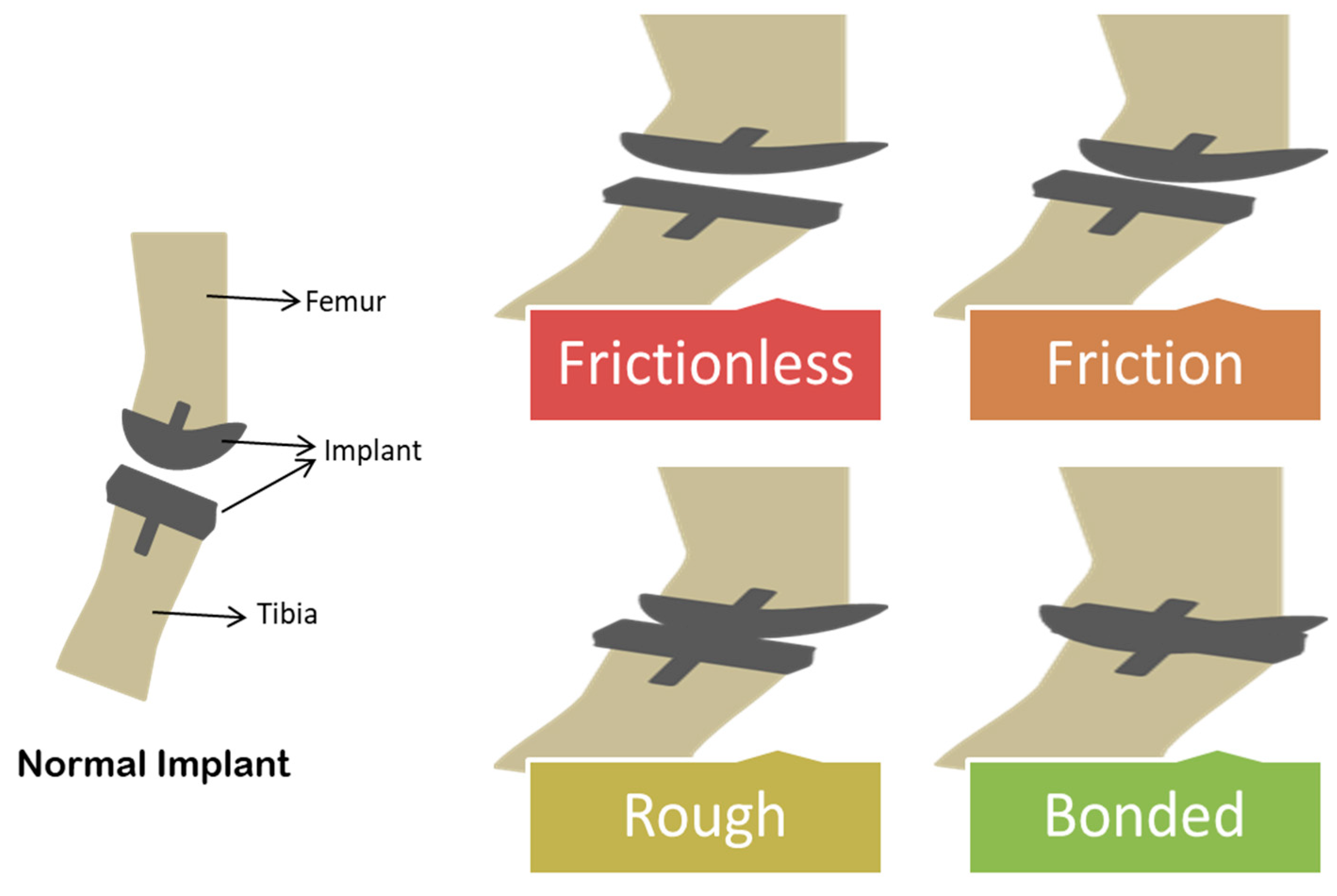
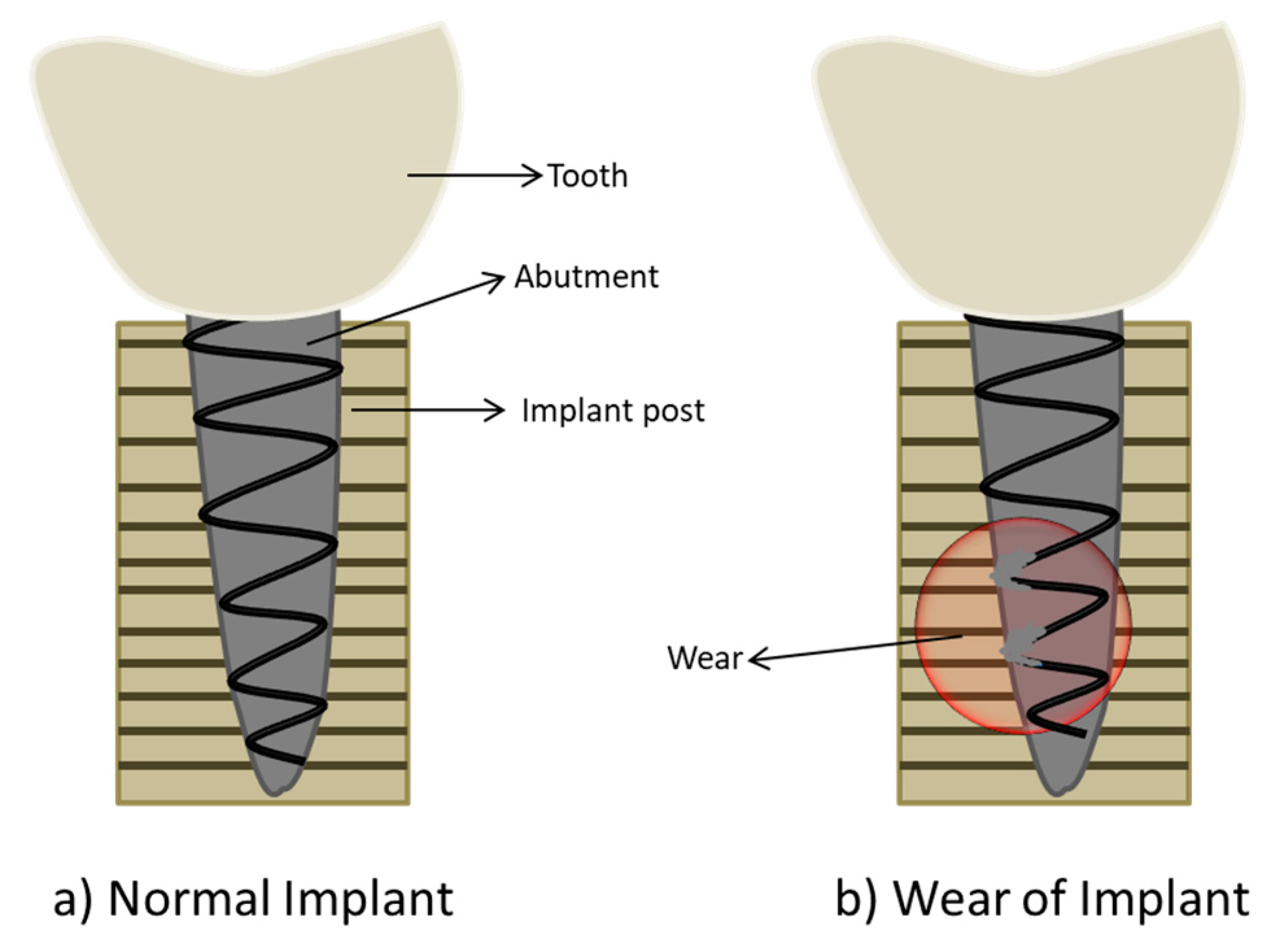
4. Implant Failure: Friction and Wear of Biomaterial Surface
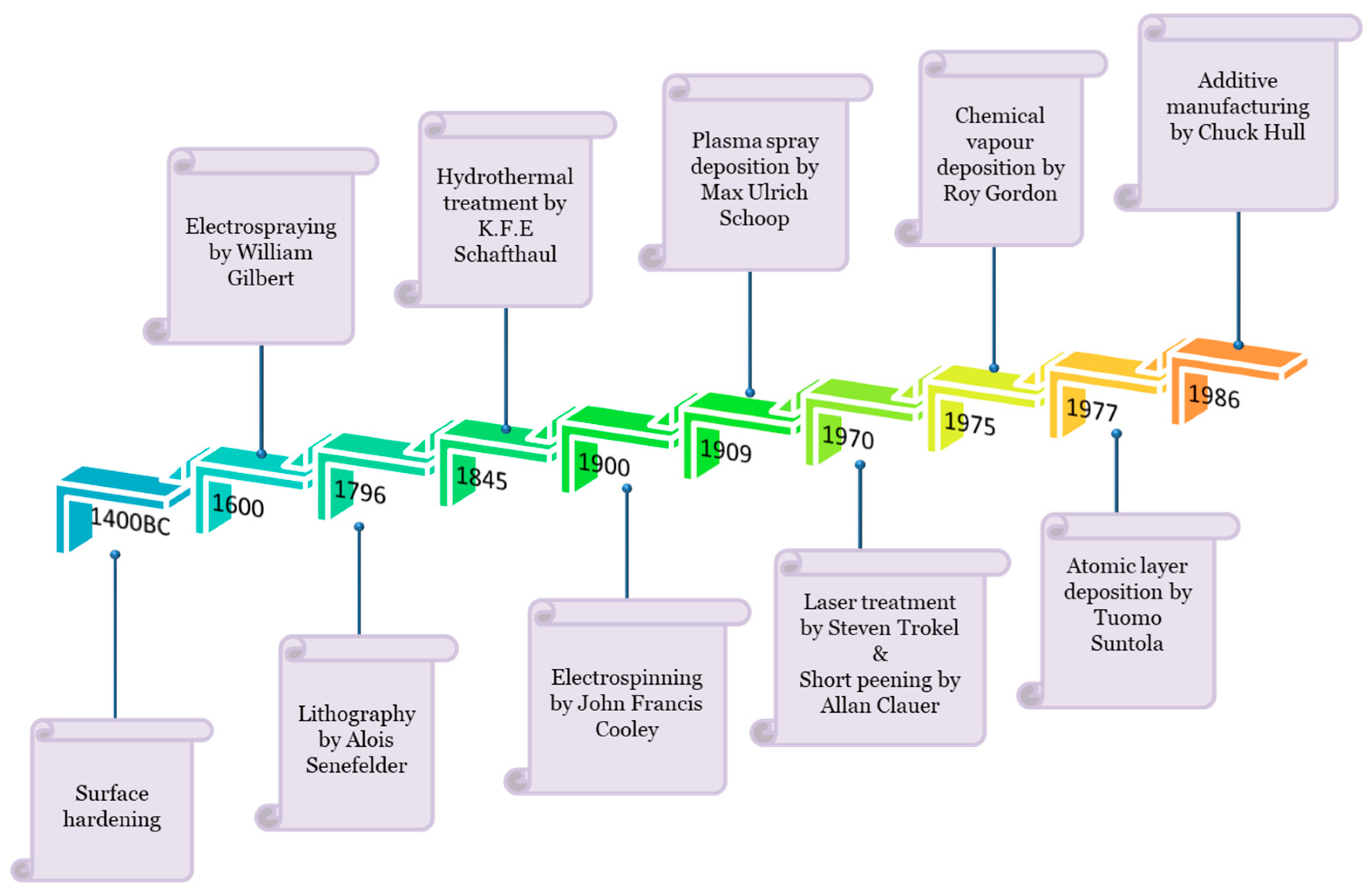
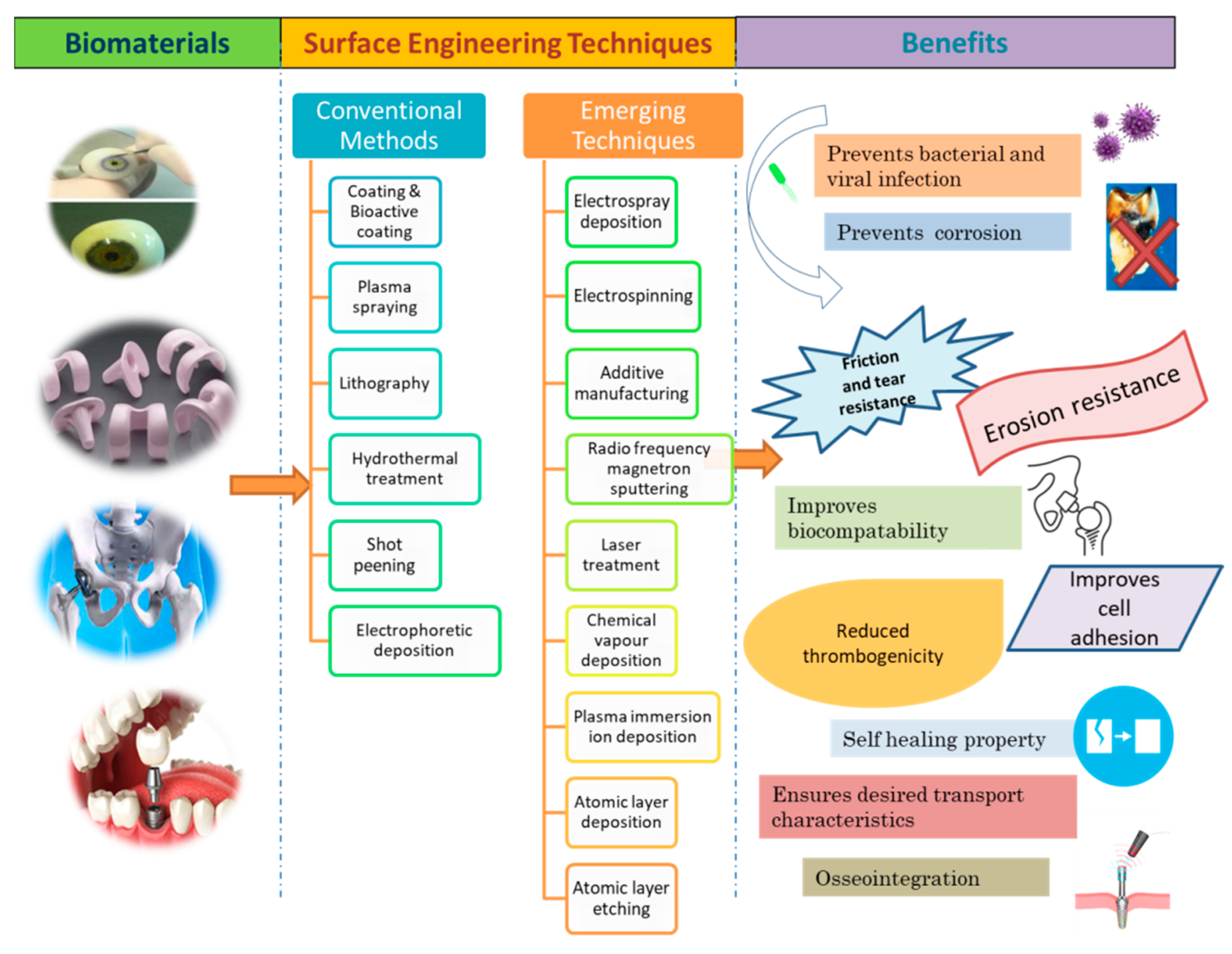
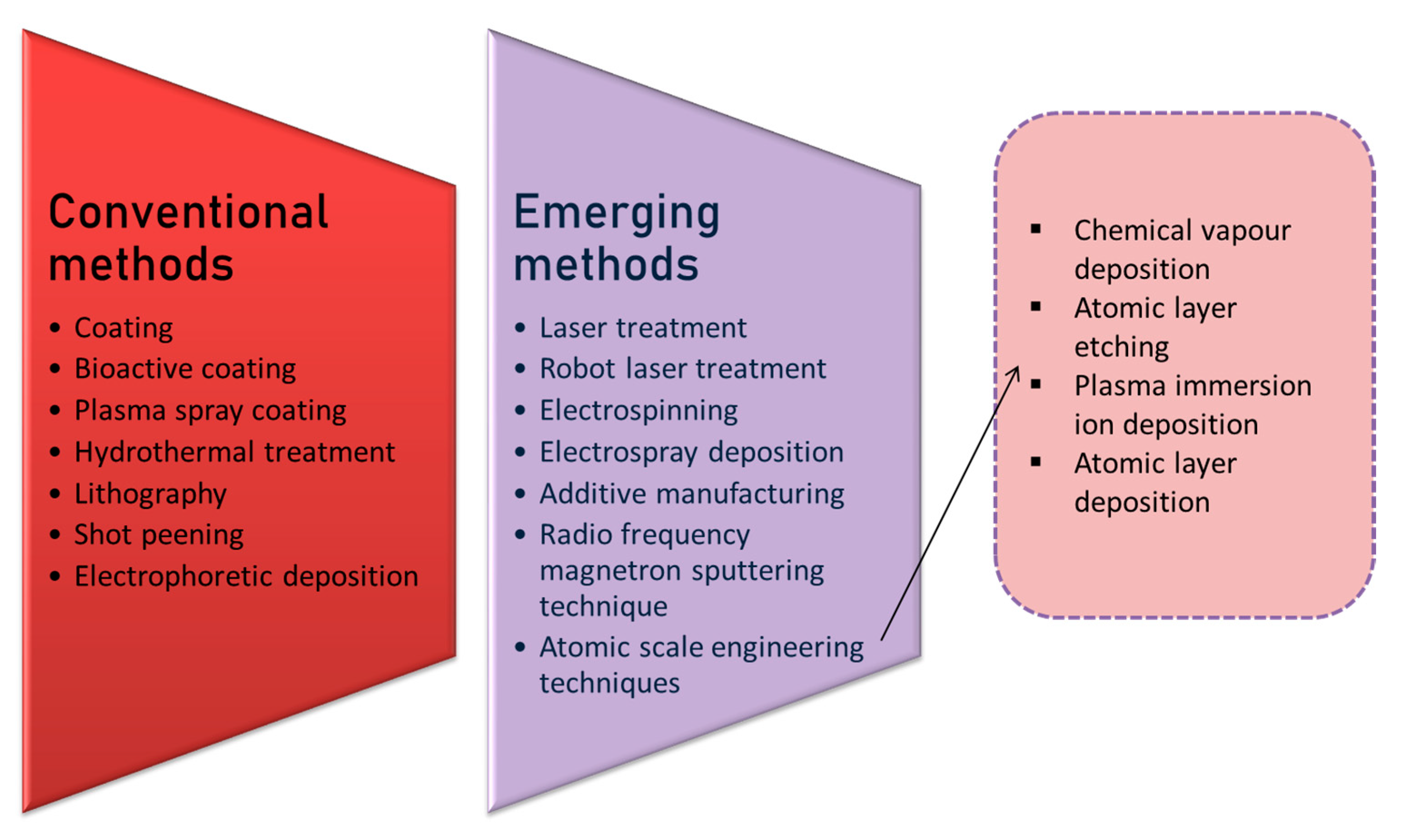
5. Surface Engineering Strategies
5.1. Conventional Surface Engineering Method
5.1.1. Coating
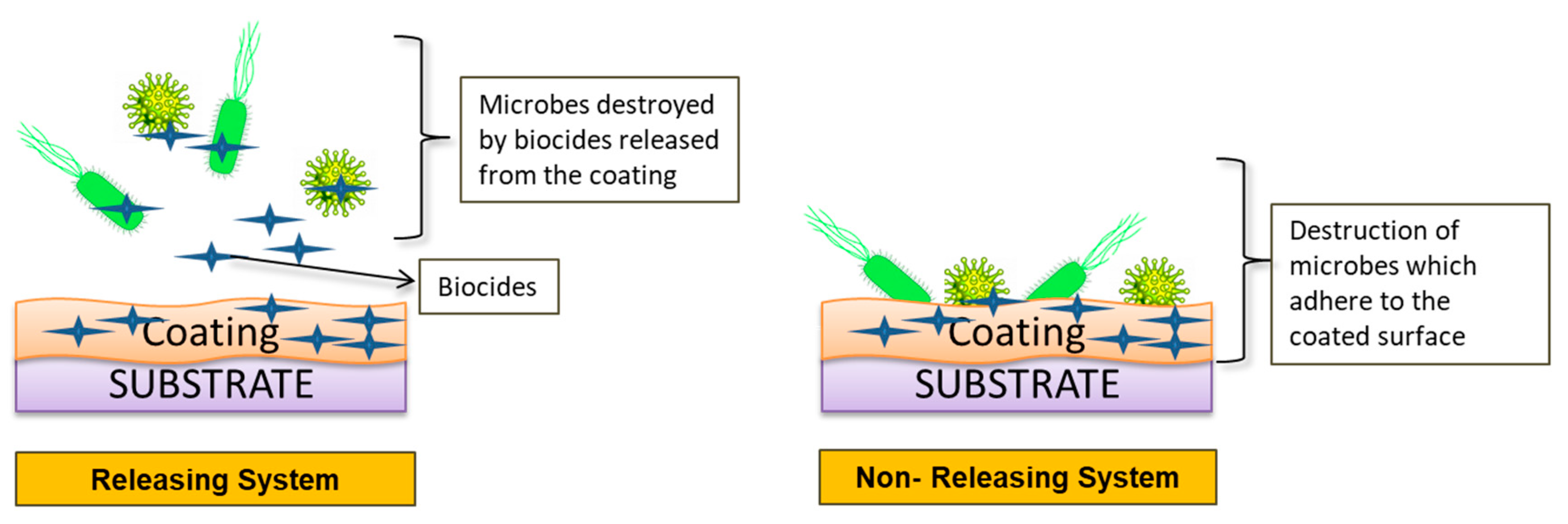
Bioactive Coating (Incorporation of Antimicrobial Agents to Prevent Biocontamination)
- Releasing system:
- Non-releasing system:
5.1.2. Plasma Spraying Coating
5.1.3. Lithography
5.1.4. Hydrothermal Treatment
5.1.5. Shot Peening
5.1.6. Electrophoretic Deposition
5.2. Emerging Surface Modification Techniques
5.2.1. Laser Treatment
5.2.2. Robot Laser Hardening
5.2.3. Electrospray
5.2.4. Radio Frequency Magnetron Sputtering Technique
5.2.5. Atomic Scale Engineering Techniques
Chemical Vapour Deposition
Atomic Layer Deposition
Plasma Immersion Ion Deposition
6. Future Scope of Surface Engineered Biomaterials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majumdar, J.D.; Manna, I. Laser surface engineering of titanium and its alloys for improved wear, corrosion and high-temperature oxidation resistance. In Laser Surface Engineering; Woodhead Publishing: Cambridge, UK, 2015; pp. 483–521. [Google Scholar]
- Hildebrand, H.F. Biomaterials–a history of 7000 years. BioNanoMaterials 2013, 14, 119–133. [Google Scholar] [CrossRef]
- Ratner, B.D.; Zhang, G. A history of biomaterials. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2020; pp. 21–34. [Google Scholar]
- Crubézy, E.; Murail, P.; Girard, L.; Bernadou, J.-P. False teeth of the Roman world. Nat. Cell Biol. 1998, 391, 29. [Google Scholar] [CrossRef]
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Huang, J.; Best, S.M. Ceramic biomaterials. In Tissue Engineering Using Ceramics and Polymers; Woodhead Publishing: Cambridge, UK, 2007; pp. 3–31. [Google Scholar]
- Ramakrishna, S.; Ramalingam, M.; Kumar, T.S.; Soboyejo, W.O. Biomaterials: A Nano Approach; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Srivastav, A. An Overview of Metallic Biomaterials for Bone Support and Replacement. Biomed. Eng. Trends Mater. Sci. 2011, 153–168. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khademhosseini, A. Make better, safer biomaterials. Nat. News 2016, 540, 335. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Lakes, R.S. Composites as biomaterials. Biomaterials 2007, 207–224. [Google Scholar] [CrossRef]
- Webber, M.; Appel, E.A.; Meijer, E.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2015, 15, 13–26. [Google Scholar] [CrossRef]
- Axpe, E.; Orive, G.; Franze, K.; Appel, E.A. Towards brain-tissue-like biomaterials. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Gu, L.; Mooney, D.J. Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment. Nat. Rev. Cancer 2015, 16, 56–66. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Pant, A.; Gupta, S.; Pant, V. Release and toxicity of dental resin composite. Toxicol. Int. Former. Indian J. Toxicol. 2012, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pittet, B.; Montandon, D.; Pittet, D. Infection in breast implants. Lancet Infect. Dis. 2005, 5, 94–106. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Yan, J.; Xiong, P.; Li, Q.; Cheng, Y.; Zheng, Y. Construction of Self-defensive Antibacterial and Osteogenic AgNPs/Gentamicin Coatings with Chitosan as Nanovalves for Controlled release. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A mini re-view. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Mayotte, J.; Bonner, M.; Holbrook, R.; Stromberg, K.; Sohail, M.R. Reduced bacterial adhesion with parylene coating: Potential implications for Micra transcatheter pacemakers. J. Cardiovasc. Electrophysiol. 2020, 31, 712–717. [Google Scholar] [CrossRef]
- Wu, B.; Jin, L.; Ding, K.; Zhou, Y.; Yang, L.; Lei, Y.; Guo, Y.; Wang, Y. Extracellular matrix coating improves the biocompatibility of polymeric heart valves. J. Mater. Chem. B 2020, 8, 10616–10629. [Google Scholar] [CrossRef]
- Pasculli, A.; Gurrado, A.; De Luca, G.M.; Mele, A.; Marzullo, A.; Mangone, A.; Cellamare, S.; Ferraro, V.; Maqoud, F.; Caggiani, M.C.; et al. Bridging repair of the abdominal wall in a rat experimental model. Comparison between uncoated and polyethylene oxide-coated equine pericardium meshes. Sci. Rep. 2020, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, J.Y.; Park, C.H. Fabrication of transparent hemispherical 3D nanofibrous scaffolds with radially aligned patterns via a novel electrospinning method. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Ishihara, K. Highly lubricated polymer interfaces for advanced artificial hip joints through biomimetic design. Polym. J. 2015, 47, 585–597. [Google Scholar] [CrossRef]
- Bi, P.; Liu, X.; Yang, Y.; Wang, Z.; Shi, J.; Liu, G.; Kong, F.; Zhu, B.; Xiong, R. Silver-Nanoparticle-Modified Polyimide for Multiple Artificial Skin-Sensing Applications. Adv. Mater. Technol. 2019, 4, 1900426. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Jiang, T.; Liang, Y.-D.; Zhang, Z.; Wang, Y.-N. Evaluation of the osseointegration of dental implants coated with calcium carbonate: An animal study. Int. J. Oral Sci. 2017, 9, 133–138. [Google Scholar] [CrossRef]
- Jiang, J.; Ai, C.; Zhan, Z.; Zhang, P.; Wan, F.; Chen, J.; Hao, W.; Wang, Y.; Yao, J.; Shao, Z.; et al. Enhanced fibroblast cellular liga-mentization process to polyethylene terepthalate artificial ligament by silk fibroin coating. Artif. Organs 2016, 40, 385–393. [Google Scholar] [CrossRef]
- Himmelfarb, J.; Ratner, B. Wearable artificial kidney: Problems, progress and prospects. Nat. Rev. Nephrol. 2020, 16, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Sein, H.; Jackson, M.; Rego, C.; Hassan, I.U.; Subramani, K. Surface engineering of dental tools with diamond for en-hanced life and performance. In Emerging Nanotechnologies in Dentistry; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 251–288. [Google Scholar]
- Berebichez-Fridman, R.; Montero-Olvera, P.; Gómez-García, R.; Berebichez-Fastlicht, E. An intramedullary nail coated with an-tibiotic and growth factor nanoparticles: An individualized state-of-the-art treatment for chronic osteomyelitis with bone defects. Med. Hypotheses 2017, 105, 63–68. [Google Scholar] [CrossRef]
- Monteiro, R.V.; Dos Santos, D.M.; Bernardon, J.K.; De Souza, G.M. Effect of surface treatment on the retention of zirconia crowns to tooth structure after aging. J. Esthet. Restor. Dent. 2020, 32, 699–706. [Google Scholar] [CrossRef]
- Bohl, A.; Eickner, T.; Petersen, S.; Schmitz, K.-P.; Sternberg, K. Specific Surface Modification of Cochlear Implant Electrode Carriers for Enhancement Of Spiral Ganglion Cell Growth. Biomed. Tech. Eng. 2013, 58 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Ardila, D.C.; Liou, J.J.; Maestas, D.; Slepian, M.J.; Badowski, M.; Wagner, W.R.; Harris, D.; Vande Geest, J.P. Surface modification of electrospun scaffolds for endothelialization of tissue-engineered vascular grafts using human cord blood-derived endothe-lial cells. J. Clin. Med. 2019, 8, 185. [Google Scholar] [CrossRef]
- Bandeira, A.M.; dos Santos, M.P.; Pulitini, G.; Elias, C.N.; da Costa, M.F. Influence of thermal or chemical degradation on the fric-tional force of an experimental coated NiTi wire. Angle Orthod. 2011, 81, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kwon, J.; Ahn, C.J.; Esmaeli, B.; Kim, G.B.; Kim, N.; Sa, H.-S. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018, 32, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-M.; Huang, C.-C.; Tsai, P.-I.; Yang, K.-Y.; Huang, S.-I.; Shen, H.-H.; Lai, H.-J.; Huang, S.-W.; Chen, S.-Y.; Lin, F.-H.; et al. Three-Dimensional Printed Porous Titanium Screw with Bioactive Surface Modification for Bone–Tendon Healing: A Rabbit Animal Model. Int. J. Mol. Sci. 2020, 21, 3628. [Google Scholar] [CrossRef]
- Cools, P.; Mota, C.; Lorenzo-Moldero, I.; Ghobeira, R.; De Geyter, N.; Moroni, L.; Morent, R. Acrylic acid plasma coated 3D scaf-folds for cartilage tissue engineering applications. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparti-cles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Samson, A.; Nicole, B.; Vargas, H.S.; Sushila, M.; Ruiz-Esparza, G.U.; de Paula Mirian, M.M.; Webster, T.J.; Roberta, T.C.; Cruz, V.B.; Dan-quan, W.; et al. Engineering multifunctional bactericidal nanofibers for abdominal hernia repair. Commun. Biol. 2021, 4, 1–4. [Google Scholar] [CrossRef]
- Sandle, T. Biocontamination Control for Pharmaceuticals and Healthcare; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Koubali, H.; Latrache, H.; Zahir, H.; Soufiani, S.; El Louali, M. Application of theoretical prediction to prevent the biocontamina-tion of medical materials. In Proceedings of the 2020 IEEE 6th International Conference on Optimization and Applications (ICOA), Beni Mellal, Morocco, 20–21 April 2020; IEEE: New York, NY, USA, 2020; pp. 1–5. [Google Scholar]
- Walker, B.; Amato, C.; Palyvoda, O.; Vangipuram, S.; Weaver, M.; Sayeed, Z.; Padela, M.T.; Yassir, W.K. Prevalence of Bacterial Contamination of Casting Material in a Pediatric Population. Int. J. Pediatr. 2020, 2020, 4717385-5. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Zou, S.; Dodd, R.Y.; Stramer, S.L.; Strong, D.M. Probability of Viremia with HBV, HCV, HIV, and HTLV among Tissue Donors in the United States. N. Engl. J. Med. 2004, 351, 751–759. [Google Scholar] [CrossRef]
- Trobos, M.; Johansson, M.L.; Jonhede, S.; Peters, H.; Hoffman, M.; Omar, O.; Thomsen, P.; Hultcrantz, M. The clinical outcome and microbiological profile of bone-anchored hearing systems (BAHS) with different abutment topographies: A prospective pi-lot study. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1395–1408. [Google Scholar] [CrossRef]
- Banche, G.; Bracco, P.; Bistolfi, A.; Allizond, V.; Boffano, M.; Costa, L.; Cimino, A.; Cuffini, A.M.; del Prever, E.M.B. Vitamin E blended Uhmwpe may have the potential to reduce bacterial adhesive ability. J. Orthop. Res. 2011, 29, 1662–1667. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Bracco, P.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach del Prever, E.M.; Cuffini, A.M. Interplay be-tween surface properties of standard, vitamin E blended and oxidised ultrahigh molecular weight polyethylene used in to-tal joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Jt. J. 2014, 96B, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Bracco, P.; Allizond, V.; Bistolfi, A.; Boffano, M.; Cimino, A.; Brach del Prever, E.M.; Cuffini, A.M. Do crosslink-ing and vitamin E stabilization influence microbial adhesions on UHMWPE-based biomaterials? Clin. Orthop. Relat. Res. 2015, 473, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.Y. Risk of Disease Transmission with Bone Allograft. Orthopedics 2012, 35, 679–681. [Google Scholar] [CrossRef]
- Cairns, L.S.; Marlow, V.L.; Bissett, E.; Ostrowski, A.; Stanley-Wall, N.R. A mechanical signal transmitted by the flagellum controls signalling in B acillus subtilis. Mol. Microbiol. 2013, 90, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Belas, R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014, 22, 517–527. [Google Scholar] [CrossRef]
- Ellison, C.K.; Kan, J.; Dillard, R.S.; Kysela, D.T.; Ducret, A.; Berne, C.; Hampton, C.M.; Ke, Z.; Wright, E.R.; Biais, N.; et al. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 2017, 358, 535–538. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef]
- Crawford, R.; Webb, H.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimi-crobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 26, 50969. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.; Truong, V.K. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J. Colloid Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers. mBio 2019, 10, e01908-19. [Google Scholar] [CrossRef]
- Viljoen, A.; Mignolet, J.; Viela, F.; Mathelié-Guinlet, M.; Dufrêne, Y.F. How microbes use force to control adhesion. J. Bac-Teriol. 2020, 202, e00125-20. [Google Scholar] [CrossRef]
- Kokare, C.R.; Chakraborty, S.; Khopade, A.N.; Mahadik, K.R. Biofilm: Importance and Applications. Indian J. Biotechnol. 2009, 8, 159–168. [Google Scholar]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Strategies for the prevention of microbial biofilm formation on silicone rubber voice prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 358–370. [Google Scholar] [CrossRef]
- Dohnt, K.; Sauer, M.; Müller, M.; Atallah, K.; Weidemann, M.; Gronemeyer, P.; Rasch, D.; Tielen, P.; Krull, R. An in vitro urinary tract catheter system to investigate biofilm development in catheter-associated urinary tract infections. J. Microbiol. Methods 2011, 87, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the Surface Nanoscale Roughness of Stainless Steel on Bacterial Adhesion and Microcolony Formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef]
- Straub, H.; Bigger, C.M.; Valentin, J.; Abt, D.; Qin, X.H.; Eberl, L.; Maniura-Weber, K.; Ren, Q. Bacterial adhesion on soft materials: Passive physicochemical interactions or active bacterial mechanosensing? Adv. Healthc. Mater. 2019, 8, 1801323. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Balani, K. Adhesion force of staphylococcus aureus on various biomaterial surfaces. J. Mech. Behav. Biomed. Mater. 2017, 65, 872–880. [Google Scholar] [CrossRef]
- Gigante, A.; Bottegoni, C.; Ragone, V.; Banci, L. Effectiveness of Vitamin-E-Doped Polyethylene in Joint Replacement: A Literature Review. J. Funct. Biomater. 2015, 6, 889–900. [Google Scholar] [CrossRef]
- Allion, A.; Herry, J.-M.; Bellon-Fontaine, M.-N. Material influence on biocontamination level and adhering cell physiology. MATEC Web Conf. 2013, 7, 04004. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Maki, D.G.; Graves, N. The International Nosocomial Infection Control Consortium (INICC): Goals and objec-tives, description of surveillance methods, and operational activities. Am. J. Infect. Control 2008, 36, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Persat, A. Bacterial mechanotransduction. Curr. Opin. Microbiol. 2017, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Tsoi, J.K.H.; Rodrigues, F.; Leprince, J.G.; Palin, W. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar] [CrossRef]
- Schuldt, L.; Bi, J.; Owen, G.; Shen, Y.; Haapasalo, M.; Häkkinen, L.; Larjava, H. Decontamination of rough implant surfaces colo-nized by multispecies oral biofilm by application of leukocyte-and platelet-rich fibrin. J. Periodontol. 2021, 92, 875–885. [Google Scholar] [CrossRef]
- Zare, M.; Zare, M.; Butler, J.A.; Ramakrishna, S. Nanoscience-Led Antimicrobial Surface Engineering to Prevent Infections. ACS Appl. Nano Mater. 2021, 4, 4269–4283. [Google Scholar] [CrossRef]
- Różalska, B.; Sadowska, B. Pet-To-Man Travelling Staphylococci; Academic Press: Cambridge, MA, USA, 2018; pp. 237–251. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, H.-C.F.J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Genet. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Rimondini, L.; Fini, M.; Giardino, R. The microbial infection of biomaterials: A challenge for clinicians and researchers. A short review. J. Appl. Biomater. Biomech. 2005, 3, 1–10. [Google Scholar] [PubMed]
- Singh, A.; Tiwari, A.; Bajpai, J.; Bajpai, A.K. Biomaterials: From Action to Application. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2017; p. 27. [Google Scholar]
- Zhou, Y.; Xiao, Y.; Qiu, Y.; Yuan, H.; Van Blitterswijk, C.A.; Zhou, X.; Xu, X.; Bao, C. Adhesion and proliferation of cells and bacteria on microchip with different surfaces microstructures. Biomed. Tech. Eng. 2015, 61, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Parreira, P.; Magalhães, A.; Gonçalves, I.C.; Gomes, J.; Vidal, R.; Reis, C.A.; Leckband, D.E.; Martins, M.C.L. Effect of surface chemistry on bacterial adhesion, viability, and morphology. J. Biomed. Mater. Res. Part A 2011, 99A, 344–353. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive man-ufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Arjunan, A.; Robinson, J.; Al Ani, E.; Heaselgrave, W.; Baroutaji, A.; Wang, C. Mechanical performance of additively manufactured pure silver antibacterial bone scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 112, 104090. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, S.; Xie, Y.-Y. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Langer, R. New Challenges in Biomaterials. Science 1994, 263, 1715–1720. [Google Scholar] [CrossRef]
- Biswal, T.; BadJena, S.K.; Pradhan, D. Sustainable biomaterials and their applications: A short review. Mater. Today Proc. 2020, 30, 274–282. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterialecell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1e18. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Review: Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. Contact Activation: A Revision. Thromb. Haemost. 1997, 78, 101–107. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef]
- Zhuo, R.; Siedlecki, C.A.; Vogler, E.A. Competitive-protein adsorption in contact activation of blood factor XII. Biomaterials 2007, 28, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Babensee, J.E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cameron, A.; Kelly, D.; Kearney, C.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, M.R.; Arabbeiki, M. Implant stability in different implantation stages: Analysis of various interface conditions. Inform. Med. Unlocked 2020, 19, 100317. [Google Scholar] [CrossRef]
- Damm, P.; Bender, A.; Duda, G.; Bergmann, G. In vivo measured joint friction in hip implants during walking after a short rest. PLoS ONE 2017, 12, e0174788. [Google Scholar] [CrossRef] [PubMed]
- Al Jabbari, Y.; Fournelle, R.; Ziebert, G.; Toth, J.; Iacopino, A. Mechanical Behavior and Failure Analysis of Prosthetic Retaining Screws after Long-Term Use In Vivo. Part 2: Metallurgical and Microhardness Analysis. J. Prosthodont. 2008, 17, 181–191. [Google Scholar] [CrossRef]
- Kumar, R.M.; Gupta, P.; Sharma, S.K.; Mittal, A.; Shekhar, M.; Kumar, V.; Kumar, B.M.; Roy, P.; Lahiri, D. Sustained drug release from surface modified UHMWPE for acetabular cup lining in total hip implant. Mater. Sci. Eng. C 2017, 77, 649–661. [Google Scholar] [CrossRef]
- Ranuša, M.; Wimmer, M.; Fullam, S.; Vrbka, M.; Křupka, I. Analysis of Friction in Total Knee Prosthesis during a Standard Gait Cycle. Lubricants 2021, 9, 36. [Google Scholar] [CrossRef]
- Memtsoudis, S.G.; Besculides, M.C.; Gaber, L.; Liu, S.; Della Valle, A.G. Risk factors for pulmonary embolism after hip and knee arthroplasty: A population-based study. Int. Orthop. 2008, 33, 1739–1745. [Google Scholar] [CrossRef]
- Rasool, G.; El Shafei, Y.; Stack, M. Mapping Tribo-Corrosion Behaviour of TI-6AL-4V Eli in Laboratory Simulated Hip Joint Environments. Lubricants 2020, 8, 69. [Google Scholar] [CrossRef]
- Wos, S.; Koszela, W.; Pawlus, P. Selected Methods and Applications of Anti-Friction and Anti-Wear Surface Texturing. Materials 2021, 14, 3227. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Qin, G.; Duan, J.; Wang, H. A graded nano-TiN coating on biomedical Ti alloy: Low friction coefficient, good bonding and biocompatibility. Mater. Sci. Eng. C 2017, 71, 520–528. [Google Scholar] [CrossRef]
- Dorner-Reisel, A.; Schürer, C.; Svoboda, S. Harsh Sliding Wear of a Zirconia Ball against a-C:H Coated CoCrMo Disc in Hyaluronic Gel. Lubricants 2020, 8, 35. [Google Scholar] [CrossRef]
- Fullam, S.; He, J.; Scholl, C.S.; Schmid, T.M.; Wimmer, M.A. Competitive Binding of Bilirubin and Fatty Acid on Serum Albumin Affects Wear of UHMWPE. Lubricants 2020, 8, 53. [Google Scholar] [CrossRef]
- Swift, K.; Booker, J. Manufacturing Process Selection Handbook; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Rashidi, H.; Yang, J.; Shakesheff, K.M. Surface engineering of synthetic polymer materials for tissue engineering and regenera-tive medicine applications. Biomater. Sci. 2014, 2, 1318–1331. [Google Scholar] [CrossRef]
- Abela, S. Physical vapour deposition on Mg alloys for biomedical applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 81–100. [Google Scholar]
- Bollino, F.; Catauro, M. Sol-Gel Technology to Prepare Advanced Coatings. In Photoenergy and Thin Film Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 321–378. [Google Scholar]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Kannan, M.B. Biodegradable polymeric coatings for surface modification of magnesium-based biomaterials. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 355–376. [Google Scholar] [CrossRef]
- Asmatulu, R. Nanocoatings for corrosion protection of aerospace alloys. In Corrosion Protection and Control Using Nanomaterials; Woodhead Publishing: Cambridge, UK, 2012; pp. 357–374. [Google Scholar]
- Prodana, M.; Stoian, A.B.; Burnei, C.; Ionita, D. Innovative Coatings of Metallic Alloys Used as Bioactive Surfaces in Implantology: A Review. Coatings 2021, 11, 649. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Bin Sahari, B.; Edwards, K.; Farahmand, F.; Arumugam, M. Aseptic loosening of femoral components—Materials engineering and design considerations. Mater. Des. 2012, 44, 155–163. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Goldschmidt, G.-M.; Krok-Borkowicz, M.; Zybała, R.; Pamuła, E.; Telle, R.; Conrads, G.; Schickle, K. Biomimetic in situ precipitation of calcium phosphate containing silver nanoparticles on zirconia ceramic materials for surface functionalization in terms of antimicrobial and osteoconductive properties. Dent. Mater. 2020, 37, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Zhao, L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Slate, A.J.; Wickens, D.J.; El Mohtadi, M.; Dempsey-Hibbert, N.; West, G.; Banks, C.E.; Whitehead, K.A. Antimicrobial activity of Ti-ZrN/Ag coatings for use in biomaterial applications. Sci. Rep. 2018, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Baek, S.M.; Polyakov, A.; Semenova, I.P.; Valiev, R.Z.; Hwang, W.-B.; Hahn, S.K.; Kim, H.S. Molybdenum Disulfide Surface Modification of Ultrafine-Grained Titanium for Enhanced Cellular Growth and Antibacterial Effect. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and Characterization of Superhydrophobic and Antibacterial Coated Fabrics Utilizing ZnO Nanocatalyst. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron ox-ide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pipattanachat, S.; Qin, J.; Rokaya, D.; Thanyasrisung, P.; Srimaneepong, V. Biofilm inhibition and bactericidal activity of NiTi alloy coated with graphene oxide/silver nanoparticles via electrophoretic deposition. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Hassanzadeh-Tabrizi, S.A.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electropho-retic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coat. Technol. 2017, 321, 171–179. [Google Scholar] [CrossRef]
- Webber, J.L.; Namivandi-Zangeneh, R.; Drozdek, S.; Wilk, K.A.; Boyer, C.; Wong, E.H.H.; Bradshaw-Hajek, B.H.; Krasowska, M.; Beattie, D.A. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sousa, D.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Burnett-Boothroyd, S.; McCarthy, B. Antimicrobial treatments of textiles for hygiene and infection control applications: An industrial perspective. In Textiles for Hygiene and Infection Control; Woodhead Publishing: Cambridge, UK, 2011; pp. 196–209. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Loozen, L.; Paganelli, F.L.; Bakhshandeh, S.; Lietaert, K.; Groot, J.A.; Fluit, A.C.; Boel, C.H.; Alblas, J.; Vogely, H.C.; et al. Antibacterial behavior of additively manufactured porous titanium with nanotubular surfaces releasing silver ions. ACS Appl. Mater. Interfaces 2016, 8, 17080–17089. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacte-rial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are medi-ated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Elbourne, A.; Coyle, V.E.; Truong, V.K.; Sabri, Y.M.; Kandjani, A.E.; Bhargava, S.K.; Ivanova, E.P.; Crawford, R.J. Multi-directional elec-trodeposited gold nanospikes for antibacterial surface applications. Nanoscale Adv. 2019, 1, 203–212. [Google Scholar] [CrossRef]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2015, 18, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Rainsford, K.D.; Powanda, M.C.; Whitehouse, M.W. Preface. Novel Natural Products: Therapeutic Effects in Pain Arthritis and Gastro-intestinal Diseases. Prog. Drug Res. Fortschr. Arzneimittelforschung. Prog. Rech. Pharm. 2015, 70, 200–215. [Google Scholar]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Stepnova, E.A.; Tikhonov, V.E.; Babushkina, T.A.; Klimova, T.P.; Vorontsov, E.V.; Babak, V.G.; Lopatin, S.A.; Yamskov, I.A. New approach to the quaternization of chitosan and its amphiphilic derivatives. Eur. Polym. J. 2007, 43, 2414–2421. [Google Scholar] [CrossRef]
- Barros, J.; Dias, A.; Rodrigues, M.A.; Pina-Vaz, C.; Lopes, M.A.; Pina-Vaz, I. Antibiofilm and Antimicrobial Activity of Polyethyl-enimine: An Interesting Compound for Endodontic Treatment. J. Contemp. Dent. Pract. 2015, 16, 427–432. [Google Scholar]
- Leyland, N.S.; Podporska-Carroll, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly Efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef]
- Makhlouf, A. Current and advanced coating technologies for industrial applications. In Nanocoatings and Ultra-Thin Films; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–23. [Google Scholar]
- Fauchais, P.; Vardelle, A.; Dussoubs, B. Quo vadis thermal spraying? J. Therm. Spray Technol. 2001, 10, 44–66. [Google Scholar] [CrossRef]
- Pillai, R.S.; Frasnelli, M.; Sglavo, V.M. HA/β-TCP plasma sprayed coatings on Ti substrate for biomedical applications. Ceram. Int. 2018, 44, 1328–1333. [Google Scholar] [CrossRef]
- Liang, R.; Xu, Y.; Zhao, M.; Han, G.; Li, J.; Wu, W.; Dong, M.; Yang, J.; Liu, Y. Properties of silver contained coatings on CoCr alloys prepared by vacuum plasma spraying. Mater. Sci. Eng. C 2019, 106, 110156. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.; Yusof, N.M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Mardanikivi, T. Deposition of duplex MAO layer/nanostructured titanium dioxide composite coatings on Mg–1% Ca alloy using a combined technique of air plasma spraying and micro arc oxidation. J. Alloys Compd. 2015, 649, 591–605. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Comprehensive Review of Bioactive Glass Coatings: State of the Art, Challenges and Future Perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Gowan, A.; Meggs, P.B.; Ashwin, C. A History of Graphic Design. Des. Issues 1984, 1, 87. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W. Fabrication of PEG Hydrogel Micropatterns by Soft-Photolithography and PEG Hydrogel as Guided Bone Regeneration Membrane in Dental Implantology. In Emerging Nanotechnologies in Dentistry; William Andrew Publishing: Norwich, NY, USA, 2012; pp. 171–187. [Google Scholar]
- Tran, K.T.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Arango-Santander, S.; Pelaez-Vargas, A.; Freitas, S.C.; García, C. A novel approach to create an antibacterial surface using tita-nium dioxide and a combination of dip-pen nanolithography and soft lithography. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Xu, Q.-T.; Li, J.-C.; Xue, H.-G.; Guo, S.-P. Binary iron sulfides as anode materials for rechargeable batteries: Crystal structures, syntheses, and electrochemical performance. J. Power Source 2018, 379, 41–52. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified bio-material surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 1–6. [Google Scholar]
- Zuldesmi, M.; Waki, A.; Kuroda, K.; Okido, M. High Osteoconductive Surface of Pure Titanium by Hydrothermal Treatment. J. Biomater. Nanobiotechnol. 2013, 4, 284–290. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, X. Hydrothermal Synthesis of Hydroxyapatite Assisted by Gemini Cationic Surfactant. J. Nanomater. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Kirk, D. Shot peening. Aircr. Eng. Aerosp. Technol. 1999, 71, 349–361. [Google Scholar] [CrossRef]
- Nino-Barrera, J.; Sanchez-Aleman, J.; Acosta-Humanez, M.; Gamboa-Martinez, L.; Cortes-Rodriguez, C. Shot peening increases resistance to cyclic fatigue fracture of endodontic files. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Verdian, M. 3.13 Finishing and Post-Treatment of Thermal Spray Coatings; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–206. [Google Scholar] [CrossRef]
- Irizalp, S.G.; Saklakoglu, N. 1.14 Laser Peening of Metallic Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 408–440. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kim, D.M.; Lee, C.; Da Silva, J.; Nagai, S.; Nojiri, T.; Nagai, M. Biological efficacy of perpendicular type-I collagen pro-truded from TiO 2-nanotubes. Int. J. Oral Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Bensebaa, F. Nanoparticle fundamentals. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 19, pp. 1–84. [Google Scholar]
- Char, D.S.; Shah, N.H.; Magnus, D. Implementing Machine Learning in Health Care—Addressing Ethical Challenges. N. Engl. J. Med. 2018, 378, 981–983. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Raee, M.J.; Manafi, Z.; Jahromi, A.S.; Ghasemi, Y. Ancient and Novel Forms of Silver in Medicine and Biomedicine. J. Adv. Med Sci. Appl. Technol. 2016, 2, 122–128. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Nene, A.G.; Galluzzi, M.; Luo, H.; Somani, P.; Ramakrishna, S.; Yu, X.F. Synthetic preparations and atomic scale engineering of sil-ver nanoparticles for biomedical applications. Nanoscale 2021, 13, 13923–13942. [Google Scholar] [CrossRef]
- Abi-Gerges, A.; Fischmeister, R. Ion Channels: New Tools to Track Cyclic Nucleotide Changes in Living Cells. Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bandyopadhyay, A.; Balla, V.K.; Roy, M.; Bose, S. Laser surface modification of metallic biomaterials. JOM 2011, 63, 94–99. [Google Scholar] [CrossRef]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2019, 124, 105973. [Google Scholar] [CrossRef]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Babić, M.; Balic, J.; Milfelner, M.; Belič, I.; Kokol, P.; Zorman, M.; Panjan, P. Robot laser hardening and the problem of overlapping laser beam. Adv. Prod. Eng. Manag. 2013, 8, 25–32. [Google Scholar] [CrossRef]
- Narayan, R.; Goering, P. Laser micro- and nanofabrication of biomaterials. MRS Bull. 2011, 36, 973–982. [Google Scholar] [CrossRef][Green Version]
- Babič, M. Modeling surface roughness of point robot laser hardening, with emphasis on the surface. Politehnika 2021, 5, 6–9. [Google Scholar] [CrossRef]
- Avilez, H.R.; Casadiego, D.C.; Avila, A.V.; Perez, O.P.; Almodovar, J. Production of chitosan coatings on metal and ceramic bio-materials. In Chitosan Based Biomaterials; Woodhead Publishing: Cambridge, UK, 2017; Volume 1, pp. 255–293. [Google Scholar]
- Lei, L.; Gamboa, A.R.; Kuznetsova, C.; Littlecreek, S.; Wang, J.; Zou, Q.; Zahn, J.D.; Singer, J.P. Self-limiting electrospray deposition on polymer templates. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-Y.; Kim, K.-M.; Kwon, J.-S. Antibacterial and Osteogenic Activity of Titania Nanotubes Modified with Electrospray-Deposited Tetracycline Nanoparticles. Nanomaterials 2020, 10, 1093. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antibacterial surfaces prepared by electrospray coating of photocatalytic na-noparticles. Chem. Eng. J. 2018, 334, 1108–1118. [Google Scholar] [CrossRef]
- Buga, C.; Chen, C.-C.; Hunyadi, M.; Csík, A.; Hegedűs, C.; Ding, S.-J. Electrosprayed calcium silicate nanoparticle-coated titanium implant with improved antibacterial activity and osteogenesis. Colloids Surf. B Biointerfaces 2021, 202, 111699. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, X.; Yin, H.; Wang, L.; Pei, Y.; Lv, X. Controlled release of vancomycin hydrochloride from a composite structure of pol-ymeric films and porous fibers on implants. Chem. Eng. J. 2017, 325, 601–610. [Google Scholar] [CrossRef]
- Mutlu, E.C.; Yıldırım, A.B.; Yıldırım, M.; Ficai, A.; Ficai, D.; Oktar, F.N.; Ţîţu, M.; Çetinkaya, A.; Demir, A. Improvement of antibacteri-al and biocompatibility properties of electrospray biopolymer films by ZnO and MCM-41. Polym. Bull. 2020, 77, 3657–3675. [Google Scholar] [CrossRef]
- Jaworek, A. Electrohydrodynamic microencapsulation technology. Encapsulations 2016, 1–45. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Sridhar, R.; Ramakrishna, S. Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter 2013, 3, e24281. [Google Scholar] [CrossRef]
- Surmenev, R.; Vladescu, A.; Surmeneva, M.; Ivanova, M.B.A.; Grubova, I.; Cotrut, C.M. Radio Frequency Magnetron Sputter Deposition as a Tool for Surface Modification of Medical Implants. In Modern Technologies for Creating the Thin-Film Systems and Coatings; InTech: London, UK, 2017; pp. 213–248. [Google Scholar]
- Hassan, M.M. Antimicrobial Coatings for Textiles. In Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2018; pp. 321–355. [Google Scholar] [CrossRef]
- Hao, J.; Li, Y.; Liao, R.; Liu, G.; Liao, Q.; Tang, C. Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect. Polymers 2017, 9, 502. [Google Scholar] [CrossRef]
- Piedade, A.; Pinho, A.C.; Branco, R.; Morais, P. Evaluation of antimicrobial activity of ZnO based nanocomposites for the coating of non-critical equipment in medical-care facilities. Appl. Surf. Sci. 2020, 513, 145818. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical va-pour deposition. Nat. Rev. Methods Primers 2021, 1, 1–20. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition: Principles, Technology and Applications; William Andrew Publishing: Norwich, NY, USA, 1999. [Google Scholar]
- Sanders, S.; Stümmler, D.; Pfeiffer, P.; Ackermann, N.; Simkus, G.; Heuken, M.; Baumann, P.K.; Vescan, A.; Kalisch, H. Chemical Vapor Deposition of Organic-Inorganic Bismuth-Based Perovskite Films for Solar Cell Application. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.M.; Sathasivam, S.; Nair, S.P.; Parkin, I.P. Antibacterial properties of Cu–ZrO2thin films prepared via aerosol assisted chemical vapour deposition. J. Mater. Chem. B 2015, 4, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Hu, Y.; Song, Q.; Ye, Y.; Gao, L.; Li, P.; Ye, T. Initiated chemical vapor deposition of graded polymer coatings enabling anti-bacterial, antifouling, and biocompatible surfaces. ACS Appl. Mater. Interfaces 2020, 12, 18978–18986. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cao, Y.; Wu, D.; Li, A. Thermal atomic layer etching: Mechanism, materials and prospects. Prog. Nat. Sci. Mater. Int. 2018, 28, 667–675. [Google Scholar] [CrossRef]
- Antoun, G.; Tillocher, T.; Lefaucheux, P.; Faguet, J.; Maekawa, K.; Dussart, R. Mechanism understanding in cryo atomic layer etching of SiO2 based upon C4F8 physisorption. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef]
- Gu, M.; Lv, L.; Du, F.; Niu, T.; Chen, T.; Xia, D.; Wang, S.; Zhao, X.; Liu, J.; Liu, Y.; et al. Effects of thermal treatment on the adhesion strength and osteoinductive activity of single-layer graphene sheets on titanium substrates. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Geng, H.; Dai, J.; Li, J.; Di, Z.; Liu, X. Antibacterial ability and hemocompatibility of graphene functionalized germanium. Sci. Rep. 2016, 6, 37474. [Google Scholar] [CrossRef]
- Arellano, D.L.G.; Kolewe, K.W.; Champagne, V.K.; Kurtz, I.S.; Burnett, E.K.; Zakashansky, J.; Arisoy, F.D.; Briseno, A.L.; Schiffman, J.D. Gecko-Inspired Biocidal Organic Nanocrystals Initiated from a Pencil-Drawn Graphite Template. Sci. Rep. 2018, 8, 11618. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.N.; Fisher, E.R. Investigation of Antibacterial 1,8-Cineole-Derived Thin Films Formed via Plasma-Enhanced Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2017, 9, 36548–36560. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.M.; Seong, H.; Im, S.G.; Sung, B.H.; Kim, S.C.; Jeong, K.J. Coating of an antimicrobial peptide on solid substrate via initiated chemical vapor deposition. J. Ind. Eng. Chem. 2018, 58, 51–56. [Google Scholar] [CrossRef]
- Getnet, T.G.; da Silva, G.F.; S Duarte, I.; Kayama, M.E.; Rangel, E.C.; Cruz, N.C. Atmospheric Pressure Plasma Chemical Vapor Depo-sition of Carvacrol Thin Films on Stainless Steel to Reduce the Formation of E. Coli and S. Aureus Biofilms. Materials 2020, 13, 3166. [Google Scholar] [CrossRef] [PubMed]
- Raiford, J.A.; Oyakhire, S.T.; Bent, S.F. Applications of atomic layer deposition and chemical vapor deposition for perovskite solar cells. Energy Environ. Sci. 2020, 13, 1997–2023. [Google Scholar] [CrossRef]
- Rockett, A. The Materials Science of Semiconductors. Mater. Sci. Semicond. 2008, 573–609. [Google Scholar] [CrossRef]
- Yan, X.T.; Xu, Y. Chemical Vapour Deposition: An Integrated Engineering Design for Advanced Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Malekimoghadam, R.; Rafiee, R. Carbon nanotubes processing. In Carbon Nanotube-Reinforced Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–59. [Google Scholar]
- Karfa, P.; Majhi, K.C.; Madhuri, R. Synthesis of two-dimensional nanomaterials. In Two-Dimensional Nanostructures for Biomedical Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–71. [Google Scholar]
- Lee, J.H.; Kim, U.J.; Han, C.H.; Rha, S.K.; Lee, W.J.; Park, C.O. Investigation of silicon oxide thin films prepared by atomic layer dep-osition using SiH2Cl2 and O3 as the precursors. Jpn. J. Appl. Phys. 2004, 43, L328. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Hu, L.; Qi, W.; Li, Y. Coating strategies for atomic layer deposition. Nanotechnol. Rev. 2018, 6, 527–547. [Google Scholar] [CrossRef]
- Narayan, R.J.; Adiga, S.P.; Pellin, M.J.; A Curtiss, L.; Stafslien, S.; Chisholm, B.; A Monteiro-Riviere, N.; Brigmon, R.L.; Elam, J.W. Atomic layer deposition of nanoporous biomaterials. Mater. Today 2010, 13, 60–64. [Google Scholar] [CrossRef]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility and antimi-crobial activity for orthopedic implants. Int. J. Nanomed. 2017, 12, 8711. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.R.; Hoogland, S.; Adachi, M.M.; Kanjanaboos, P.; Wong, C.T.O.; McDowell, J.J.; Xu, J.; Voznyy, O.; Ning, Z.; Houtepen, A.J.; et al. Perovskite Thin Films via Atomic Layer Deposition. Adv. Mater. 2014, 27, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef]
- Muñoz-Rojas, D.; MacManus-Driscoll, J. Spatial atmospheric atomic layer deposition: A new laboratory and industrial tool for low-cost photovoltaics. Mater. Horiz. 2014, 1, 314–320. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Anders, A. Metal plasma immersion ion implantation and deposition: A review. Surf. Coat. Technol. 1997, 93, 158–167. [Google Scholar] [CrossRef]
- Mantese, J.V.; Brown, I.G.; Cheung, N.W.; Collins, G.A. Plasma-immersion ion implantation. Mrs Bull. 1996, 21, 52–56. [Google Scholar] [CrossRef]
- Harrasser, N.; Jüssen, S.; Obermeir, A.; Kmeth, R.; Stritzker, B.; Gollwitzer, H.; Burgkart, R. Antibacterial potency of different depo-sition methods of silver and copper containing diamond-like carbon coated polyethylene. Biomater. Res. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Shiau, D.K.; Yang, C.H.; Sun, Y.S.; Wu, M.F.; Pan, H.; Huang, H.H. Enhancing the blood response and antibacterial adhesion of tita-nium surface through oxygen plasma immersion ion implantation treatment. Surf. Coat. Technol. 2019, 365, 173–178. [Google Scholar] [CrossRef]
- Pelletier, J.; Anders, A. Plasma-based ion implantation and deposition: A review of physics, technology, and applications. IEEE Trans. Plasma Sci. 2005, 33, 1944–1959. [Google Scholar] [CrossRef]
- Wood, B.P. Fundamentals of Plasma Immersion Ion Implantation and Deposition. 2000. Available online: https://escholarship.org/uc/item/4pz675zk (accessed on 29 October 2021).
- Liu, C.-F.; Lee, T.-H.; Liu, J.-F.; Hou, W.-T.; Li, S.-J.; Hao, Y.-L.; Pan, H.; Huang, H.-H. A unique hybrid-structured surface produced by rapid electrochemical anodization enhances bio-corrosion resistance and bone cell responses of β-type Ti-24Nb-4Zr-8Sn alloy. Sci. Rep. 2018, 8, 6623. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C.; Seikh, A.H.; Alharthi, N.; Hussain, R.R. Corrosion mechanism and kinetics of Al-Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 3399. [Google Scholar] [CrossRef]
- Jones, A.; Mistry, K.; Kao, M.; Shahin, A.; Yavuz, M.; Musselman, K.P. In-situ spatial and temporal electrical characterization of ZnO thin films deposited by atmospheric pressure chemical vapour deposition on flexible polymer substrates. Sci. Rep. 2020, 10, 19947. [Google Scholar] [CrossRef]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin metal–organic framework membrane production by gel–vapour deposition. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.; dos Santos, V.; Cardoso, S.; Doria, A.; Figueira, F.; Rodrigues, B.; Testoni, G.; Fraga, M.; Marciano, F.; Lobo, A.O.; et al. TiO2 coatings via atomic layer deposition on polyurethane and polydimethylsiloxane substrates: Properties and effects on C. albicans growth and inactivation process. Appl. Surf. Sci. 2017, 422, 73–84. [Google Scholar] [CrossRef]
- Beigi, M.H.; Safaie, N.; Nasr-Esfahani, M.H.; Kiani, A. 3D titania nanofiber-like webs induced by plasma ionization: A new direc-tion for bioreactivity and osteoinductivity enhancement of biomaterials. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Yu, K.; Mei, Y.; Hadjesfandiari, N.; Kizhakkedathu, J.N. Engineering biomaterials surfaces to modulate the host response. Colloids Surf. B Biointerfaces 2014, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Fundeanu, I.; van der Mei, H.C.; Schouten, A.J.; Busscher, H.J. Microbial adhesion to surface-grafted polyacrylamide brushes after long-term exposure to PBS and reconstituted freeze-dried saliva. J. Biomed. Mater. Res. Part A 2010, 9999A, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Barnacle Cement as Surface Anchor for “Clicking” of Antifouling and Antimicrobial Polymer Brushes on Stainless Steel. Biomacromolecules 2013, 14, 2041–2051. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Defi-nitions, concepts, advances, and outlook. Bone Res. 2021, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Spaans, S.; Ning, K.; Ippel, B.D.; Yang, F.; Loomans, B.; Dankers, P.Y.W.; Leeuwenburgh, S.C.G. Self-Healing Biomaterials: From Molecular Concepts to Clinical Applications. Adv. Mater. Interfaces 2018, 5, 1800118. [Google Scholar] [CrossRef]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]

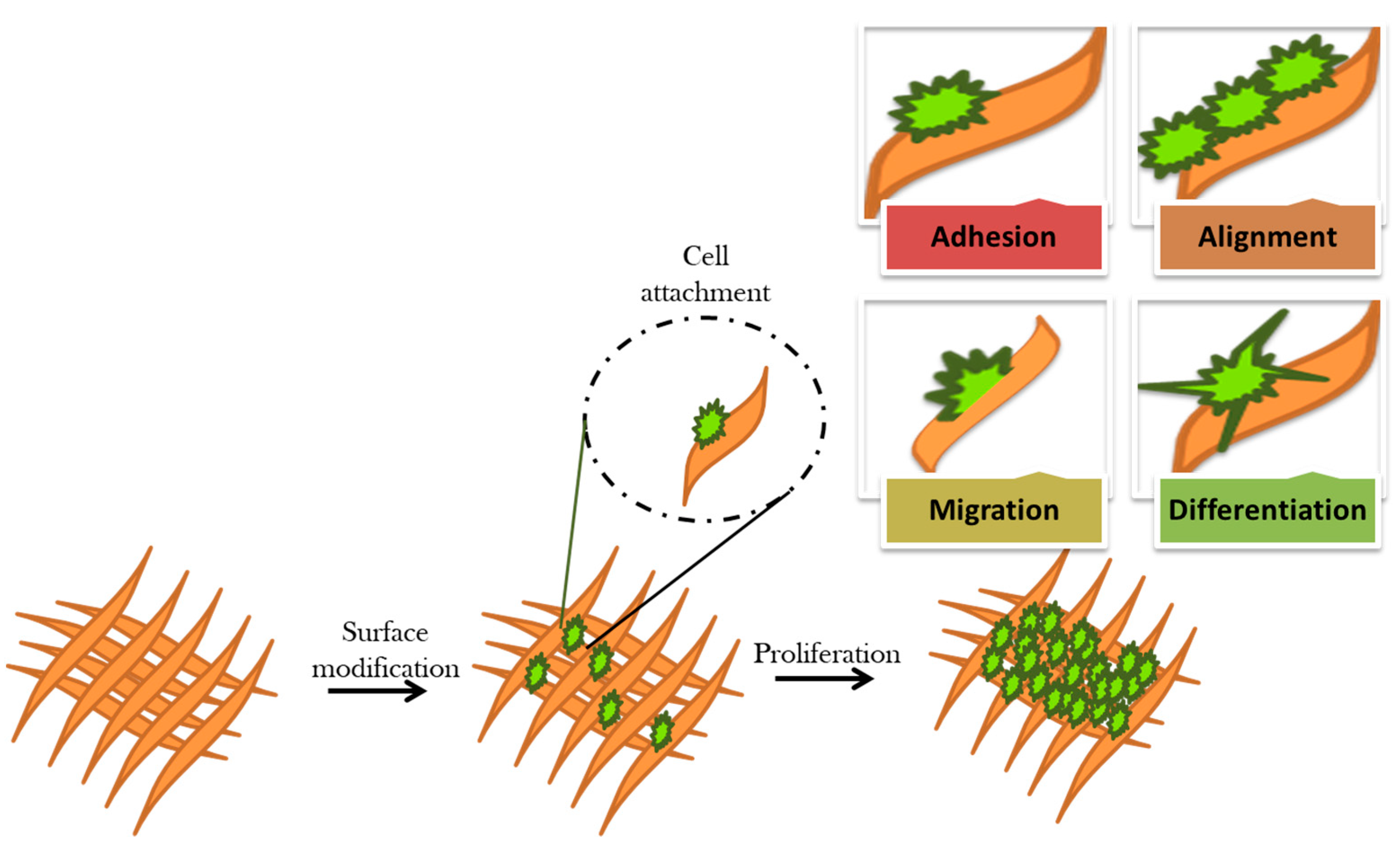
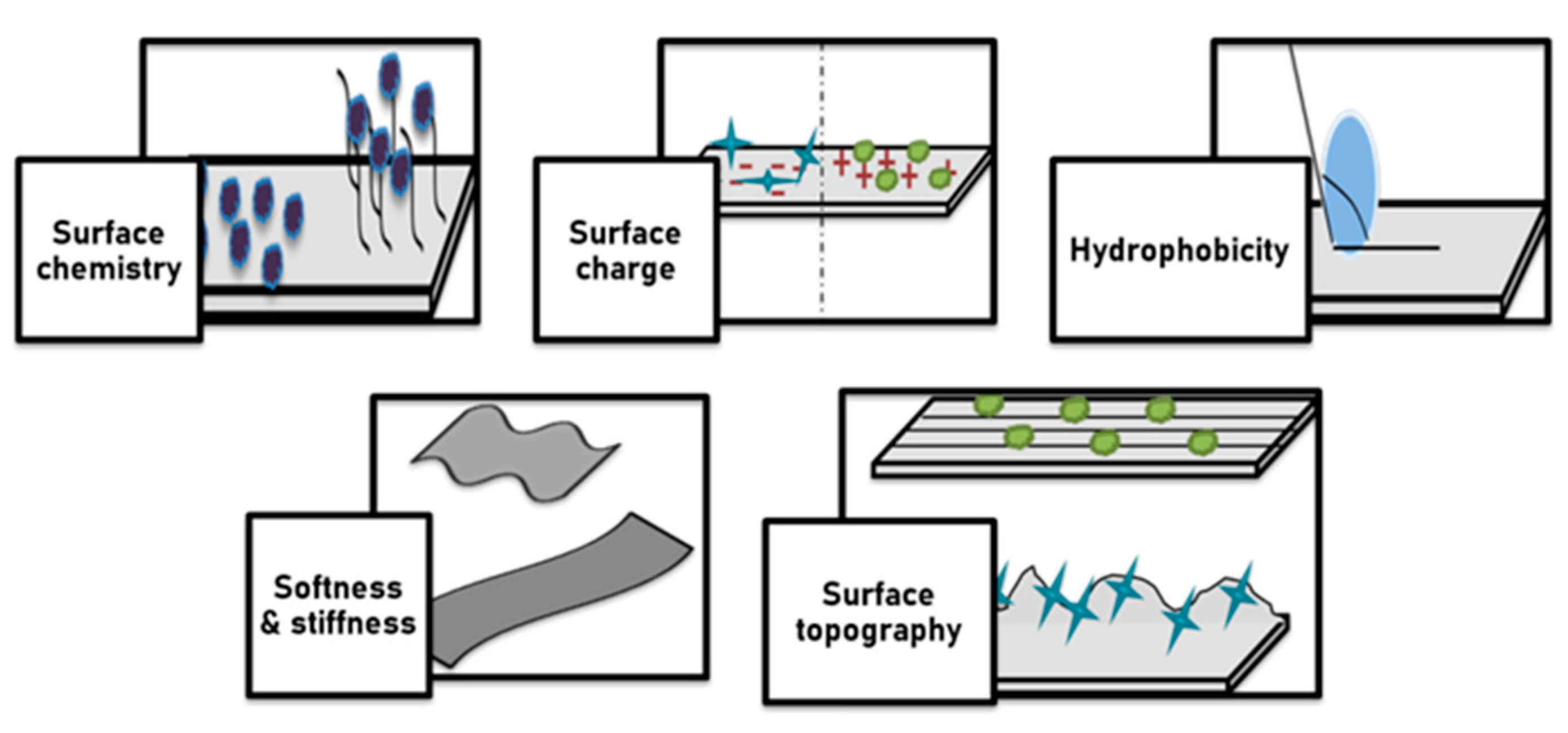
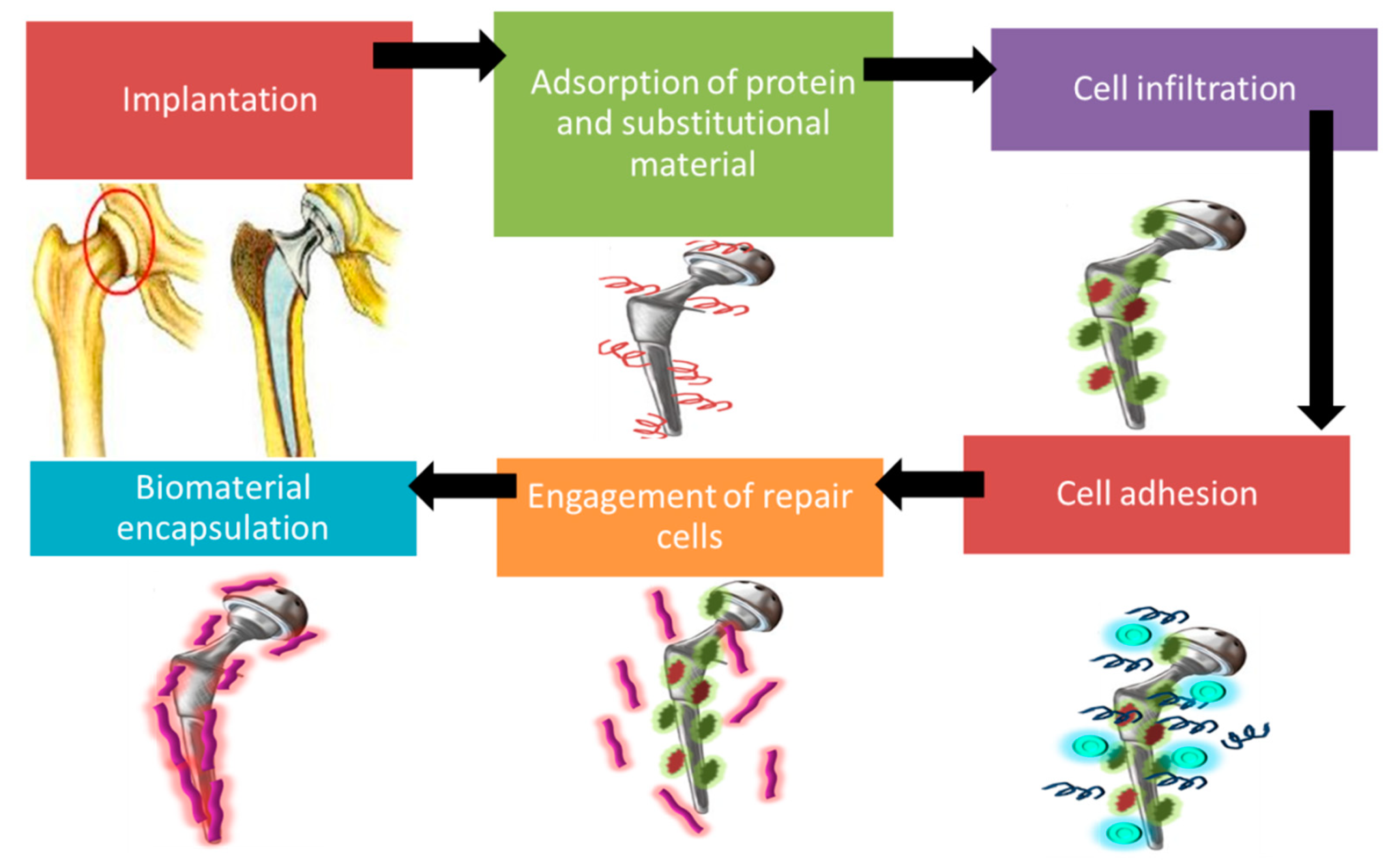
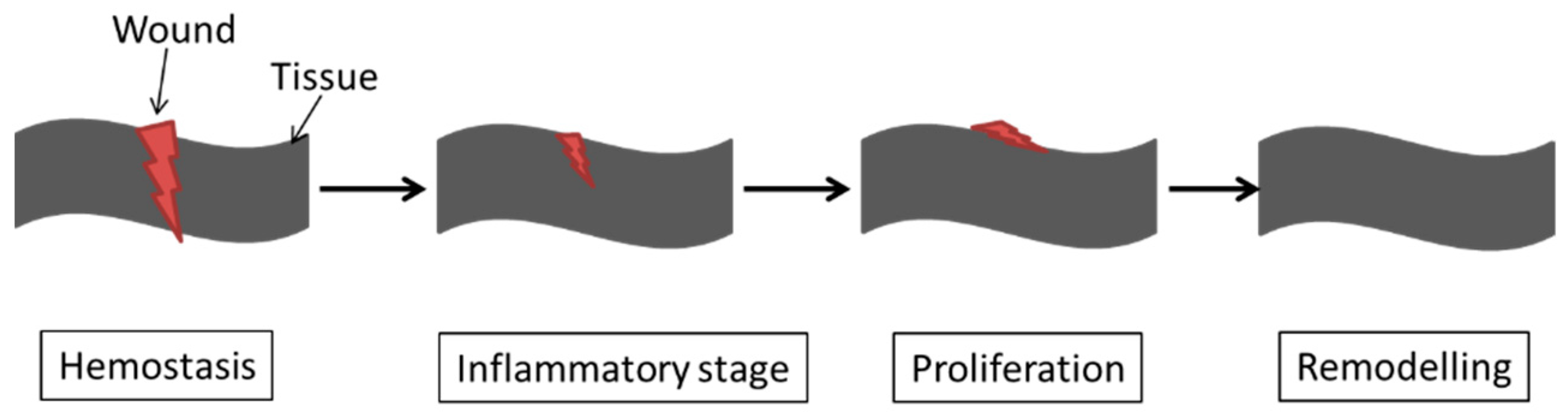
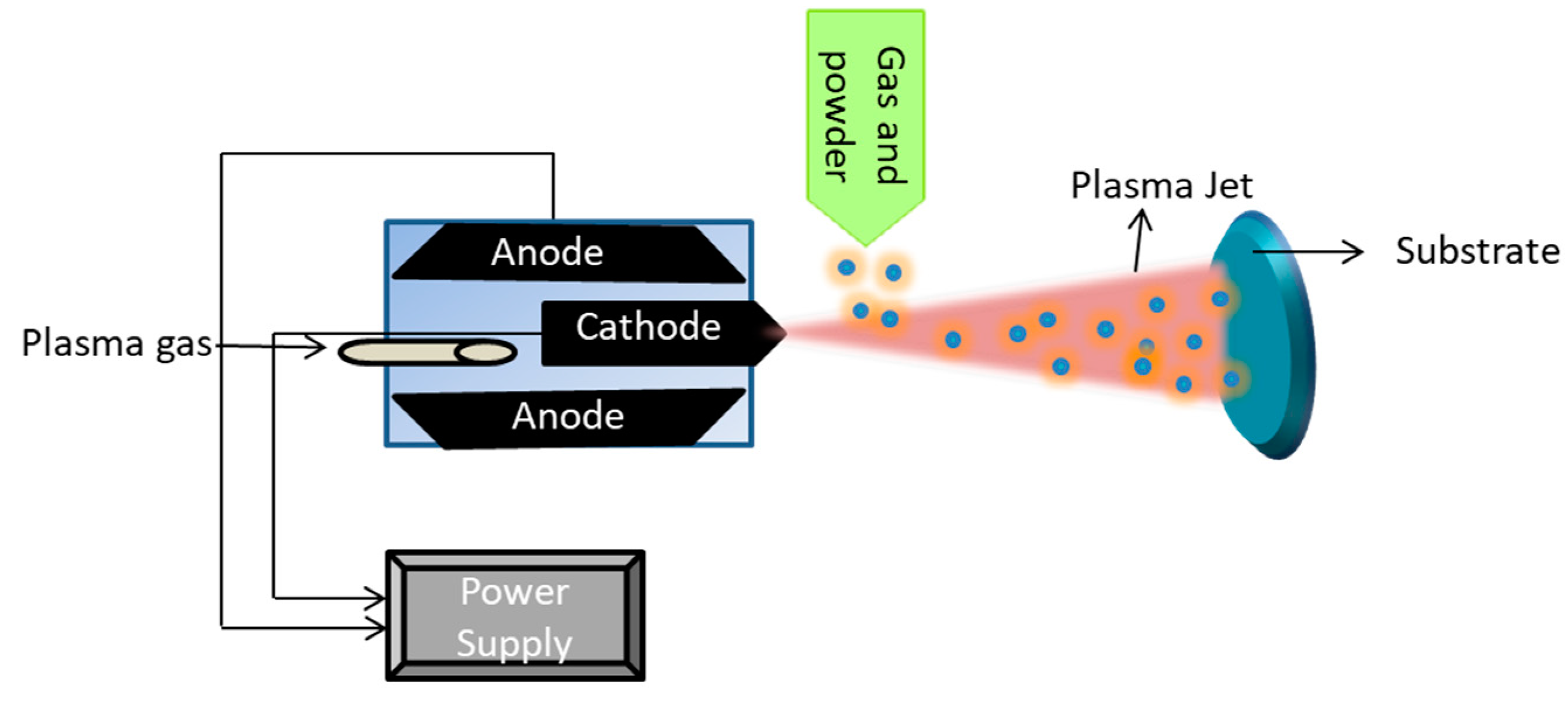
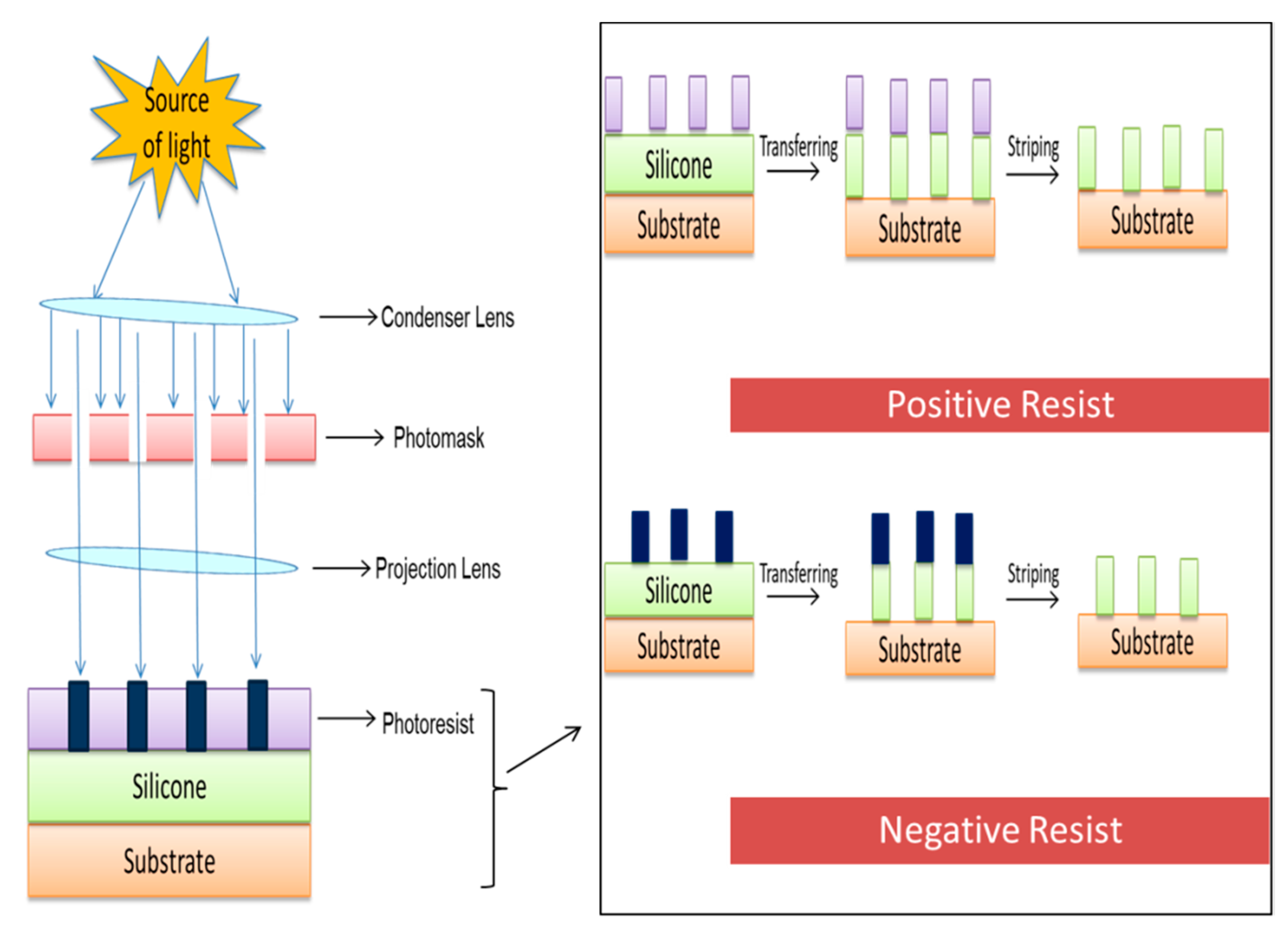
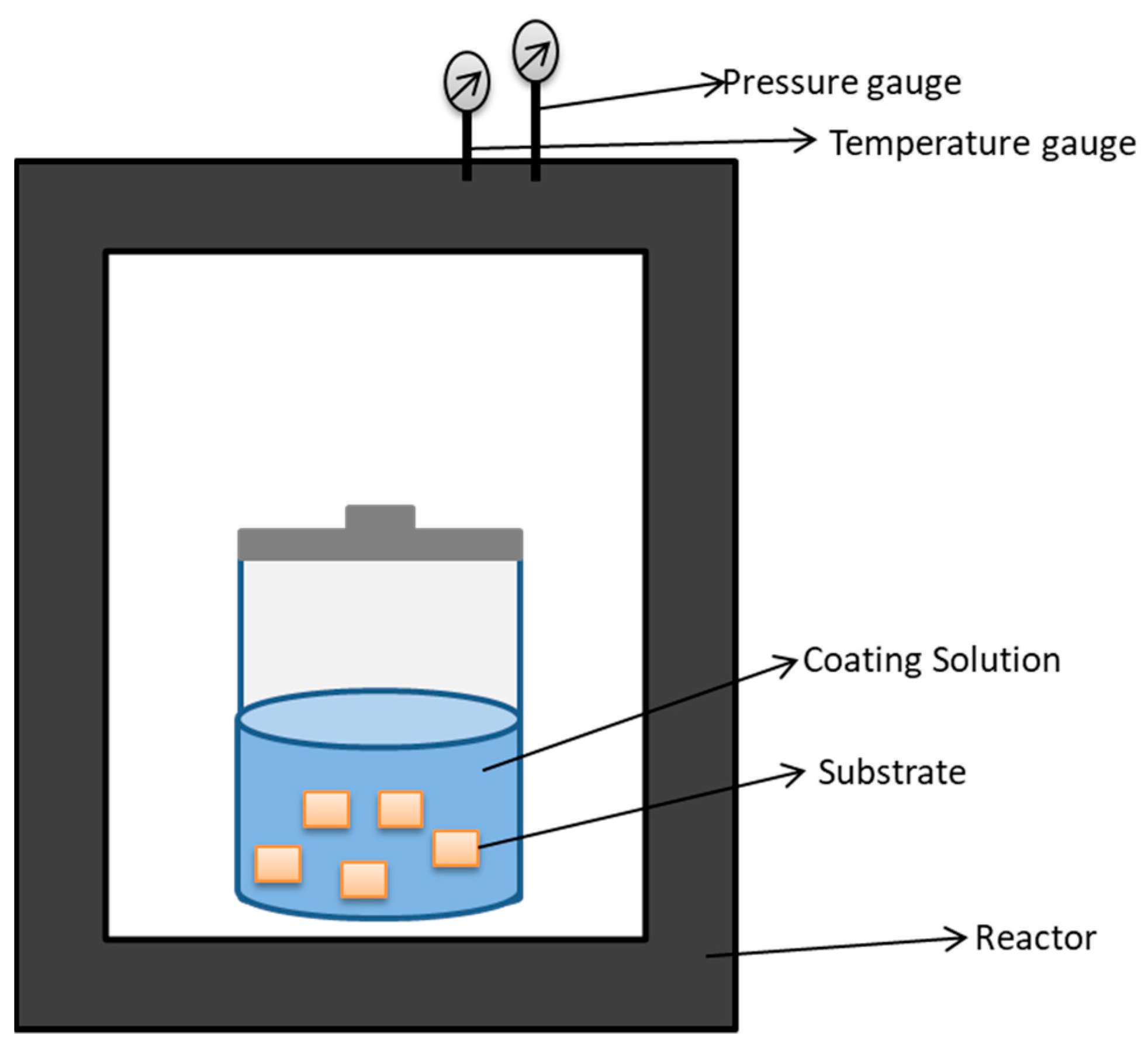
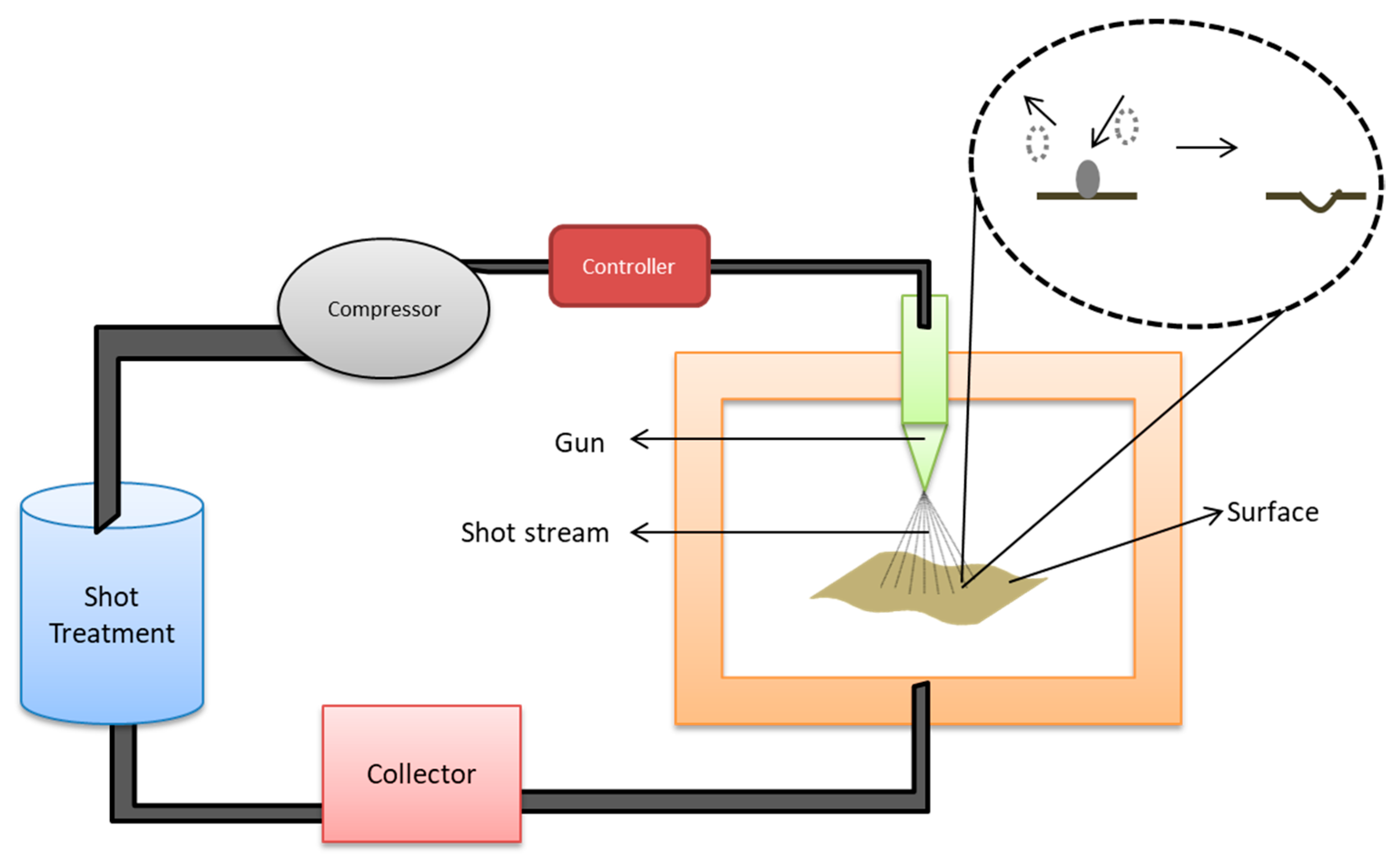

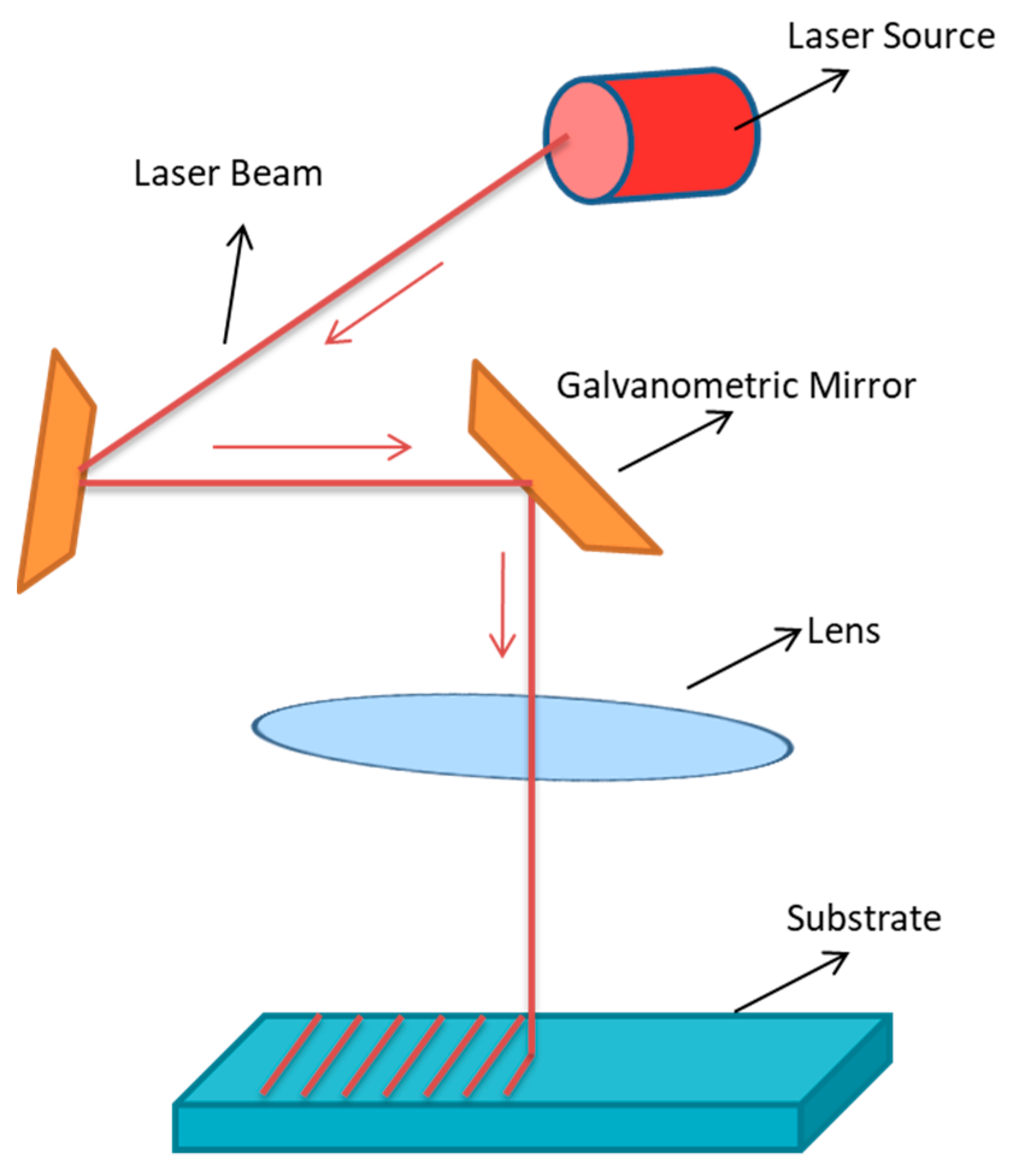
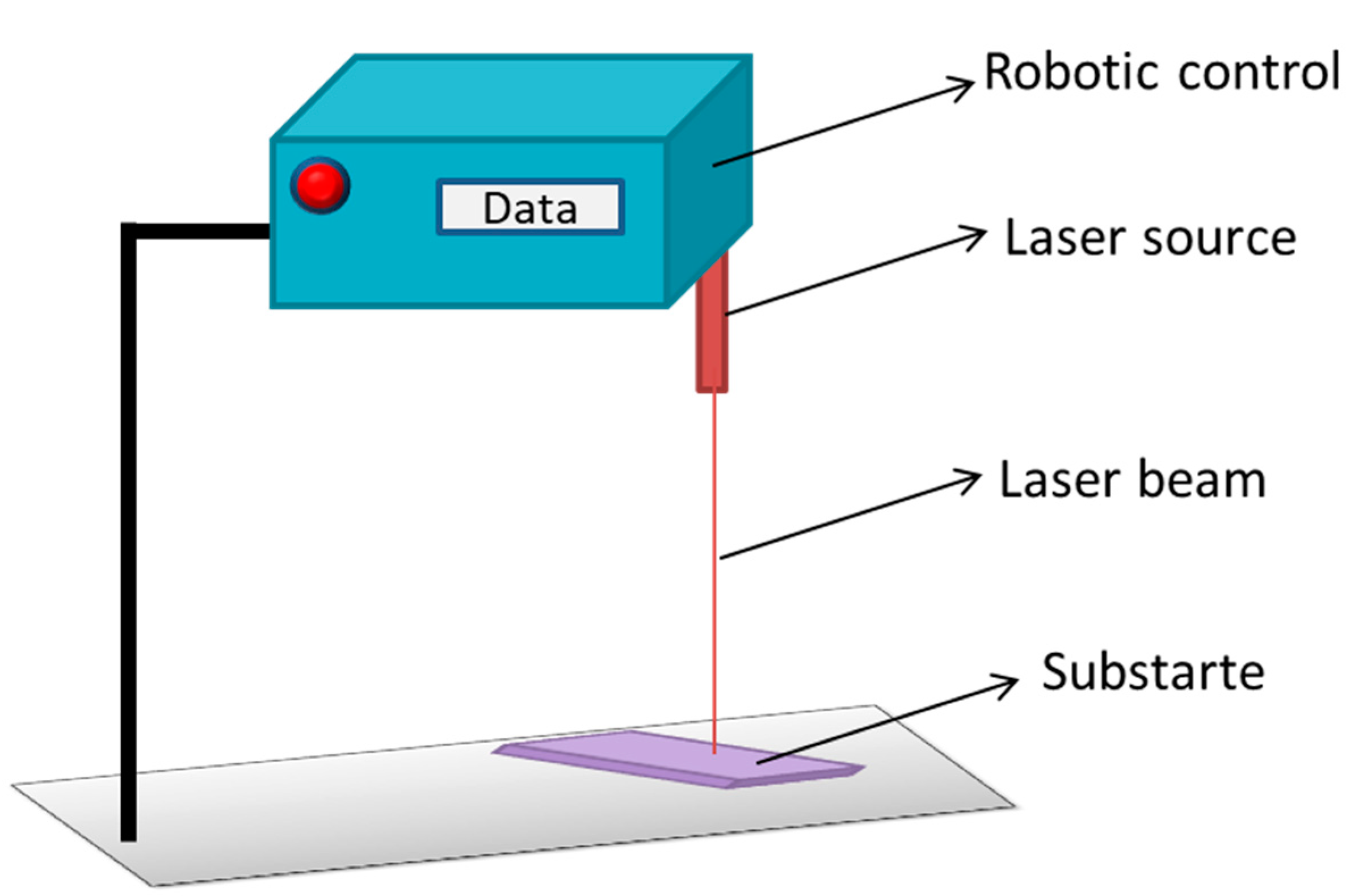
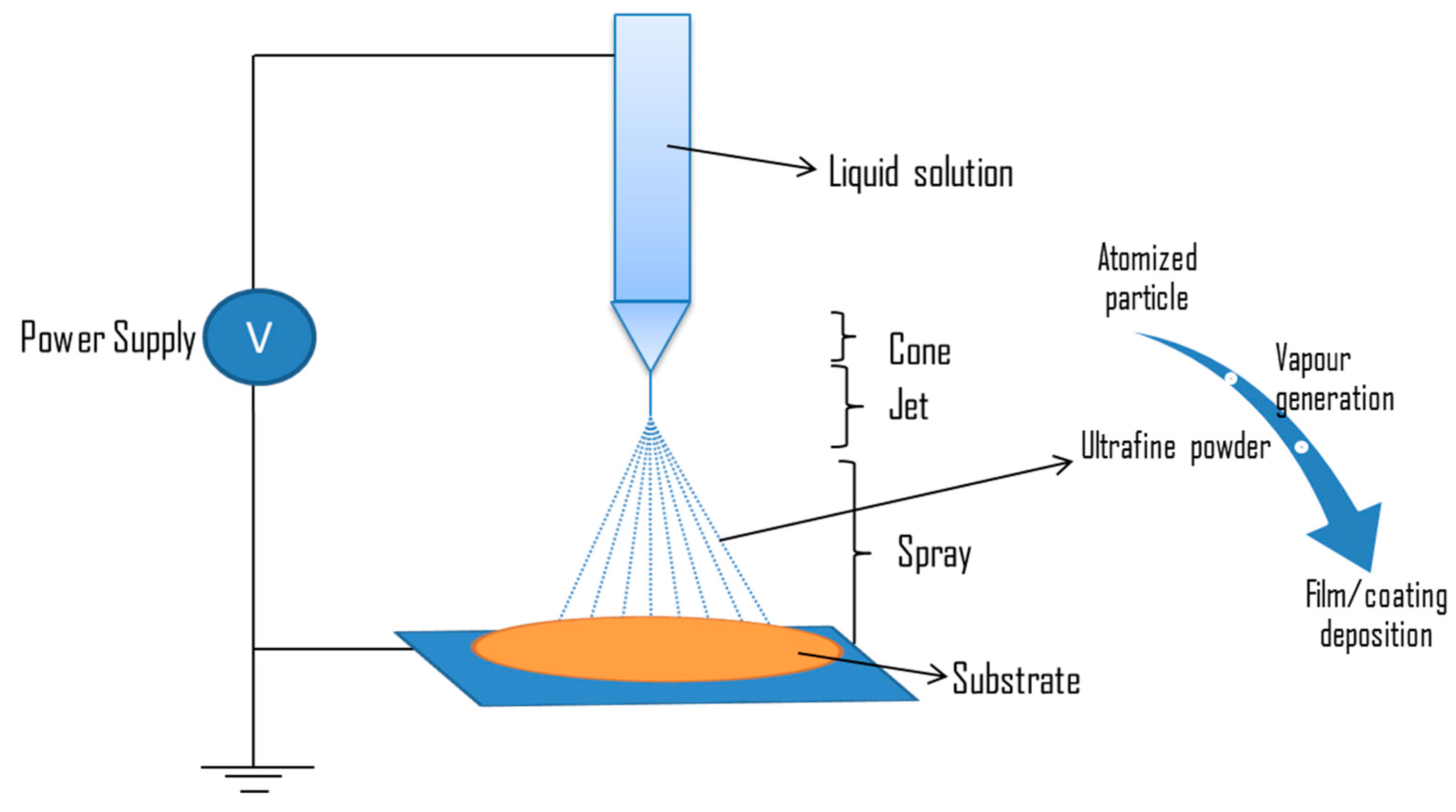
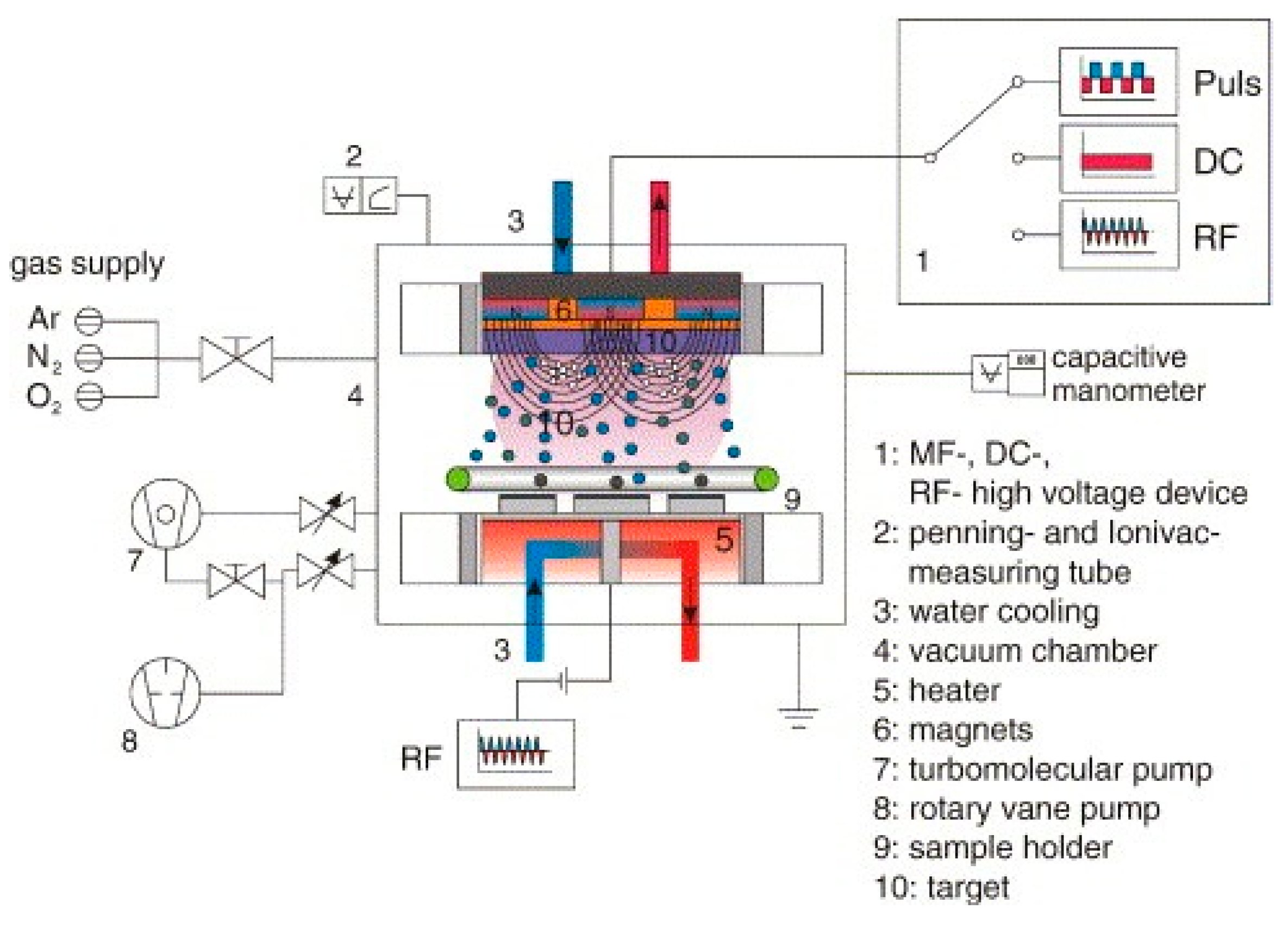
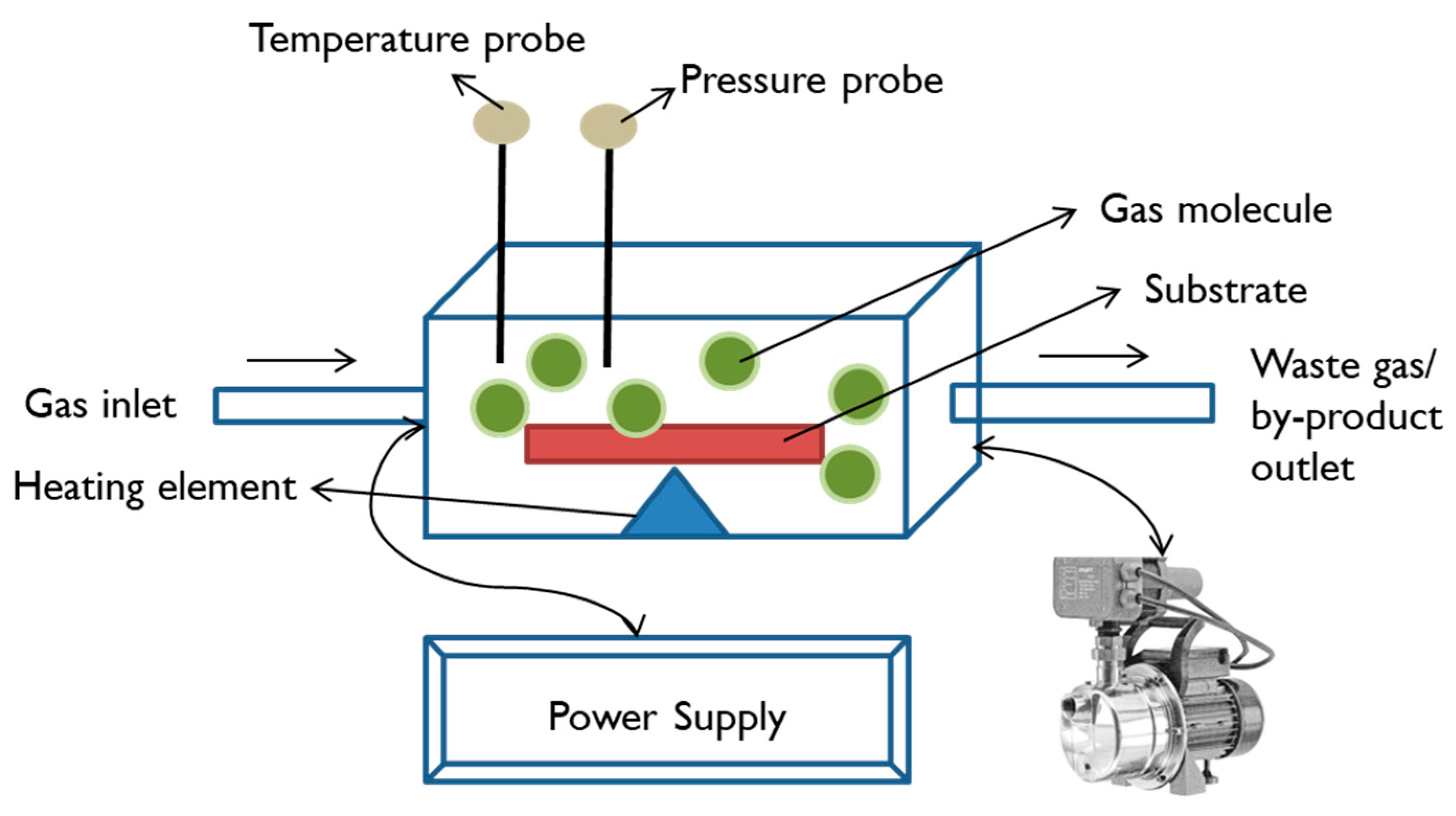
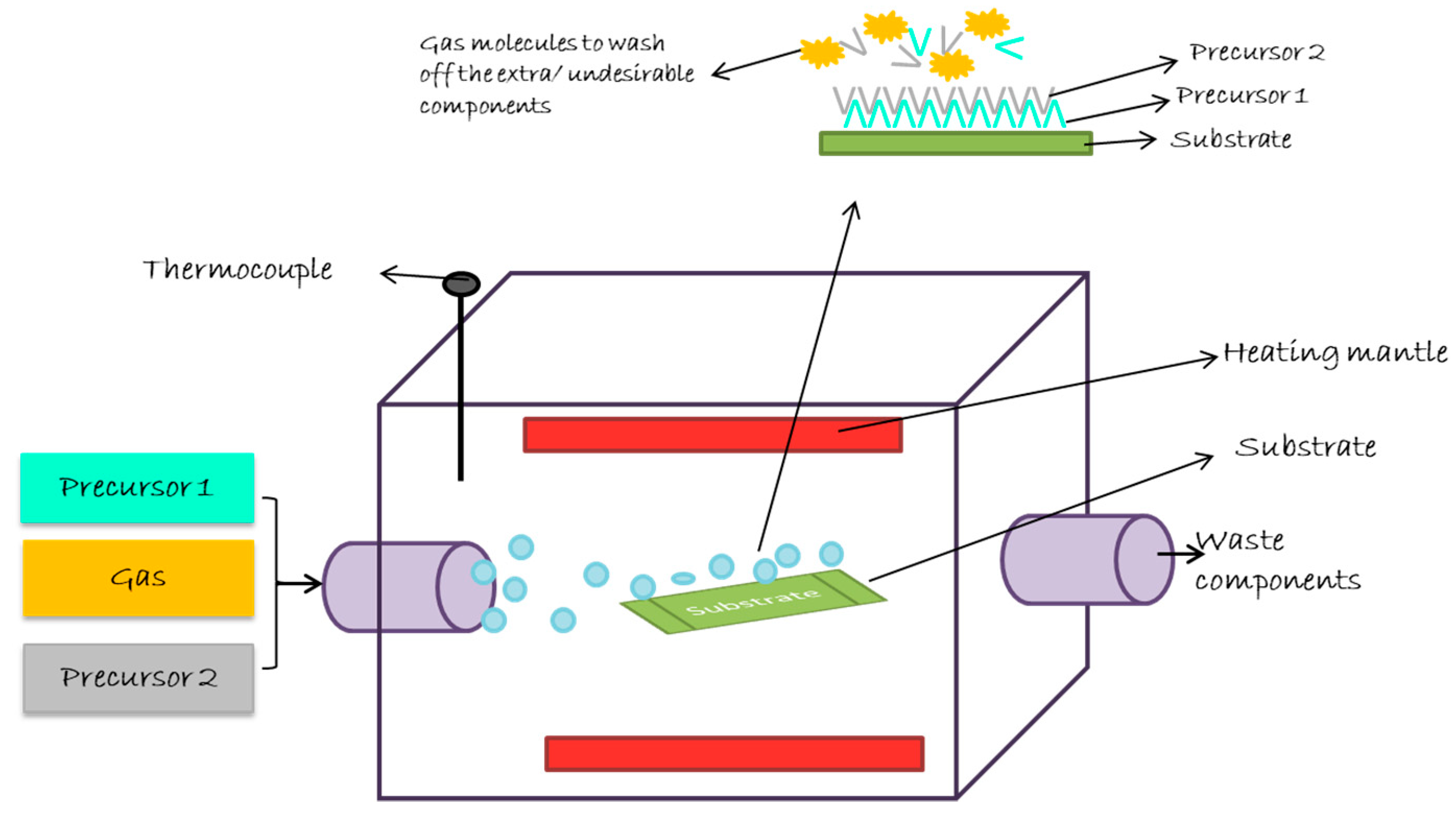
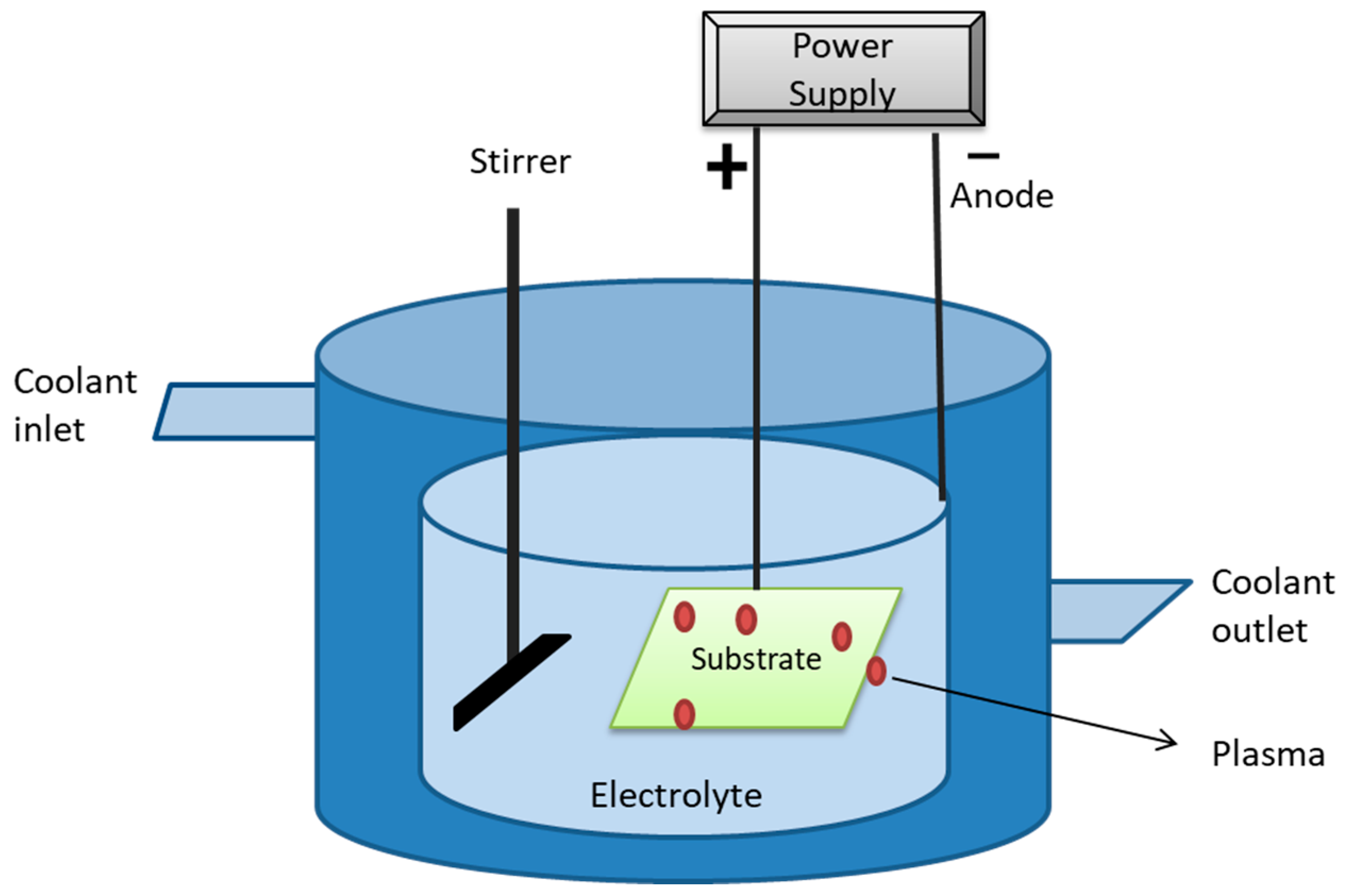
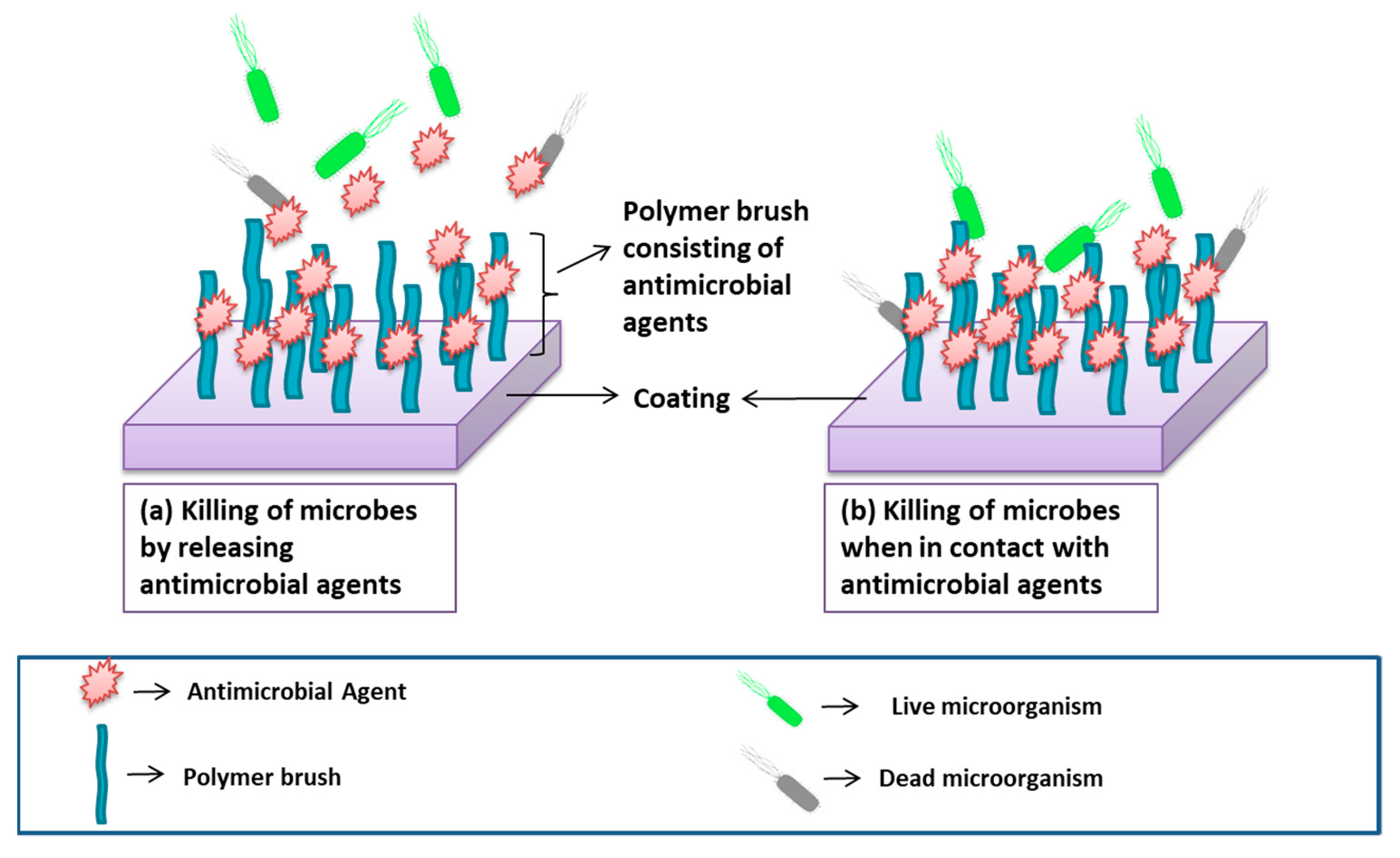

| Biomaterials | Surface Treatments | Results | References |
|---|---|---|---|
| Pacemaker | Parylene coating. | Bacterial count for coated samples was recorded as 3.69 and 5.51 log (CFU/mL) for S. aureus and E. coli, respectively. | [24] |
| Prosthetic heart valve (polystyrene-block-isobutylene-block-styrene (SIBS)) | Dip coating with extracellular matrix (derived from porcine skin). | Coated samples showed improved biocompatibility and reduced protein adhesion by increasing hydrophilicity. | [25] |
| Abdominal Prosthetic | Polyethylene oxide coating on equine pericardium mesh. | Enzymatic degradation is more common in uncoated sample. Better mesh integrity and calcification in coated sample was observed. | [26] |
| Artifical Cornea | Electrospinnig of polycaprolactone and collagen. | This technique was successful in fabrication of hemispherical scaffold. | [27] |
| Artificial hip | Poly(2-methacryloyloxyethyl phosphorylcholine) grafted on UHMWPE | Treated samples showed reduction in wear particles. | [28] |
| Artificial skin | Polyimide fibers were fabricated via electrospinning, and silver nanoparticles were incorporated into the scaffold. | Silver treated samples were able to detect pressure in the range of 5 KPa to 100 KPa. | [29] |
| Dental implant | Dip coating of calcium carbonate on titanium. | Growth of new bone was observed after 12 days of implantation. | [30] |
| Artificial ligament | Polyethylene Terephthalate was dip coated with silk fibroin. | Contact angle reduced from 132° to 50°. After 12 h, DNA content of coated sample was 13 mg/scaffold, which was higher than uncoated sample (8 mg/scaffold), suggesting better biocompatability than control. | [31] |
| Artificial kidney | Treatment of dialysis membrane via photo-reactive zwitterionic copolymers. | Improves blood compatibility. | [32] |
| Dental burs | Deposition of polycrystalline diamond films via chemical vapor deposition. | Coated samples have lesser wear compared to uncoated samples. | [33] |
| Intramedullary nail | Coating with antibiotic and growth factors (IGF-1 and rhBMPs). | Coated samples showed controlled release of antibiotic and growth factors for development of healthy bone. Reduced risk of fracture and efficient healing were observed. | [34] |
| Dental crowns | Two coatings were analyzed: tribochemical silica and alumina. | Both the coated samples showed similar retention strength of 4 MPa, and control had 0.8 MPa strength. | [35] |
| Cochlear implant | Coating of poly(4-hydroxybutyrate) with brain derived neurotrophic factor in the presence of disuccinimidyl suberate (DSS). | Coated samples showed improved spiral ganglion cell growth | [36] |
| Vascular graft | Coating of gelatin/fibrinogen/polycaprolactone scaffolds prepared via electrospinning with fibronectin and collagen. | Porosity reduced to 36% for treated samples. Number of human umbilical vein endothelial cells in treated and nontreated samples was recorded as 5.71 × 103 and 4.9 × 103, respectively | [37] |
| Nikel titanium wire | Electrostatic powder technique. | Friction force was recorded as 99.65 for coated sample to, and for control it was 105. | [38] |
| Artificial orbital wall | Three-Dimensional printing. | Tissue volumes for pre-operation and post operation were 24 and 22.31, respectively, while for the reconstructed wall, 22.31 and 22.01 were the volumes for affected and unaffected orbit. | [39] |
| Titanium bone screw | Additive manufacturing using laser melting. | The mean maximal load required for fracture was 43.3 N for control and 56.6 N for treated samples. | [40] |
| Cartilage | Additive manufacturing of poly(ethylene oxide terephthalate/poly(butylene terephthalate) and plasma coating of acrylic acid. | Plasma coated samples showed the highest cell interaction efficiency. | [41] |
| Drug delivery vehicle (Nanoparticle) | Coating of gold nanoparticles with neuron-targeted exosomes. | After 30 min, 5% of treated nanoparticles were transported across the blood–brain barrier while control samples transported were less than half of treated samples. | [42] |
| Hernia repair | Electrospinning, plasma treatment, and direct surface modification to prepare gelatin methacryloyl and polycaprolactone methacrylate. | Treated samples showed smooth and non-defected surface. Cell viability was recorded as more than 95% for all the samples except for the polycaprolactone membrane. Treated scaffolds showed inhibitory activity against S. aureus, P. aeruginosa, and MRSA. | [43] |
| Medical Implants | Types of Materials | Infection Causing Agents | References |
|---|---|---|---|
| Orthopedic devices | Stainless steel, cobalt-based alloys, titanium, silicone, polyethylene, polypropylene, and polymethyl methacrylate; aluminium oxide and calcium phosphates. | Acinetobacter pittii, Enterobacter cloacae, Micrococcus luteus, Staphylococcus epidermidis, and Staphylococcus hominis. | [46] |
| Urologic devices | Polytetrafluoroethylene, rubber, polyurethane, polyamide, silicones, and polyhydroxyalkonates. | Staphylococcus aureus and Staphylococcus epidermis. | [47] |
| Prosthetic heart valve | Titanium, graphite, pyrolytic carbon, polyethylene, polypropylene, polyamide, and diamond-like carbon. | Mycobacterium tuberculosis and hepatitis B virus. | [48] |
| Bone-anchored hearing systems | Titanium, platinum, aluminium oxide, silicone, teflon, polyethylene, and polyimides. | Staphylococcus epidermis. | [49] |
| Atheoplasty devices | Cobalt-based alloys, titanium, zirconium, nickel, and ultra-high molecular weight polyethylene. | Escherichia coli, Staphylococcus aureus, and Staphylococcus epidermidis. | [50,51,52] |
| Bone allograft | Calcium phosphate ceramic, calcium sulphate, hydroxyapatite, bioactive glasses, and magnesium. | Hepatitis, tuberculosis, and human immunodeficiency virus. | [53] |
| Contact lenses and corneal implants | Silicone hydrogel and polymethylmethacrylate. | S. epidermidis, E. coli, P. aeruginosa, S. aureus, Proteus spp., Serratia spp., and Candida spp. | [54,55,56,57,58,59,60] |
| Breast implants | Silicone gel within silicone rubber envelope and inflatable saline. | S. aureus, Enterococcus spp., S. epidermidis, P. acnes, and diphtheroids. | [61,62,63] |
| Dental implants | Acrylic resin, titanium and its alloys, zirconia, silver and silver nanoparticles, and ZnO. | Veillonella spp., F. nucleatum, A. naeslundii, Streptococcus spp., C. albicans, S. sanguinis, P. gingivalis, E. timidum, E. brachy, and P. anerobicus. | [58,59,64,65,66,67,68,69,70,71,72] |
| Type of Coating | Advantages | Disadvantages | References |
|---|---|---|---|
| Polymeric | Flexibility with biomaterial or biocompatible; resists corrosion and abrasion. | Low adhesive strength; permeability of body fluid across the coating. | [115,116] |
| Ceramic | Prevents corrosion and friction. | Comparatively heavier than organic coating; in case of cracks, corrosions occurs. | [117] |
| Metallic | High tensile strength; helps in osseointegration; decreases friction and wear. | Fails to show bioactivity; high elastic modulus is observed. | [118,119] |
| Biomaterials | Coating Materials | Method | Microorganisms Inhibited | References |
|---|---|---|---|---|
| Stainless steel | Ti-ZrN/Ag | Coating deposition | S. aureus and S. epidermidis | [124] |
| Titanium | Molybdenum disulphide | Electrostatic deposition | E. coli | [125] |
| Cotton fabrics | Zinc oxide NPs | Spin coating | K. pneumonia | [126] |
| Iron oxide NPs | Chitosan | Dip coating | Bacillus subtilis and E. coli | [127] |
| Nickle titanium alloy | Graphene oxide/AgNPs | Electrophoretic deposition | S. mutans | [128] |
| Ti6Al4V | Hydroxyapatite–copper | Electrophoretic deposition | E. coli and S. aureus | [129] |
| Carrageenan/chitosan multilayers | Nisin A | Layer by layer deposition | S. aureus and MRSA | [130] |
| Chitosan | N-acetyl cysteine | Spin coating | S. aureus | [131] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Thickness of coating can be easily modulated | [148] |
| Disadvantages | Expensive technique; may cause crystallization of bioactive glass | [149] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | High accuracy | [155,156,157] |
| Disadvantages | It is a long process; restricted to ultraviolet; and chemically sensitive material | |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Complex shapes and geometry; cost effective; less energy consuming; mild operational temperature is needed | [161,162] |
| Disadvantages | Uncontrolled dispersion of coating | [163] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Improves resistance to fatigue | [166] |
| Disadvantages | It is not suitable for small surface area | [167] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Complex shapes and geometry can be coated; low deposition cost | [170] |
| Disadvantages | Lack of uniformity | |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Requires no cooling agents and chemicals; less deformation; input of heat is low | [179] |
| Disadvantages | High capita cost; thickness of coating has a limit | [180] |
| Biomaterials | Functional Agents | Antimicrobial Effect | Results | References |
|---|---|---|---|---|
| Glass | Titanium dioxide | S. aureus | 90% of biofilm formation was inhibited, and no viable cells were grown. | [186] |
| Titanium | Calcium silicate nanoparticles | S. aureus and E. coli | Bacterial adhesions of S. aureus and E. coli were inhibited. | [187] |
| Titanium | Vancomycin hydrochloride (VH) loaded polyvinyl alcohol-borax (PVA-B) microgels | S. aureus | Inhibition zone increased when increasing immersion time in normal saline. | [188] |
| Chitosan/poly(ethylene glycol)/hyaluronic acid | Zinc oxide | S. aureus, S. epidermidis, and E. cloacea | Inhibition zone for S. epidermidis, S. aureus, and E. cloaceae were recorded as 13.8, 13.0, and 10.3 mm, respectively. | [189] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Motion of droplets can be controlled; prevents agglomeration and coagulation of droplets; can be used for bulk production | [190,191] |
| Disadvantages | May degrade macromolecules because of stress | [192] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Dense and uniform coating is achievable; heat sensitive materials can be coated; strong adhesion of coating | [196] |
| Disadvantages | Costly; deposition rate is low | |
| Biomaterials | Functional Substances | Antimicrobial Effect | Results | References |
|---|---|---|---|---|
| Titania Nanotube (TNT) | Silver Nanograins | S. aureus | TNT with 1% of silver nanograins showed 48.6% of biofilm inhibition by live/dead analysis. | [204] |
| Titanium | Graphene | S. aureus and E. coli | Number of colonies of E. coli and S. aureus was observed as less than 500 and 1000 CFU/mL, respectively. | [205] |
| Germanium | Graphene | S. aureus and E. coli | Inhibition of E. coli and S. aureus was recorded via live/dead analysis. The reports suggest that spots were visible in graphene containing samples. | [206] |
| Graphite | Zinc phthalocyanine | E. coli | 97% of E. coli were inhibited within 15 min. | [207] |
| Polystyrene | 1,8-cineole | S. aureus and E. coli | Fluorescence microscopy images reported that treated samples have a slightly greater number of attached S. aureus than E. coli. | [208] |
| Glass and latex | Antimicrobial peptide (SHAP1) | S. aureus and E. coli | More than 96% of S. aureus and E. coli were inhibited. | [209] |
| Stainless steel | Carvacrol extract | S. aureus and E. coli | More than 90% of 96% of S. aureus and E. coli were inhibited. | [210] |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Uniformity of film; high vacuum is not needed | [211,212,213,214,215] |
| Disadvantages | High capital investment is needed; chemical reactant involved can be hazardous to health; high temperature limits the use of all the substrate; highly toxic by-products; expensive instrumentation | |
| Advantages/Disadvantages | References | |
|---|---|---|
| Advantages | Requires low vacuum and low temperature; pinhole free deposition can be obtained | [217,221,222,223,224] |
| Disadvantages | Slow deposition rate; large amount of waste of energy and material; slow deposition rate; wastage of energy and 60% precursor | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultana, A.; Zare, M.; Luo, H.; Ramakrishna, S. Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering. Int. J. Mol. Sci. 2021, 22, 11788. https://doi.org/10.3390/ijms222111788
Sultana A, Zare M, Luo H, Ramakrishna S. Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering. International Journal of Molecular Sciences. 2021; 22(21):11788. https://doi.org/10.3390/ijms222111788
Chicago/Turabian StyleSultana, Afreen, Mina Zare, Hongrong Luo, and Seeram Ramakrishna. 2021. "Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering" International Journal of Molecular Sciences 22, no. 21: 11788. https://doi.org/10.3390/ijms222111788
APA StyleSultana, A., Zare, M., Luo, H., & Ramakrishna, S. (2021). Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering. International Journal of Molecular Sciences, 22(21), 11788. https://doi.org/10.3390/ijms222111788








