The Cytotoxic Effect of Copper (II) Complexes with Halogenated 1,3-Disubstituted Arylthioureas on Cancer and Bacterial Cells
Abstract
:1. Introduction
2. Results and Discussion
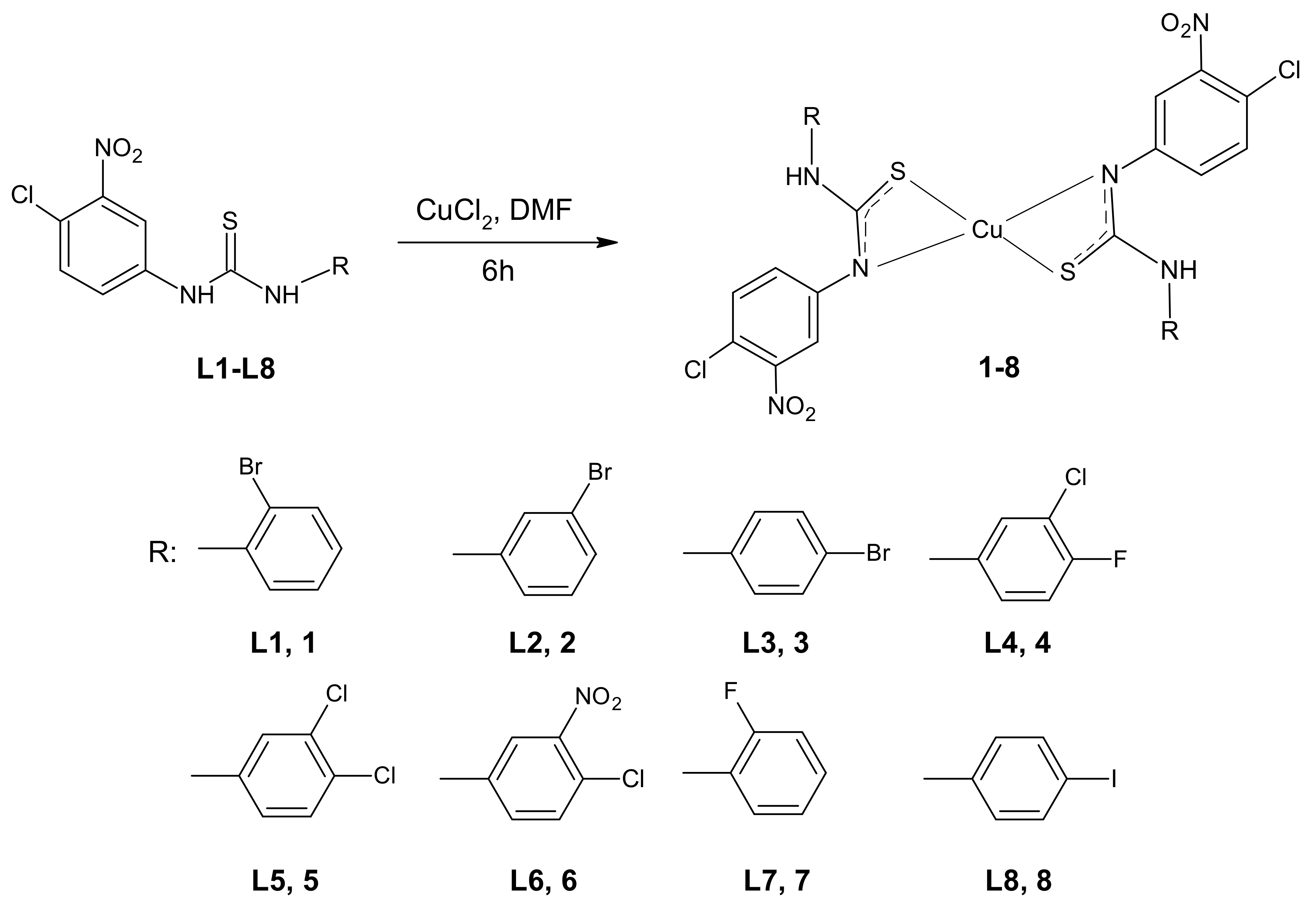
2.1. Synthesis of Complexes
2.2. Structural Characterization of Complexes
2.3. Biological Studies
2.3.1. Anticancer Activity
MTT Assay
LDH Assay
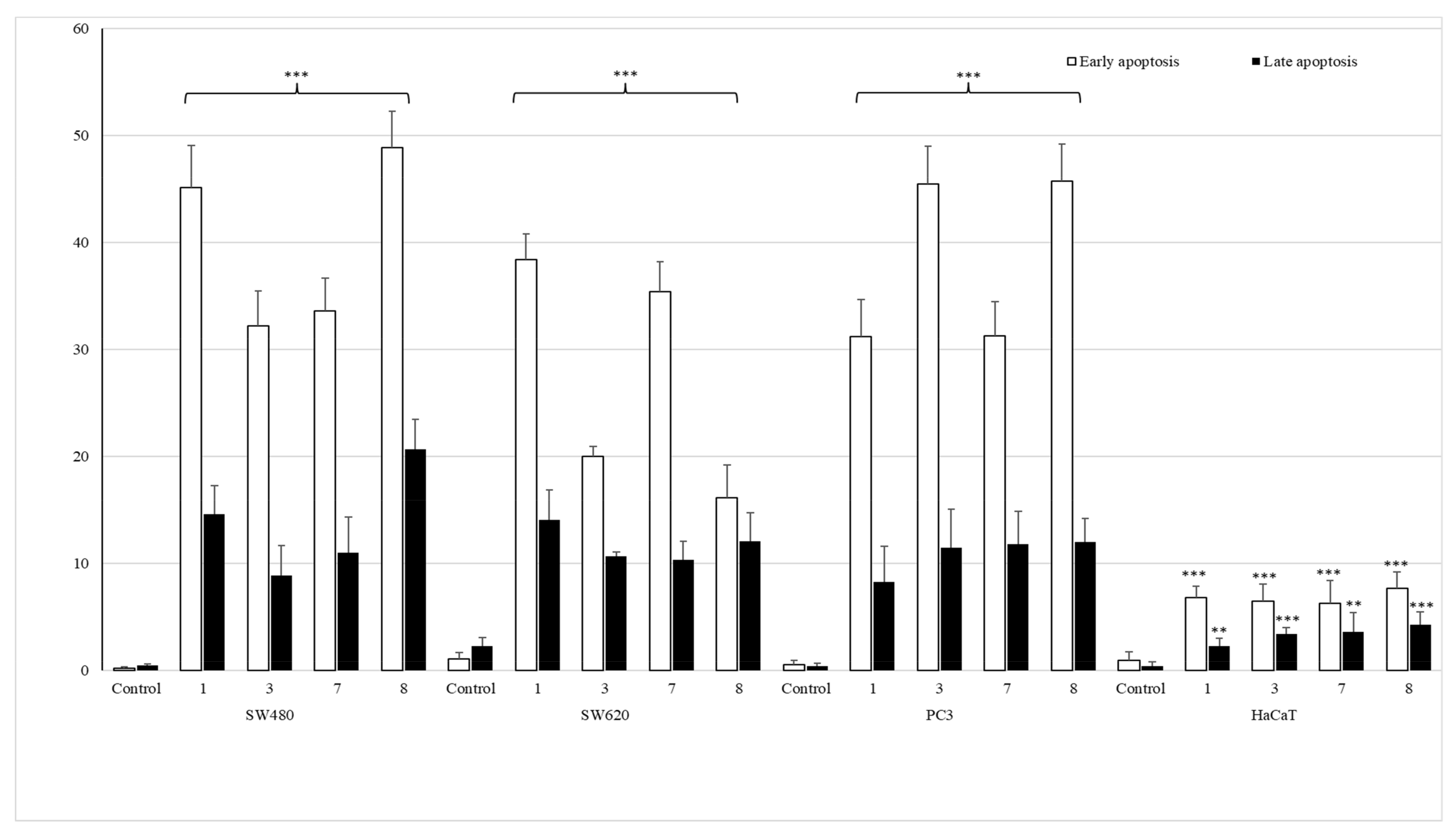
Apoptotic Activity
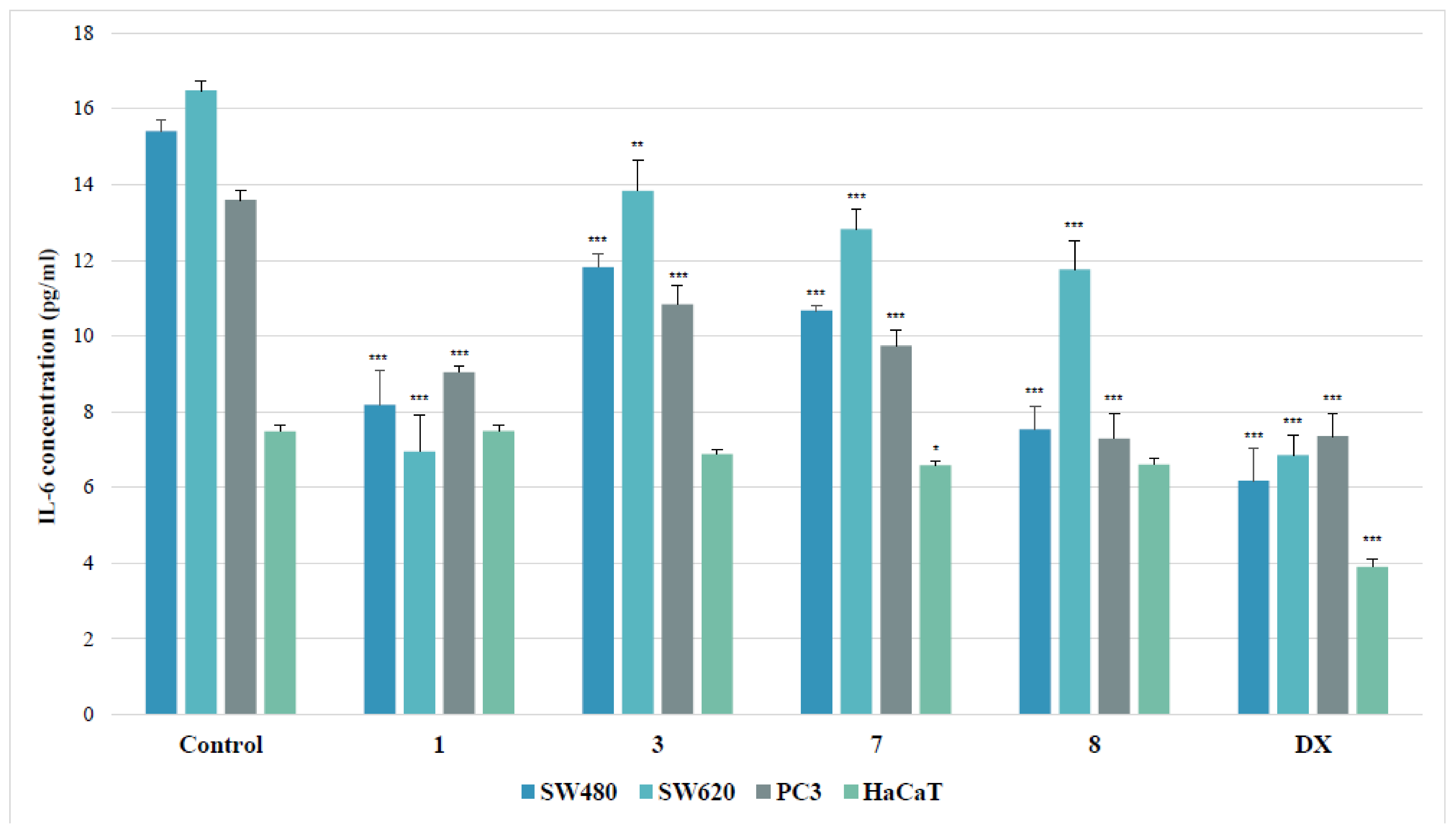
IL-6 Assay
Proteomic Analysis of Antioxidant and Detoxifying Enzymes
2.3.2. In Vitro Antimicrobial Activity
3. Materials and Methods
3.1. General Procedure
3.1.1. Synthesis of Cu (II) Complexes of 3-(4-Chloro-3-nitrophenyl)thiourea (1‒8)
- Copper (II) complex with 1-(2-bromophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
- 2.
- Copper (II) complex with 1-(3-bromophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
- 3.
- Copper (II) complex with 1-(4-bromophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
- 4.
- Copper (II) complex with 1-(3-chloro-4-fluorophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
- 5.
- Copper (II) complex with 1-(4-chloro-3-nitrophenyl)-3-(3,4-dichlorophenyl)thiourea.
- 6.
- Copper (II) complex with 1,3-bis(4-chloro-3-nitrophenyl)thiourea.
- 7.
- Copper (II) complex with 1-(2-fluorophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
- 8.
- Copper (II) complex with 1-(4-iodophenyl)-3-(4-chloro-3-nitrophenyl)thiourea.
3.1.2. Instrumentation
3.2. Cell Culture
3.3. MTT Assay
3.4. LDH Assay
3.5. Annexin V Binding Assay
3.6. Il-6 Level Assay
3.7. LC-MS Proteome Analysis
3.8. In Vitro Evaluation of Antimicrobial Activity
3.9. Genotoxicity Studies
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, I.S.; Murphy, M.E.P. Type-2 copper-containing enzymes. Cell. Mol. Life Sci. 2007, 64, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gorritti, W.-R.; Spodine-Spirinova, E.; Beyer, L.; Schröder, U.; Richter, R.; Ferreira, J.; Pavani, M. Synthesis, characterization and antitumor activity of copper (II) complexes, [CuL2] [HL1-3 = N,N-Diethyl-N′-(R-Benzoyl)Thiourea (R = H, o-Cl and p-NO2)]. Bioinorg. Chem. Appl. 2005, 3, 299–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Gao, Z.; Li, X.; Li, T.; Chen, G.; Zhou, M.; Zhang, J. DNA binding, DNA cleavage and HSA interaction of several metal complexes containing N-(2-hydroxyethyl)-N′-benzoylthiourea and 1,10-phenanthroline ligands. JBIC J. Biol. Inorg. Chem. 2016, 21, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.; Isaia, F.; Verani, G.; Cannas, C.; Serra, L.; Castellano, C.; Demartin, F.; Pilla, F.; Manca, M.; Pani, A. Mixed-1,10-phenanthroline–Cu(II) complexes: Synthesis, cytotoxic activity versus hematological and solid tumor cells and complex formation equilibria with glutathione. J. Inorg. Biochem. 2012, 114, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka-Antonik, A.; Rejmak, P.; Klepka, M.; Wolska, A.; Pietrzyk, P.; Stępień, K.; Sanna, G.; Struga, M. Synthesis, structural studies and biological activity of novel Cu (II) complexes with thiourea derivatives of 4-azatricyclo [5.2.1.0 2,6 ] dec-8-ene-3,5-dione. J. Inorg. Biochem. 2017, 176, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyng, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, structural and antimicrobial studies of type II topoisomerase-targeted copper (II) complexes of 1,3-disubstituted thiourea ligands. J. Inorg. Biochem. 2018, 182, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Repich, H.; Orysyk, V.; Palchykovska, L.; Orysyk, S.; Zborovskii, Y.L.; Vasylchenko, O.; Storozhuk, O.; Biluk, A.; Nikulina, V.; Garmanchuk, L.; et al. Synthesis, spectral characterization of novel Pd (II), Pt (II) π-coordination compounds based on N-allylthioureas. Cytotoxic properties and DNA binding ability. J. Inorg. Biochem. 2017, 168, 98–106. [Google Scholar] [CrossRef]
- Ćoćić, D.; Jovanovic, S.; Radisavljević, S.; Korzekwa, J.; Scheurer, A.; Puchta, R.; Baskić, D.; Todorovic, D.; Popovic, S.; Matić, S.; et al. New monofunctional platinum (II) and palladium (II) complexes: Studies of the nucleophilic substitution reactions, DNA/BSA interaction, and cytotoxic activity. J. Inorg. Biochem. 2018, 189, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Plutín, A.M.; Mocelo, R.; Alvarez, A.; Ramos, R.; Castellano, E.E.; Cominetti, M.R.; Graminha, A.E.; Ferreira, A.G.; Batista, A.A. On the cytotoxic activity of Pd (II) complexes of N,N-disubstituted-N′-acyl thioureas. J. Inorg. Biochem. 2014, 134, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Marverti, G.; Gozzi, G.; Lauriola, A.; Ponterini, G.; Belluti, S.; Imbriano, C.; Costi, M.P.; D’Arca, D. The 1,10-phenanthroline ligand enhances the antiproliferative activity of DNA-intercalating thiourea-Pd (II) and -Pt (II) complexes against cisplatin-sensitive and-resistant human ovarian cancer cell lines. Int. J. Mol. Sci. 2019, 20, 6122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pracharova, J.; Zerzankova, L.; Stepankova, J.; Novakova, O.; Farrer, N.J.; Sadler, P.J.; Brabec, V.; Kasparkova, J. Interactions of DNA with a new platinum (IV) azide dipyridine complex activated by UVA and visible light: Relationship to toxicity in tumor cells. Chem. Res. Toxicol. 2012, 25, 1099–1111. [Google Scholar] [CrossRef]
- Ma, Z.; Choudhury, J.R.; Wright, M.W.; Day, C.S.; Saluta, G.; Kucera, G.L.; Bierbach, U. A non-cross-linking platinum−acridine agent with potent activity in non-small-cell lung cancer. J. Med. Chem. 2008, 51, 7574–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiter, L.C.; Hall, N.W.; Day, C.S.; Saluta, G.; Kucera, G.L.; Bierbach, U. Gold (I) analogues of a platinum−acridine antitumor agent are only moderately cytotoxic but show potent activity against mycobacterium tuberculosis. J. Med. Chem. 2009, 52, 6519–6522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Ma, L.; Jin, J.; Jiang, F.; Zhou, G.; Yan, K.; Liu, Y. Mitochondrial toxicity induced by a thiourea gold (I) complex: Mitochondrial permeability transition and respiratory deficit. Toxicol. Res. 2018, 7, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, R.; de Oliveira, K.M.; Delolo, F.G.; Alvarez, A.; Mocelo, R.; Plutin, A.M.; Cominetti, M.R.; Castellano, E.E.; Batista, A.A. Ru (II)-based complexes with N-(acyl)-N′,N′-(disubstituted)thiourea ligands: Synthesis, characterization, BSA- and DNA-binding studies of new cytotoxic agents against lung and prostate tumour cells. J. Inorg. Biochem. 2015, 150, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.N.; Colina-Vegas, L.; Plutín, A.M.; da Silveira, R.G.; Honorato, J.; de Oliveira, K.M.; Cominetti, M.R.; Ferreira, A.G.; Castellano, E.E.; Batista, A.A. Hydrolysis reaction promotes changes in coordination mode of Ru (II)/acylthiourea organometallic complexes with cytotoxicity against human lung tumor cell lines. J. Inorg. Biochem. 2018, 186, 147–156. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.D.; Plutín, A.M.; Luna-Dulcey, L.; Castellano, E.E.; Cominetti, M.R.; Batista, A.A. Cytotoxicity of ruthenium-N,N-disubstituted-N′-acylthioureas complexes. Mater. Sci. Eng. C 2020, 115, 111106. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, M.; Berber, H.; Banti, C.N.; Kourkoumelis, N.; Manos, M.J.; Hadjikakou, S.K. Synthesis characterization and biological activity of mixed ligand silver (I) complex of 2-benzimidazolylurea and triphenylphosphine. Polyhedron 2017, 128, 95–103. [Google Scholar] [CrossRef]
- Perillo, B.; di Donato, M.; Pezone, A.; di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.; Jha, S.; Kesari, K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Bułdak, R.; Buldak, L.; Kukla, M.; Gabriel, A.; Żwirska-Korczala, K. Significance of selected antioxidant enzymes in cancer cell progression. Pol. J. Pathol. 2014, 3, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Hwang, P.M.; Bunz, F.; Yu, J.; Rago, C.; Chan, T.A.; Murphy, M.P.; Kelso, G.F.; Smith, R.A.J.; Kinzler, K.W.; Vogelstein, B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil–induced apoptosis in colorectal cancer cells. Nat. Med. 2001, 7, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J. Natl. Cancer Inst. 2002, 94, 1745–1757. [Google Scholar] [CrossRef]
- Zhou, W.; Liotta, L.A.; Petricoin, E.F. Cancer metabolism: What we can learn from proteomic analysis by mass spectrometry. Cancer Genom.-Proteom. 2012, 9, 373–381. [Google Scholar]
- Yu, B.; Liu, Y.; Peng, X.; Hua, S.; Zhou, G.; Yan, K.; Liu, Y. Synthesis, characterization, and antitumor properties of Au (I)–thiourea complexes. Metallomics 2020, 12, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-P.; Lai, S.; Wu, S.; Hu, S.; Zhou, L.; Chen, Y.; Wang, M.; Zhu, Y.; Lian, W.; Peng, W.; et al. Nuclear permeable ruthenium (II) β-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis. J. Med. Chem. 2010, 53, 7613–7624. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Cai, M.; Deng, J.; Yu, P.; Liang, H.; Yang, F. Anticancer function and ROS-mediated multi-targeting anticancer mechanisms of copper (II) 2-hydroxy-1-naphthaldehyde complexes. Molecules 2019, 24, 2544. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Solís, C.; Jimenez-Farfan, D.; Rodriguez-Enriquez, S.; Fernandez-Valverde, F.; Cruz-Salgado, A.; Ruiz-Azuara, L.; Sotelo, J. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer 2012, 12, 156. [Google Scholar] [CrossRef] [Green Version]
- Terbouche, A.; Ramdane-Terbouche, C.A.; Bendjilali, Z.; Berriah, H.; Lakhdri, H.; Lerari, D.; Bachari, K.; Mezaoui, D.; Bensiradj, N.E.H.; Guegan, J.-P.; et al. Synthesis, spectral characterization, molecular modeling, antibacterial and antioxidant activities and stability study of binuclear Pd (II) and Ru (III) complexes with novel bis-[1-(2-[(2-hydroxynaphthalen-1-yl)methylidene]amino}ethyl)-1-ethyl-3-phenylthiourea] ligand: Application to detection of cholesterol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 146–159. [Google Scholar] [CrossRef]
- Esmaeili, L.; Perez, M.G.; Jafari, M.; Paquin, J.; Ispas-Szabo, P.; Pop, V.; Andruh, M.; Byers, J.; Mateescu, M.A. Copper complexes for biomedical applications: Structural insights, antioxidant activity and neuron compatibility. J. Inorg. Biochem. 2019, 192, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Inci, D.; Aydın, R.; Zorlu, Y. Cu (II) complex with auxin (3-indoleacetic acid) and an aromatic planar ligand: Synthesis, crystal structure, biomolecular interactions and radical scavenging activity. Eur. Biophys. J. 2021, 50, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, Z.; Genc, M.; Yılmaz, E.; Keser, S. Synthesis and antitumor, antioxidant effects studies of N-ethylpiperazine substitute thiourea ligands and their copper (II) complexes. Spectrosc. Lett. 2013, 46, 182–190. [Google Scholar] [CrossRef]
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef]
- Bielenica, A.; Sanna, G.; Madeddu, S.; Giliberti, G.; Stefańska, J.; Kozioł, A.E.; Savchenko, O.; Strzyga-Łach, P.; Chrzanowska, A.; Kubiak-Tomaszewska, G.; et al. Disubstituted 4-chloro-3-nitrophenylthiourea derivatives: Antimicrobial and cytotoxic studies. Molecules 2018, 23, 2428. [Google Scholar] [CrossRef] [Green Version]
- Bielenica, A.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Krukowski, S.; Włodarczyk, M.; Struga, M. Synthesis and antimicrobial activity of 4-chloro-3-nitrophenylthiourea derivatives targeting bacterial type II topoisomerases. Chem. Biol. Drug Des. 2016, 87, 905–917. [Google Scholar] [CrossRef]
- Stefańska, J.; Stepien, K.; Bielenica, A.; Wrzosek, M.; Struga, M. Antistaphylococcal activity of selected thiourea derivatives. Pol. J. Microbiol. 2016, 65, 451–460. [Google Scholar] [CrossRef] [Green Version]
- John, R.P.; Sreekanth, A.; Kurup, M.R.P.; Usman, A.; Ibrahim, A.R.; Fun, H.K. Spectral studies and structure of a 2-hydroxyacetophenone 3-hexamethyleneiminyl thiosemicarbazonate (−2) copper (II) complex containing 1,10-phenanthroline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 1349–1358. [Google Scholar] [CrossRef]
- Sreekanth, A.; Kurup, M.R.P. Structural and spectral studies on four coordinate copper (II) complexes of 2-benzoylpyridine N(4), N(4)-(butane-1,4-diyl) thiosemicarbazone. Polyhedron 2003, 22, 3321–3332. [Google Scholar] [CrossRef]
- Drzewiecka-Antonik, A.; Rejmak, P.; Klepka, M.; Wolska, A.; Chrzanowska, A.; Struga, M. Structure and anticancer activity of Cu (II) complexes with (bromophenyl) thiourea moiety attached to the polycyclic imide. J. Inorg. Biochem. 2020, 212, 111234. [Google Scholar] [CrossRef] [PubMed]
- Low, M.L.; Maigre, L.; Tahir, M.I.M.; Tiekink, E.R.; Dorlet, P.; Guillot, R.; Ravoof, T.B.; Rosli, R.; Pagès, J.-M.; Policar, C.; et al. New insight into the structural, electrochemical and biological aspects of macroacyclic Cu (II) complexes derived from S-substituted dithiocarbazate schiff bases. Eur. J. Med. Chem. 2016, 120, 1–12. [Google Scholar] [CrossRef]
- Singh, D.P.; Pratap, S.; Shukla, M. Solvent induced geometry transformation of trigonal planar Cu (I) complexes of N-((2/4-methyoxy carbonyl) phenyl)-N′-(ethoxy/methoxy carbonyl) thiocarbamides to square-planar Cu (II) complexes: Synthesis, spectral, single crystal, DFT and in vitro cytotoxic study. Inorg. Chim. Acta 2014, 423, 386–396. [Google Scholar] [CrossRef]
- Singh, S. Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother. Pharmacol. 2014, 75, 1–15. [Google Scholar] [CrossRef]
- Beaumont, P.; Moore, M.J.; Ahmad, K.; Payne, M.M.; Lee, C.; Riddick, D.S. Role of glutathione S-transferases in the resistance of human colon cancer cell lines to doxorubicin. Cancer Res. 1998, 58, 947–955. [Google Scholar] [PubMed]
- Hasegawa, N.; Mizutani, K.; Suzuki, T.; Deguchi, T.; Nozawa, Y. A comparative study of protein profiling by proteomic analysis in camptothecin-resistant PC3 and camptothecin-sensitive LNCaP human prostate cancer cells. Urol. Int. 2006, 77, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lorestani, S.; Hashemy, S.I.; Mojarad, M.; Shahrestanaki, M.K.; Bahari, A.; Asadi, M.; Avval, F.Z. Increased glutathione reductase expression and activity in colorectal cancer tissue samples: An investigational study in Mashhad, Iran. Middle East J. Cancer 2018, 9, 99–104. [Google Scholar]
- Freitas, M.; Baldeiras, I.; Proença, T.; Alves, V.; Mota-Pinto, A.; Sarmento-Ribeiro, A. Oxidative stress adaptation in aggressive prostate cancer may be counteracted by the reduction of glutathione reductase. FEBS Open Bio 2012, 2, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, W.; Zhang, H.J.; Domann, F.E.; Oberley, L.W. Overexpression of copper zinc superoxide dismutase suppresses human glioma cell growth. Cancer Res. 2002, 62, 1205–1212. [Google Scholar] [PubMed]
- Satomi, A.; Murakami, S.; Hashimoto, T.; Ishida, K.; Matsuki, M.; Sonoda, M. Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: Correlation with malignant intensity. J. Gastroenterol. 1995, 30, 177–182. [Google Scholar] [CrossRef]
- Martino, T.; Kudrolli, T.A.; Kumar, B.; Salviano, I.; Mencalha, A.L.; Coelho, M.G.P.; Justo, G.; Costa, P.R.R.; Sabino, K.C.C.; Lupold, S.E. The orally active pterocarpanquinone LQB-118 exhibits cytotoxicity in prostate cancer cell and tumor models through cellular redox stress. Prostate 2018, 78, 140–151. [Google Scholar] [CrossRef]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wang, Y.; Su, Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009, 286, 154–160. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, W.-S.; Ip, C.; Chae, H.-Z.; Park, E.-M.; Park, Y.-M. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006, 66, 7136–7142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.H.; Kim, S.Y.; Park, S.K.; Jeon, H.S.; Lee, Y.M.; Jung, J.H.; Lee, S.Y.; Chae, H.B.; Jung, Y.J.; Lee, K.O.; et al. Phosphorylation and concomitant structural changes in human 2-Cys peroxiredoxin isotype I differentially regulate its peroxidase and molecular chaperone functions. FEBS Lett. 2005, 580, 351–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-F.; Lee, K.-D.; Yeh, C.-H.; Chen, W.-C.; Huang, W.-S.; Chin, C.-C.; Lin, P.-Y.; Wang, J.-Y. Role of peroxiredoxin I in rectal cancer and related to p53 status. Int. J. Radiat. Oncol. 2010, 78, 868–878. [Google Scholar] [CrossRef]
- Whitaker, H.C.; Patel, D.J.; Howat, W.J.; Warren, A.Y.; Kay, J.; Sangan, T.; Marioni, J.; Mitchell, J.F.B.; Aldridge, S.; Luxton, H.J.; et al. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br. J. Cancer 2013, 109, 983–993. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Banerjee, H.; Rojas, H.; Martinez, S.R.; Roy, S.; Jia, Z.; Lilly, M.B.; de Leon, M.; Casiano, C.A. Differential expression of peroxiredoxins in prostate cancer: Consistent upregulation of PRDX3 and PRDX4. Prostate 2011, 71, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Spałek, T.; Pietrzyk, P.; Sojka, Z. Application of the genetic algorithm joint with the powell method to nonlinear least-squares fitting of powder EPR spectra. J. Chem. Inf. Model. 2005, 45, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard M7-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline M26-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard M7-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. In CLSI Document M27-A3, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; pp. 1–25. [Google Scholar]
- Kada, T.; Hirano, K.; Shirasu, Y. Bacillus subtilis rec-assay test. In Chemical Mutagens; de Sevres, F.E., Hollaende, A., Eds.; Plenum Press: New York, NY, USA, 1980; Volume 6, p. 149. [Google Scholar]
- Sadaie, Y.; Kada, T. Recombination-deficient mutants of Bacillus subtilis. J. Bacteriol. 1976, 125, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrzanowska, A.; Roszkowski, P.; Bielenica, A.; Olejarz, W.; Stępień, K.; Struga, M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur. J. Med. Chem. 2020, 185, 111810. [Google Scholar] [CrossRef] [PubMed]
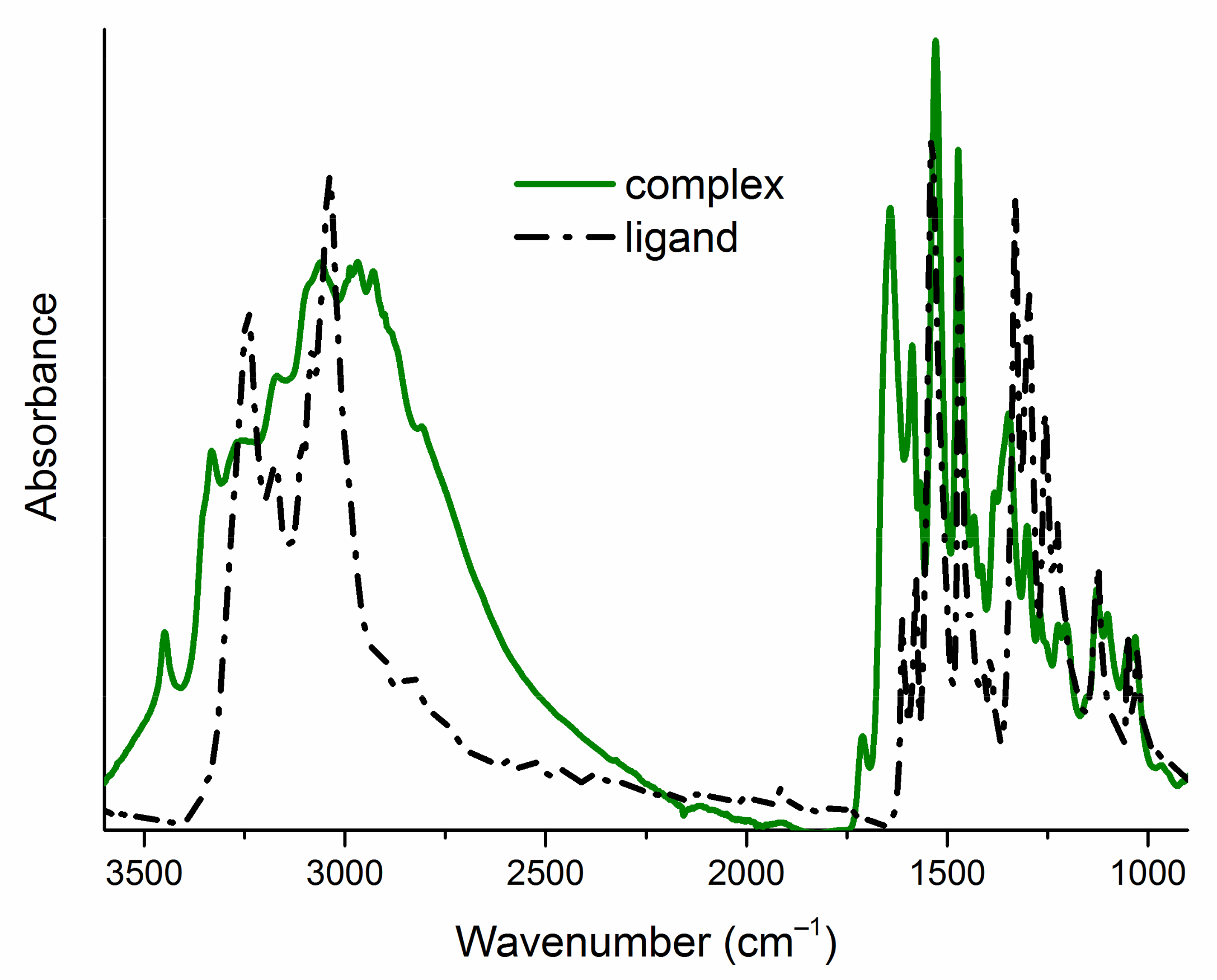
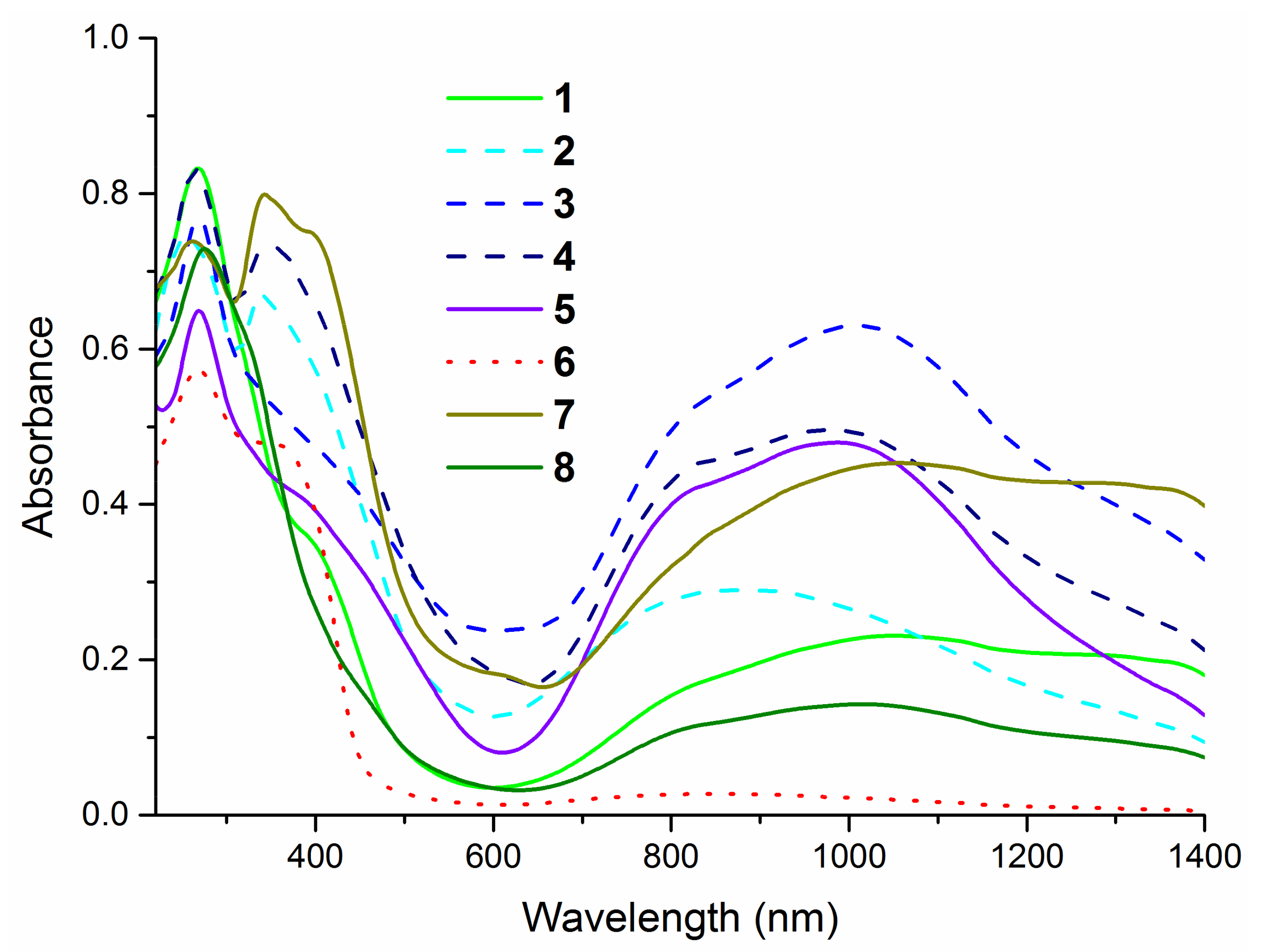
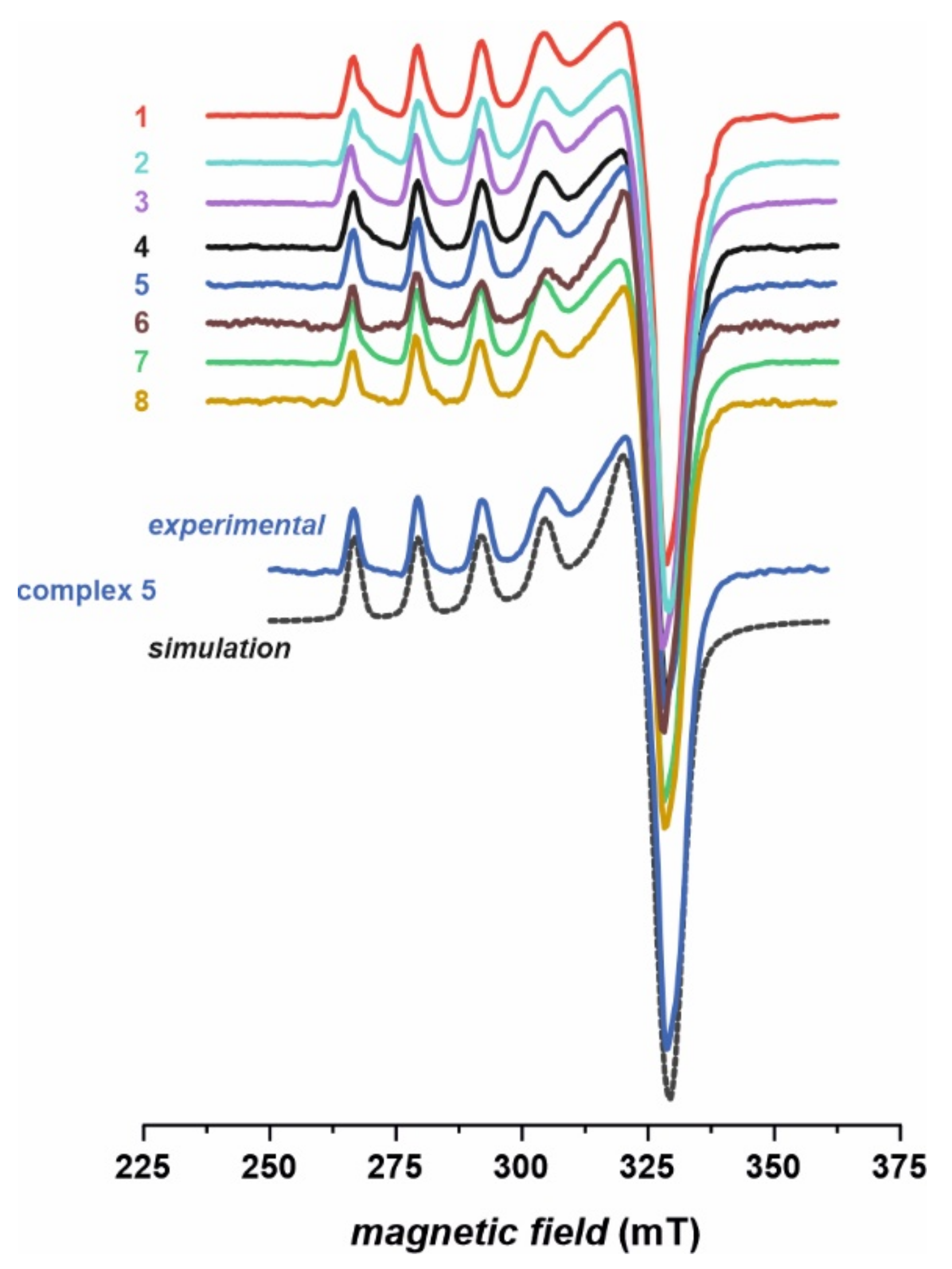

| Compound | υN−H | υasNO2 | υsNO2 | υC=S | υC−X |
|---|---|---|---|---|---|
| 1 | 3470 w, 3372 m | 1529 m * | 1347 m * | 1301 m, 782 w | 1047 w |
| L1 | 3340 w, 3209 m | 1537 sh, 1526 vs | 1347 sh | 1332 m, 832 m | 1044 m |
| 2 | 3444 w, 3395 w | 1531 m * | 1344 m * | 1306 w, 776 w | 1070 w |
| L2 | 3334 sh, 3250 m | 1547 m, 1526 m | 1352 m, 1333 s | 1332 m, 834 w | 1067 m |
| 3 | 3448 w, 3333 w | 1530 m * | 1351 m * | 1305 w, 774 sh | 1071 w |
| L3 | 3361 w, 3317 s | 1537 sh, 1525 s | 1355 m * | −, 839 w | 1070 m |
| 4 | 3449 w, 3335 m | 1531vs * | 1344 m * | 1311 m, 781 w | 1051 m |
| L4 | 3365 sh, 3244 s | 1546 m, 1525 s | 1353 m, 1335 s | 1335 m, 840 m | 1049 m |
| 5 | 3449 w, 3333 m | 1529 vs * | 1344 m * | 1300 m, 779 sh | 1050 m |
| L5 | 3348 sh, 3243 s | 1539 vs, 1509 sh | 1347 sh, 1331 s | 1331 m, 835 m | 1050 m |
| 6 | 3449 w, 3340 m | 1539 sh, 1519 vs | 1341 m * | 1313 m, 784 w | 1044 m |
| L6 | 3327 s, 3243 m | 1542 m, 1515 s | 1353 w, 1333 m | 1333 m, 837 sh | 1047 m |
| 7 | 3446 w, 3341 m | 1532 m * | 1346 m * | 1313 m, 785 w | 1236 w |
| L7 | 3325 s, 3242 m | 1539 s, 1518 vs | 1356 s, 1335 s | 1335 m, 854 w | 1232 w |
| 8 | 3448 w, 3336 w | 1532 m * | 1350 m * | 1307 w, 771 w | 1057 w |
| L8 | 3364 s, 3314 m | 1538 s, 1525 s | 1350 s * | 1331 sh, 833 sh | 1059 m |
| Compound | Cancer Cells | Normal Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| SW480 d | SW620 e | PC3 f | HaCaT g | |||||
| R | IC50 b | SI c | IC50 | SI | IC50 | SI | IC50 | |
| 1 | 2-Br-Ph | 4.7 ± 0.3 | 23.2 | 3.3 ± 0.2 | 33.2 | 9.7 ± 0.1 | 11.5 | 109.6 ± 3.4 |
| 2 | 3-Br-Ph | 24.3 ± 2.6 | 6.8 | 22.3 ± 1.8 | 7.5 | 19.2 ± 2.2 | 8.7 | 167.2 ± 2.3 |
| 3 | 4-Br-Ph | 11.9 ± 2.1 | 8.6 | 19.2 ± 2.3 | 5.3 | 8.8 ± 0.8 | 11.7 | 103.2 ± 3.2 |
| 4 | 3-Cl,4-F-Ph | 19.2 ± 1.1 | 6.3 | 21.6 ± 2.9 | 5.7 | 23.2 ± 1.6 | 5.2 | 120.9 ± 5.2 |
| 5 | 3-Cl,4-Cl-Ph | 20.6 ± 2.1 | 8.1 | 10.8 ± 2.6 | 15.2 | 11.4 ± 2.4 | 14.4 | 164.9 ± 4.7 |
| 6 | 3-NO2,4-Cl-Ph | 26.8 ± 2.3 | 4.6 | 21.5 ± 1.4 | 5.7 | 20.3 ± 3.1 | 6.1 | 123.2 ± 2.1 |
| 7 | 2-F-Ph | 15.5 ± 2.6 | 8.9 | 9.1 ± 0.8 | 12.8 | 10.8 ± 1.3 | 5.5 | 138.3 ± 4.4 |
| 8 | 4-I-Ph | 3.9 ± 0.8 | 26.3 | 17.8 ± 1.3 | 5.7 | 4.3 ± 0.5 | 23.8 | 102.7 ± 3.4 |
| Doxorubicin h | 0.75 ± 0.1 | 0.4 | 0.26 ± 0.1 | 1.1 | 0.31 ± 0.1 | 0.9 | 0.29 ± 0.1 | |
| Cisplatin i | 10.4 ± 0.9 | 0.6 | 6.7 ± 1.1 | 0.9 | 13.2 ± 2.1 | 0.5 | 6.3 ± 0.7 | |
| CuCl2 j | 109.4 ± 7.6 | 1.0 | 96.3 ± 5.2 | 1.2 | 106.5 ± 6.3 | 1.1 | 114.3 ± 4.8 | |
| Protein Intensity, % | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession | Name of Enzyme | SW480 | SW620 | PC3 | ||||||||||||
| Control | 1 | 3 | 7 | 8 | Control | 1 | 3 | 7 | 8 | Control | 1 | 3 | 7 | 8 | ||
| GSTA1_HUMAN | Glutathione S-transferase A1 OS = Homo sapiens OX = 9606 GN = GSTA1 PE = 1 SV = 3 | 100 | 78.3 | 86.6 | 75.6 | 91.6 | 100 | 50.2 | 45.2 | 36.4 | 54.3 | 100 | 84.4 | 70.6 | 79.2 | 73.5 |
| GSTO1_HUMAN | Glutathione S-transferase omega-1 OS = Homo sapiens OX = 9606 GN = GSTO1 PE = 1 SV = 2 | 100 | 70.9 | 66.0 | 45.1 | 52.4 | 100 | 85.5 | 77.0 | 52.5 | 65.0 | 100 | 65.0 | 64.0 | 68.6 | 31.6 |
| GSTP1_HUMAN | Glutathione S-transferase P OS = Homo sapiens OX = 9606 GN = GSTP1 PE = 1 SV = 2 | 100 | 84.8 | 75.9 | 80.9 | 83.0 | 100 | 74.4 | 64.5 | 67.3 | 57.5 | 100 | 95.0 | 51.1 | 35.8 | 64.1 |
| GSHR_HUMAN | Glutathione reductase, mitochondrial OS = Homo sapiens OX = 9606 GN = GSR PE = 1 SV = 2 | 100 | 58.1 | 63.1 | 91.7 | 74.3 | 100 | 69.1 | 79.2 | 67.3 | 79.3 | 100 | 71.1 | 58.9 | 61.2 | 68.5 |
| SODC_HUMAN | Superoxide dismutase [Cu-Zn] OS = Homo sapiens OX = 9606 GN = SOD1 PE = 1 SV = 2 | 100 | 68.2 | 75.3 | 40.6 | 88.2 | 100 | 72.7 | 78.2 | 44.7 | 64.5 | 100 | 58.5 | 30.9 | 15.4 | 33.0 |
| SODM_HUMAN | Superoxide dismutase [Mn], mitochondrial OS = Homo sapiens OX = 9606 GN = SOD2 PE = 1 SV = 3 | 100 | 67.8 | 77.7 | 65.1 | 94.3 | 100 | 88.8 | 77.1 | 45.3 | 92.7 | 100 | 29.1 | 31.2 | 25.2 | 29.4 |
| PRDX1_HUMAN | Peroxiredoxin-1 OS = Homo sapiens OX = 9606 GN = PRDX1 PE = 1 SV = 1 | 100 | 95.9 | 88.5 | 71.6 | 84.4 | 100 | 96.1 | 89.0 | 83.2 | 93.5 | 100 | 73.9 | 47.8 | 46.5 | 52.3 |
| PRDX2_HUMAN | Peroxiredoxin-2 OS = Homo sapiens OX = 9606 GN = PRDX2 PE = 1 SV = 5 | 100 | 95.5 | 93.7 | 69.4 | 78.3 | 100 | 92.1 | 71.6 | 58.6 | 88.6 | 100 | 42.3 | 47.3 | 32.2 | 39.9 |
| PRDX4_HUMAN | Peroxiredoxin-4 OS = Homo sapiens OX = 9606 GN = PRDX4 PE = 1 SV = 1 | 100 | 67.6 | 60.1 | 58.3 | 78.2 | 100 | 79.4 | 76.4 | 73.0 | 93.9 | 100 | 72.6 | 61.1 | 35.7 | 30.8 |
| PRDX5_HUMAN | Peroxiredoxin-5, mitochondrial OS = Homo sapiens OX = 9606 GN = PRDX5 PE = 1 SV = 4 | 100 | 77.3 | 84.8 | 91.0 | 81.7 | 100 | 88.3 | 82.4 | 81.4 | 74.4 | 100 | 29.3 | 40.9 | 36.7 | 42.0 |
| PRDX6_HUMAN | Peroxiredoxin-6 OS = Homo sapiens OX = 9606 GN = PRDX6 PE = 1 SV = 3 | 100 | 75.1 | 81.3 | 68.3 | 71.1 | 100 | 83.2 | 66.4 | 67.5 | 77.6 | 100 | 66.6 | 71.1 | 35.0 | 53.7 |
| PRDX3_HUMAN | Thioredoxin-dependent peroxide reductase, mitochondrial OS = Homo sapiens OX = 9606 GN = PRDX3 PE = 1 SV = 3 | 100 | 95.2 | 85.4 | 90.4 | 96.3 | 100 | 69.9 | 67.5 | 61.2 | 95.2 | 100 | 69.5 | 57.3 | 12.4 | 24.8 |
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Ref. * | Ref. ** |
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus NCTC 4163 | 8 | 16 | 8 | 4 | 32 | 8 | 32 | 4 | 0.25 | - |
| S. aureus ATCC 25923 | 8 | 16 | 8 | 4 | 32 | 8 | 32 | 4 | 0.5 | - |
| S. aureus ATCC 6538 | 8 | 16 | 8 | 4 | 32 | 8 | 32 | 4 | 0.25 | - |
| S. aureus ATCC 29213 | 8 | 16 | 16 | 4 | 32 | 8 | 32 | 4 | 0.25 | - |
| S. epidermidis ATCC 12228 | 16 | 16 | 16 | 4 | 32 | 8 | 32 | 8 | 0.25 | - |
| S. epidermidis ATCC 35984 | 8 | 16 | 16 | 4 | 32 | 8 | 32 | 4 | ≤0.125 | - |
| E. coli NCTC 10538 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | ≤0.125 | - |
| E. coli ATCC 25922 | 256 | 128 | >256 | 128 | 128 | 128 | 256 | 128 | ≤0.125 | - |
| P. aeruginosa ATCC 15442 | 128 | 128 | 128 | 128 | 128 | 128 | >256 | 128 | 0.5 | - |
| P. aeruginosa ATCC 27853 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 0.5 | - |
| C. albicans ATCC 10231 | ≥256 | 64 | 64 | 128 | 128 | 64 | 128 | 64 | - | 0.5 |
| C. albicans ATCC 90028 | ≥256 | 64 | 64 | 128 | 128 | 64 | 128 | 128 | - | 0.5 |
| C. parapsilosis ATCC 22019 | ≥256 | 64 | 64 | 64 | 64 | 64 | 128 | 64 | - | 0.5 |
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Ref. * |
|---|---|---|---|---|---|---|---|---|---|
| S. aureus 498 | 8 | 8 | 4 | 4 | 16 | 32 | 4 | 8 | 0.5 |
| S. aureus 537 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 8 | 256 |
| S. aureus 567 | 8 | 8 | 4 | 4 | 16 | 32 | 4 | 8 | 0.5 |
| S. aureus 568 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 8 | 0.5 |
| S. aureus 573 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 8 | 128 |
| S. aureus 585 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 8 | 256 |
| S. aureus 586 | 4 | 8 | 4 | 4 | 16 | 32 | 8 | 8 | 0.5 |
| S. aureus 495 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 4 | 0.5 |
| S. aureus 496 | 8 | 8 | 4 | 8 | 32 | 32 | 8 | 8 | 0.25 |
| S. aureus 497 | 8 | 8 | 4 | 8 | 16 | 32 | 8 | 8 | 256 |
| S. aureus 514 | 8 | 8 | 4 | 4 | 32 | 32 | 8 | 4 | 128 |
| S. aureus 522 | 8 | 8 | 4 | 4 | 16 | 32 | 8 | 4 | 256 |
| S. aureus 572 | 8 | 8 | 4 | 4 | 16 | 32 | 4 | 4 | 256 |
| S. aureus 481 | 8 | 8 | 4 | 4 | 16 | 32 | 4 | 4 | 256 |
| S. epidermidis 420 | 4 | 8 | 8 | 4 | 32 | 64 | 8 | 8 | 0.5 |
| S. epidermidis 423 | 8 | 8 | 4 | 4 | 32 | 64 | 8 | 8 | 0.5 |
| S. epidermidis 424 | 8 | 8 | 4 | 8 | 32 | 64 | 8 | 8 | 16 |
| S. epidermidis 469 | 8 | 8 | 4 | 4 | 32 | 64 | 8 | 8 | 0.5 |
| S. epidermidis 471 | 8 | 8 | 8 | 8 | 32 | 64 | 8 | 8 | 32 |
| S. epidermidis 510 | 8 | 8 | 4 | 8 | 16 | 64 | 8 | 8 | 0.5 |
| S. epidermidis 511 | 8 | 8 | 4 | 4 | 32 | 64 | 8 | 8 | 32 |
| S. epidermidis 515 | 4 | 8 | 8 | 4 | 32 | 64 | 8 | 8 | 32 |
| S. epidermidis 431 | 8 | 8 | 4 | 8 | 16 | 64 | 8 | 8 | 8 |
| S. epidermidis 432 | 8 | 8 | 4 | 8 | 32 | 64 | 8 | 8 | 64 |
| S. epidermidis 433 | 4 | 8 | 4 | 4 | 32 | 64 | 8 | 4 | 64 |
| S. epidermidis 435 | 8 | 8 | 4 | 8 | 32 | 128 | 8 | 8 | 0.25 |
| S. epidermidis 436 | 8 | 8 | 4 | 8 | 32 | 128 | 8 | 8 | ≤ 0.125 |
| S. epidermidis 437 | 8 | 8 | 8 | 8 | 32 | 128 | 8 | 8 | 0.5 |
| S. epidermidis 438 | 8 | 8 | 4 | 8 | 32 | 128 | 8 | 8 | ≤ 0.125 |
| S. epidermidis 513 | 8 | 8 | 4 | 8 | 16 | 64 | 8 | 8 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzanowska, A.; Drzewiecka-Antonik, A.; Dobrzyńska, K.; Stefańska, J.; Pietrzyk, P.; Struga, M.; Bielenica, A. The Cytotoxic Effect of Copper (II) Complexes with Halogenated 1,3-Disubstituted Arylthioureas on Cancer and Bacterial Cells. Int. J. Mol. Sci. 2021, 22, 11415. https://doi.org/10.3390/ijms222111415
Chrzanowska A, Drzewiecka-Antonik A, Dobrzyńska K, Stefańska J, Pietrzyk P, Struga M, Bielenica A. The Cytotoxic Effect of Copper (II) Complexes with Halogenated 1,3-Disubstituted Arylthioureas on Cancer and Bacterial Cells. International Journal of Molecular Sciences. 2021; 22(21):11415. https://doi.org/10.3390/ijms222111415
Chicago/Turabian StyleChrzanowska, Alicja, Aleksandra Drzewiecka-Antonik, Katarzyna Dobrzyńska, Joanna Stefańska, Piotr Pietrzyk, Marta Struga, and Anna Bielenica. 2021. "The Cytotoxic Effect of Copper (II) Complexes with Halogenated 1,3-Disubstituted Arylthioureas on Cancer and Bacterial Cells" International Journal of Molecular Sciences 22, no. 21: 11415. https://doi.org/10.3390/ijms222111415
APA StyleChrzanowska, A., Drzewiecka-Antonik, A., Dobrzyńska, K., Stefańska, J., Pietrzyk, P., Struga, M., & Bielenica, A. (2021). The Cytotoxic Effect of Copper (II) Complexes with Halogenated 1,3-Disubstituted Arylthioureas on Cancer and Bacterial Cells. International Journal of Molecular Sciences, 22(21), 11415. https://doi.org/10.3390/ijms222111415






