Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation
Abstract
1. Introduction
2. Role of Estrogen Signaling through ERα
2.1. Lipoprotein Lipase
2.2. Adipogenesis via Peroxisome Proliferator-Activated Receptor Gamma (PPARγ)
2.3. Lipolysis/Lipogenesis via Adrenergic Receptors (ARs)
2.4. Glucose Transporter 4 (GLUT 4) and Glucose Translocation
2.5. Angiogenesis via Vascular Endothelial Growth Factors (VEGF)
3. Role of Estrogen Signaling through ERß
3.1. Inhibitor of ERα
3.2. PPARγ Activity and Adipogenesis
4. Coregulators of the Estrogen Receptor
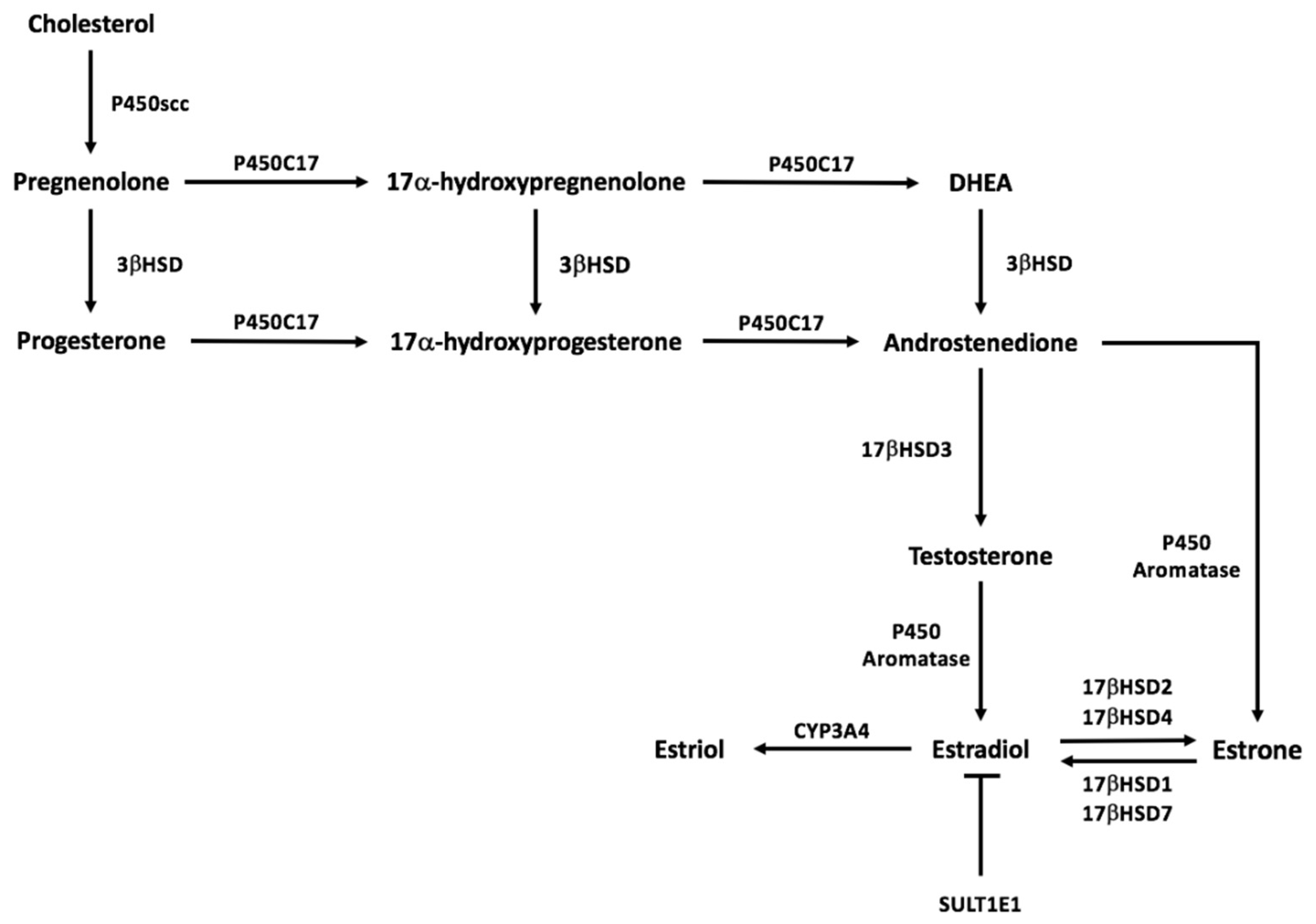
5. Estrogen Synthesis Pathway
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Forner-Cordero, I.; Szolnoky, G.; Forner-Cordero, A.; Kemeny, L. Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome—Systematic review. Clin. Obes. 2012, 2, 86–95. [Google Scholar] [CrossRef]
- Lontok, E.; Briggs, L.; Donlan, M.; Kim, Y.; Mosley, E.; Riley, E.A.U.; Stevens, M. Lipedema—A Giving Smarter Guide; Milken Institute Center for Strategic Philanthropy: Washington, DA, USA, 2017; pp. 1–40. [Google Scholar]
- Buso, G.; Depairon, M.; Tomson, D.; Raffoul, W.; Vettor, R.; Mazzolai, L. Lipedema: A Call to Action! Obesity 2019, 27, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Romeijn, J.R.M.; de Rooij, M.J.M.; Janssen, L.; Martens, H. Exploration of Patient Characteristics and Quality of Life in Patients with Lipoedema Using a Survey. Dermatol. Ther. 2018, 8, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, A.; Fetzer, S. Lipoedema UK Big Survey 2014 Research Report; Lipoedema: London, UK, 2016; pp. 2–8. [Google Scholar]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med. Genet. A 2010, 152, 970–976. [Google Scholar] [CrossRef]
- Paolacci, S.; Precone, V.; Acquaviva, F.; Chiurazzi, P.; Fulcheri, E.; Pinelli, M.; Buffelli, F.; Michelini, S.; Herbst, K.L.; Unfer, V.; et al. Genetics of lipedema: New perspectives on genetic research and molecular diagnoses. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5581–5594. [Google Scholar] [CrossRef]
- Halk, A.B.; Damstra, R.J. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebology 2017, 32, 152–159. [Google Scholar] [CrossRef]
- Marshall, M.; Schwahn-Schreiber, C. Prevalence of lipoedema in professional women in Germany. (Lipoedema-3-study). Phlebologie 2011, 40, 127–133. [Google Scholar]
- Al-Ghadban, S.; Herbst, K.L.; Bunnell, B.A. Lipedema: A Painful Adipose Tissue Disorder. In Adipose Tissue—An Update; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Mayes, J.S.; Watson, G.H. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes. Rev. 2004, 5, 197–216. [Google Scholar] [CrossRef]
- Szél, E.; Kemény, L.; Groma, G.; Szolnoky, G. Pathophysiological dilemmas of lipedema. Med. Hypotheses 2014, 83, 599–606. [Google Scholar] [CrossRef]
- Priglinger, E.; Wurzer, C.; Steffenhagen, C.; Maier, J.; Hofer, V.; Peterbauer, A.; Nuernberger, S.; Redl, H.; Wolbank, S.; Sandhofer, M. The adipose tissue-derived stromal vascular fraction cells from lipedema patients: Are they different? Cytotherapy 2017, 19, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef]
- Frank, A.P.; de Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar] [CrossRef]
- Björnström, L.; Sjöberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, T.H.; Choi, K.C. Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse. Lab. Anim. Res. 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Barros, R.P.; Gabbi, C.; Morani, A.; Warner, M.; Gustafsson, J.A. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E124–E133. [Google Scholar] [CrossRef]
- Blüher, M. Importance of estrogen receptors in adipose tissue function. Mol. Metab. 2013, 2, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: Regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 2004, 286, C655–C661. [Google Scholar] [CrossRef] [PubMed]
- Krotkiewski, M.; Björntorp, P.; Sjöström, L.; Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Investig. 1983, 72, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Carr, M.C.; Murdoch, S.J.; Mitchell, E.; Woods, N.F.; Wener, M.H.; Chandler, W.L.; Boyko, E.J.; Brunzell, J.D. Adipokines, inflammation, and visceral adiposity across the menopausal transition: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 1104–1110. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Toth, M.J.; Gardner, A.W. Changes in energy balance and body composition at menopause: A controlled longitudinal study. Ann. Intern. Med. 1995, 123, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef]
- Gavin, K.M.; Cooper, E.E.; Raymer, D.K.; Hickner, R.C. Estradiol effects on subcutaneous adipose tissue lipolysis in premenopausal women are adipose tissue depot specific and treatment dependent. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1167–E1174. [Google Scholar] [CrossRef]
- Martin, M.L.; Jensen, M.D. Effects of body fat distribution on regional lipolysis in obesity. J. Clin. Investig. 1991, 88, 609–613. [Google Scholar] [CrossRef]
- Rebuffé-Scrive, M.; Eldh, J.; Hafström, L.O.; Björntorp, P. Metabolism of mammary, abdominal, and femoral adipocytes in women before and after menopause. Metabolism 1986, 35, 792–797. [Google Scholar] [CrossRef]
- Wahrenberg, H.; Lönnqvist, F.; Arner, P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J. Clin. Investig. 1989, 84, 458–467. [Google Scholar] [CrossRef]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef]
- Yagyu, H.; Chen, G.; Yokoyama, M.; Hirata, K.; Augustus, A.; Kako, Y.; Seo, T.; Hu, Y.; Lutz, E.P.; Merkel, M.; et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Investig. 2003, 111, 419–426. [Google Scholar] [CrossRef]
- Weigt, C.; Hertrampf, T.; Kluxen, F.M.; Flenker, U.; Hülsemann, F.; Fritzemeier, K.H.; Diel, P. Molecular effects of ER alpha- and beta-selective agonists on regulation of energy homeostasis in obese female Wistar rats. Mol. Cell Endocrinol. 2013, 377, 147–158. [Google Scholar] [CrossRef]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef]
- Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Høst, C.; Hjerrild, B.E.; Pedersen, S.B.; Nielsen, S.; Christiansen, J.S.; Gravholt, C.H. Estradiol acutely inhibits whole body lipid oxidation and attenuates lipolysis in subcutaneous adipose tissue: A randomized, placebo-controlled study in postmenopausal women. Eur. J. Endocrinol. 2012, 167, 543–551. [Google Scholar] [CrossRef]
- Santosa, S.; Jensen, M.D. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes 2013, 62, 775–782. [Google Scholar] [CrossRef]
- Arner, P.; Lithell, H.; Wahrenberg, H.; Bronnegard, M. Expression of lipoprotein lipase in different human subcutaneous adipose tissue regions. J. Lipid Res. 1991, 32, 423–429. [Google Scholar] [CrossRef]
- Price, T.M.; O’Brien, S.N.; Welter, B.H.; George, R.; Anandjiwala, J.; Kilgore, M. Estrogen regulation of adipose tissue lipoprotein lipase--possible mechanism of body fat distribution. Am. J. Obstet. Gynecol. 1998, 178, 101–107. [Google Scholar] [CrossRef]
- Lindberg, U.B.; Crona, N.; Silfverstolpe, G.; Bjorntorp, P.; Rebuffe-Scrive, M. Regional adipose tissue metabolism in postmenopausal women after treatment with exogenous sex steroids. Horm. Metab. Res. 1990, 22, 345–351. [Google Scholar] [CrossRef]
- Ferrara, C.M.; Lynch, N.A.; Nicklas, B.J.; Ryan, A.S.; Berman, D.M. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 4166–4170. [Google Scholar] [CrossRef]
- Ferré, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53 (Suppl. 1), S43–S50. [Google Scholar] [CrossRef]
- Foryst-Ludwig, A.; Clemenz, M.; Hohmann, S.; Hartge, M.; Sprang, C.; Frost, N.; Krikov, M.; Bhanot, S.; Barros, R.; Morani, A.; et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008, 4, e1000108. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Chu, R.; van Hasselt, A.; Vlantis, A.C.; Ng, E.K.; Liu, S.Y.; Fan, M.D.; Ng, S.K.; Chan, A.B.; Liu, Z.; Li, X.Y.; et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer 2014, 120, 142–153. [Google Scholar] [CrossRef]
- Hong, L.; Colpan, A.; Peptan, I.A.; Daw, J.; George, A.; Evans, C.A. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007, 13, 1197–1203. [Google Scholar] [CrossRef]
- Sato, H.; Sugai, H.; Kurosaki, H.; Ishikawa, M.; Funaki, A.; Kimura, Y.; Ueno, K. The effect of sex hormones on peroxisome proliferator-activated receptor gamma expression and activity in mature adipocytes. Biol. Pharm. Bull. 2013, 36, 564–573. [Google Scholar] [CrossRef]
- Luglio, H.F. Estrogen and body weight regulation in women: The role of estrogen receptor alpha (ER-α) on adipocyte lipolysis. Acta Med. Indones. 2014, 46, 333–338. [Google Scholar]
- Carmen, G.Y.; Víctor, S.M. Signalling mechanisms regulating lipolysis. Cell. Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Bessesen, D.H.; Stotz, S.; Peelor, F.F.; Miller, B.F.; Horton, T.J. Adrenergic control of lipolysis in women compared with men. J. Appl. Physiol. 2014, 117, 1008–1019. [Google Scholar] [CrossRef]
- Masuo, K.; Lambert, G.W. Relationships of adrenoceptor polymorphisms with obesity. J. Obes. 2011, 2011, 609485. [Google Scholar] [CrossRef]
- Watson, R.T.; Pessin, J.E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001, 56, 175–193. [Google Scholar] [CrossRef]
- Barros, R.P.; Machado, U.F.; Warner, M.; Gustafsson, J.A. Muscle GLUT4 regulation by estrogen receptors ERbeta and ERalpha. Proc. Natl. Acad. Sci. USA 2006, 103, 1605–1608. [Google Scholar] [CrossRef]
- Campello, R.S.; Fatima, L.A.; Barreto-Andrade, J.N.; Lucas, T.F.; Mori, R.C.; Porto, C.S.; Machado, U.F. Estradiol-induced regulation of GLUT4 in 3T3-L1 cells: Involvement of ESR1 and AKT activation. J. Mol. Endocrinol. 2017, 59, 257–268. [Google Scholar] [CrossRef]
- Barreto-Andrade, J.N.; de Fatima, L.A.; Campello, R.S.; Guedes, J.A.C.; de Freitas, H.S.; Machado, M. Estrogen Receptor 1 (ESR1) Enhances Slc2a4/GLUT4 Expression by a SP1 Cooperative Mechanism. Int. J. Med. Sci. 2018, 15, 1320–1328. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef][Green Version]
- Fatima, L.A.; Campello, R.S.; Santos, R.S.; Freitas, H.S.; Frank, A.P.; Machado, U.F.; Clegg, D.J. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Sci. Rep. 2017, 7, 16716. [Google Scholar] [CrossRef]
- Applanat, M.P.; Buteau-Lozano, H.; Herve, M.A.; Corpet, A. Vascular endothelial growth factor is a target gene for estrogen receptor and contributes to breast cancer progression. Adv. Exp. Med. Biol. 2008, 617, 437–444. [Google Scholar] [CrossRef]
- Garvin, S.; Nilsson, U.W.; Huss, F.R.; Kratz, G.; Dabrosin, C. Estradiol increases VEGF in human breast studied by whole-tissue culture. Cell Tissue Res. 2006, 325, 245–251. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic activation of estrogen receptor beta increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J. 2017, 31, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Cooper, E.E.; Hickner, R.C. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism 2013, 62, 1180–1188. [Google Scholar] [CrossRef]
- Shin, J.H.; Hur, J.Y.; Seo, H.S.; Jeong, Y.A.; Lee, J.K.; Oh, M.J.; Kim, T.; Saw, H.S.; Kim, S.H. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids 2007, 72, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.C.; Frasor, J.; Komm, B.; Katzenellenbogen, B.S. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 2006, 147, 4831–4842. [Google Scholar] [CrossRef]
- Pinton, G.; Nilsson, S.; Moro, L. Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: Importance of KDM6B and SIRT1 for ERβ expression and functionality. Oncogenesis 2018, 7, 15. [Google Scholar] [CrossRef]
- Bartella, V.; Rizza, P.; Barone, I.; Zito, D.; Giordano, F.; Giordano, C.; Catalano, S.; Mauro, L.; Sisci, D.; Panno, M.L.; et al. Estrogen receptor beta binds Sp1 and recruits a corepressor complex to the estrogen receptor alpha gene promoter. Breast Cancer Res. Treat. 2012, 134, 569–581. [Google Scholar] [CrossRef]
- Lonard, D.M.; Lanz, R.B.; O’Malley, B.W. Nuclear receptor coregulators and human disease. Endocr. Rev. 2007, 28, 575–587. [Google Scholar] [CrossRef]
- Onate, S.A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Paramanik, V.; Krishnan, H.; Kumar Thakur, M. Estrogen Receptor alpha- and beta-Interacting Proteins Contain Consensus Secondary Structures: An Insilico Study. Ann. Neurosci. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Manavathi, B.; Samanthapudi, V.S.; Gajulapalli, V.N. Estrogen receptor coregulators and pioneer factors: The orchestrators of mammary gland cell fate and development. Front. Cell Dev. Biol. 2014, 2, 34. [Google Scholar] [CrossRef]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Tchernof, A.; Mansour, M.F.; Pelletier, M.; Boulet, M.M.; Nadeau, M.; Luu-The, V. Updated survey of the steroid-converting enzymes in human adipose tissues. J. Steroid Biochem. Mol. Biol. 2015, 147, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 1987, 45, 277–282. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Fluck, C.E.; Miller, W.L.; Auchus, R.J. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 2003, 88, 3762–3766. [Google Scholar] [CrossRef] [PubMed]
| Type | Fat Location |
|---|---|
| Type 1 | Pelvis, buttocks, hips |
| Type 2 | Buttocks to knees, with formations of folds of fat around the inner side of the knee |
| Type 3 | Buttocks to ankles |
| Type 4 a–c |
|
| Type 5 | Knees to ankles |
| Stage | Disease Progression |
|---|---|
| Stage 1 | Normal skin surface with enlarged subcutaneous tissue; fat tissue is soft with noticeable small nodules |
| Stage 2 | Uneven skin with enlarged subcutaneous tissue; larger fat nodules present |
| Stage 3 | Large extrusions of tissue causing deformations, especially on the thighs and around the knees; fat nodules of varying sizes are palpable |
| Stage 4 | Development of lipolymphedema with large overhangs of tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. Int. J. Mol. Sci. 2021, 22, 11720. https://doi.org/10.3390/ijms222111720
Katzer K, Hill JL, McIver KB, Foster MT. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. International Journal of Molecular Sciences. 2021; 22(21):11720. https://doi.org/10.3390/ijms222111720
Chicago/Turabian StyleKatzer, Kaleigh, Jessica L. Hill, Kara B. McIver, and Michelle T. Foster. 2021. "Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation" International Journal of Molecular Sciences 22, no. 21: 11720. https://doi.org/10.3390/ijms222111720
APA StyleKatzer, K., Hill, J. L., McIver, K. B., & Foster, M. T. (2021). Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. International Journal of Molecular Sciences, 22(21), 11720. https://doi.org/10.3390/ijms222111720






