Enhanced Bone Regeneration in Variable-Type Biphasic Ceramic Phosphate Scaffolds Using rhBMP-2
Abstract
:1. Introduction
2. Results
2.1. Compression Test of Block Scaffold
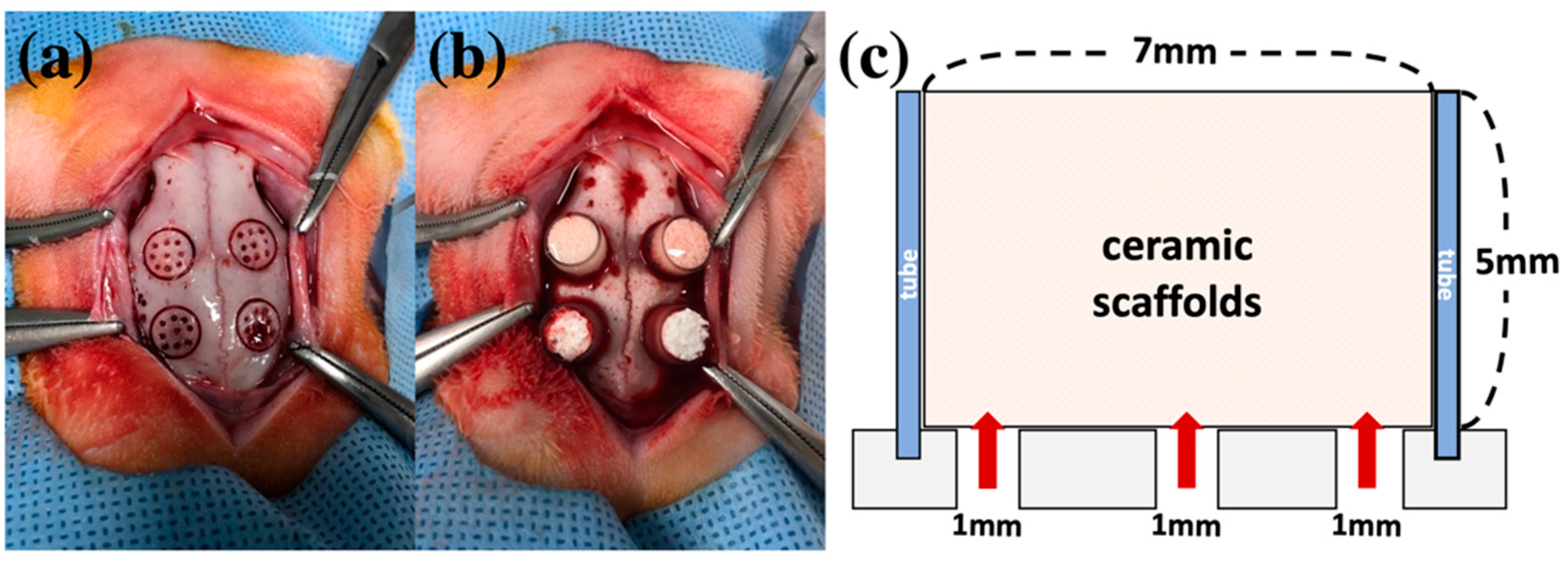
2.2. Clinical Findings in Animal Experiments
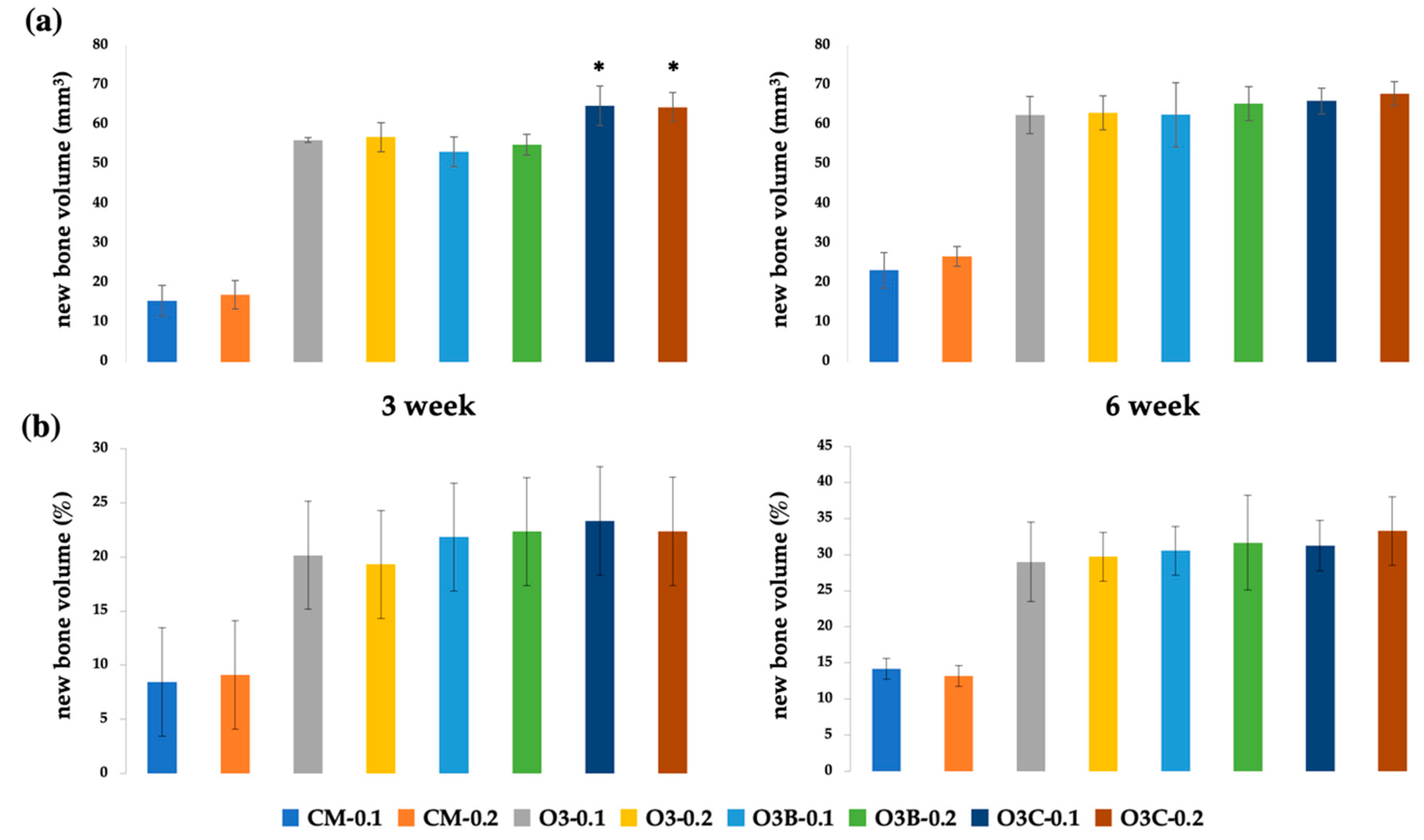
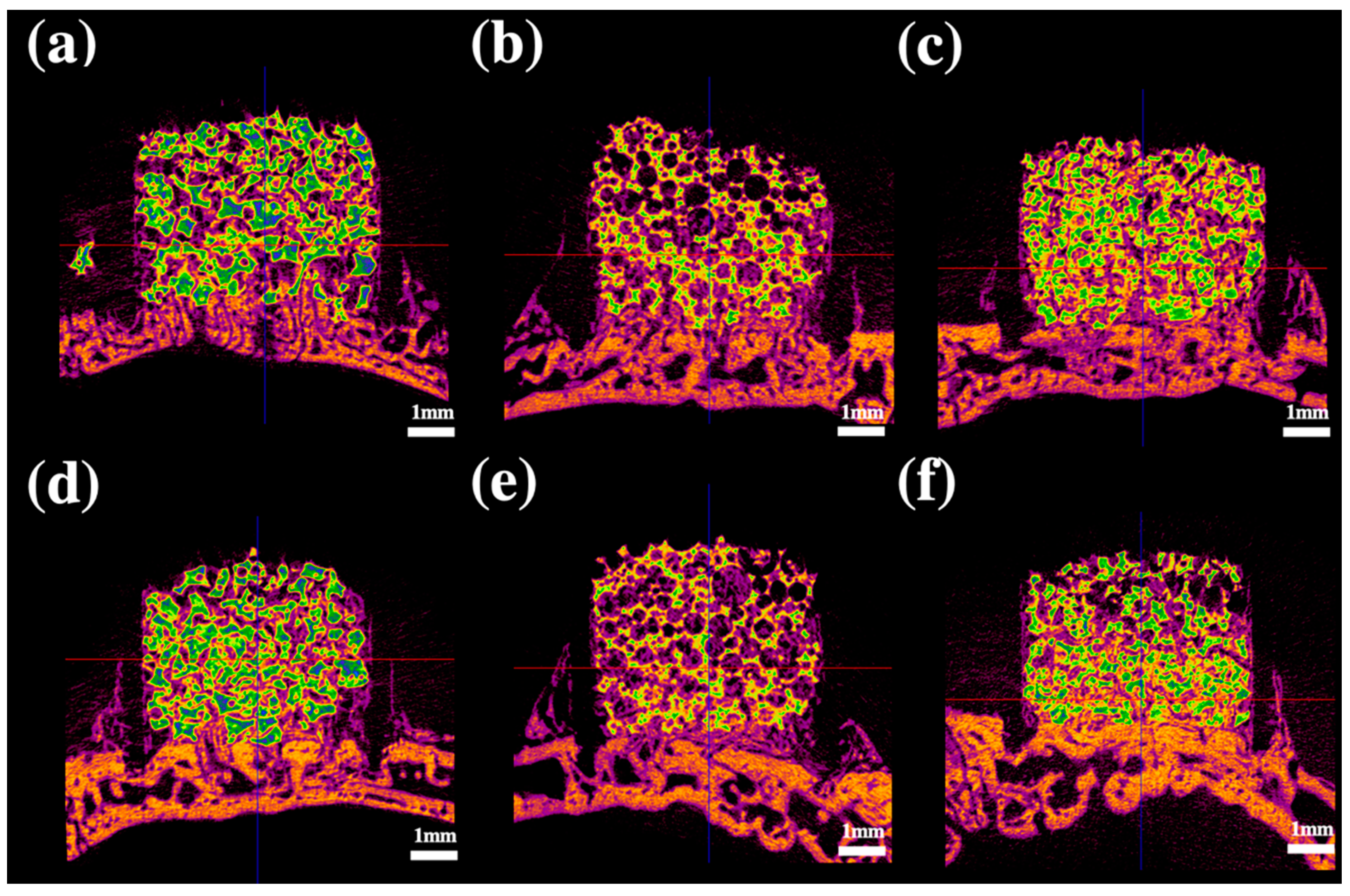
2.3. Radiological Analysis
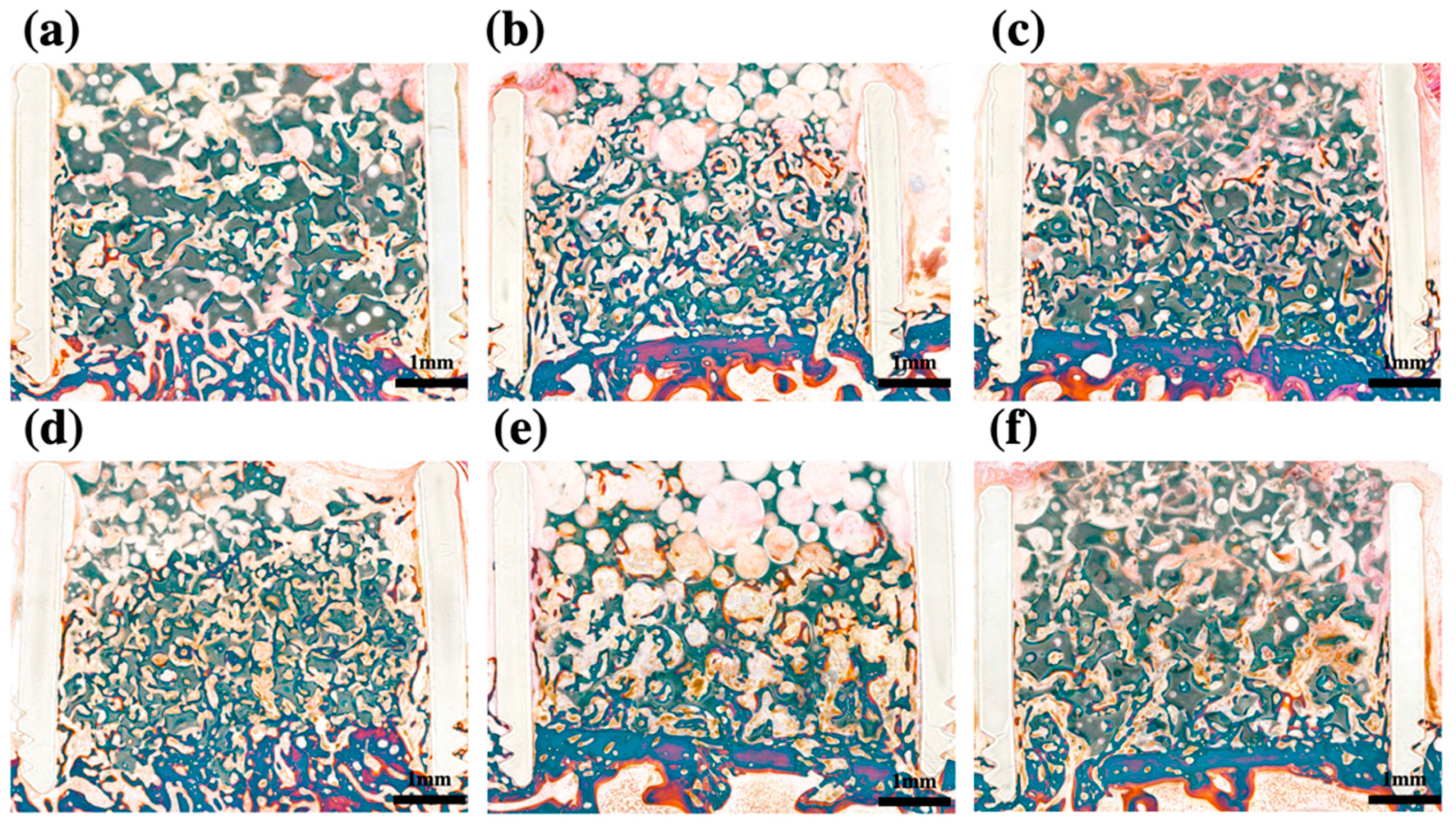
2.4. Histomorphometric Analysis
3. Discussion
4. Materials and Methods
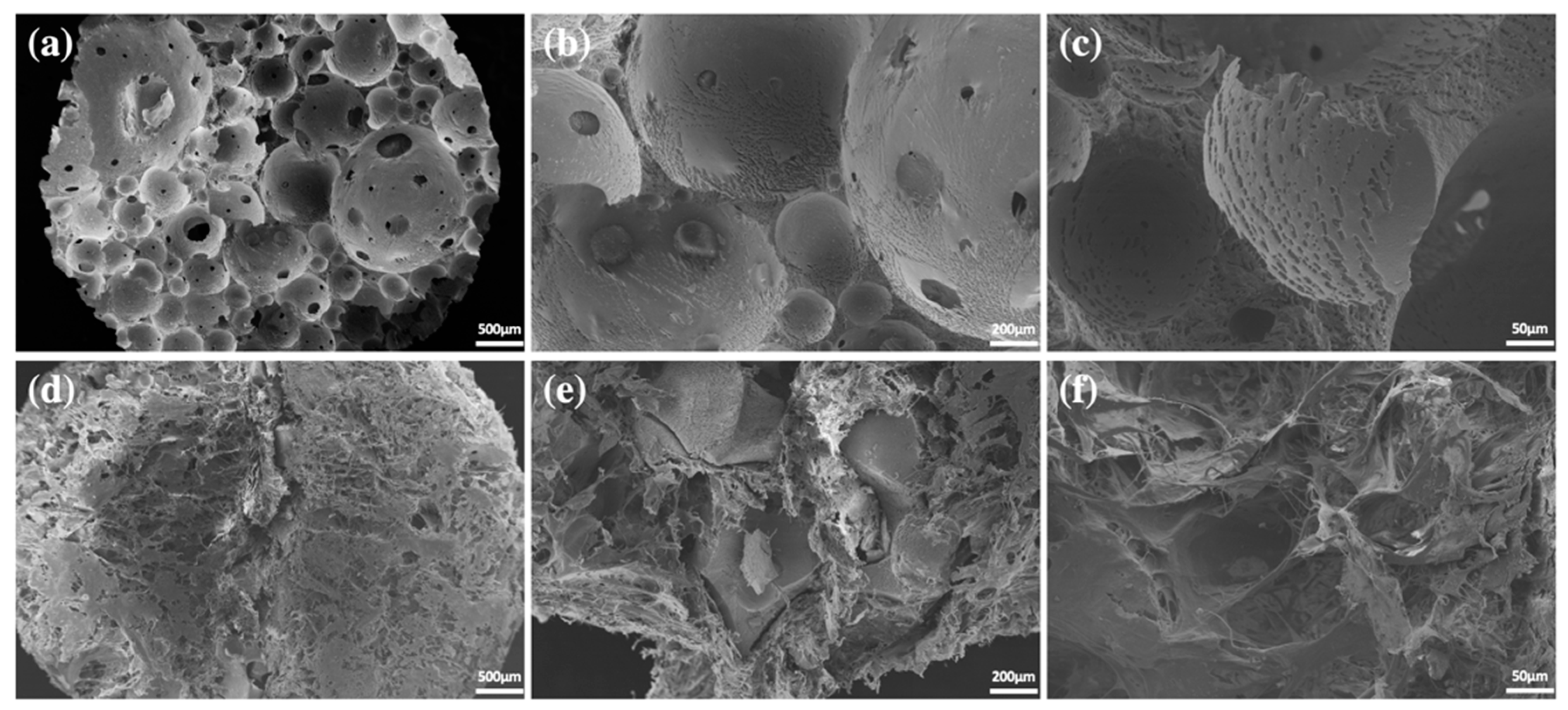
4.1. Preparation of Ceramic Scaffolds
4.2. Compression Test of Block-Type Scaffold
4.3. Animal Experiments
4.4. Radiological Analysis
4.5. Histological Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thuaksuban, N.; Nuntanaranont, T.; Pripatnanont, P. A comparison of autogenous bone graft combined with deproteinized bovine bone and autogenous bone graft alone for treatment of alveolar cleft. Int. J. Oral Maxillofac. Surg. 2010, 39, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int. J. Nanomed. 2013, 8, 47–59. [Google Scholar] [CrossRef]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Ueda, M.; Naiki, T.; Takahashi, M.; Hata, K.; Nagasaka, T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Eng. 2004, 10, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Chen, C.H.; Tsai, C.L.; Hsiue, G.H. Targeted delivery system for juxtacrine signaling growth factor based on rhBMP-2-mediated carrier-protein conjugation. Bone 2006, 39, 825–836. [Google Scholar] [CrossRef]
- Sigurdsson, T.J.; Nguyen, S.; Wikesjo, U.M. Alveolar ridge augmentation with rhBMP-2 and bone-to-implant contact in induced bone. Int. J. Periodontics Restor. Dent. 2001, 21, 461–473. [Google Scholar]
- Kim, H.S.; Park, J.C.; Yun, P.Y.; Kim, Y.K. Evaluation of bone healing using rhBMP-2 soaked hydroxyapatite in ridge augmentation: A prospective observational study. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 40. [Google Scholar] [CrossRef]
- Hwang, D.Y.; On, S.W.; Song, S.I. Bone regenerative effect of recombinant human bone morphogenetic protein-2 after cyst enucleation. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 22. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, V.; Sinha, M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 904–925. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Zhang, Y.; Pippenger, B.E.; Miron, R.J. In vitro evaluation of an injectable biphasic calcium phosphate (BCP) carrier system combined with recombinant human bone morphogenetic protein (rhBMP)-9. Bio-Med. Mater. Eng. 2017, 28, 293–304. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.K.; Hong, I.; Lee, B.K.; Yun, P.Y.; Lee, J.K. Dental alloplastic bone substitutes currently available in Korea. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, J.K.; Kim, Y.K.; Yun, P.Y. Influence of biodegradable polymer membrane on new bone formation and biodegradation of biphasic bone substitutes: An animal mandibular defect model study. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 34. [Google Scholar] [CrossRef]
- Zhang, R.; Li, S.; Yin, Y.; Guo, J.; Chen, W.; Hou, Z.; Zhang, Y. Open-Wedge HTO with Absorbable beta-TCP/PLGA Spacer Implantation and Proximal Fibular Osteotomy for Medial Compartmental Knee Osteoarthritis: New Technique Presentation. J. Investig. Surg. 2021, 34, 653–661. [Google Scholar] [CrossRef]
- Salih, V.; Georgiou, G.; Knowles, J.C.; Olsen, I. Glass reinforced hydroxyapatite for hard tissue surgery--part II: In vitro evaluation of bone cell growth and function. Biomaterials 2001, 22, 2817–2824. [Google Scholar] [CrossRef]
- Kakar, A.; Kakar, K.; Sripathi Rao, B.H.; Lindner, A.; Nagursky, H.; Jain, G.; Patney, A. Lateral alveolar ridge augmentation procedure using subperiosteal tunneling technique: A pilot study. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 3. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.M.; Jung, I.K.; Yoon, B.H.; Choi, B.R.; Kim, D.M.; Jang, J.S. Evaluation of Different Combinations of Biphasic Calcium Phosphate and Growth Factors for Bone Formation in Calvarial Defects in a Rabbit Model. Int. J. Periodontics Restor. Dent. 2016, 36, s49–s59. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.K.; Kwon, Y.J.; Hong, S.J.; Choi, H.G.; Chung, S.M.; Yang, B.E.; Lee, J.H.; Byun, S.H. Bone regeneration in ceramic scaffolds with variable concentrations of PDRN and rhBMP-2. Sci. Rep. 2021, 11, 11470. [Google Scholar] [CrossRef]
- Ventur, R.D.; Padalhin, A.R.; Kim, B.; Park, M.; Lee, B.T. Evaluation of bone regeneration potential of injectable extracellular matrix (ECM) from porcine dermis loaded with biphasic calcium phosphate (BCP) powder. Mater. Sci. Eng. C Mater. Biol Appl. 2020, 110, 110663. [Google Scholar] [CrossRef]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbuhl, R.; Bohner, M.; Richter, W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.C.; Kang, J.H.; Cha, J.K.; Lee, J.S.; Paik, J.W.; Jung, U.W.; Choi, S.H. Bone regeneration using three-dimensional hexahedron channel structured BCP block in rabbit calvarial defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B.T. Comparative Bone Regeneration Potential Studies of Collagen, Heparin, and Polydopamine-Coated Multichannelled BCP Granules. ASAIO J. 2018, 64, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Jeon, J.H.; Lee, S.Y.; An, H.W.; Park, K.O.; Park, K.B.; Kim, S. Effects of Collagen Grafting on Cell Behaviors in BCP Scaffold with Interconnected Pore Structure. Biomater. Res. 2016, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706, discussion 706–708. [Google Scholar] [CrossRef]
- Kido, H.; Schulz, E.E.; Kakura, K.; Yamamoto, K.; Morinaga, K.; Matsuura, M. Human mandibular trabecular bone density correlation with mechanical strength: Implications for implant dentistry. Implant. Dent. 2011, 20, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Breitwieser, T.; Breusch, S.J. Mechanical stability of structured bone grafts from the anterior iliac crest. Clin. Biomech. 2008, 23, 955–960. [Google Scholar] [CrossRef]
- Fujita, R.; Yokoyama, A.; Nodasaka, Y.; Kohgo, T.; Kawasaki, T. Ultrastructure of ceramic-bone interface using hydroxyapatite and beta-tricalcium phosphate ceramics and replacement mechanism of beta-tricalcium phosphate in bone. Tissue Cell 2003, 35, 427–440. [Google Scholar] [CrossRef]
- Lim, H.C.; Zhang, M.L.; Lee, J.S.; Jung, U.W.; Choi, S.H. Effect of different hydroxyapatite:beta-tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int. J. Oral Maxillofac. Implant. 2015, 30, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Pripatnanont, P.; Monmaturapoj, N.; Suttapreyasri, S. Fabrication and characterization of novel nano hydroxyapatite/beta-tricalcium phosphate scaffolds in three different composition ratios. J. Biomed. Mater. Res. Part A 2012, 100, 2260–2268. [Google Scholar] [CrossRef]
- Yun, P.Y.; Kim, Y.K.; Jeong, K.I.; Park, J.C.; Choi, Y.J. Influence of bone morphogenetic protein and proportion of hydroxyapatite on new bone formation in biphasic calcium phosphate graft: Two pilot studies in animal bony defect model. J. Cranio-Maxillofac. Surg. 2014, 42, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Lee, J.H.; Byun, S.H. 3D-Printed Ceramic Bone Scaffolds with Variable Pore Architectures. Int. J. Mol. Sci. 2020, 21, 6942. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of particle size and porosity on in vivo remodeling of settable allograft bone/polymer composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1641–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.C.; Song, K.H.; You, H.; Lee, J.S.; Jung, U.W.; Kim, S.Y.; Choi, S.H. Effectiveness of biphasic calcium phosphate block bone substitutes processed using a modified extrusion method in rabbit calvarial defects. J. Periodontal Implant. Sci. 2015, 45, 46–55. [Google Scholar] [CrossRef]
- Bae, S.Y.; Park, J.C.; Shin, H.S.; Lee, Y.K.; Choi, S.H.; Jung, U.W. Tomographic and histometric analysis of autogenous bone block and synthetic hydroxyapatite block grafts without rigid fixation on rabbit calvaria. J. Periodontal Implant. Sci. 2014, 44, 251–258. [Google Scholar] [CrossRef]
- Kim, S.Y.; Bae, E.B.; Huh, J.W.; Ahn, J.J.; Bae, H.Y.; Cho, W.T.; Huh, J.B. Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects. Materials 2019, 12, 2435. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Choi, K.H.; Yun, J.H.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Cho, K.S. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 298–306. [Google Scholar] [CrossRef]
- Hwang, K.S.; Choi, J.W.; Kim, J.H.; Chung, H.Y.; Jin, S.; Shim, J.H.; Yun, W.S.; Jeong, C.M.; Huh, J.B. Comparative Efficacies of Collagen-Based 3D Printed PCL/PLGA/beta-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials 2017, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Ab, T.K.; Chaitra, N.T.; Ps, G.D.; Triveni, M.G.; Mehta, D.S. A clinico-radiographic and histomorphometric analysis of alveolar ridge preservation using calcium phosphosilicate, PRF, and collagen plug. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.C.; Song, J.M.; Kim, C.J.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Shin, S.H. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 16. [Google Scholar] [CrossRef] [Green Version]
- Schaller, B.; Fujioka-Kobayashi, M.; Zihlmann, C.; Schuler, V.C.; Katagiri, H.; Lang, N.P.; Saulacic, N. Effects of additional collagen in biphasic calcium phosphates: A study in a rabbit calvaria. Clin. Oral Investig. 2020, 24, 3093–3103. [Google Scholar] [CrossRef]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B. Incorporation of BMP-2 loaded collagen conjugated BCP granules in calcium phosphate cement based injectable bone substitutes for improved bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 713–724. [Google Scholar] [CrossRef]
- Bucchi, C.; Borie, E.; Arias, A.; Dias, F.J.; Fuentes, R. Radiopacity of alloplastic bone grafts measured with cone beam computed tomography: An analysis in rabbit calvaria. Bosn. J. Basic Med. Sci. 2016, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; You, C.; Kim, K.H. Combined Effect of a Microporous Layer and Type I Collagen Coating on a Biphasic Calcium Phosphate Scaffold for Bone Tissue Engineering. Materials 2015, 8, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; Lee, J.S.; Kim, M.S.; Choi, S.H.; Chai, J.K.; Jung, U.W. Comparison of collagen membrane and bone substitute as a carrier for rhBMP-2 in lateral onlay graft. Clin. Oral Implant. Res. 2015, 26, e13–e19. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Asahina, I.; Ohmamiuda, K.; Enomoto, S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J. Biomed. Mater. Res. 2001, 54, 129–138. [Google Scholar] [CrossRef]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.U.; Lim, H.C.; Hong, J.Y.; Lee, J.S.; Jung, U.W.; Choi, S.H. Bone regenerative efficacy of biphasic calcium phosphate collagen composite as a carrier of rhBMP-2. Clin. Oral Implant. Res. 2016, 27, e91–e99. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 4, e100115. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaller, S.R.; Hoyt, J.; Borjeson, K.; Dart, A.; Tesluk, H. Reconstruction of calvarial defects with anorganic bovine bone mineral (Bio-Oss) in a rabbit model. J. Craniofacial Surg. 1993, 4, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Humber, C.C.; Sandor, G.K.; Davis, J.M.; Peel, S.A.; Brkovic, B.M.; Kim, Y.D.; Holmes, H.I.; Clokie, C.M. Bone healing with an in situ-formed bioresorbable polyethylene glycol hydrogel membrane in rabbit calvarial defects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 372–384. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-K.; Kwon, I.-J.; On, S.-W.; Hong, S.-J.; Yang, B.-E.; Kim, S.-M.; Lee, J.-H.; Byun, S.-H. Enhanced Bone Regeneration in Variable-Type Biphasic Ceramic Phosphate Scaffolds Using rhBMP-2. Int. J. Mol. Sci. 2021, 22, 11485. https://doi.org/10.3390/ijms222111485
Lim H-K, Kwon I-J, On S-W, Hong S-J, Yang B-E, Kim S-M, Lee J-H, Byun S-H. Enhanced Bone Regeneration in Variable-Type Biphasic Ceramic Phosphate Scaffolds Using rhBMP-2. International Journal of Molecular Sciences. 2021; 22(21):11485. https://doi.org/10.3390/ijms222111485
Chicago/Turabian StyleLim, Ho-Kyung, Ik-Jae Kwon, Sung-Woon On, Seok-Jin Hong, Byoung-Eun Yang, Soung-Min Kim, Jong-Ho Lee, and Soo-Hwan Byun. 2021. "Enhanced Bone Regeneration in Variable-Type Biphasic Ceramic Phosphate Scaffolds Using rhBMP-2" International Journal of Molecular Sciences 22, no. 21: 11485. https://doi.org/10.3390/ijms222111485
APA StyleLim, H.-K., Kwon, I.-J., On, S.-W., Hong, S.-J., Yang, B.-E., Kim, S.-M., Lee, J.-H., & Byun, S.-H. (2021). Enhanced Bone Regeneration in Variable-Type Biphasic Ceramic Phosphate Scaffolds Using rhBMP-2. International Journal of Molecular Sciences, 22(21), 11485. https://doi.org/10.3390/ijms222111485








