Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity
Abstract
:1. Introduction
2. Results
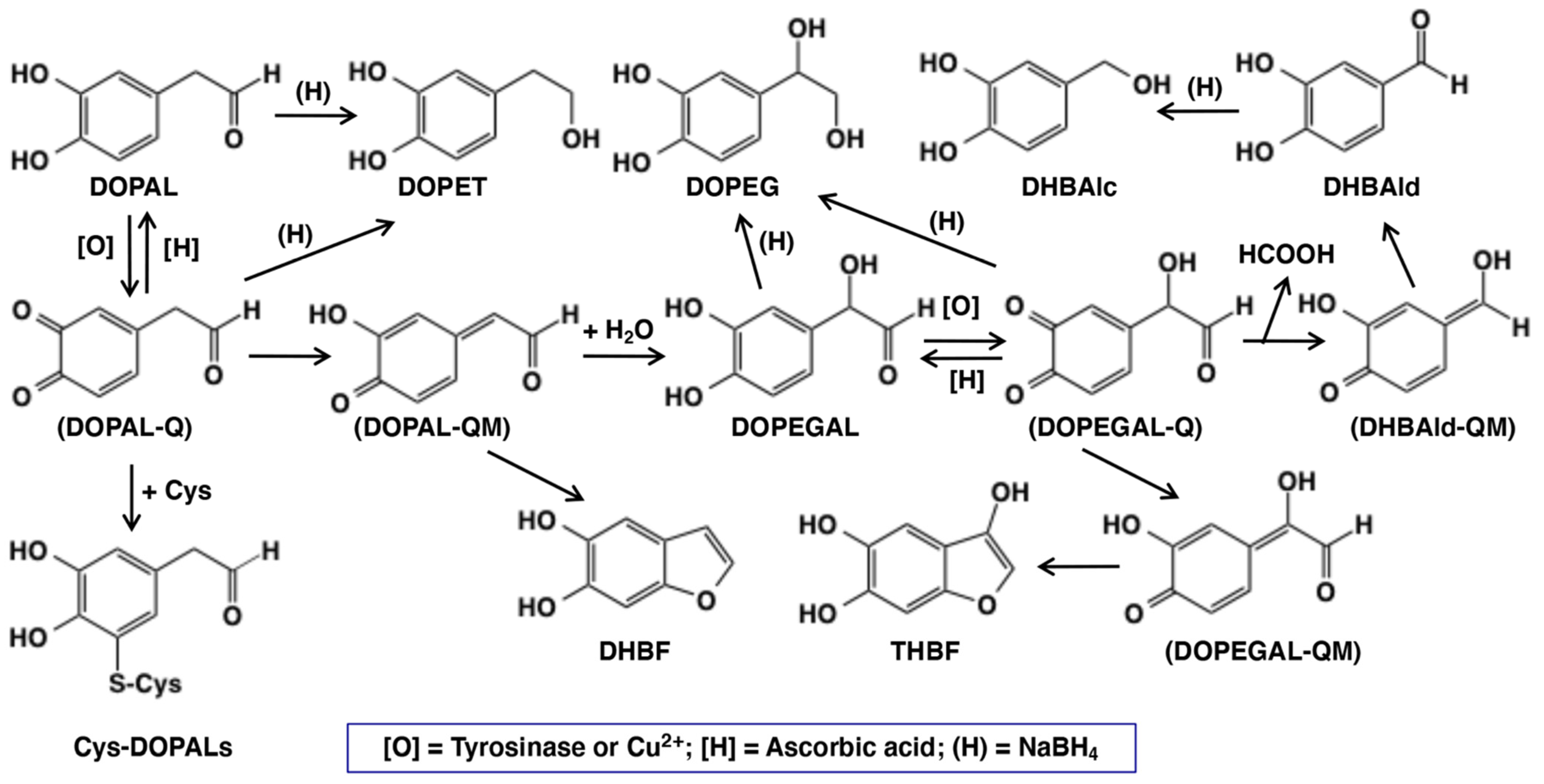
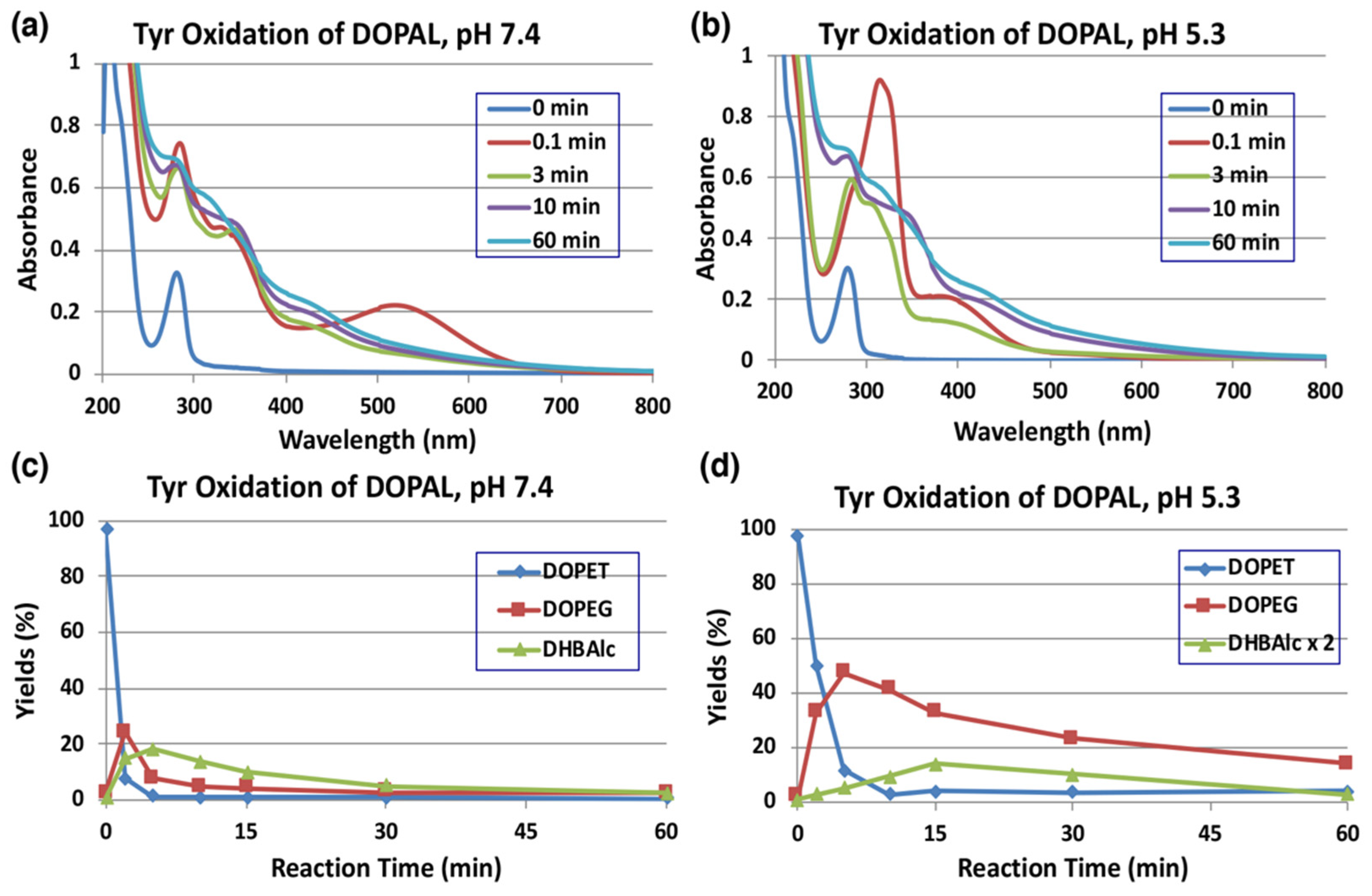
2.1. Oxidative Transformation of DOPAL by Tyrosinase-Catalyzed Reaction
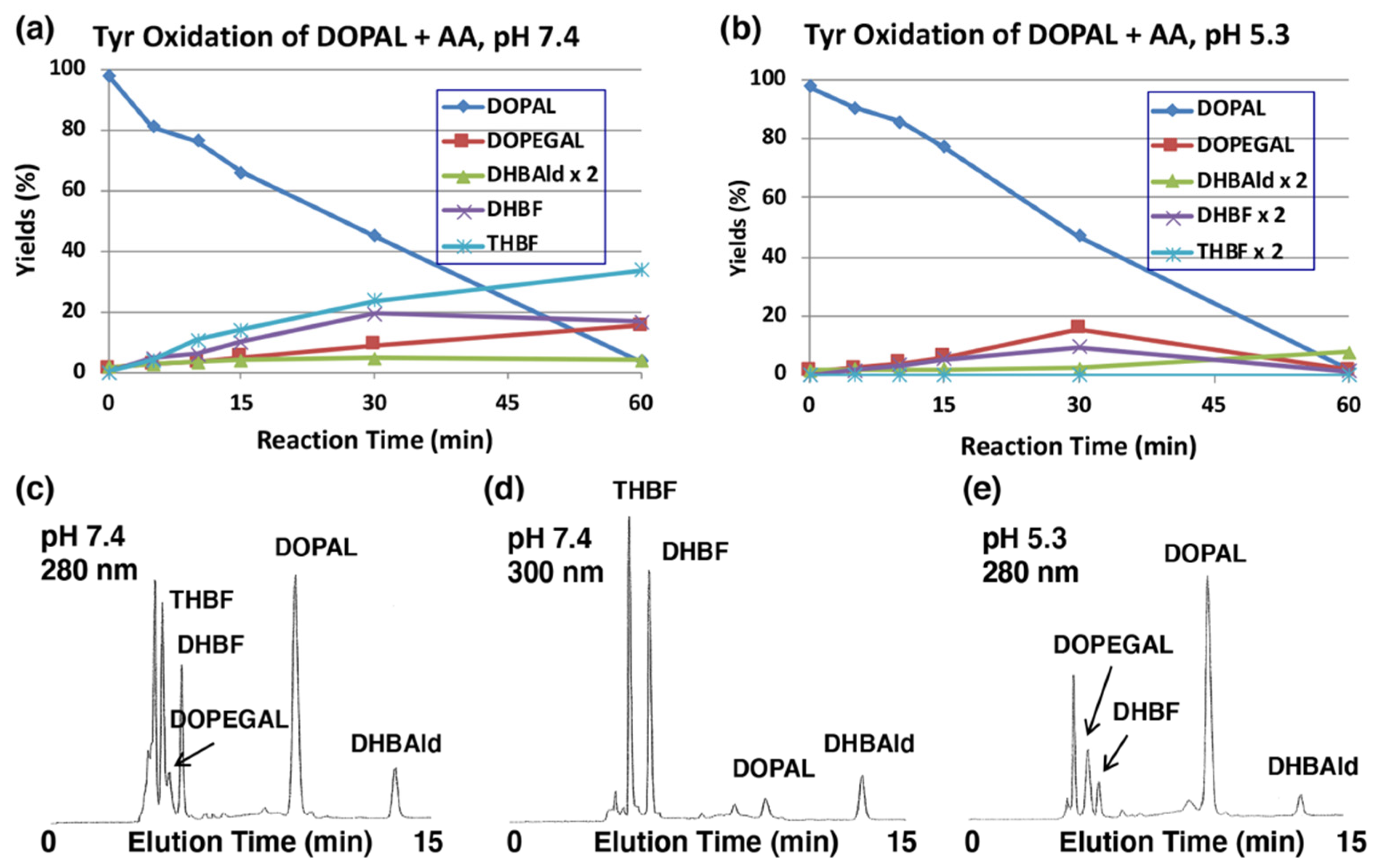
2.2. Oxidative Transformation of DOPAL by Tyrosinase-Catalyzed Reaction in the Presence of Ascorbic Acid (AA)
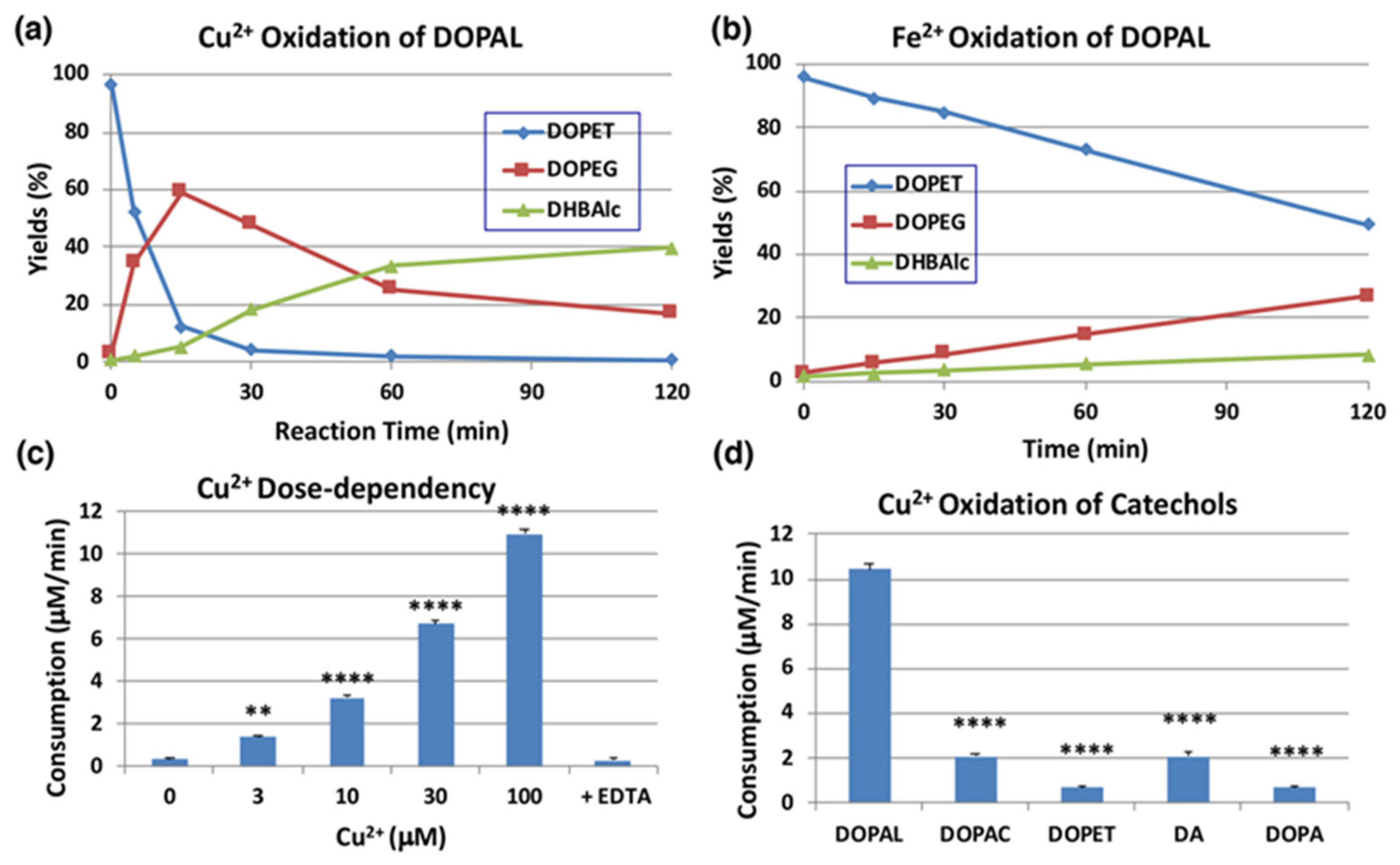
2.3. Copper (II)-Catalyzed Oxidation of DOPAL Produces DOPEGAL and DHBAld
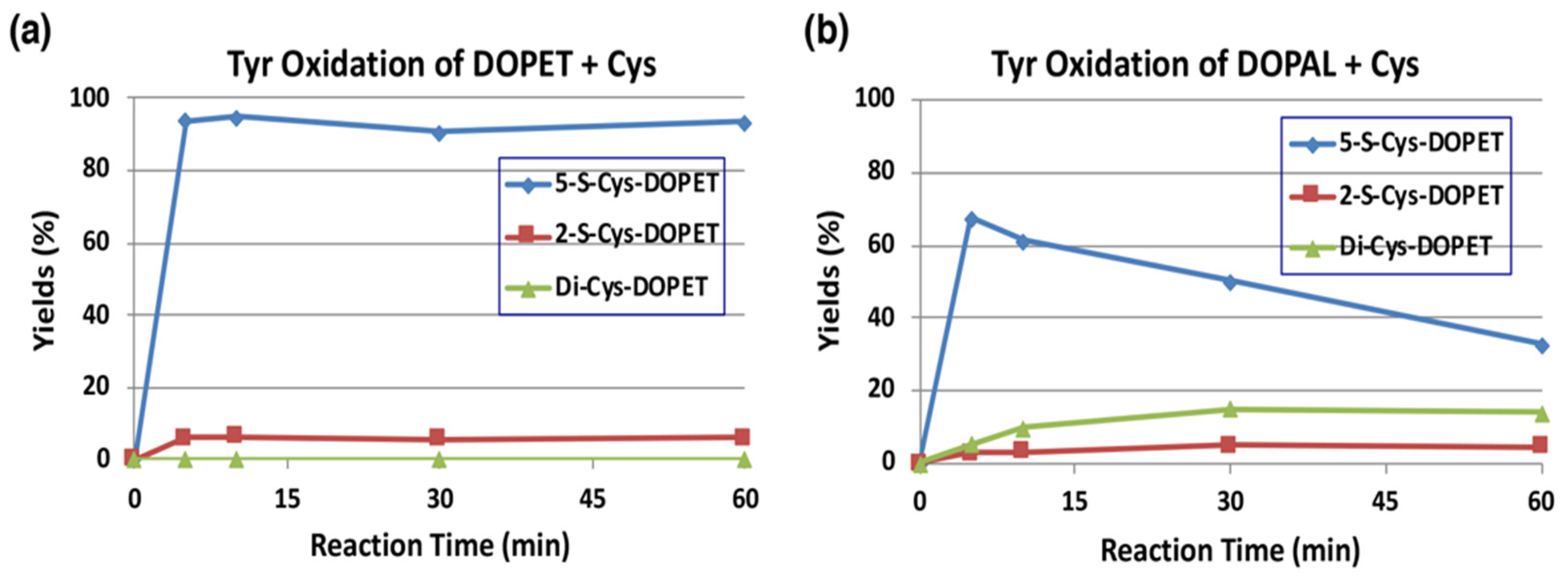
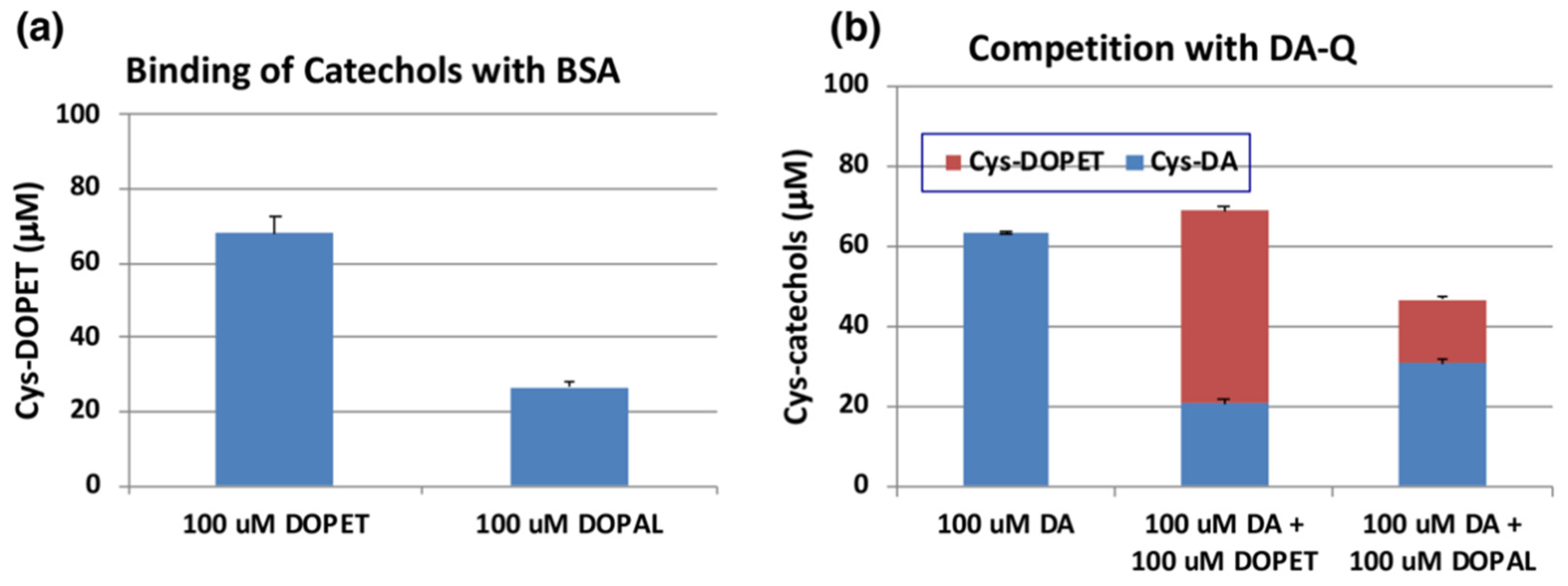
2.4. Reaction of DOPAL-Q with L-Cysteine (Cys) and Thiol-Proteins
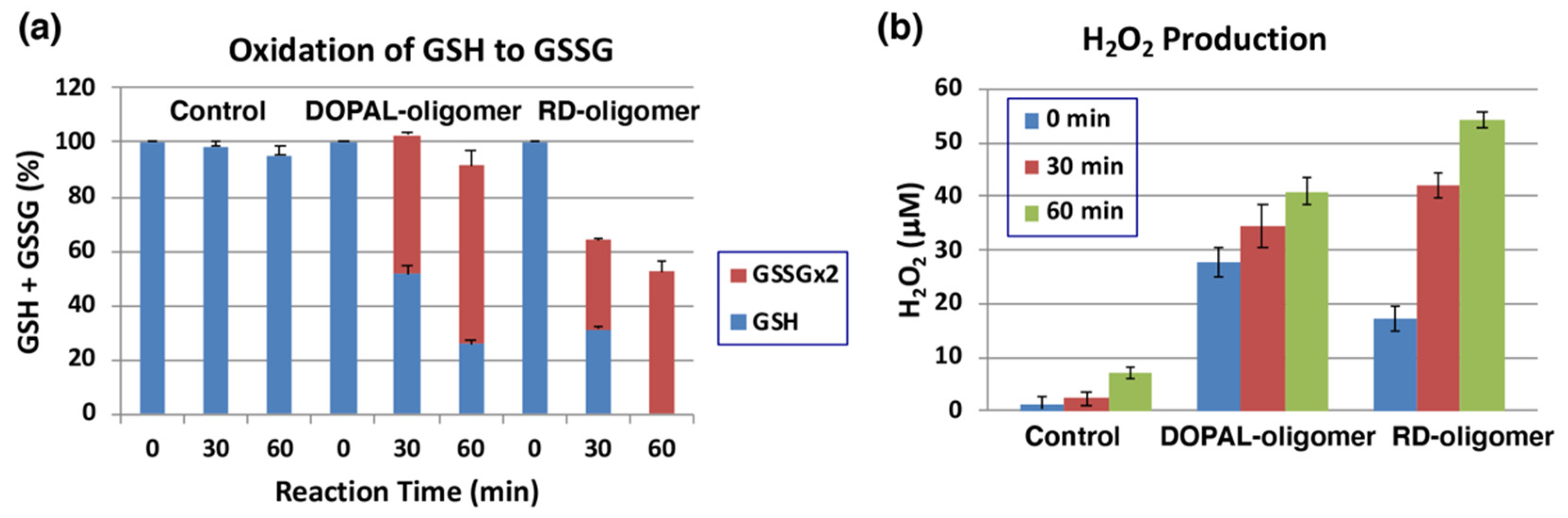
2.5. Pro-Oxidant Activity of DOPAL-Oligomer
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Analytical Methods
4.3. Oxidation of DOPAL by Tyrosinase or Metal Ions in the Absence or Presence of L-Ascorbic Acid (AA) or Cys
4.4. Oxidation of DOPAL, DOPET, or DA in the Presence of BSA and HPLC Estimates of Binding through the Cys Residue
4.5. Synthesis of Cys-DOPET and Cys-DOPET Derivatives
4.6. Pro-Oxidant Activity of the DOPAL Oxidation Product, DOPAL-Oligomer
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| Cys | L-cysteine |
| DA | dopamine |
| DHBAlc | 3,4-dihydroxybenzylalcohol |
| DHBAld | 3,4-dihydroxybenzaldehyde |
| DHBF | 5,6-dihydroxybenzofuran |
| DOMA | 3,4-dihydroxymandelic acid |
| DOPA | L-3,4-dihydroxyphenylalanine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DOPAL | 3,4-dihydroxyphenylacetaldehyde |
| DOPEGAL | 3,4-dihydroxyphenylglycolaldehyde |
| DOPET | 2-(3,4-dihydroxyphenyl)ethanol |
| DOPEG | 3,4-dihydroxyphenylethylene glycol |
| MAO | monoamine oxidase |
| NE | norepinephrine |
| RD | rhododendrol |
| THBF | 3,5,6-trihydroxybenzofuran |
References
- Hornykiewicz, O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol. Rev. 1966, 18, 925–964. [Google Scholar]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Alter, S.; Strong, R.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J. Neurochem. 2013, 126, 591–603. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Kopin, I.J.; Sharabi, Y.; Mash, D.C. Decreased vesicular storage and aldehyde dehydrogenase activity in multiple system atrophy. Parkinsonism Relat. Disord. 2015, 21, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Mattammal, M.B.; Haring, J.H.; Chung, H.D.; Raghu, G.; Strong, R. An endogenous dopaminergic neurotoxin: Implication for Parkinson’s disease. Neurodegeneration 1995, 4, 271–281. [Google Scholar] [CrossRef]
- Cagle, B.S.; Crawford, R.A.; Doorn, J.A. Biogenetic aldehyde-mediated mechanisms of toxicity in neurodegenerative disease. Curr. Opin. Toxicol. 2019, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Jinsmaa, Y.; Sullivan, P.; Sharabi, Y. N-Acetylcysteine prevents the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochem. Res. 2017, 42, 3289–3295. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Williams, E.A.; Nonneman, R.; Zahm, D.S. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res. 2003, 989, 205–213. [Google Scholar] [CrossRef]
- Panneton, W.M.; Kumar, V.B.; Gan, Q.; Burke, W.J.; Galvin, J.E. The neurotoxicity of DOPAL: Behavioral and stereological evidence for its role in Parkinson disease pathogenesis. PLoS ONE 2010, 5, e15251. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J.; Sharabi, Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 144, 268–282. [Google Scholar] [CrossRef] [Green Version]
- Jinsmaa, Y.; Isonaka, R.; Sharabi, Y.; Goldstein, D.S. 3,4-Dihydroxyphenylacetaldehyde is more efficient than dopamine in oligomerizing and quinonizing α-synuclein. J. Pharmacol. Exp. Ther. 2020, 372, 157–165. [Google Scholar] [CrossRef]
- Sun, J.; He, C.; Yan, Q.-X.; Wang, H.-D.; Li, K.-X.; Sun, X.; Feng, Y.; Zha, R.-R.; Cui, C.-P.; Xiong, X.; et al. Parkinson-like early autonomic dysfunction induced by vagal application of DOPAL in rats. CNS Neurosci. Ther. 2021, 27, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Jinsmaa, Y.; Sharabi, Y.; Sullivan, P.; Isonaka, R.; Goldsteain, D.S. 3,4-Dihydroxyphenylacetaldehyde-induced protein modifications and their mitigation by N-acetylcysteine. J. Pharmacol. Exp. Ther. 2018, 366, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S. Biomarkers, mechanisms, and potential prevention of catecholamine neuron loss in Parkinson disease. Adv. Pharmacol. 2013, 68, 235–272. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. The catecholaldehyde hypothesis: Where MAO fits in. J. Neural Transm. 2020, 127, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. The catecholaldehyde hypothesis for the pathogenesis of catecholaminergic neurodegeration: What we know and we do not know. Int. J. Mol. Sci. 2021, 22, 5999. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.N.; Florang, V.R.; Eckert, L.L.; Doorn, J.A. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. 2009, 22, 1256–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner-Allen, J.W.; DuMond, J.F.; Levine, R.L.; Bax, A. Toxic dopamine metabolite DOPAL forms an unexpected dicatechol pyrrole adduct with lysines of α-synuclein. Angew. Chem. Int. Ed. Engl. 2016, 55, 7374–7378. [Google Scholar] [CrossRef] [Green Version]
- Werner-Allen, J.W.; Monti, S.; DuMond, J.F.; Levine, R.L.; Bax, A. Isoindole linkages provide a pathway for DOPAL-mediated cross-linking of α-synuclein. Biochemistry 2018, 57, 1462–1474. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinoid products formed by tyrosinase and phenoloxidase action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of quinone methides versus o-quinones in catecholamine metabolism and eumelanin biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef] [Green Version]
- Vermeer, L.M.; Florang, V.R.; Doorn, J.A. Catechol and aldehyde moieties of 3,4-dihydroxyphenylacetaldehyde contribute to tyrosine hydroxylase inhibition and neurotoxicity. Brain Res. 2012, 1474, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.G.; Mariappan, S.V.; Buettner, G.R.; Doorn, J.A. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011, 286, 26978–26986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Yamanaka, Y.; Ojika, M.; Wakamatsu, K. The metabolic fate of ortho-quinones derived from catecholamine metabolites. Int. J. Mol. Sci. 2016, 17, 164. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M. Tyrosinase catalyzes an unusual oxidative decarboxylation of 3,4-dihydroxymandelate. Biochemistry 1986, 25, 4489–4492. [Google Scholar] [CrossRef]
- Sugumaran, M.; Dali, H.; Semensi, V.; Hannigan, B. Tyrosinase catalyzed unusual oxidative dimerization of 1,2-dehydro-N-acetyldopamine. J. Biol. Chem. 1987, 262, 10546–10549. [Google Scholar] [CrossRef]
- Sugumaran, M.; Semensi, V.; Dali, H.; Mitchell, W. Novel transformations of enzymatically generated carboxymethyl-o-benzoquinone to 2,5,6-trihydroxybenzofuran and 3,4-dihydroxymandelic acid. Bioorg. Chem. 1989, 17, 86–95. [Google Scholar] [CrossRef]
- Nakamura, T. On the process of enzymatic oxidation of hydroquinone. Biochem. Biophys. Res. Commun. 1960, 2, 111–113. [Google Scholar] [CrossRef]
- Sugumaran, M.; Semensi, V.; Kalyanaraman, B.; Bruce, M.J.; Land, E.J. Evidence for the formation of a quinone methide during the oxidation of the insect cuticular sclerotizing precursor, 1,2-dehydro-N-acetyldopamine. J. Biol. Chem. 1992, 267, 10355–10361. [Google Scholar] [CrossRef]
- Sugumaran, M. Oxidation chemistry of 1,2-dehydro-N-acetyldopamines: Direct evidence for the formation of 1,2-dehydro-N-acetyldopamine quinone. Arch. Biochem. Biophys. 2000, 378, 404–410. [Google Scholar] [CrossRef]
- Bolton, J.L.; Acay, N.M.; Vukomanovic, V. Evidence that 4-allyl-o-quinones spontaneously rearrange to their more electrophilic quinone methides: Potential bioactivation mechanism for the hepatocarcinogen safrole. Chem. Res. Toxicol. 1994, 7, 443–450. [Google Scholar] [CrossRef]
- Sugumaran, M.; Bolton, J. Direct evidence for quinone–quinone methide tautomerization during tyrosinase catalyzed oxidation of 4-allylcatechol. Biochem. Biophys. Res. Commun. 1995, 213, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Duggaraju, P.; Jayachandran, E.; Kirk, K.L. Formation of a new quinone methide intermediate during the oxidative transformation of 3,4-dihydroxyphenylacetic acids: Implications for melanin biosynthesis. Arch. Biochem. Biophys. 1999, 371, 98–106. [Google Scholar] [CrossRef]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of the leukoderma-inducing agent raspberry ketone produces (E)-4-(3-oxo-1-butenyl)-1,2-benzoquinone: Implications for melanocyte toxicity. Chem. Res. Toxicol. 2017, 20, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Dali, H.; Semensi, V. Mechanistic studies on tyrosinase-catalysed oxidative decarboxylation of 3,4-dihydroxymandelic acid. Biochem. J. 1992, 281, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Burke, W.J.; Schmitt, C.A.; Gillespie, K.N.; Li, S.W. Norepinephrine transmitter metabolite is a selective cell death messenger in differentiated rat pheochromocytoma cells. Brain Res. 1996, 722, 232–235. [Google Scholar] [CrossRef]
- Bagha, U.K.; Satpathy, J.K.; Mukherjee, G.; Sastri, C.V.; de Visser, S.P. A comprehensive insight into aldehyde deformylation: Mechanistic implications from biology and chemistry. Org. Biomol. Chem. 2021, 19, 1879–1899. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Sullivan, P.; Gross, D.; Cooney, A.; Sharabi, Y.; Goldstein, D.S. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci. Lett. 2014, 569, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G. A facile one-step synthesis of cysteinyldopas using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef]
- Ito, S.; Fujita, K.; Yoshioka, M.; Sienko, D.; Nagatsu, T. Identification of 5-S- and 2-S-cysteinyldopamine and 5-S-glutathionyldopamine formed from dopamine by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. 1986, 375, 134–140. [Google Scholar] [CrossRef]
- Kato, T.; Ito, S.; Fujita, K. Tyrosinase-catalyzed binding of 3,4-dihydroxyphenylalanine with proteins through the sulfhydryl group. Biochim. Biophys. Acta 1986, 881, 415–421. [Google Scholar] [CrossRef]
- Ito, S.; Kato, T.; Fujita, K. Covalent binding of catechols to proteins through the sulphydryl group. Biochem. Pharmacol. 1988, 37, 1707–1710. [Google Scholar] [CrossRef]
- Ito, S.; Okura, M.; Nakanishi, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T. Tyrosinase-catalyzed metabolism of rhododendrol (RD) in B16 melanoma cells: Production of RD-pheomelanin and covalent binding with thiol proteins. Pigment Cell Melanoma Res. 2015, 28, 295–306. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Tabuchi, K.; Ojika, M.; Zucca, F.A.; Zecca, L.; Ito, S. Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 2015, 135, 768–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Okura, M.; Wakamatsu, K.; Yamashita, T. The potent pro-oxidant activity of rhododendrol-eumelanin induces cysteine depletion in B16 melanoma cells. Pigment Cell Melanoma Res. 2017, 30, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Fujiki, Y.; Matsui, N.; Ojika, M.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of resveratrol produces a highly reactive ortho-quinone: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2019, 32, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ito, S.; Ojika, M.; Nishimaki-Mogami, T.; Kondo, K.; Wakamatsu, K. The oxidation of equol by tyrosinase produces a unique di-ortho-quinone: Possible implications for melanocyte toxicity. Int. J. Mol. Sci. 2021, 22, 9145. [Google Scholar] [CrossRef]
- Matsunaga, K.; Suzuki, K.; Ito, A.; Tanemura, A.; Abe, Y.; Suzuki, T.; Yoshikawa, M.; Sumikawa, Y.; Yagami, A.; Masui, Y.; et al. Rhododendrol-induced leukoderma update I: Clinical findings and treatment. J. Dermatol. 2021, 48, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ito, S.; Fujita, K. Determination of natural thiols by liquid chromatography after derivatization with 3,5-di-tert.-butyl-1,2-benzoquinone. J. Chromatogr. 1987, 420, 404–410. [Google Scholar] [CrossRef]
- Thompson, E.A., Jr.; Siiteri, P.K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J. Biol. Chem. 1974, 249, 5364–5372. [Google Scholar] [CrossRef]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef]
- Sugumaran, M.; Evans, J.; Ito, S.; Wakamatsu, K. Nonenzymatic spontaneous oxidative transformation of 5,6-dihydroxyindole. Int. J. Mol. Sci. 2020, 21, 7321. [Google Scholar] [CrossRef]
- Kang, S.S.; Liu, X.; Ahn, E.H.; Xiang, J.; Manfredsson, F.P.; Yang, X.; Luo, H.R.; Liles, C.; Weinshenker, D.; Ye, K. Norepinephrine metabolite DOPEGAL activates AEP and pathological Tau aggregation in locus coeruleus. J. Clin. Investig. 2020, 130, 422–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, W.J.; Li, S.W.; Schmitt, C.A.; Xia, P.; Chung, H.D.; Gillespie, K.N. Accumulation of 3,4-dihydroxyphenylglycolaldehyde, the neurotoxic monoamine oxidase A metabolite of norepinephrine, in locus ceruleus cell bodies in Alzheimer’s disease: Mechanism of neuron death. Brain Res. 1999, 816, 633–637. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Chung, H.D.; Ruggiero, D.A.; Kristal, B.S.; Johnson, E.M.; Lampe, P.; Kumar, V.B.; Franko, M.; Williams, E.A.; et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: Role in neurodegenerative diseases. Neurotoxicology 2004, 25, 101–115. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, S.-H. Protocatechuic aldehyde inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells via PKA/CREB-associated MITF downregulation. Int. J. Mol. Sci. 2021, 22, 3861. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, K.; Nagatsu, I.; Ito, S.; King, R.A.; Nishimura, A.; Nagatsu, T. Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 1998, 253, 198–200. [Google Scholar] [CrossRef]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A.; et al. Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat. Commum. 2019, 10, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.M.; Bohic, S.; Carmona, A.; Ortega, R.; Cottam, V.; Hare, D.J.; Finberg, J.P.M.; Reyes, S.; Halliday, G.M.; Mercer, J.F.B.; et al. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging 2014, 35, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.G.; Ball, H.J.; Hare, D.J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.; Kaur, P.; Gupta, G.D.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, M.; Church, S.J.; Xu, J.; Patassini, S.; Roncaroli, F.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Widespread decreases in cerebral copper are common to Parkinson’s disease dementia and Alzheimer’s disease dementia. Front. Aging Neurosci. 2021, 13, 641222. [Google Scholar] [CrossRef]
- Crawfold, R.A.; Gilardoni, E.; Monroe, T.B.; Regazzoni, L.; Anderson, E.J.; Doorn, J.A. Characterization of catecholaldehyde adducts with carnosine and L-cysteine reveals their potential as biomarkers of catecholaminergic stress. Chem. Res. Toxicol. 2021, 34, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Florang, V.R.; Schamp, J.H.; Buettner, G.R.; Doorn, J.A. Antioxidant-mediated modulation of protein reactivity for 3,4-dihydroxyhenylacetaldehyde, a toxic dopamine metabolite. Chem. Res. Toxicol. 2016, 29, 1098–1107. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Nakao, K.; Tanaka, H.; Kitahori, Y.; Tanaka, Y.; Ojika, M.; Ito, S. The oxidative pathway to dopamine-protein conjugates and their pro-oxidant activities: Implications for the neurodegeneration of Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Panchuk-Voloshina, N. A one-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal. Biochem. 1997, 253, 169–174. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Tanaka, H.; Ojika, M.; Wakamatsu, K.; Sugumaran, M. Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity. Int. J. Mol. Sci. 2021, 22, 11751. https://doi.org/10.3390/ijms222111751
Ito S, Tanaka H, Ojika M, Wakamatsu K, Sugumaran M. Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity. International Journal of Molecular Sciences. 2021; 22(21):11751. https://doi.org/10.3390/ijms222111751
Chicago/Turabian StyleIto, Shosuke, Hitomi Tanaka, Makoto Ojika, Kazumasa Wakamatsu, and Manickam Sugumaran. 2021. "Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity" International Journal of Molecular Sciences 22, no. 21: 11751. https://doi.org/10.3390/ijms222111751
APA StyleIto, S., Tanaka, H., Ojika, M., Wakamatsu, K., & Sugumaran, M. (2021). Oxidative Transformations of 3,4-Dihydroxyphenylacetaldehyde Generate Potential Reactive Intermediates as Causative Agents for Its Neurotoxicity. International Journal of Molecular Sciences, 22(21), 11751. https://doi.org/10.3390/ijms222111751








