Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics?
Abstract
:1. Introduction
2. Results
2.1. Piceatannol at Micromolar Concentrations Reduced the Senescence of MSCs
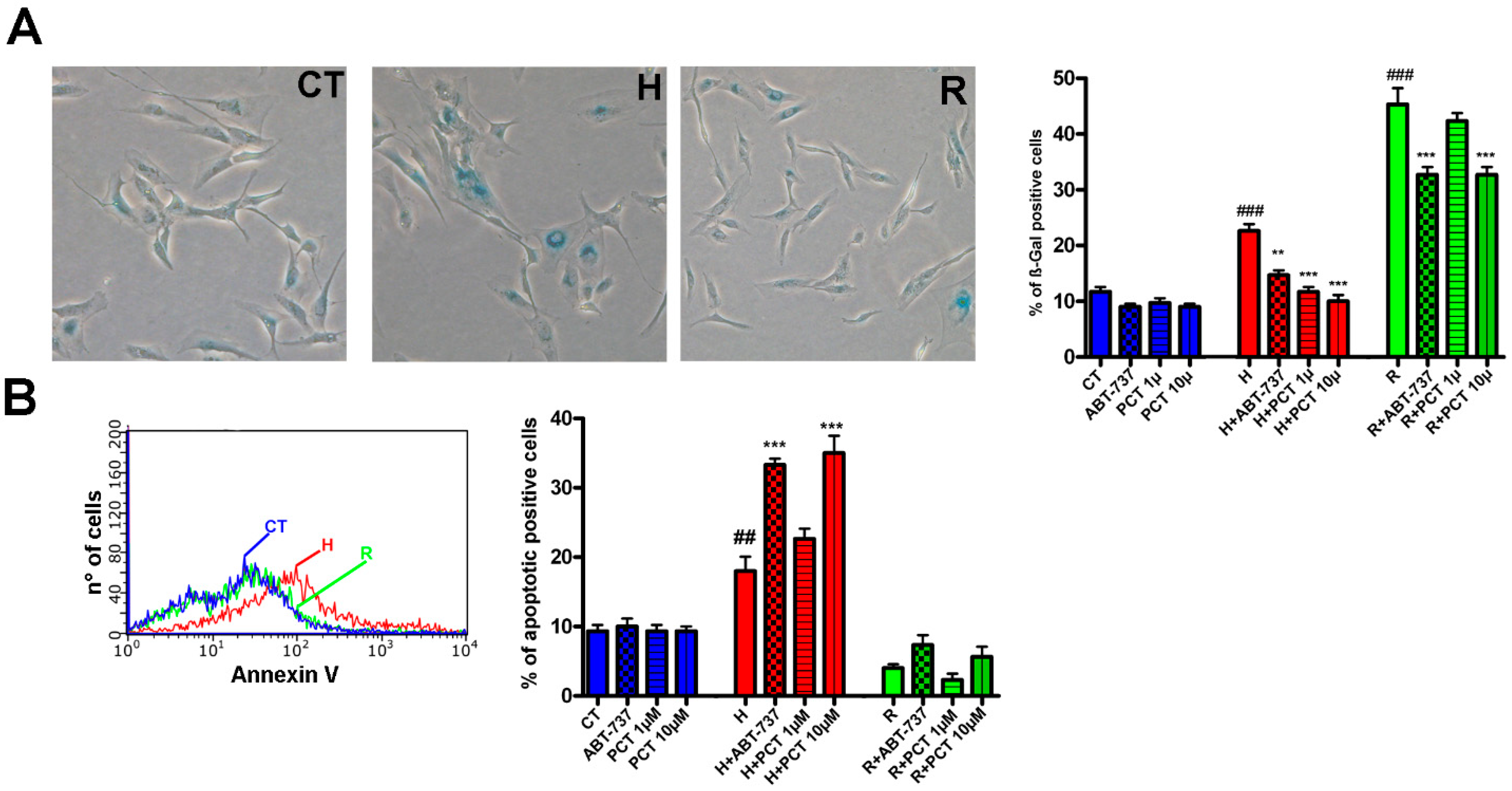
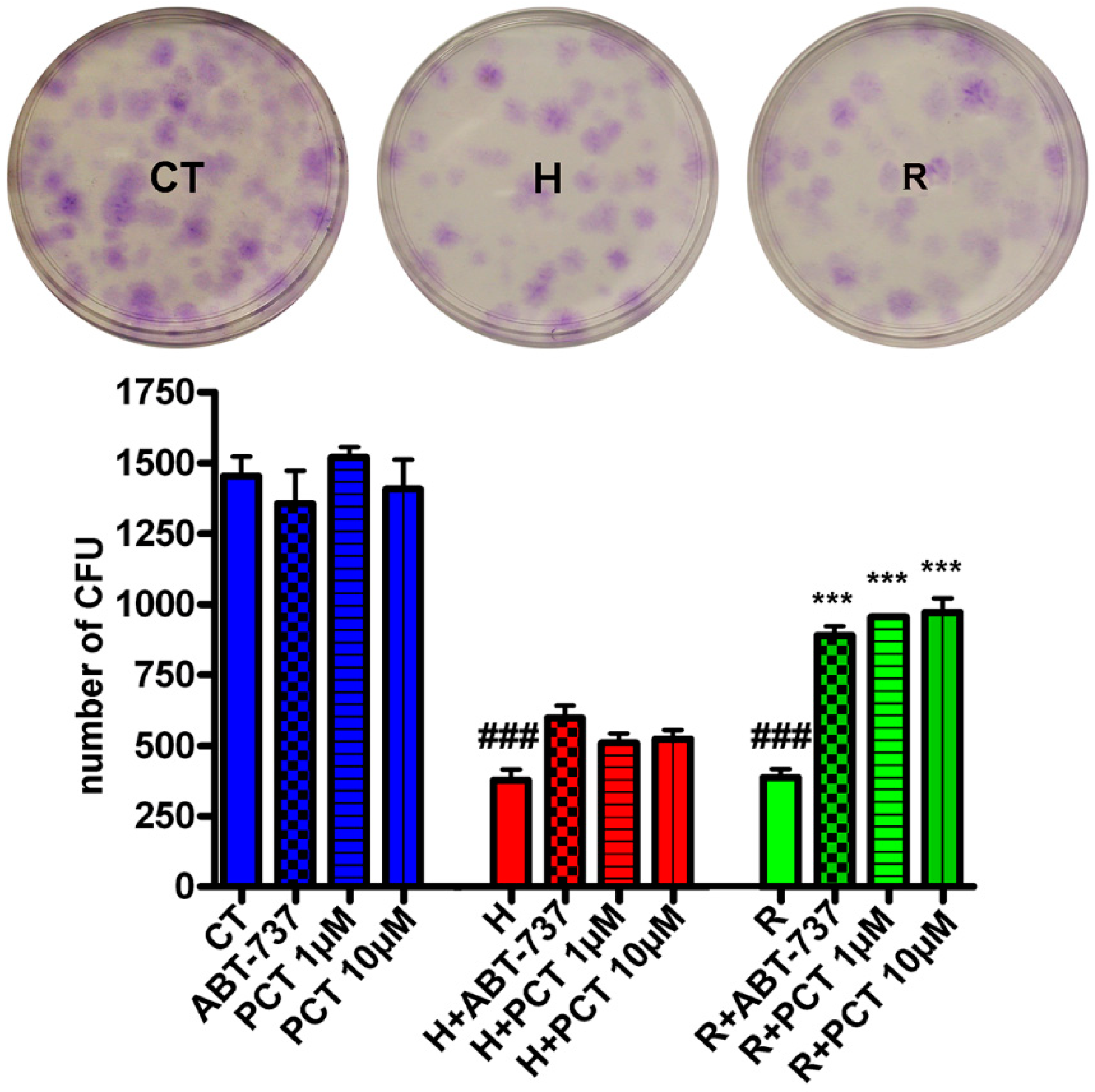
2.2. Piceatannol Effectively Act Both on Acute and Chronic Senescence
2.3. Piceatannol Affect Molecular Pathways Associated with Acute and Chronic Senescence
2.4. How Does Piceatannol Interfere with Senescence Signaling Pathways?
3. Discussion
3.1. Healthy Aging and Senotherapeutics
3.2. Piceatannol Counteracts Acute and Chronic Senescence
3.3. Molecular Execution Pathways Associated with Piceatannol’s Antisenescent Activity
3.4. Piceatannol Reinforces SIRT1 Decrease in Senescent Cells
3.5. Senolytics or Senomorphics?
3.6. Final Remarks
4. Materials and Methods
4.1. MSC Cultures
4.2. Acute and Chronic Senescent MSCs
4.3. In Situ Senescence-Associated Beta-Gal Assay
4.4. Cell Proliferation and Cytotoxicity Assay
4.5. Cell Cycle Analysis
4.6. Apoptosis Detection
4.7. Colony Forming Unit (CFU) Assay
4.8. Western Blot (WB) Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessio, N.; Aprile, D.; Squillaro, T.; Di Bernardo, G.; Finicelli, M.; Melone, M.A.; Peluso, G.; Galderisi, U. The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging 2019, 11, 5817–5828. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Alessio, N.; Acar, M.B.; Toprak, G.; Gonen, Z.B.; Peluso, G.; Galderisi, U. Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity. Oncotarget 2015, 6, 39482–39492. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Kim, J.R. Senotherapeutics: Emerging strategy for healthy aging and age-related disease. BMB Rep. 2019, 52, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Malavolta, M.; Bracci, M.; Santarelli, L.; Sayeed, M.A.; Pierpaoli, E.; Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Cardelli, M.; et al. Inducers of Senescence, Toxic Compounds, and Senolytics: The Multiple Faces of Nrf2-Activating Phytochemicals in Cancer Adjuvant Therapy. Mediat. Inflamm. 2018, 2018, 4159013. [Google Scholar] [CrossRef] [Green Version]
- Akinwumi, B.C.; Bordun, K.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [Green Version]
- Cipolletti, M.; Solar Fernandez, V.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: The Modulation of Estrogen Receptors (ERs) Signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef] [Green Version]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef] [PubMed]
- Kilic Eren, M.; Kilincli, A.; Eren, O. Resveratrol Induced Premature Senescence Is Associated with DNA Damage Mediated SIRT1 and SIRT2 Down-Regulation. PLoS ONE 2015, 10, e0124837. [Google Scholar] [CrossRef] [PubMed]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2021, 1–14. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Birar, V.C.; Sheerin, A.N.; Ostler, E.L.; Faragher, R.G.A. Novel resveratrol derivatives have diverse effects on the survival, proliferation and senescence of primary human fibroblasts. Biogerontology 2020, 21, 817–826. [Google Scholar] [CrossRef]
- Costa, F.P.D.; Puty, B.; Nogueira, L.S.; Mitre, G.P.; Santos, S.M.D.; Teixeira, B.J.B.; Kataoka, M.; Martins, M.D.; Barboza, C.A.G.; Monteiro, M.C.; et al. Piceatannol Increases Antioxidant Defense and Reduces Cell Death in Human Periodontal Ligament Fibroblast under Oxidative Stress. Antioxidants 2019, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Alessio, N.; Capasso, S.; Ferone, A.; Di Bernardo, G.; Cipollaro, M.; Casale, F.; Peluso, G.; Giordano, A.; Galderisi, U. Misidentified Human Gene Functions with Mouse Models: The Case of the Retinoblastoma Gene Family in Senescence. Neoplasia 2017, 19, 781–790. [Google Scholar] [CrossRef]
- Capasso, S.; Alessio, N.; Squillaro, T.; Di Bernardo, G.; Melone, M.A.; Cipollaro, M.; Peluso, G.; Galderisi, U. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget 2015, 6, 39457–39468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galderisi, U.; Cipollaro, M.; Giordano, A. The retinoblastoma gene is involved in multiple aspects of stem cell biology. Oncogene 2006, 25, 5250–5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.R.; Han, D.R.; Kim, S.H.; Ohn, T.; Jung, K.H.; Chai, Y.G. Resveratrol relieves hydrogen peroxide-induced premature senescence associated with SIRT1 in human mesenchymal stem cells. Mol. Cell. Toxicol. 2014, 10, 29–39. [Google Scholar] [CrossRef]
- Kao, C.L.; Chen, L.K.; Chang, Y.L.; Yung, M.C.; Hsu, C.C.; Chen, Y.C.; Lo, W.L.; Chen, S.J.; Ku, H.H.; Hwang, S.J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010, 17, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Brooks, C.L.; Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer 2009, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Wu, Z.; Mazur, S.J.; Borges, H.; Rossi, M.; Lin, T.; Wang, J.Y.; Anderson, C.W.; Appella, E.; Xu, Y. Acetylation of mouse p53 at lysine 317 negatively regulates p53 apoptotic activities after DNA damage. Mol. Cell. Biol. 2006, 26, 6859–6869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.; Luo, J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim. Biophys. Acta 2010, 1804, 1684–1689. [Google Scholar] [CrossRef] [Green Version]
- Peel, N.M.; McClure, R.J.; Bartlett, H.P. Behavioral determinants of healthy aging. Am. J. Prev. Med. 2005, 28, 298–304. [Google Scholar] [CrossRef]
- Hosoda, R.; Hamada, H.; Uesugi, D.; Iwahara, N.; Nojima, I.; Horio, Y.; Kuno, A. Different Antioxidative and Antiapoptotic Effects of Piceatannol and Resveratrol. J. Pharmacol. Exp. Ther. 2021, 376, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Parichatikanond, W.; Eroglu, E.; Klec, C.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+ Coupling between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016, 39, 1404–1420. [Google Scholar] [CrossRef]
- Xu, M.; Feng, M.; Peng, H.; Qian, Z.; Zhao, L.; Wu, S. Epigenetic regulation of chondrocyte hypertrophy and apoptosis through Sirt1/P53/P21 pathway in surgery-induced osteoarthritis. Biochem. Biophys. Res. Commun. 2020, 528, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Qu, S.; Wei, X.; Zhu, H.; Luo, Q.; Liu, M.; Chen, G.; Xiao, X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011, 90, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Kishino, K.; Satoh, R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Shirataki, Y.; Sakagami, H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005, 25, 2055–2063. [Google Scholar] [PubMed]



| Piceatannol Treatment | Percentage of Live Cells |
|---|---|
| CT | 100% |
| 0.001 μM PCT | 94 ± 4% |
| 0.01 μM PCT | 96 ± 2% |
| 0.1 μM PCT | 93 ± 3% |
| 1 μM PCT | 95 ± 3% |
| 10 μM PCT | 92 ± 3% * |
| 100 μM PCT | 38 ± 3% *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessio, N.; Squillaro, T.; Lettiero, I.; Galano, G.; De Rosa, R.; Peluso, G.; Galderisi, U.; Di Bernardo, G. Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? Int. J. Mol. Sci. 2021, 22, 11619. https://doi.org/10.3390/ijms222111619
Alessio N, Squillaro T, Lettiero I, Galano G, De Rosa R, Peluso G, Galderisi U, Di Bernardo G. Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? International Journal of Molecular Sciences. 2021; 22(21):11619. https://doi.org/10.3390/ijms222111619
Chicago/Turabian StyleAlessio, Nicola, Tiziana Squillaro, Ida Lettiero, Giovanni Galano, Roberto De Rosa, Gianfranco Peluso, Umberto Galderisi, and Giovanni Di Bernardo. 2021. "Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics?" International Journal of Molecular Sciences 22, no. 21: 11619. https://doi.org/10.3390/ijms222111619
APA StyleAlessio, N., Squillaro, T., Lettiero, I., Galano, G., De Rosa, R., Peluso, G., Galderisi, U., & Di Bernardo, G. (2021). Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? International Journal of Molecular Sciences, 22(21), 11619. https://doi.org/10.3390/ijms222111619










