Abstract
Poly (ADP-ribose) polymerase-1 (PARP-1) is a nuclear enzyme involved in processes of cell cycle regulation, DNA repair, transcription, and replication. Hyperactivity of PARP-1 induced by changes in cell homeostasis promotes development of chronic pathological processes leading to cell death during various metabolic disorders, cardiovascular and neurodegenerative diseases. In contrast, tumor growth is accompanied by a moderate activation of PARP-1 that supports survival of tumor cells due to enhancement of DNA lesion repair and resistance to therapy by DNA damaging agents. That is why PARP inhibitors (PARPi) are promising agents for the therapy of tumor and metabolic diseases. A PARPi family is rapidly growing partly due to natural polyphenols discovered among plant secondary metabolites. This review describes mechanisms of PARP-1 participation in the development of various pathologies, analyzes multiple PARP-dependent pathways of cell degeneration and death, and discusses representative plant polyphenols, which can inhibit PARP-1 directly or suppress unwanted PARP-dependent cellular processes.
1. Introduction
Poly (ADP-ribose) polymerase-1 (PARP-1) is a widespread nuclear protein with a spectrum of different activities due to its DNA-binding and enzymatic properties [1,2,3,4,5]. PARP-1 uses β-NAD+ as a substrate to synthesize branched polymers of ADP-ribose (PAR) and to covalently modifies more than 40 nuclear proteins and transcription factors, including PARP-1 itself. Under conditions of moderate genotoxic stress, the functioning of PARP-1 maintains integrity and activity of cell genome, while during severe genotoxic stress PARP-1 coordinates multiple pathways of cell death. General enhancement of PARP-1 activity is associated with the development of tumor, cardiovascular and neurodegenerative diseases, and pharmacological inhibition of PARP-1 is a promising strategy for their therapy. Several inhibitors of the enzymatic activity of PARP-1 (PARPi) are already used in clinical practice for the treatment of cancer [6,7]. A search for more active and less toxic PARPi, as well as for compounds that block the development of unwanted PARP-dependent cellular processes is in progress. In particular, a search for PARPi is carried out among natural compounds, since they might have a higher bioavailability, more effective cell penetration, higher pharmacological activity and fewer side effects than synthetic agents. Polyphenols are the largest and most studied group of plant metabolites, among which a considerable number of compounds were found to have therapeutic potential due to antiviral, antibacterial, antioxidant or antitumor activities. Some polyphenols were demonstrated to be effective PARPi or/and can affect signaling pathways that regulate cell survival under adverse conditions of oxidative/nitrosative or genotoxic stress. Many of these signaling pathways are closely related to molecular processes that are under the control of PARP-1. Accordingly, in the first part of the review, data are systematized on PARP-1-dependent molecular mechanisms that contribute to the development of diseases and therefore are targets for therapeutic intervention; in the second part, the polyphenols are discussed, which affect PARP-1 or (and) the signaling pathways under its control.
2. Relationship of PARP-1 with Inflammatory and Metabolic Diseases
Hyperactivation of PARP-1 plays an important role in the development of diseases, which are associated with or caused by chronic inflammation [8]. These include diabetes, neurodegenerative disorders (Alzheimer’s disease (AD), Parkinson’s disease (PD)), and cardiovascular diseases (Figure 1) [9,10,11,12].
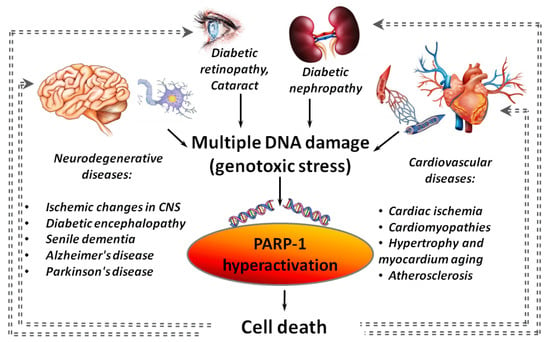
Figure 1.
PARP-1 hyperactivation as an aggravating factor in the development of various diseases. Gray dotted lines indicate “vicious circles” when PARP-1 hyperactivation initiated by inflammation, cardiovascular, neurodegenerative or diabetic pathology leads to an increase in the severity of the disease.
Hyperactivation of PARP-1 was found in various cardiovascular diseases (ischemic heart disease, atherosclerosis, cardiomyopathies of various origins, hypertrophy and aging of the myocardium) [13,14] and in many models of a central nervous system (CNS) damage (stroke [15], traumatic brain injury [16], neurodegeneration [17] and senile dementia [18]). A negative role of the PARP-1 hyperactivation in cardiac ischemia/reperfusion is known for a long time [13,19]. It was shown that the strongest activation of PARP-1 is observed in a peri-infarction zone and areas of necrotic damage during a heart attack [20]. PARP-1 hyperactivation is involved in a cascade of events initiated by β-amyloid peptides (Aβ), the accumulation of which leads to the death of brain cells in AD. A significant increase in PARP-1 expression and accumulation of PAR polymers was found in the cerebral cortex at the early (3.5 months) and intermediate (6 months) stages of Aβ-aggregation in mouse models of AD [21]. Hyperglycemia is also associated with PARP-1 hyperactivation [22,23,24] that is usually an aggravating factor in the development of systemic diabetic dysfunctions. In particular, PARP-1 hyperactivation is involved in the death of insulin-producing pancreatic β-cells [25].
Inflammatory processes, hypoxia, hypo- and hyperglycemia are often accompanied by an uncontrolled increase in the levels of reactive oxygen (ROS) and nitrogen species (RNS), which cause DNA damage. As a consequence, an increase in PARP-1 activity is required for DNA repair. In turn, PARP-1 hyperactivation can initiate parthanatos—a programmed caspase-independent cell death (Figure 2) [26,27]. PAR and PARylated proteins that are formed in large quantities migrate from a nucleus to cytoplasm and cause the release of apoptosis-inducing factor (AIF) from mitochondria [26,28,29]. Released AIF is transported to a nucleus due to a nuclear localization signal (NLS) and activates endonucleases, which cause DNA fragmentation followed by cell death. PARP-1-mediated parthanatos is observed in neurons during PD, excitotoxicity of glutamate and cerebral ischemia [30,31].
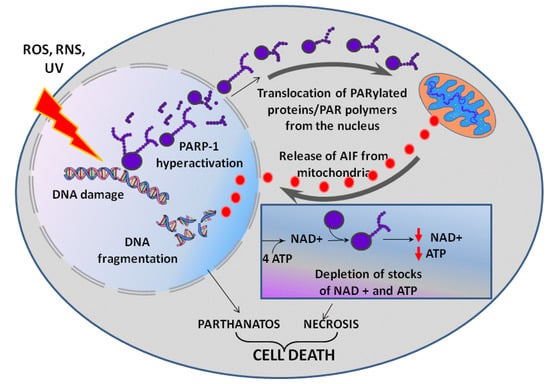
Figure 2.
PARP-1 dependent cell death. UV—ultraviolet light, ROS—reactive oxygen species overproduced in oxidative stress, RNS—reactive nitrogen species (e.g., nitric oxide NO) overproduced in nitrosative stress. See text for detail.
The PARP-1-dependent cell death can also occur after PARP-1 hyperactivation due to an energy crisis caused by the depletion of cellular reserves of macroergic compounds [32,33]. A synthesis of a NAD+ molecule requires four ATP molecules, and intense consumption of NAD+ by PARP-1 can result in a rapid depletion of ATP and NAD+ stocks, lead to suppression of energy-dependent cellular processes followed by cell necrosis [34]. Suppression of energy-dependent processes is additionally enhanced by PAR metabolism. Free and protein-bound PAR is intensely cleaved by poly(ADP-ribose)glycohydrolase to ADP-ribose, which is then metabolized by NUDIX-hydrolases (NUDIX - NUcleoside DIphosphates linked to any other moiety X) to AMP [35]. A high AMP/ATP ratio is interpreted by a cell as an energy stress, and AMP-activated protein kinase corrects this apparent energy misbalance by blocking the mammalian target of rapamycin (mTOR) signaling pathway with a subsequent down-regulation of ATP consuming processes [36].
PARP-1 itself promotes the development of inflammatory processes by up-regulating expression of various inflammatory mediators such as tumor necrosis factor α (TNFα), inducible isoform of nitrite oxide synthase (iNOS), cyclooxygenase 2 (COX2), monocyte chemoattractant protein 1 (MCP1), interleukins 1β and 6 (IL-1β, IL-6). Here PARP-1 acts as a co-activator of transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator proteins 1 and 2 (AP1, AP2) that regulate immune and inflammatory responses (Figure 3) [37,38,39,40]. PARP-1 was shown to be acetylated at lysine residues (K498, K505, K508, K521, K524) by the p300/CREB-binding protein complex (CREB - cAMP-response element binding protein) and phosphorylated at Y829 by mitogen-activated protein kinases (MAPKs) in response to pro-inflammatory stimuli [37,38]. Modified in this way, PARP-1 stimulates transcription of NF-κB-dependent genes of inflammatory mediators (Figure 3) [37,38,39,40]. Interestingly, neither the enzymatic activity of PARP-1 nor its DNA-binding activity were required for full activation of NF-kB in response to various stimuli [37]. PAR polymers can act as alarmins releasing from a cell during stress and activating production of inflammatory cytokines by the cells of an innate immunity system [41].
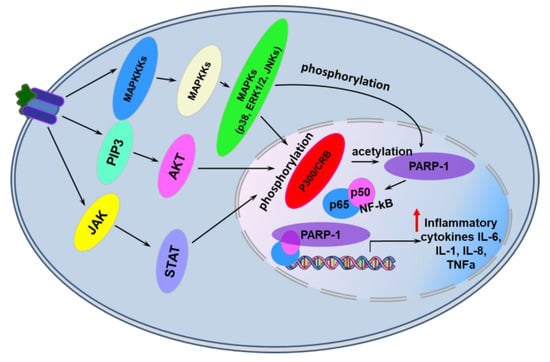
Figure 3.
PARP-1-dependent transcriptional activation of genes encoding pro-inflammatory cytokines in eukaryotic cells. See text for details. Abbreviations: JAK—Janus kinase; PIP3—phosphatidylinositol (3,4,5)-trisphosphate; MAPKKKs—Mitogen-Activated Protein (MAP) kinase kinase kinases; MAPKKs—Mitogen-activated protein kinase kinases; MAPKs—mitogen-activated protein kinases; STAT—members of the signal transducer and activator of transcription protein family; AKT—subfamily of serine/threonine kinases; p38—p38 mitogen-activated protein kinases; JNK—c-Jun N-terminal kinases; ERKs—extracellular signal-regulated kinases; IL-l, IL6, IL-8—interleukins 1, 6, 8, p300/CRB—p300/CREB-binding protein complex, TNFα—tumor necrosis factor α.
During apoptosis, which may accompany the development of some pathologies, PARP-1 is cleaved by caspases 3 and 7 into DNA-binding and catalytically active fragments [42,43], but retains its ability to activate NF-κB and enhance transcription of inflammatory mediator genes [44]. The C-terminal fragment preserves catalytic activity, but is not stimulated by DNA damage. The N-terminal fragment remains associated with DNA injuries blocking access of repair factors to them [45,46].
As a co-activator of NF-κB and activator protein 1, PARP-1 was suggested to be responsible for accelerated aging during chronic inflammatory diseases [47].
An important role of PARP-1 in the development of inflammatory diseases was confirmed by experiments with PARP-1 knockout mice. These mice are better protected from diabetic and septic complications associated with inflammation such as multiple organ dysfunction syndromes [37,48].
In summary, PARP-1 hyperactivation, which occurs during oxidative/nitrosative stress, chronic inflammation and irreversible genotoxic damage, leads to massive cell death that at the level of the organism promotes development of metabolic syndrome, multiple organ dysfunction syndrome, cardiovascular and neurodegenerative diseases.
3. PARP-1 and Oncological Diseases
PARP-1 is involved in pathogenesis of oncological diseases in a complex way as described in several excellent comprehensive reviews [49,50,51,52]. Here we will only briefly describe the effect of PARP-1 on tumor cell metabolism, referring readers to the published reviews for more details.
In contrast to the negative role of severe hyperactivation of PARP-1 in inflammatory processes, a moderate activation of PARP-1 occurring during transformation of cells does not lead to a cell death. On the contrary, it contributes to cell survival. The pro-tumor activity of PARP-1 is mediated by PARP-1-dependent deregulation of factors involved in the cell cycle, mitosis, apoptosis and autophagy [53]. PARP-1 impedes with cell differentiation thus enhancing tumor malignancy [54], and moderate activation of PARP-1 caused by the accumulation of DNA damage during intensive cell division increases DNA repair efficiency and cell viability.
Malignancy of cancer cells and its ability to metastasize strongly depend on the tumor microenvironment [55]. PARP-1 plays an important role in the functioning of the tumor microenvironment, participating in angiogenesis as well as in the formation of a tumor-associated stroma [56]. PARP-1 is involved in the process of epithelial-mesenchymal transition (EMT) during the acquisition of the ability of tumor cells to metastasize [52]. PARP-1 knockdown leads to EMT reversal through inhibitory transcription factors such as Smad4, p65 and ZEB1 [57].
PARP-1 participates in several processes responsible for the resistance of tumor cells to therapy (Figure 4) [25,30,58,59,60]. As a key repair enzyme, PARP-1 ensures the stability of a tumor cell genome after treatment with DNA damaging chemotherapy agents [61,62,63]. PARP-1 is able to promote (directly or indirectly) epigenetic modifications, creating conditions for development of heterogeneity of tumor cells and formation of super-resistant clones in a heterogeneous population [64]. Another PARP-1-mediated mechanism of drug resistance is a non-lethal autophagy [65,66,67]. PARP-1 is also known to control the expression of heat shock protein 70 [68,69], which makes a significant contribution to the survival of tumor cells and their resistance to antitumor agents [70].
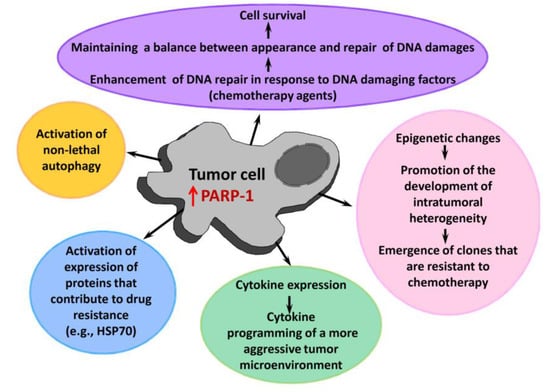
Figure 4.
A role of PARP-1 in the tumor progression and development of its drug resistance. HSP70—heat shock protein 70.
In general, an increased level of PARP-1 expression is considered to be a prognostic marker associated with an aggressive phenotype of malignant tumors and a worse prognosis of patient survival [71,72,73].
4. Synthetic PARP-1 Inhibitors in Treatment of Diseases
PARPi are considered to be promising antitumor agents since the increased activity of PARP-1 is a key factor contributing to growth of tumors, to an increase in their malignancy and to the development of drug resistance [74]. Most PARPi that are currently in antitumor preclinical and clinical trials are nicotinamide mimetics. They act by competing with NAD+ for the binding to a catalytic domain of PARP-1 and suppressing PAR synthesis [74]. Several PARPi are already used in clinical practice (Figure 5), and their combined administration with chemotherapy agents is promising for overcoming the drug resistance of tumor cells [75]. Inhibition of PARP1 is especially toxic to cells lacking functional forms of the tumor suppressors, breast cancer type 1 susceptibility protein (BRCA1) or breast cancer type 2 susceptibility protein (BRCA2) [76].
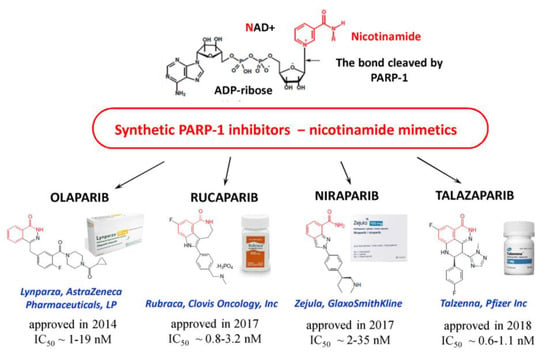
Figure 5.
Synthetic PARPi approved for use in oncology. IC50 values (PARPi concentrations inducing 50% inhibition of PARP-1 activity) are cited from [19].
PARPi apparently can find wide application in the treatment of diseases related to inflammation. There are numerous examples of pharmacological or genetic inactivation of PARP-1 leading to a powerful anti-inflammatory effect that were demonstrated using different models of respiratory, gastrointestinal, osteochondral, cardiovascular and neurological pathologies (Table 1).

Table 1.
Inhibition of PARP-1 in the treatment of non-cancer diseases.
Importantly, PARPi block NF-κB-mediated transcription of genes encoding pro-inflammatory cytokines, but do not reduce the transcription of anti-inflammatory cytokine-encoding genes of IL-10 and IL-13 [99]. At the same time, even clinically approved PARPi are characterized by side effects that demand the search for safer drugs that target PARP-1 [100].
5. Polyphenols as PARP-1 Inhibitors
An alternative to synthetic PARPi can be found among natural compounds—plant metabolites. Polyphenolic compounds (flavonoids and non-flavonoids, Figure 6), along with terpenoids and alkanoids, are the most common secondary plant metabolites. Flavonoids, which are subdivided into flavones, flavonols, flavanones, catechins (flavan-3-ols), isoflavonoids, and anthocyanidins (Figure 6) are the most widespread and studied natural polyphenols.
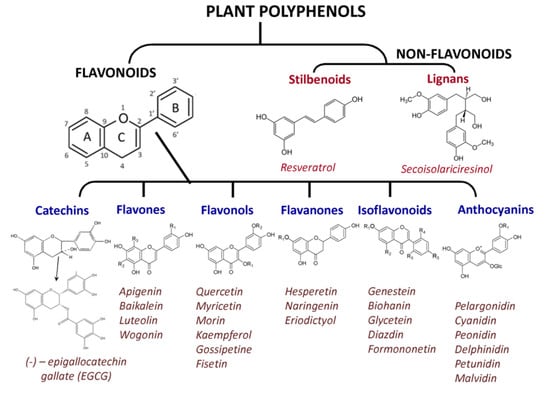
Figure 6.
Plant polyphenols with known pharmacological properties.
Various types of plant polyphenols have different anti-inflammatory, antioxidant, anti-allergic, antiviral and/or antitumor activities [101,102]. Some natural polyphenols are epigenetically active compounds and may play an important role in the regulation of gene expression, including PARP-dependent genes [103]. Some of polyphenols were shown to have PARP-1 inhibiting activity [104,105,106]. Many polyphenols have high bioavailability, efficiently penetrate cells and induce biological effects at micromolar concentrations [107,108,109,110] that makes them good candidates for the search of new PARPi. Thus, a search in the flavonoid library led to the discovery of PARPi such as myricetin, quercetin, fisetin, tricetin, gossipetin and delphinidin [104]. Functional screening of the library of polyphenols used in traditional medicine resulted in identification of 11 compounds interacting with PARP-1 with the dissociation constants of the complexes ranging from 0.32 to 79 µM [111]. The most active PARPi among the polyphenols was 2”-hydroxygenkwanol A isolated from the plant Daphne linearifolia that has long been used to treat inflammation and rheumatism in Arab traditional medicine. This polyphenol is structurally similar to talazaparib, the strongest synthetic PARPi. Computer screening technologies also predict an existence of PARPi among polyphenols that may have affinities higher than clinically approved synthetic PARPi [112].
Below the features of PARP-1 inhibition by some representatives of polyphenols are considered in more detail.
5.1. Flavonols
Flavonols are the most abundant species of flavonoids existing in nature. Their distinct feature is the presence of 3-hydroxyflavone in the structure. Flavonols are often found as O-glycoside, glucuronide, methyl, and sulfate conjugates.
The flavonol quercetin (QC) is found in large quantities in plants, predominantly red and orange (sea buckthorn, cranberries, raspberries, blueberries, onions), as well as in food products such as buckwheat, tea, red wine, and olive oil. QC is usually found in plants in conjunction with glycosylated forms—isoquercetin and rutin [113]. These flavonols perform a wide range of physiological functions in plants, the most important of which is antioxidant. QC is able to inhibit PARP-1 in the micromolar concentration range, and its activity is approximately seven times higher than that of 3-aminobenzamide (3-AB) [114]. It was found that glycosylation improves the solubility of QC derivatives, but decreases their inhibitory activity. It was shown that QC at a concentration of more than 30 µM exhibits genotoxicity. Glycosylated analogs have less cyto- and genotoxicity, but this might be due to their lower cell permeability.
Regular consumption of citrus fruit reduces the risk of cancer, and this effect is likely associated with inhibition of PARP-1 by flavonols naringenin (NG), hesperetin (GP) and their O-glycoside forms naringin and hesperidin, which are contained in citrus fruit [115]. GP was found to be more active than QC and cytotoxic for both wild-type V79 cells and mutant cells deficient in the BRCA2 protein involved in DNA repair (100% cytotoxicity at 30 µM GP) [116]. In turn, QC selectively induces the death of BRCA2 mutant cells (40% cytotoxicity at 30 µM QC).
Glycosylated isoquercetin, rutin, naringin, and hesperidin have less cytotoxicity than the corresponding non-glycosylated flavonoids, but at the same time exhibit selectivity towards BRCA2 mutant cells [115]. The death of more than 80% of the mutant cells was observed at 100 µM rutin and isoquercetin and at ~1 mM naringin and hesperidin.
In hepatocytes stimulated by the pro-inflammatory cytokine IL-1, QC reduces NO production through suppression of iNOS expression, which in turn, can block the enhancement of the inflammatory cascade [116]. Similarly, QC inhibits the LPS-induced iNOS gene expression in various models [117,118,119]. It is believed that the anti-inflammatory effect of QC is based on a combination of antioxidant and anti-PARP activities. Other flavonoids such as naringenin, apigenin, and resveratrol also block iNOS expression in macrophages [120,121].
Flavonoids are able to attenuate NAD+ depletion by inhibiting PARP-1 hyperactivation both in vitro and in vivo [104], therefore reducing the likelihood of cell death and exerting a pleiotropic protective effect at high glucose levels [122]. This may play an important role in preventing the development of diabetic complications caused by increased PARP-1 activity, including those associated with massive neuronal death [123]. The molecular mechanisms of the protective action of flavonoids in the suppression of diabetic complications remain the subject of active study [124]. QC can up-regulate expression of neural and synapse-associated proteins (nerve growth factor, brain-derived neurotrophic factor, post synaptic density 93 and 95 proteins) and inhibit neurodegeneration [125]. QC increases the level of SIRT1 (NAD+-dependent histone deacetylase) and inhibits the stress proteins of the endoplasmic reticulum (RNA-like endoplasmic reticulum kinase, inositol-requiring enzyme-1α, activating transcription factor 6α, eukaryotic initiation factor 2, binding immunoglobulin protein and protein disulfide isomerase). An increase in SIRT1 activity was shown to have a positive effect on the metabolism of mammals, leading to inhibition of aging and longevity [126,127]. PARP-1 knockout increases the NAD + content and, accordingly, SIRT1 activity in brown adipose tissue and muscles [128]. A similar effect is caused by PARPi. In aging rats, QC activates SIRT1, promotes monoamine synthesis and improves animal cognitive functions. QC improves learning and memory in diabetic rats [124,129,130].
5.2. Flavones
Flavones are flavonoids that have a 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) group. 4’-Methoxyflavone (4MF) and 3’, 4’-dimethoxyflavone (DMF) were reported to prevent parthanatos in cells treated with a DNA-alkylating agent and possess neuroprotective activity [131]. It was concluded that the anti-parthanatos effect of 4MF and DMF is related to the suppression of PARP-1 activity and is not associated with antioxidant or free radical scavenging properties. Both compounds almost equally prevented parthanatos in HeLa cells, but 4MF demonstrated higher neuroprotection than DMF.
It should be noted that some flavones (e.g., apigenin and luteolin) inhibit tankyrases (TNK), the proteins of the PARP family, which are attractive targets in cancer treatment [132]. The antitumor therapeutic potential of TNK is determined by their participation in telomere homeostasis, mitosis, and Wnt signaling pathways [133]. Interestingly, flavones do not contain the nicotinamide-like moiety that is characteristic for most PARP-1 inhibitors, and the flavone-based pharmacophore model was designed for TNK inhibitors [134,135].
5.3. Catechins
Flavan derivatives catechins include a wide variety of biologically active compounds. A feature of their structure is the absence of a double bond between the second and third carbon atoms leading to emergence of two chiral centers and four diastereoisomers. The trans and cis isomers are called catechins and epicatechins, respectively. Catechins are present in large quantities in tea leaves and cocoa beans. Green tea contains epigallocatechin gallate (EGCG), which is considered one of the most powerful dietary antioxidants [136]. During the production of black tea (enzymatic oxidation), catechin is oxidized to quinone, which is further condensed into several other chemical compounds, one of which is the theaflavin polyphenol (TF). Tea polyphenols affect regulatory systems of cells and may produce an inhibitory effect on various stages of carcinogenesis: inflammatory processes, cell transformation, proliferation, apoptosis, metastasis, and invasion [107,137,138,139,140]. It was found that EGCG and TF cause synthetic lethality in BRCA2-deficient cells through a PARP-dependent mechanism [141]. EGCG inhibits PARP-1 more effectively than TF, which is probably due to the presence of a haloyl group. Moreover PARP-1, the targets of tea polyphenols are histone deacetylases [142], transcription factors [143], DNA topoisomerase II [109] and ABC transporters responsible for the development of multidrug resistance [144,145].
Other catechins that affect PARP-1 include epicatechin, myricetin, epigallocatechin, catechin gallate, epicatechin gallate, gallocatechin, and gallocatechin gallate [146].
5.4. Resveratrol
The representative of stilbenoids, resveratrol (RSV), exists in the form of cis- and trans-stereoisomers and is often glycosylated. RSV is found in grapes, nuts and cocoa beans, as well as in berries, leaves and flowers of orchids, eucalyptus, gnetum and some other plants. Numerous studies have shown that the RSV containing extracts reduce thrombus formation, improve the rheological properties of blood, relax the vascular endothelium, lower cholesterol and triglyceride levels in the blood preventing atherosclerosis development, exhibit antioxidant and anti-inflammatory activity [147,148,149]. Such properties are strongly associated with the RSV-mediated blocking of the mTOR (mammalian target of rapamycin) signaling pathway [150]. mTOR is known to integrate various signaling pathways, including the pathways of insulin, growth factors, and mitogens. It functions as a sensor for redox status and cellular nutrient and energy levels. Dysregulation of mTOR pathway leads to the development of various metabolic and oncological diseases. The mTOR pathway can intersect with PARP-1 during partanotosis. In this case, SIRT1, involved in the regulation of the intracellular level of NAD +, can play an important role as a factor that binds PARP-1 and mTOR pathway [151]. It was demonstrated that RSV directly binds to PARP-1 and induces its dose-dependent inhibition (IC50 = 0.65 μM) [152]. Treatment of cells damaged by hyperglycemia with RSV reduces the production of ROS, improves the ratio of reduced/oxidized glutathione (GSH/GSSG), restoration mitochondrial membrane potential [153]. Studies on the suppression of metabolic stress leading to the onset of diabetic cataracts revealed that the administration of RSV led to a significant decrease in cataractogenesis. This effect may be associated with both the activation of antioxidant protection and the inhibition of PARP-1.
6. Conclusions
A moderate level of PARP-1 activation provides an effective reparation of DNA lesions supporting survival of cells under the action of genotoxic factors. A hyperactivation of PARP-1, which often occurs at the inflammation, hypoxia, hypo- and hyperglycemia, modulates or activates multiple cellular pathways leading to cell death or degeneration. Synthetic PARPi are already implemented in anticancer therapy, and might also be useful in treatment of metabolic syndrome, multiple organ dysfunction syndrome, diabetic complications, cardiovascular and neurodegenerative diseases.
Natural polyphenols capable of inhibiting PARP-1 directly or indirectly (Figure 7) can become a supplement or even an alternative to synthetic drugs, because besides a pronounced pharmacological activity they could have low systemic toxicity and minor side effects. As an adjunct to standard drug therapy, polyphenols can allow one to reduce a concentration of toxic drugs, providing a synergistic effect.
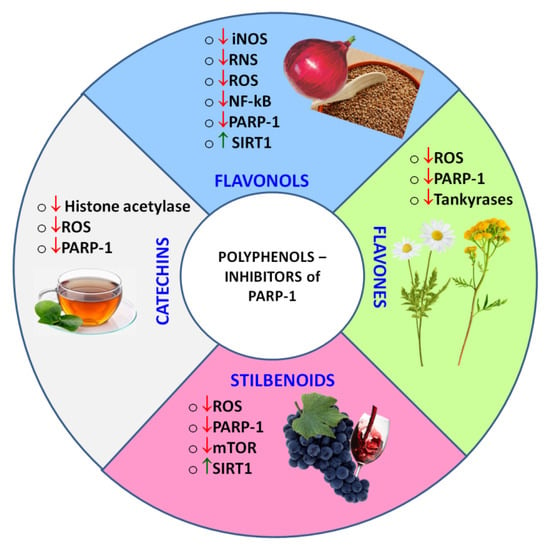
Figure 7.
Classes of polyphenols, whose representatives were found to act as PARPi, and the observed polyphenol-induced regulatory effects. ↑ - up-regulated, ↓- down-regulated.
Extending a search for natural PARPi among the secondary plant metabolites, terpenoids should be also considered. Terpenoids, like polyphenols, have a wide spectrum of biological activities [154,155] and some of them were reported to be PARPi [21,140].
Author Contributions
Conceptualization, N.V.M., A.V.F. and V.M.S.; formal analysis N.V.M. and A.V.F.; writing—original draft N.V.M. and A.V.F.; writing—review and editing A.V.F. and V.M.S.; project administration V.M.S.; funding acquisition V.M.S.; supervision V.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
Studies were financially supported by Russian Science Foundation (grant 21-74-20018).
Acknowledgments
The authors acknowledge the use of facilities of the Interdisciplinary Scientific and Educational School of Moscow University «Molecular Technologies of the Living Systems and Synthetic Biology».
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| PARP-1 | poly (ADP-ribose) polymerase-1 |
| PARPi | PARP inhibitors |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| UV | ultraviolet light |
| ROS | reactive oxygen species overproduced in oxidative stress |
| RNS | reactive nitrogen species |
| TNFα | tumor necrosis factor α |
| iNOS | inducible isoform of nitrite oxide synthase |
| COX2 | cyclooxygenase 2 |
| MCP1 | monocyte chemoattractantprotein 1 |
| IL-1β, 6, 8 | interleukins 1β, 6, 8 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| AP1, AP2 | activator proteins 1 and 2 |
| JAK | Janus kinase |
| PIP3 | phosphatidylinositol (3,4,5)-trisphosphate |
| MAPKKKs | mitogen-activated protein (MAP) kinase kinase kinases |
| MAPKKs | mitogen-activated protein kinase kinases |
| MAPKs | mitogen-activated protein kinases |
| STAT | members of the signal transducer and activator of transcription protein family |
| AKT | subfamily of serine/threonine kinases |
| p38 | p38 mitogen-activated protein kinases |
| JNK | c-Jun N-terminal kinases |
| ERKs | extracellular signal-regulated kinases |
| p300/CRBP | p300/CREB-binding protein complex |
| EMT | epithelial-mesenchymal transition |
| HSP70 | heat shock protein 70 |
| AIF | apoptosis inducing factor |
| HDAC | histone deacetylases |
| SIRT-1 | sirtuin-1 |
| AMPK | AMP-activated protein kinase |
| TNBS | trinitrobenzenesulfonic acid |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-α |
| TIMP-2 | tissue inhibitor of metalloproteinases 2, I |
| ICAM-1 | inter-cellular adhesion molecule 1 |
| ALDH2 | aldehyde dehydrogenase 2 |
| TC | total cholesterol |
| VLDL | very low density lipoproteins |
| LDL | low density lipoproteins |
| ACAT1 | acetyl-CoA acetyltransferase 1 |
| SMC | smooth muscle cell content |
| CRP | C-reactive protein |
| MPO | myeloperoxidase |
| BRCA | BRCA1 (breast cancer type 1 susceptibility protein) and BRCA2 (breast cancer type 2 susceptibility protein) |
| LPS | lipopolysaccharide |
| MMPs | matrix metalloproteinases |
| MIP-1a and 2 | macrophage inflammatory proteins 1a and 2 |
| CXCLs | C-X-C motif chemokine ligands |
| GBP2 | guanylate binding protein 2 |
| IigP1 | interferon-inducible GTPase 1 |
| CD274 | programmed death-ligand 1 or PD-L1 |
| Gpx1,4 | glutathione peroxidase 1, 4 |
| SOD1 | superoxidedismutase 1 |
| mt-Nd1 | mitochondrially encoded NADH |
| Sdha | succinate dehydrogenasecomplexflavoproteinsubunit A |
| mt-Cytb | mitochondrially encoded cytochrome B |
| FOXO1 | forkhead box protein O1 |
| Nrf1 | nuclear respiratory factor 1 |
| STAT6 | signal transducer and activator of transcription 6 |
| TLR4 | toll-like receptor 4 |
| VCAM-1 | vascular cell adhesion molecule 1 |
| QC | quercetin |
| RSV | resveratrol |
| NG | naringenin |
| GP | hesperetin |
| 4MF | 4’-methoxyflavone |
| DMF | 3’, 4’-dimethoxyflavone |
| 3-AB | 3-aminobenzamide |
| TNK | tankyrases |
| TF | theaflavin polyphenol |
| EGCG | epigallocatechin gallate |
References
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological out-comes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef]
- Kraus, W.L. PARPs and ADP-ribosylation: 60 years on. Genes Dev. 2020, 34, 251–253. [Google Scholar] [CrossRef]
- Eisemann, T.; Pascal, J.M. Poly(ADP-ribose) polymerase enzymes and the maintenance of genome integrity. Cell. Mol. Life Sci. 2019, 77, 19–33. [Google Scholar] [CrossRef]
- Maluchenko, N.V.; Kulaeva, O.I.; Kotova, E.J.; Chupyrkina, A.A.; Nikitin, D.V.; Kirpichnikov, M.P.; Studitsky, V.M. Mo-lecular Mechanisms of Transcriptional Regulation by Poly(ADP-Ribose) Polymerase. Mol. Biol. 2015, 49, 1–15. [Google Scholar] [CrossRef]
- Hottiger, M.O. Nuclear ADP-Ribosylation and Its Role in Chromatin Plasticity, Cell Differentiation, and Epigenetics. Annu. Rev. Biochem. 2015, 84, 227–263. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmana, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Annals of oncology: Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.N.; Goel, A.; Johnson, M.A. Poly(ADP-Ribose) Polymerases in Host-Pathogen Interactions, Inflammation, and Immunity. Microbiol. Mol. Biol. Rev. 2019, 83, e00038-18. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.; Pomilio, C.; Gregosa, A.; Bentivegna, M.; Presa, J.; Bellotto, M.; Saravia, F.; Beauquis, J. Inflammation and Insulin Resistance as Risk Factors and Potential Therapeutic Targets for Alzheimer’s Disease. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial Effect of Pistachio Consumption on Glucose Metabolism, Insulin Resistance, Inflammation, and Related Metabolic Risk Markers: A Randomized Clinical Trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef]
- Ministrini, S.; Carbone, F.; Montecucco, F. Updating concepts on atherosclerotic inflammation: From pathophysiology to treatment. Eur. J. Clin. Investig. 2020, 51, e13467. [Google Scholar] [CrossRef]
- Ajala, O.N.; Everett, B.M. Targeting Inflammation to Reduce Residual Cardiovascular Risk. Curr. Atheroscler. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ordog, K.; Horvath, O.; Eros, K.; Bruszt, K.; Toth, S.; Kovacs, D.; Kalman, N.; Radnai, B.; Deres, L.; Gallyas, F.; et al. Mitochondrial protective effects of PARP-inhibition in hypertension-induced myocardial remodeling and in stressed cardiomyocytes. Life Sci. 2021, 268, 118936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nemoto, M.; Xu, Z.; Yu, S.W.; Shimoji, M.; Andrabi, S.A.; Haince, J.F.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L.; et al. Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apopto-sis-inducing factor to the nucleus. Neuroscience 2007, 144, 56–65. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Chen, J.; Graham, S.H.; Du, L.; Kochanek, P.; Draviam, R.; Guo, F.; Nathaniel, P.D.; Szabo, C.; Watkins, S.; et al. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale dna fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 2002, 82, 181–191. [Google Scholar] [CrossRef]
- Yu, S.W.; Wang, H.; Dawson, T.M.; Dawson, V.L. Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in neuro-toxicity. Neurobiol. Dis. 2003, 14, 303–317. [Google Scholar] [CrossRef]
- Koh, D.W.; Dawson, T.M.; Dawson, V.L. Poly(ADP-ribosyl)ation regulation of life and death in the nervous system. Cell. Mol. Life Sci. CMLS 2005, 62, 760–768. [Google Scholar] [CrossRef]
- Antolin, A.A.; Ameratunga, M.; Banerji, U.; Clarke, P.A.; Workman, P.; Al-Lazikani, B. The kinase polypharmacology landscape of clinical PARP inhibitors. Sci. Rep. 2020, 10, 2585. [Google Scholar] [CrossRef]
- Szabó, G.; Liaudet, L.; Hagl, S.; Szabó, C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc. Res. 2004, 61, 471–480. [Google Scholar] [CrossRef]
- Luccarini, I.; Pantano, D.; Nardiello, P.; Cavone, L.; Lapucci, A.; Miceli, C.; Nediani, C.; Berti, A.; Stefani, M.; Casamenti, F. The Polyphenol Oleuropein Aglycone Modulates the PARP1-SIRT1 Interplay: An In Vitro and In Vivo Study. J. Alzheimer’s Dis. 2016, 54, 737–750. [Google Scholar] [CrossRef]
- Andreone, T.; Meares, G.P.; Hughes, K.J.; Hansen, P.A.; Corbett, J.A. Cytokine-mediated beta-cell damage in PARP-1-deficient islets. Am. J. Physiology. Endocrinol. Metab. 2012, 303, E172–E179. [Google Scholar] [CrossRef]
- Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Experiment. Biol. Med. 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Pacher, P.; Szabo, C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: Endo-thelial dysfunction, as a common underlying theme. Antioxid. Redox Signal. 2005, 7, 1568–1580. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug resistance in cancer: Mechanisms and tackling strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Dawson, V.; Dawson, T.M. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharmacol. 2014, 171, 2000–2016. [Google Scholar] [CrossRef] [PubMed]
- Harrision, D.; Gravells, P.; Thompson, R.; Bryant, H.E. Poly(ADP-Ribose) Glycohydrolase (PARG) vs. Poly(ADP-Ribose) Polymerase (PARP)—Function in Genome Maintenance and Relevance of Inhibitors for Anti-cancer Therapy. Front. Mol. Biosci. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatos. Exp. Neurol. 2009, 218, 193–202. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef] [PubMed]
- Santagostino, S.F.; Assenmacher, C.-A.; Tarrant, J.C.; Adedeji, A.O.; Radaelli, E. Mechanisms of Regulated Cell Death: Current Perspectives. Vet. Pathol. 2021. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.-I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-ribose) (PAR)-dependent cell death in neurodegenerative diseases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Umanah, G.K.E.; Chang, C.; Stevens, D.A.; Karuppagounder, S.; Gagné, J.-P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef]
- Berger, N.A.; Sims, J.L.; Catino, D.M.; Berger, S.J. Poly(ADP-ribose) Polymerase Mediates the Suicide Response to Massive DNA Damage: Studies in Normal and DNA-repair Defective Cells. Princess Takamatsu Symp. 2020, 219–226. [Google Scholar] [CrossRef]
- Chiarugi, A. Poly(ADP-ribose) polymerase: Killer or conspirator? The ‘suicide hypothesis’ revisited. Trends Pharmacol. Sci. 2002, 23, 122–129. [Google Scholar] [CrossRef]
- Formentini, L.; Macchiarulo, A.; Cipriani, G.; Camaioni, E.; Rapizzi, E.; Pellicciari, R.; Moroni, F.; Chiarugi, A. Poly(ADP-ribose) Catabolism Triggers AMP-dependent Mitochondrial Energy Failure. J. Biol. Chem. 2009, 284, 17668–17676. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Sanders, M.J.; Woods, A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 2008, 32, S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in in-flammatory disorders. Cell. Mol. Life Sci. 2002, 59, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Zerfaoui, M.; Errami, Y.; Naura, A.S.; Suzuki, Y.; Kim, H.; Ju, J.; Liu, T.; Hans, C.P.; Kim, J.G.; Abd Elmageed, Z.Y. Poly(ADP-ribose) polymerase-1 is a determining factor in crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J. Immunol. 2010, 185, 1894–1902. [Google Scholar] [CrossRef]
- Hassa, P.O.; Haenni, S.S.; Buerki, C.; Meier, N.I.; Lane, W.S.; Owen, H.; Gersbach, M.; Imhof, R.; Hottiger, M.O. Acetylation of PARP-1 by p300/CBP regulates coactivation of NF-kappa B-dependent transcription. J. Biol. Chem. 2005, 280, 40450–40464. [Google Scholar] [CrossRef]
- Wasyluk, W.; Zwolak, A. PARP Inhibitors: An Innovative Approach to the Treatment of Inflammation and Metabolic Dis-orders in Sepsis. J. Inflamm. Res. 2021, 14, 1827–1844. [Google Scholar] [CrossRef]
- Krukenberg, K.A.; Kim, S.; Tan, E.S.; Maliga, Z.; Mitchison, T.J. Extracellular Poly(ADP-Ribose) Is a Pro-inflammatory Signal for Macrophages. Chem. Biol. 2015, 22, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Molinete, M.; Boeuf, H.; De Murcia, G.; Murcia, J.M.-D. The human poly(ADP-ribose) polymerase nuclear localization signal is a bipartite element functionally separate from DNA binding and catalytic activity. EMBO J. 1992, 11, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Scovassi, A.I.; Diederic, M. Modulation of poly(ADP-ribosylation) in apoptotic cells. Biochem. Pharmacol. 2004, 68, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Pétrilli, V.; Herceg, Z.; Hassa, P.; Patel, N.; Di Paola, R.; Cortes, U.; Dugo, L.; Filipe, H.-M.; Thiemermann, C.; Hottiger, M.; et al. Noncleavablepoly(ADP-ribose) polymerase-1 regulates the inflammation response in mice. J. Clin. Invest. 2004, 114, 1072–1081. [Google Scholar] [CrossRef]
- Yung, T.M.C.; Satoh, M.S. Functional Competition between Poly(ADP-ribose) Polymerase and Its 24-kDa Apoptotic Fragment in DNA Repair and Transcription. J. Biol. Chem. 2001, 276, 11279–11286. [Google Scholar] [CrossRef]
- Soldani, C.; Scovassi, A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002, 7, 321–328. [Google Scholar] [CrossRef]
- Boesten, D.M.; de Vos-Houben, J.M.; Timmermans, L.; den Hartog, G.J.; Bast, A.; Hageman, G.J. Accelerated aging during chronic oxidative stress: A role for PARP-1. Oxidative Med. Cell. Longev. 2013, 2013, 680414. [Google Scholar] [CrossRef]
- Oliver, F.J.; Menissier-de Murcia, J.; Nacci, C.; Decker, P.; Andriantsitohaina, R.; Muller, S.; de la Rubia, G.; Stoclet, J.C.; de Murcia, G. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymer-ase-1 deficient mice. EMBO J. 1999, 18, 4446–4454. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tan, K.V.; Cornelissen, B. PARP Inhibitors in Cancer Diagnosis and Therapy. Clin. Cancer Res. 2020, 27, 1585–1594. [Google Scholar] [CrossRef]
- Singh, N.; Pay, S.L.; Bhandare, S.B.; Arimpur, U.; Motea, E.A. Therapeutic Strategies and Biomarkers to Modulate PARP Activity for Targeted Cancer Therapy. Cancers 2020, 12, 972. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Kraus, W.L. Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance. Exp. Mol. Med. 2021, 53, 42–51. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, R.K.; Yadava, P.K.; Singh, R.P. An assessment of poly (ADP-ribose) polymerase-1 role in normal and cancer cells. BioFactors 2020, 46, 894–905. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Ly, D.H.; Rosenthal, D.S.; Konopka, G.; Luo, R.; Wang, Z.Q.; Schultz, P.G.; Smulson, M.E. Mis-regulation of gene expression in primary fibroblasts lacking poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. USA 2000, 97, 11274–11279. [Google Scholar] [CrossRef]
- Liu, Z.; Kraus, W.L. Catalytic-Independent Functions of PARP-1 Determine Sox2 Pioneer Activity at Intractable Genomic Loci. Mol. Cell 2017, 65, 589–603.e9. [Google Scholar] [CrossRef]
- Neophytou, C.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Marti, J.M.; Fernandez-Cortes, M.; Serrano-Saenz, S.; Zamudio-Martinez, E.; Delgado-Bellido, D.; Garcia-Diaz, A.; Oliver, F.J. The Multifactorial Role of PARP-1 in Tumor Microenvironment. Cancers 2020, 12, 739. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jaiswal, R.K.; Prasad, R.; Yadav, S.S.; Kumar, A.; Yadava, P.K.; Singh, R.P. PARP-1 induces EMT in non-small cell lung carcinoma cells via modulating the transcription factors Smad4, p65 and ZEB1. Life Sci. 2021, 269, 118994. [Google Scholar] [CrossRef]
- Balaji, S.; Kim, U.; Muthukkaruppan, V.; Vanniarajan, A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-mesenchymal transition. Life Sci. 2021, 280, 119750. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Plummer, R.; Azad, N.S.; Helleday, T. The DNA Damaging Revolution: PARP Inhibitors and Beyond. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 185–195. [Google Scholar] [CrossRef] [PubMed]
- de Haan, R.; Pluim, D.; van Triest, B.; van den Heuvel, M.; Peulen, H.; van Berlo, D.; George, J.; Verheij, M.; Schellens, J.H.M.; Vens, C. Improved pharmacodynamic (PD) assessment of low dose PARP inhibitor PD activity for radiotherapy and chem-otherapy combination trials. Radiother. Oncol. 2018, 126, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Monk, B.J. PARP inhibitor and chemotherapy combination trials for the treatment of advanced malignancies: Does a development pathway forward exist? Ann. Oncol. 2017, 28, 443–447. [Google Scholar] [CrossRef]
- Yang, X.-D.; Kong, F.-E.; Qi, L.; Lin, J.-X.; Yan, Q.; Loong, J.H.C.; Xi, S.-Y.; Zhao, Y.; Zhang, Y.; Yuan, Y.-F.; et al. PARP inhibitor Olaparib overcomes Sorafenib resistance through reshaping the pluripotent transcriptome in hepatocellular carcinoma. Mol. Cancer 2021, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, W.; Liao, W.; Hao, Q.; Tang, D.; Wang, D.; Wang, Y.; Ge, G. Green tea polyphenol epigallocatechin-3-gallate alleviates nonalcoholic fatty liver disease and ameliorates intestinal immunity in mice fed a high-fat diet. Food Funct. 2020, 11, 9924–9935. [Google Scholar] [CrossRef]
- Karunarathna, K.H.T.; Mewan, K.M.; Weerasena, O.V.D.S.J.; Perera, S.A.C.N.; Edirisinghe, E.N.U. A functional molecular marker for detecting blister blight disease resistance in tea (Camellia sinensis L.). Plant Cell Rep. 2020, 40, 351–359. [Google Scholar] [CrossRef]
- Yang, M.; Liu, L.; Xie, M.; Sun, X.; Yu, Y.; Kang, R.; Yang, L.; Zhu, S.; Cao, L.; Tang, D. Poly-ADP-ribosylation of HMGB1 regulates TNFSF10/TRAIL resistance through autophagy. Autophagy 2015, 11, 214–224. [Google Scholar] [CrossRef]
- Tulin, A.; Spradling, A. Chromatin Loosening by Poly(ADP)-Ribose Polymerase (PARP) at Drosophila Puff Loci. Science 2003, 299, 560–562. [Google Scholar] [CrossRef]
- Petesch, S.J.; Lis, J.T. Rapid, Transcription-Independent Loss of Nucleosomes over a Large Chromatin Domain at Hsp70 Loci. Cell 2008, 134, 74–84. [Google Scholar] [CrossRef]
- Leu, J.I.; Pimkina, J.; Frank, A.; Murphy, M.E.; George, D.L. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 2009, 36, 15–27. [Google Scholar] [CrossRef]
- Rojo, F.; García-Parra, J.; Zazo, S.; Tusquets, I.; Ferrer-Lozano, J.; Menendez, S.; Eroles, P.; Chamizo, C.; Servitja, S.; Ramírez-Merino, N.; et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 2011, 23, 1156–1164. [Google Scholar] [CrossRef]
- Domagala, P.; Huzarski, T.; Lubinski, J.; Gugala, K.; Domagala, W. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: Possible implications for PARP-1 inhibitor therapy. Breast Cancer Res. Treat. 2011, 127, 861–869. [Google Scholar] [CrossRef]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef]
- Malyuchenko, N.V.; Kotova, E.Y.; Kulaeva, O.I.; Kirpichnikov, M.P.; Studitskiy, V.M. PARP1 Inhibitors: Antitumor drug design. Acta Nat. 2015, 7, 27–37. [Google Scholar] [CrossRef]
- Singh, M.P.; Cho, H.J.; Kim, J.-T.; Baek, K.E.; Lee, H.G.; Kang, S.C.; Cho, K.; Baek, L. Morin Hydrate Reverses Cisplatin Resistance by Impairing PARP1/HMGB1-Dependent Autophagy in Hepatocellular Carcinoma. Cancers 2019, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ledermann, J.A.; Kohn, E.C. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann. Oncol. 2014, 25, 32–40. [Google Scholar] [CrossRef]
- Liaudet, L.; Pacher, P.; Mabley, J.G.; Virag, L.; Soriano, F.G.; Hasko, G.; Szabo, C. Activation of poly(ADP-Ribose) poly-merase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am. J. Respir. Crit. Care Med. 2002, 165, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Zerfaoui, M.; Naura, A.S.; Errami, Y.; Hans, C.P.; Rezk, B.M.; Park, J.W.; Elsegeiny, W.; Kim, H.; Lord, K.; Kim, J.G.; et al. Effects of PARP-1 deficiency on airway inflammatory cell recruitment in response to LPS or TNF: Differential effects on CXCR2 ligands and Duffy antigen receptor for chemokines. J. Leukoc. Biol. 2009, 86, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Cakala, M.; Gajkowska, B.; Strosznajder, J.B. Poly(ADP-ribose) polymerase-1 inhibition protects the brain against systemic inflammation. Neurochem. Int. 2006, 49, 751–755. [Google Scholar] [CrossRef]
- Altmeyer, M.; Barthel, M.; Eberhard, M.; Rehrauer, H.; Hardt, W.-D.; Hottiger, M.O. Absence of Poly(ADP-Ribose) Polymerase 1 Delays the Onset of Salmonella enterica Serovar Typhimurium-Induced Gut Inflammation. Infect. Immun. 2010, 78, 3420–3431. [Google Scholar] [CrossRef]
- Zingarelli, B.; Szabó, C.; Salzman, A.L. Blockade of poly(ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology 1999, 116, 335–345. [Google Scholar] [CrossRef]
- Ullrich, O.; Diestel, A.; Eyupoglu, I.Y.; Nitsch, R. Regulation of microglial expression of integrins by poly(ADP-ribose) poly-merase-1. Nat. Cell Biol. 2001, 3, 1035–1042. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I.; Sanchez-Fidalgo, S. Poly(ADP-ribose) polymerase inhibitors: New pharmacological functions and potential clinical implications. Curr. Pharm. Des. 2007, 13, 933–962. [Google Scholar] [CrossRef]
- Matsuura, S.; Egi, Y.; Yuki, S.; Horikawa, T.; Satoh, H.; Akira, T. MP-124, a novel poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor, ameliorates ischemic brain damage in a non-human primate model. Brain Res. 2011, 1410, 122–131. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.S.; Noh, M.-Y.; Lee, H.; Joe, B.; Kim, H.Y.; Kim, J.; Kim, S.H.; Park, J. Neuroprotective effects of a novel poly (ADP-ribose) polymerase-1 inhibitor, JPI-289, in hypoxic rat cortical neurons. Clin. Exp. Pharmacol. Physiol. 2017, 44, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Sharma, U.; Grewal, A.K.; Singh, T.G. Poly (ADP-ribose) polymerase-1 as a promising drug target for neurodegenerative diseases. Life Sci. 2020, 267, 118975. [Google Scholar] [CrossRef]
- Martire, S.; Fuso, A.; Rotili, D.; Tempera, I.; Giordano, C.; De Zottis, I.; Muzi, A.; Vernole, P.; Graziani, G.; Lococo, E.; et al. PARP-1 Modulates Amyloid Beta Peptide-Induced Neuronal Damage. PLoS ONE 2013, 8, e72169. [Google Scholar] [CrossRef]
- Bayrakdar, E.T.; Uyanikgil, Y.; Kanit, L.; Koylu, E.; Yalcin, A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1-42)-induced rat model of Alzheimer’s disease. Free Radic. Res. 2014, 48, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Cieslik, M.; Wencel, P.L.; Wojtowicz, S.; Strosznajder, R.P.; Strosznajder, J.B. Inhibition of poly(ADP-ribose) polymerase-1 alters expression of mitochondria-related genes in PC12 cells: Relevance to mitochondrial homeostasis in neurodegenerative disorders. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 281–288. [Google Scholar] [CrossRef]
- Kim, T.W.; Cho, H.M.; Choi, S.Y.; Suguira, Y.; Hayasaka, T.; Setou, M.; Koh, H.C.; Hwang, E.M.; Park, J.Y.; Kang, S.J.; et al. (ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson’s disease. Cell Death Dis. 2013, 4, e919. [Google Scholar] [CrossRef] [PubMed]
- Cosi, C.; Marien, M. Decreases in mouse brain NAD+ and ATP induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): Prevention by the poly(ADP-ribose) polymerase inhibitor, benzamide. Brain Res. 1998, 809, 58–67. [Google Scholar] [CrossRef]
- Lee, Y.; Karuppagounder, S.S.; Shin, J.H.; Lee, Y.I.; Ko, H.S.; Swing, D.; Jiang, H.; Kang, S.U.; Lee, B.D.; Kang, H.C. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neu-ronal loss. Nat. Neurosci. 2013, 16, 1392–1400. [Google Scholar] [CrossRef]
- Genovese, T.; Mazzon, E.; Muià, C.; Patel, N.S.A.; Threadgill, M.D.; Bramanti, P.; De Sarro, A.; Thiemermann, C.; Cuzzocrea, S. Inhibitors of Poly(ADP-Ribose) Polymerase Modulate Signal Transduction Pathways and Secondary Damage in Experimental Spinal Cord Trauma. J. Pharmacol. Exp. Ther. 2004, 312, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Kornwoski, R.; Aravot, D.; Arad, M.; Hochhauser, E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp. Cell Res. 2018, 373, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Lee, K.; Jeon, J.; Kim, J.-R.; Lee, J.E.; Kwon, G.Y.; Kim, Y.-G.; Kim, D.J.; Ko, J.-W.; Huh, W. Poly (ADP-Ribose) Polymerase Inhibitor Treatment as a Novel Therapy Attenuating Renal Ischemia-Reperfusion Injury. Front. Immunol. 2020, 11, 564288. [Google Scholar] [CrossRef]
- Ji, Z.; Zeng, L.; Cheng, Y.; Liang, G. Poly(ADP-ribose) polymerases-1 inhibitor MRL-45696 alleviates DNA damage after myocardial ischemia-reperfusion in diabetic rats. Nan Fang Yike Da Xuexue Bao 2018, 38, 830–835. [Google Scholar]
- Liu, Z.; Wang, H.; Wang, S.; Gao, J.; Niu, L. PARP-1 inhibition attenuates the inflammatory response in the cartilage of a rat model of osteoarthritis. Bone Jt. Res. 2021, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Attia, S.M.; Zoheir, K.M.; Ashour, A.E.; Bakheet, S.A. Attenuation of the progression of adjuvant-induced arthritis by 3-aminobenzamide treatment. Int. Immunopharmacol. 2014, 19, 52–59. [Google Scholar] [CrossRef]
- Idowu, S.O.; Fatokun, A.A. Artificial Intelligence (AI) to the Rescue: Deploying Machine Learning to Bridge the Biorelevance Gap in Antioxidant Assays. SLAS Technol. Transl. Life Sci. Innov. 2020, 26, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nilov, D.; Maluchenko, N.; Kurgina, T.; Pushkarev, S.; Lys, A.; Kutuzov, M.; Gerasimova, N.; Feofanov, A.; Švedas, V.; Lavrik, O.; et al. Molecular Mechanisms of PARP-1 Inhibitor 7-Methylguanine. Int. J. Mol. Sci. 2020, 21, 2159. [Google Scholar] [CrossRef] [PubMed]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Pre-vention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Pandey, S.N.; Khawaja, H.; Brown, K.J.; Hathout, Y.; Chen, Y.-W. PARP1 Differentially Interacts with Promoter region of DUX4 Gene in FSHD Myoblasts. J. Genet. Syndr. Gene Ther. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Moonen, H.J.J.; Brauers, K.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Dietary Flavones and Flavonoles Are Inhibitors of Poly(ADP-ribose)polymerase-1 in Pulmonary Epithelial Cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [CrossRef]
- Maeda, J.; Roybal, E.J.; Brents, C.A.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2013, 31, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Narwal, M.; Haikarainen, T.; Fallarero, A.; Vuorela, P.M.; Lehtiö, L. Screening and Structural Analysis of Flavones Inhibiting Tankyrases. J. Med. Chem. 2013, 56, 3507–3517. [Google Scholar] [CrossRef]
- Qadir, M.I. Role of Green Tea Flavonoids and Other Related Contents in Cancer Prevention. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 163–171. [Google Scholar] [CrossRef]
- Arts, I.C.W. A Review of the Epidemiological Evidence on Tea, Flavonoids, and Lung Cancer. J. Nutr. 2008, 138, 1561S–1566S. [Google Scholar] [CrossRef]
- Amin, A.; Buratovich, M. The anti-cancer charm of flavonoids: A cup-of-tea will do! Recent Pat. Anti-Cancer Drug Discov. 2007, 2, 109–117. [Google Scholar] [CrossRef]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary Polyphenols as Topoisomerase II Poisons: B Ring and C Ring Substituents Determine the Mechanism of Enzyme-Mediated DNA Cleavage Enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef]
- Piaz, F.D.; Ferro, P.; Vassallo, A.; Vasaturo, M.; Forte, G.; Chini, M.G.; Bifulco, G.; Tosco, A.; De Tommasi, N. Identification and mechanism of action analysis of the new PARP-1 inhibitor 2″-hydroxygenkwanol A. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1806–1814. [Google Scholar] [CrossRef]
- Inyang, O.K.; Omotuyi, O.I.; Ogunleye, A.J.; Eniafe, G.O.; Adewumi, B.; Metibemu, D.S. Molecular Interaction and Inhibitory Potential of Polyphenol on DNA Repair Pathway in Small Cell Lung Cancer: A Computational Study. J. Anal. Pharm. Res. 2017, 6, 00178–00186. [Google Scholar] [CrossRef][Green Version]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Engen, A.; Maeda, J.; Wozniak, D.E.; Brents, C.A.; Bell, J.J.; Uesaka, M.; Aizawa, Y.; Kato, T.A. Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 784–785, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Haskins, A.H.; Omata, C.; Aizawa, Y.; Kato, T.A. PARP Inhibition by Flavonoids Induced Selective Cell Killing to BRCA2-Deficient Cells. Pharmaceuticals 2017, 10, 80. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Cui, S.; Wu, Q.; Wang, J.; Li, M.; Qian, J.; Li, S. Quercetin inhibits LPS-induced macrophage migration by suppressing the iNOS/FAK/paxillin pathway and modulating the cytoskeleton. Cell Adhes. Migr. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.-D.; et al. Quercetin Inhibits LPS-Induced Inflammation and ox-LDL-Induced Lipid Deposition. Front. Pharmacol. 2017, 8, 40. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.-J.; Frei, B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016, 9, 104–113. [Google Scholar] [CrossRef]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Ikeda, A.; Iso, H.; Yamagishi, K.; Iwasaki, M.; Yamaji, T.; Miura, T.; Sawada, N.; Inoue, M.; Tsugane, S. Plasma tea catechins and risk of cardiovascular disease in middle-aged Japanese subjects: The JPHC study. Atherosclerosis 2018, 277, 90–97. [Google Scholar] [CrossRef]
- Yu, S.-W.; Wang, H.; Poitras, M.F.; Coombs, C.; Bowers, W.J.; Federoff, H.J.; Poirier, G.G.; Dawson, T.M.; Dawson, V.L. Mediation of Poly(ADP-Ribose) Polymerase-1-Dependent Cell Death by Apoptosis-Inducing Factor. Science 2002, 297, 259–263. [Google Scholar] [CrossRef]
- Oh, C.M.; Oh, I.H.; Choe, B.K.; Yoon, T.Y.; Choi, J.M.; Hwang, J. Consuming Green Tea at Least Twice Each Day Is As-sociated with Reduced Odds of Chronic Obstructive Lung Disease in Middle-Aged and Older Korean Adults. J. Nutr. 2018, 148, 70–76. [Google Scholar] [CrossRef]
- Kuriyama, S. The Relation between Green Tea Consumption and Cardiovascular Disease as Evidenced by Epidemiological Studies. J. Nutr. 2008, 138, 1548S–1553S. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Tome, M.; Smith, R.A.; Darlington, L.G.; Stone, T.W. Protection by the flavonoids quercetin and luteolin against peroxide- or menadione-induced oxidative stress in MC3T3-E1 osteoblast cells. Nat. Prod. Res. 2015, 29, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Uberti, D.; Ferrari-Toninelli, G.; Bonini, S.A.; Sarnico, I.; Benarese, M.; Pizzi, M.; Benussi, L.; Ghidoni, R.; Binetti, G.; Spano, P.; et al. Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor DR5 prevents beta-amyloid neurotoxicity. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 872–880. [Google Scholar] [CrossRef]
- Huang, Y.; Xing, K.; Qiu, L.; Wu, Q.; Wei, H. Therapeutic implications of functional tea ingredients for ameliorating inflam-matory bowel disease: A focused review. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Guo, Y.; Bian, Z.; Si, J.; Yang, L.; Chen, Y.; Ren, X.; Jiang, G.; Chen, J.; et al. Tea consumption and risk of ischaemic heart disease. Heart 2017, 103, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Mineharu, Y.; Koizumi, A.; Wada, Y.; Iso, H.; Watanabe, Y.; Date, C.; Yamamoto, A.; Kikuchi, S.; Inaba, Y.; Toyoshima, H.; et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J. Epidemiol. Community Health 2009, 65, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fatokun, A.A.; O Liu, J.; Dawson, V.L.; Dawson, T.M. Identification through high-throughput screening of 4’-methoxyflavone and 3’,4’-dimethoxyflavone as novel neuroprotective inhibitors of parthanatos. Br. J. Pharmacol. 2013, 169, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Chen, H.H.; Lin, C.A.; Wu, H.C.; Sheu, J.J.; Chen, H.J. Apigenin-induced lysosomal degradation of beta-catenin in Wnt/beta-catenin signaling. Sci. Rep. 2017, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Lehtiö, L.; Chi, N.-W.; Krauss, S. Tankyrases as drug targets. FEBS J. 2013, 280, 3576–3593. [Google Scholar] [CrossRef]
- Zhang, X.H.; Gao, Y.T.; Rashid, A.; Deng, J.; Liu, E.J.; Wu, K.; Sun, L.; Cheng, J.R.; Gridley, G.; Hsing, A.W. Tea consumption and risk of biliary tract cancers and gallstone disease: A population-based case-control study in Shanghai, China. ZhonghuaZhongliu Za Zhi 2005, 27, 667–671. [Google Scholar]
- Alam, S.; Khan, F. 3D-QSAR, Docking, ADME/Tox studies on Flavone analogs reveal anticancer activity through Tankyrase inhibition. Sci. Rep. 2019, 9, 5414. [Google Scholar] [CrossRef]
- Stewart, A.J.; Mullen, W.; Crozier, A. On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol. Nutr. Food Res. 2004, 49, 52–60. [Google Scholar] [CrossRef]
- Larsen, C.A.; Dashwood, R.H.; Bisson, W.H. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacol. Res. 2010, 62, 457–464. [Google Scholar] [CrossRef]
- Fang, J.; Sureda, A.; Silva, A.S.; Khan, F.; Xu, S.; Nabavi, S.M. Trends of tea in cardiovascular health and disease: A critical review. Trends Food Sci. Technol. 2019, 88, 385–396. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, J.; Yang, X.; Chen, J.; Cao, J.; Wu, X.; Lu, X.; Huang, J.; Li, Y.; et al. Tea consumption and the risk of atherosclerotic cardiovascular disease and all-cause mortality: The China-PAR project. Eur. J. Prev. Cardiol. 2020, 27, 1956–1963. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Alqahtani, S.; Welton, K.; Gius, J.P.; Elmegerhi, S.; Kato, T.A. The Effect of Green and Black Tea Polyphenols on BRCA2 Deficient Chinese Hamster Cells by Synthetic Lethality through PARP Inhibition. Int. J. Mol. Sci. 2019, 20, 1274. [Google Scholar] [CrossRef]
- Bassett, S.A.; Barnett, M.P.G. The Role of Dietary Histone Deacetylases (HDACs) Inhibitors in Health and Disease. Nutrients 2014, 6, 4273–4301. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Polyphenol Compound as a Transcription Factor Inhibitor. Nutrients 2015, 7, 8987–9004. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wei, D.; Zhang, Q.; Yang, S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (–)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005, 59, 64–69. [Google Scholar] [CrossRef]
- Albassam, A.A.; Markowitz, J.S. An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis). Planta Med. 2017, 83, 496–508. [Google Scholar] [CrossRef]
- Formentini, L.; Arapistas, P.; Pittelli, M.; Jacomelli, M.; Pitozzi, V.; Menichetti, S.; Romani, A.; Giovannelli, L.; Moroni, F.; Chiarugi, A. Mono-galloyl glucose derivatives are potent poly(ADP-ribose) glycohydrolase (PARG) inhibitors and partially reduce PARP-1-dependent cell death. Br. J. Pharmacol. 2008, 155, 1235–1249. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- de Vries, K.; Strydom, M.; Steenkamp, V. A Brief Updated Review of Advances to Enhance Resveratrol’s Bioavailability. Molecules 2021, 26, 4367. [Google Scholar] [CrossRef]
- Fraiz, G.M.; da Conceição, A.R.; Vilela, D.L.D.S.; Rocha, D.M.U.P.; Bressan, J.; Hermsdorff, H.H.M. Can resveratrol modulate sirtuins in obesity and related diseases? A systematic review of randomized controlled trials. Eur. J. Nutr. 2021, 60, 2961–2977. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.; Kurscheid, S.; Nekrasov, M.; Domaschenz, R.; Vera, D.L.; Dennis, J.H.; Tremethick, D.J. Multiple roles of H2A.Z in regulating promoter chromatin architecture in human cells. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-R.; Song, J.-Y.; Fan, B.; Wang, Y.; Che, L.; Zhang, S.-M.; Chang, Y.-X.; He, C.; Li, G.-Y. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun. Signal. 2020, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.; Alshehri, A.S.; Ahmad, M.; Guo, W.W. Promising anti- cervical carcinoma and inflammatory agent, Resveratrol targets poly (ADP-ribose) polymerase 1 (PARP-1) induced premature ovarian failure with a potent enzymatic modulatory activity. J. Reprod. Immunol. 2021, 144, 103272. [Google Scholar] [CrossRef]
- Fatokun, C.; Girma, G.; Abberton, M.; Gedil, M.; Unachukwu, N.; Oyatomi, O.; Yusuf, M.; Rabbi, I.; Boukar, O. Genetic di-versity and population structure of a mini-core subset from the world cowpea (Vigna unguiculata (L.) Walp.) germplasm col-lection. Sci. Rep. 2018, 8, 16035. [Google Scholar] [CrossRef]
- Jaeger, R.; Cuny, E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Nabavi, S.M.; Daglia, M.; Jafari, S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016, 68, 671–679. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).