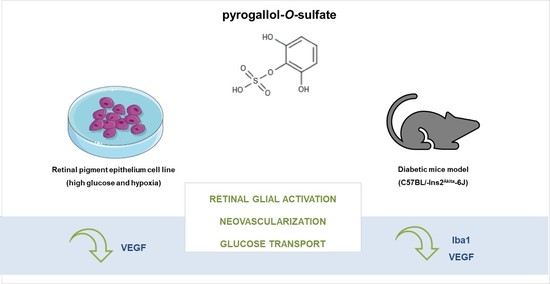
Polyphenol Metabolite Pyrogallol-O-Sulfate Decreases Microglial Activation and VEGF in Retinal Pigment Epithelium Cells and Diabetic Mouse Retina
Abstract
1. Introduction
2. Results
2.1. Metabolic Activity of RPE Cells Is Not Affected by Treatment with Pyrogallol-O-Sulfate
2.2. Pre-Treatment of RPE Cells with Pyrogallol-O-Sulfate before Hypoxia Has No Significant Effect on the Expression of Neovascularization-Related Proteins
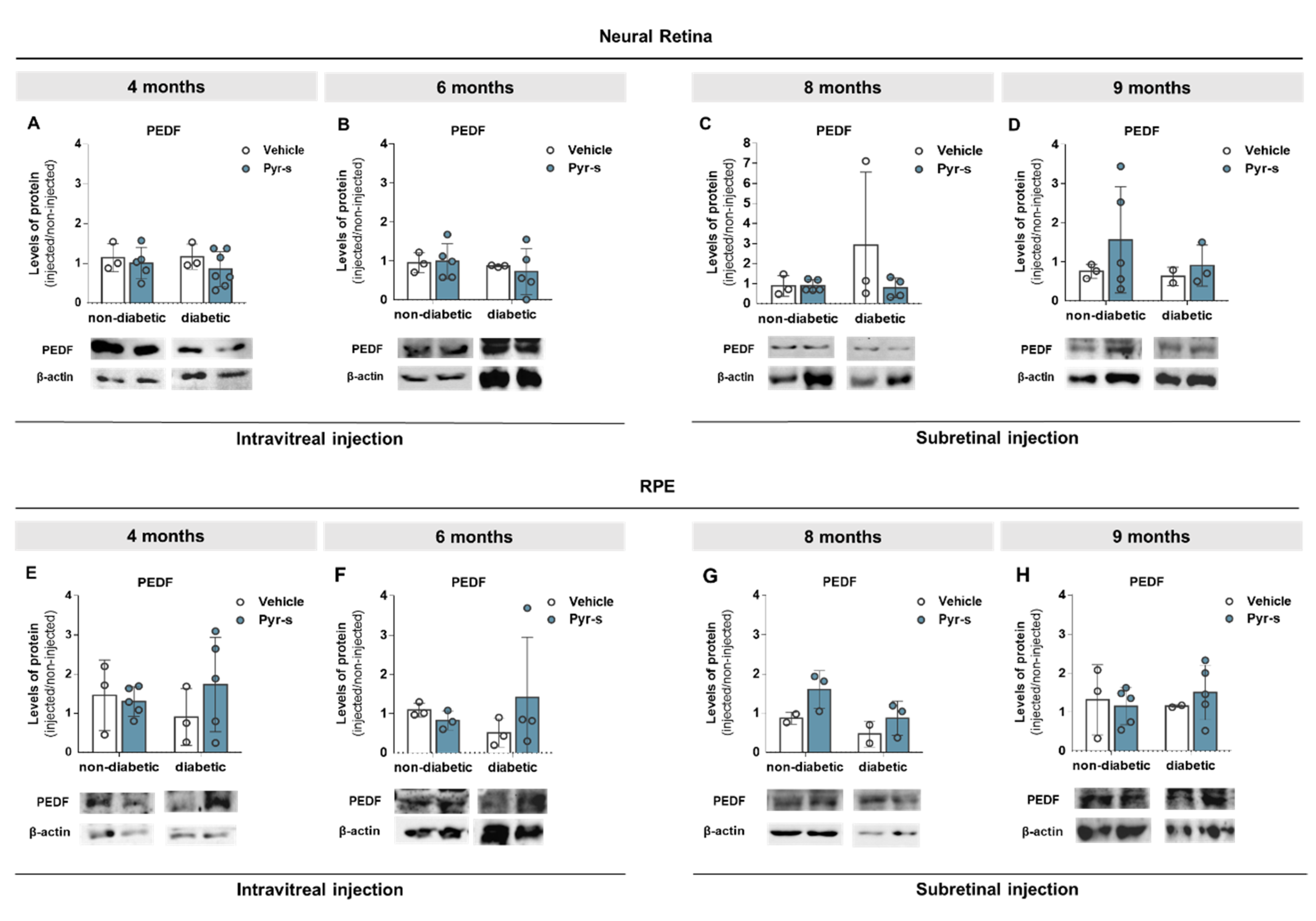
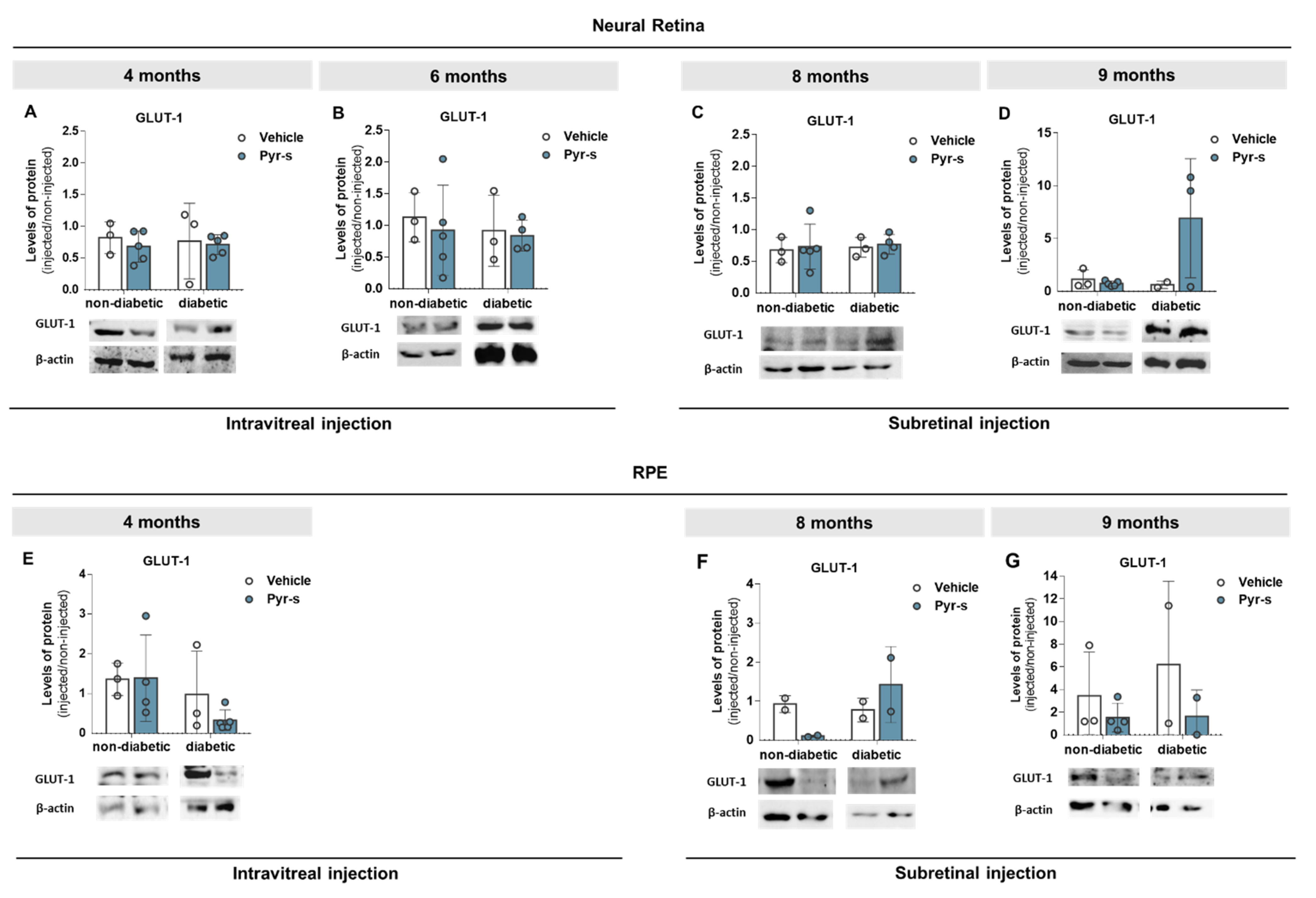
2.3. Treatment of RPE Cells with Pyrogallol-O-Sulfate after Hypoxia Does Not Compromise the Expression of PEDF, VEGF, Or GLUT1
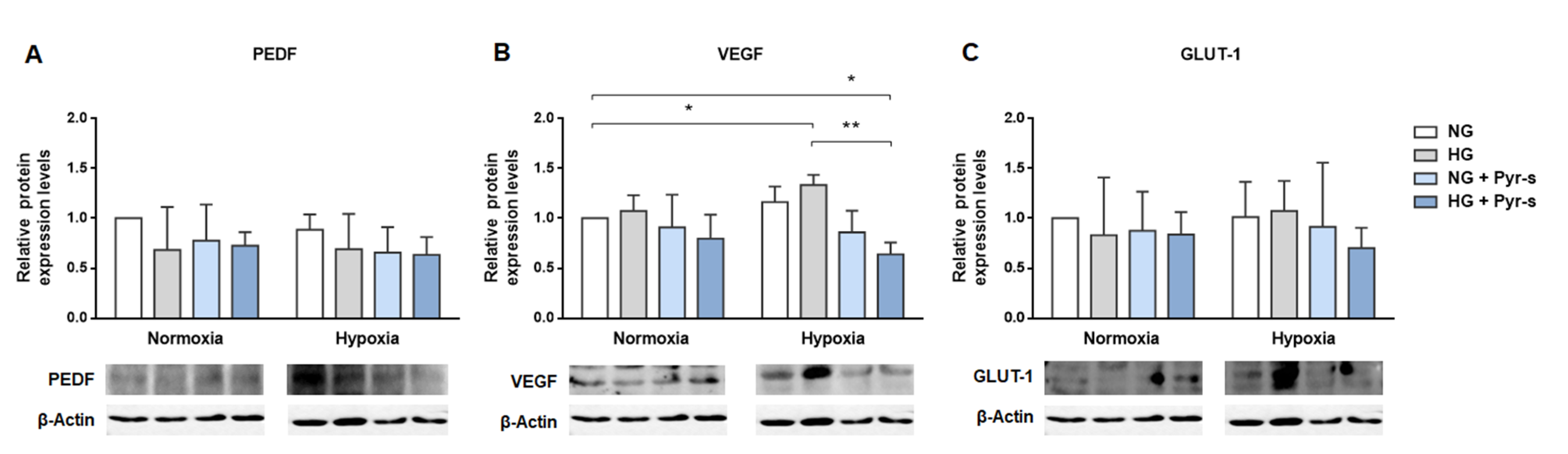
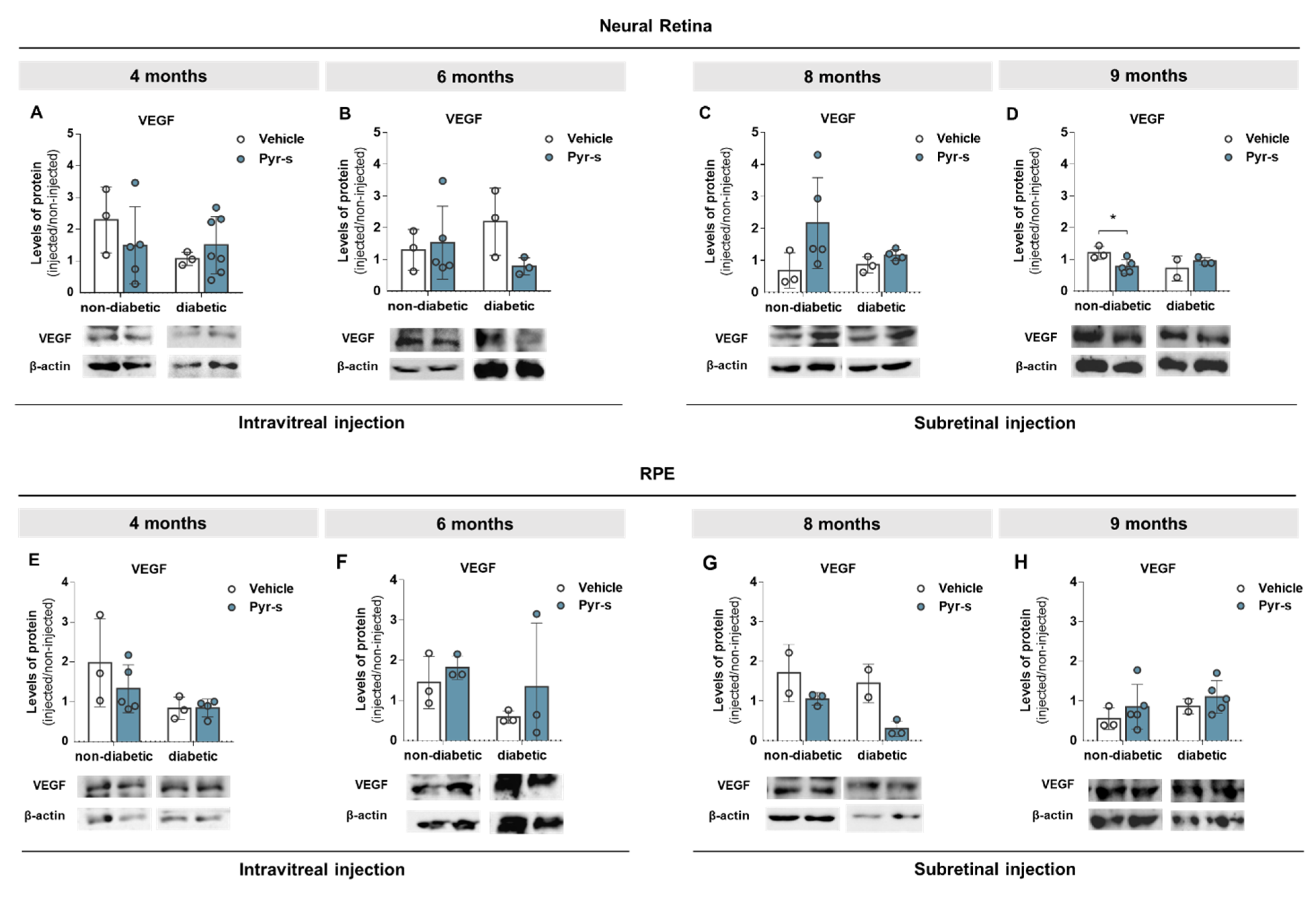
2.4. Treatment of RPE Cells with Pyrogallol-O-Sulfate for 24 H Decreases the Expression of Pro-Angiogenic VEGF
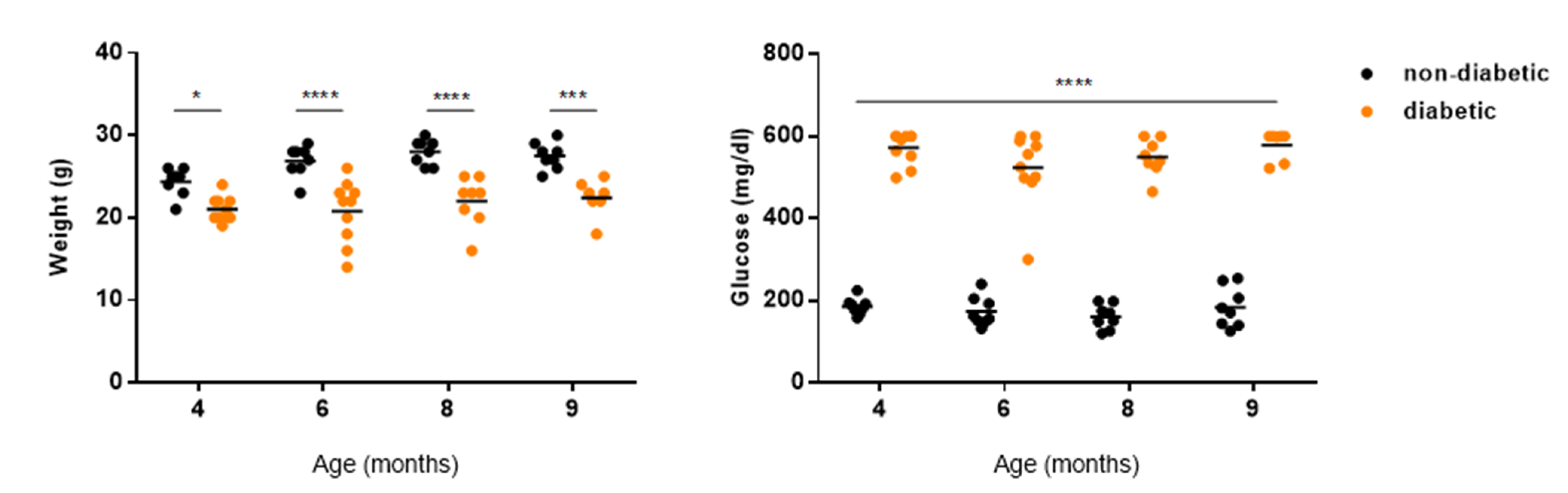
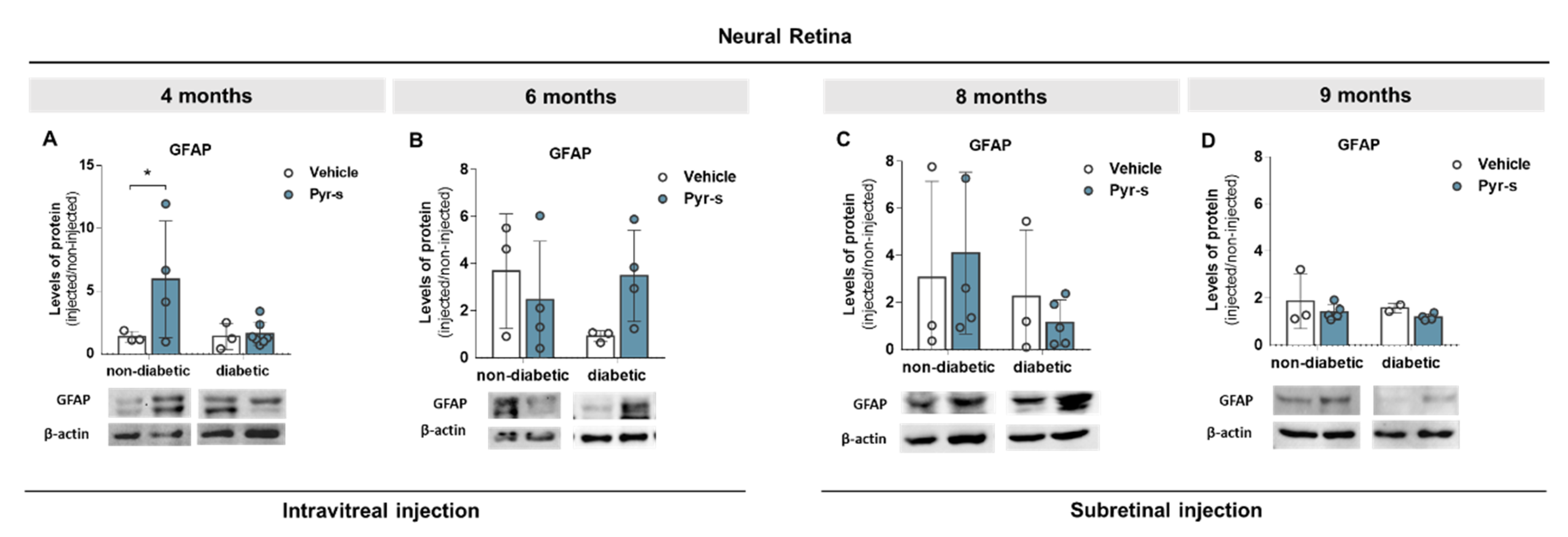
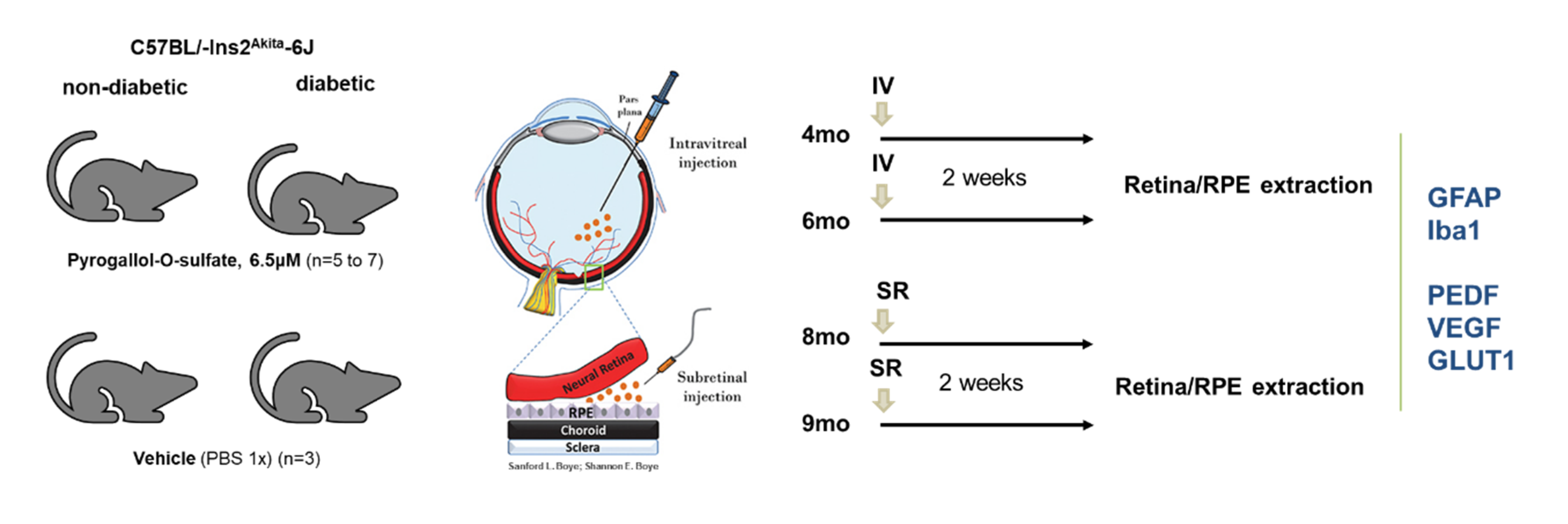
2.5. A Single Intraocular Injection of Pyrogallol-O-Sulfate Decreases the Expression of Microglial Marker Iba1 in The Retina of a Diabetic Mice
2.6. A Single Subretinal Injection of Pyrogallol-O-Sulfate Decreases the Expression of Pro-Angiogenic VEGF in the RPE of a Diabetic Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Glucose and Hypoxia
4.3. Treatment with (Poly)Phenol-Derived Metabolites
4.4. Protein Extraction and Quantification
4.4.1. Whole Cell Lysates
4.4.2. Protein Quantification
4.5. MTT Assay
4.6. Immunoblotting
4.7. Animals
4.7.1. Housing
4.7.2. Intraocular Injections of Pyrogallol-O-Sulfate in C57BL/Ins2Akita Mice
4.7.3. Retina and RPE Protein Extraction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.-Q.; Wu, B.-J.; Pan, W.H.; Zhang, X.-M.; Liu, J.-H.; Chen, M.-M.; Chao, F.-P.; Chao, H.-M. Resveratrol Mitigates Rat Retinal Ischemic Injury: The Roles of Matrix Metalloproteinase-9, Inducible Nitric Oxide, and Heme Oxygenase-1. J. Ocul. Pharmacol. Ther. 2013, 29, 33–40. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol Improves Diabetic Retinopathy Possibly through Oxidative Stress—Nuclear Factor ΚB—Apoptosis Pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols Journey through Blood-Brain Barrier towards Neuronal Protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Tavares, L.; Jardim, C.E.C.G.; Costa, I.; Terrasso, A.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.; et al. Blood-Brain Barrier Transport and Neuroprotective Potential of Blackberry-Digested Polyphenols: An in Vitro Study. Eur. J. Nutr. 2019, 58, 113–130. [Google Scholar] [CrossRef]
- Dias-Pedroso, D.; Guerra, J.; Gomes, A.; Oudot, C.; Brenner, C.; Santos, C.N.; Vieira, H.L.A. Phenolic Metabolites Modulate Cardiomyocyte Beating in Response to Isoproterenol. Cardiovasc. Toxicol. 2019, 19, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic Sulfates as New and Highly Abundant Metabolites in Human Plasma after Ingestion of a Mixed Berry Fruit Purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Dew, T.; Figueira, M.E.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N.; Williamson, G. Urinary Metabolite Profiling Identifies Novel Colonic Metabolites and Conjugates of Phenolics in Healthy Volunteers. Mol. Nutr. Food Res. 2014, 58, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Kerimi, A.; Tumova, S.; Williamson, G. Inhibition of Intestinal Glucose Transport by Polyphenols: A Mechanism for Indirect Attenuation of Cholesterol Absorption? Food Funct. 2019, 10, 3127–3134. [Google Scholar] [CrossRef]
- Lee, H.; Jun, J.-H.; Jung, E.-H.; Koo, B.; Kim, Y. Epigalloccatechin-3-Gallate Inhibits Ocular Neovascularization and Vascular Permeability in Human Retinal Pigment Epithelial and Human Retinal Microvascular Endothelial Cells via Suppression of MMP-9 and VEGF Activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef]
- Ola, M.S.; Al-Dosari, D.; Alhomida, A.S. Role of Oxidative Stress in Diabetic Retinopathy and the Beneficial Effects of Flavonoids. Curr. Pharm. Des. 2018, 24, 2180–2187. [Google Scholar] [CrossRef]
- O’Donovan, M. A Critique of Methods to Measure Cytotoxicity in Mammalian Cell Genotoxicity Assays. Mutagenesis 2012, 27, 615–621. [Google Scholar] [CrossRef][Green Version]
- Araújo, R.S.; Silva, M.S.; Santos, D.F.; Silva, G.A. Dysregulation of Trophic Factors Contributes to Diabetic Retinopathy in the Ins2Akita Mouse. Exp. Eye Res. 2020, 194, 108027. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in Diabetes. Diabetes Care 2004, 27 (Suppl. 1), S84–S87. [Google Scholar] [CrossRef] [PubMed]
- Calado, S.M.; Alves, L.S.; Simão, S.; Silva, G.A. GLUT1 Activity Contributes to the Impairment of PEDF Secretion by the RPE. Mol. Vis. 2016, 22, 761–770. [Google Scholar]
- Kern, T.S. Contributions of Inflammatory Processes to the Development of the Early Stages of Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of Microglia-Mediated Neuroinflammation to Retinal Degenerative Diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Chao, C.C.; Hu, S.; Molitor, T.W.; Shaskan, E.G.; Peterson, P.K. Activated Microglia Mediate Neuronal Cell Injury via a Nitric Oxide Mechanism. J. Immunol. 1992, 149, 2736–2741. [Google Scholar]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase Signalling Controls Microglia Activation and Neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef]
- Rivera, J.C.; Sitaras, N.; Noueihed, B.; Hamel, D.; Madaan, A.; Zhou, T.; Honoré, J.-C.; Quiniou, C.; Joyal, J.-S.; Hardy, P.; et al. Microglia and Interleukin-1β in Ischemic Retinopathy Elicit Microvascular Degeneration through Neuronal Semaphorin-3A. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1881–1891. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.N.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218. [Google Scholar] [CrossRef]
- Han, Z.; Guo, J.; Conley, S.M.; Naash, M.I. Retinal Angiogenesis in the Ins2Akita Mouse Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 574–584. [Google Scholar] [CrossRef]
- Nwanyanwu, K.H.; Talwar, N.; Gardner, T.W.; Wrobel, J.S.; Herman, W.H.; Stein, J.D. Predicting Development of Proliferative Diabetic Retinopathy. Diabetes Care 2013, 36, 1562–1568. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of Diabetic Retinopathy, Diabetic Macular Edema and Related Vision Loss. Eye Vis. Lond. Engl. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Molecular Basis of the Inner Blood-Retinal Barrier and Its Breakdown in Diabetic Macular Edema and Other Pathological Conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Giurdanella, G.; Lupo, G.; Gennuso, F.; Conti, F.; Furno, D.L.; Mannino, G.; Anfuso, C.D.; Drago, F.; Salomone, S.; Bucolo, C. Activation of the VEGF-A/ERK/PLA2 Axis Mediates Early Retinal Endothelial Cell Damage Induced by High Glucose: New Insight from an In Vitro Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 7528. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Carrasco, E.; García-Ramírez, M.; Hernández, C. Angiogenic and Antiangiogenic Factors in Proliferative Diabetic Retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef]
- Spranger, J.; Osterhoff, M.; Reimann, M.; Mohlig, M.; Ristow, M.; Francis, M.K.; Cristofalo, V.; Hammes, H.-P.; Smith, G.; Boulton, M.; et al. Loss of the Antiangiogenic Pigment Epithelium-Derived Factor in Patients with Angiogenic Eye Disease. Diabetes 2001, 50, 2641–2645. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Patel, A.; Vingerling, J.R.; Cruickshank, J.K.; Hughes, A.D.; Stanton, A.; Lu, J.; McG Thom, S.A.; Grobbee, D.E.; et al.; on behalf of the AdRem* project team and ADVANCE management committee. Effects of Blood Pressure Lowering and Intensive Glucose Control on the Incidence and Progression of Retinopathy in Patients with Type 2 Diabetes Mellitus: A Randomised Controlled Trial. Diabetologia 2009, 52, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; for the DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Gardlik, R.; Fusekova, I. Pharmacologic Therapy for Diabetic Retinopathy. Semin. Ophthalmol. 2015, 30, 252–263. [Google Scholar] [CrossRef]
- EURODIAB ACE Study Group. Variation and Trends in Incidence of Childhood Diabetes in Europe. Lancet 2000, 355, 873–876. [Google Scholar]
- Gillespie, K.M.; Bain, S.C.; Barnett, A.H.; Bingley, P.J.; Christie, M.R.; Gill, G.V.; Gale, E.A.M. The Rising Incidence of Childhood Type 1 Diabetes and Reduced Contribution of High-Risk HLA Haplotypes. Lancet 2004, 364, 1699–1700. [Google Scholar] [CrossRef]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic Effects of a Phenolic Acid Fraction of Rice Bran and Ferulic Acid in C57BL/KsJ-Db/Db Mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Rizvi, S.I. Quercetin Modulates Na(+)/K(+) ATPase and Sodium Hydrogen Exchanger in Type 2 Diabetic Erythrocytes. Cell. Mol. Biol. 2012, 58, 148–152. [Google Scholar] [PubMed]
- Popescu, M.; Bogdan, C.; Pintea, A.; Rugină, D.; Ionescu, C. Antiangiogenic Cytokines as Potential New Therapeutic Targets for Resveratrol in Diabetic Retinopathy. Drug Des. Dev. Ther. 2018, 12, 1985–1996. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, Y.; Xu, Y. Taxifolin Shows Anticataractogenesis and Attenuates Diabetic Retinopathy in STZ-Diabetic Rats via Suppression of Aldose Reductase, Oxidative Stress, and MAPK Signaling Pathway. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 599–608. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (Poly)Phenol Metabolites Correlate with Improvements in Vascular Function: A Double-Blind, Randomized, Controlled, Dose-Response, Crossover Study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and Time Dependence of Blueberry Flavonoid–Induced Improvements in Vascular Function: A Randomized, Controlled, Double-Blind, Crossover Intervention Study with Mechanistic Insights into Biological Activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R.; Bråkenhielm, E. Antiangiogenic Mechanisms of Diet-Derived Polyphenols. J. Nutr. Biochem. 2002, 13, 380–390. [Google Scholar] [CrossRef]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.M.; Brites, D.; et al. Bioaccessible (Poly)Phenol Metabolites from Raspberry Protect Neural Cells from Oxidative Stress and Attenuate Microglia Activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef]
- Rungger-Brändle, E.; Dosso, A.A.; Leuenberger, P.M. Glial Reactivity, an Early Feature of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1971–1980. [Google Scholar]
- Zeng, H.; Green, W.R.; Tso, M.O.M. Microglial Activation in Human Diabetic Retinopathy. Arch. Ophthalmol. 2008, 126, 227–232. [Google Scholar] [CrossRef]
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; LaNoue, K.F.; Levison, S.W. Diabetic Retinopathy: More than Meets the Eye. Surv. Ophthalmol. 2002, 47 (Suppl. 2), S253–S262. [Google Scholar] [CrossRef]
- Davis, A.A.; Bernstein, P.S.; Bok, X.D.; Turner, J.; Nachtigal, M.; Hunt, R.C. Human Retinal Pigment Epithelial Cell Line That Etains Epithelial Characteristics After Prolonged Culture. Investig. Ophthalmol. 1995, 36, 955–964. [Google Scholar]
- Drugbank Online. Mannitol. Available online: https://go.drugbank.com/drugs/DB00742 (accessed on 28 November 2020).
- Aprelikova, O.; Chandramouli, G.V.R.; Wood, M.; Vasselli, J.R.; Riss, J.; Maranchie, J.K.; Linehan, W.M.; Barrett, J.C. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J. Cell. Biochem. 2004, 92, 491–501. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Induction and Testing of Hypoxia in Cell Culture. J. Vis. Exp. JoVE 2011, 54, e2899. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wei, H.; Xun, Z.; Granado, H.; Wu, A.; Handa, J.T. An Easy, Rapid Method to Isolate RPE Cell Protein from the Mouse Eye. Exp. Eye Res. 2016, 145, 450–455. [Google Scholar] [CrossRef]




| Primary Antibody | Dilution | Reference | Brand |
|---|---|---|---|
| VEGF-A | 1:500–1:1000 | ab46154 | Abcam, Cambridge, UK |
| PEDF | 1:500–1:1000 | 07-280 | Merck Millipore, Burlington, MA, USA |
| Iba1 | 1:500 | SAB2500041 | Sigma-Aldrich, St. Louis, MO, USA |
| GFAP | 1:10,000 | ab7260 | Abcam, Cambridge, UK |
| GLUT-1 | 1:2000 | ab32551 | Abcam, Cambridge, UK |
| β-actin | 1:5000 | A5441 | Sigma-Aldrich, St. Louis, MO, USA |
| Goat anti-mouse igG-HRP | 1:5000 | sc-516162 | Santa Cruz Biotechnology, Dallas, TX, USA |
| Goat anti-rabbit igG-HRP | 1:5000 | sc-2004 | Santa Cruz Biotechnology, Dallas, TX, USA |
| Donkey anti-goat igG-HRP | 1:5000 | sc-2020 | Santa Cruz Biotechnology, Dallas, TX, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.F.; Pais, M.; Santos, C.N.; Silva, G.A. Polyphenol Metabolite Pyrogallol-O-Sulfate Decreases Microglial Activation and VEGF in Retinal Pigment Epithelium Cells and Diabetic Mouse Retina. Int. J. Mol. Sci. 2021, 22, 11402. https://doi.org/10.3390/ijms222111402
Santos DF, Pais M, Santos CN, Silva GA. Polyphenol Metabolite Pyrogallol-O-Sulfate Decreases Microglial Activation and VEGF in Retinal Pigment Epithelium Cells and Diabetic Mouse Retina. International Journal of Molecular Sciences. 2021; 22(21):11402. https://doi.org/10.3390/ijms222111402
Chicago/Turabian StyleSantos, Daniela F., Mariana Pais, Cláudia N. Santos, and Gabriela A. Silva. 2021. "Polyphenol Metabolite Pyrogallol-O-Sulfate Decreases Microglial Activation and VEGF in Retinal Pigment Epithelium Cells and Diabetic Mouse Retina" International Journal of Molecular Sciences 22, no. 21: 11402. https://doi.org/10.3390/ijms222111402
APA StyleSantos, D. F., Pais, M., Santos, C. N., & Silva, G. A. (2021). Polyphenol Metabolite Pyrogallol-O-Sulfate Decreases Microglial Activation and VEGF in Retinal Pigment Epithelium Cells and Diabetic Mouse Retina. International Journal of Molecular Sciences, 22(21), 11402. https://doi.org/10.3390/ijms222111402