Viral Infection Drives the Regulation of Feeding Behavior Related Genes in Salmo salar
Abstract
1. Introduction
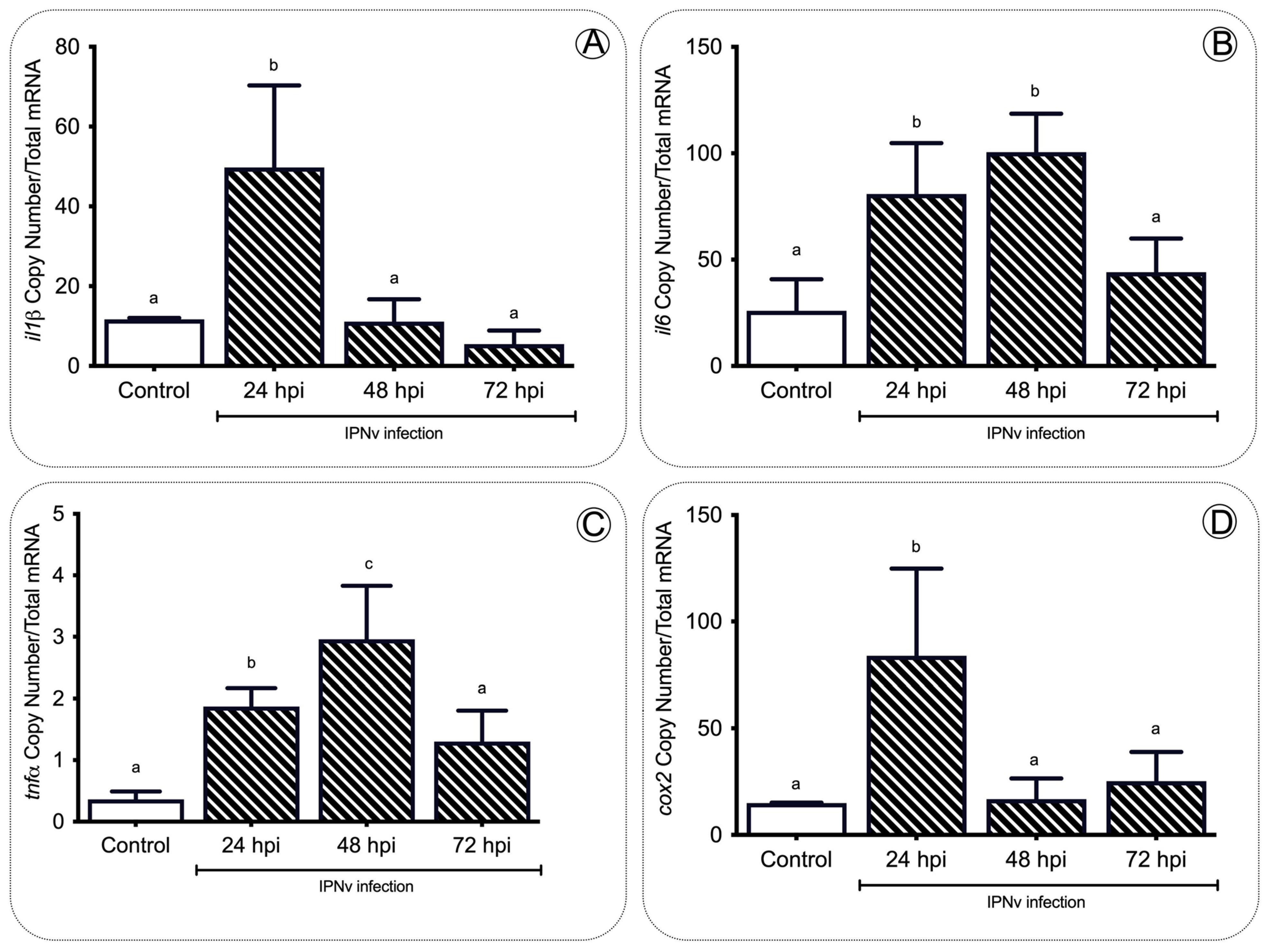
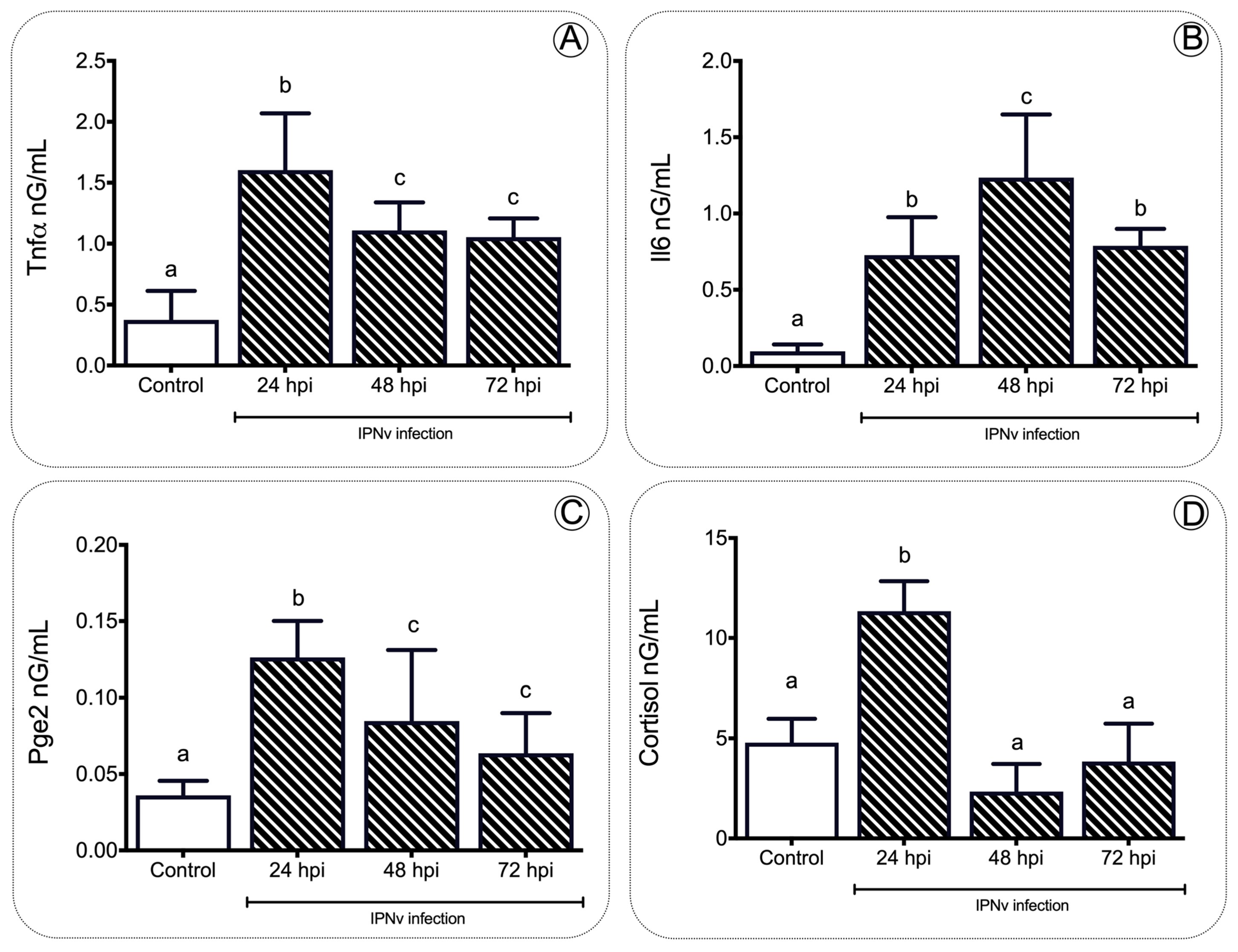
2. Results
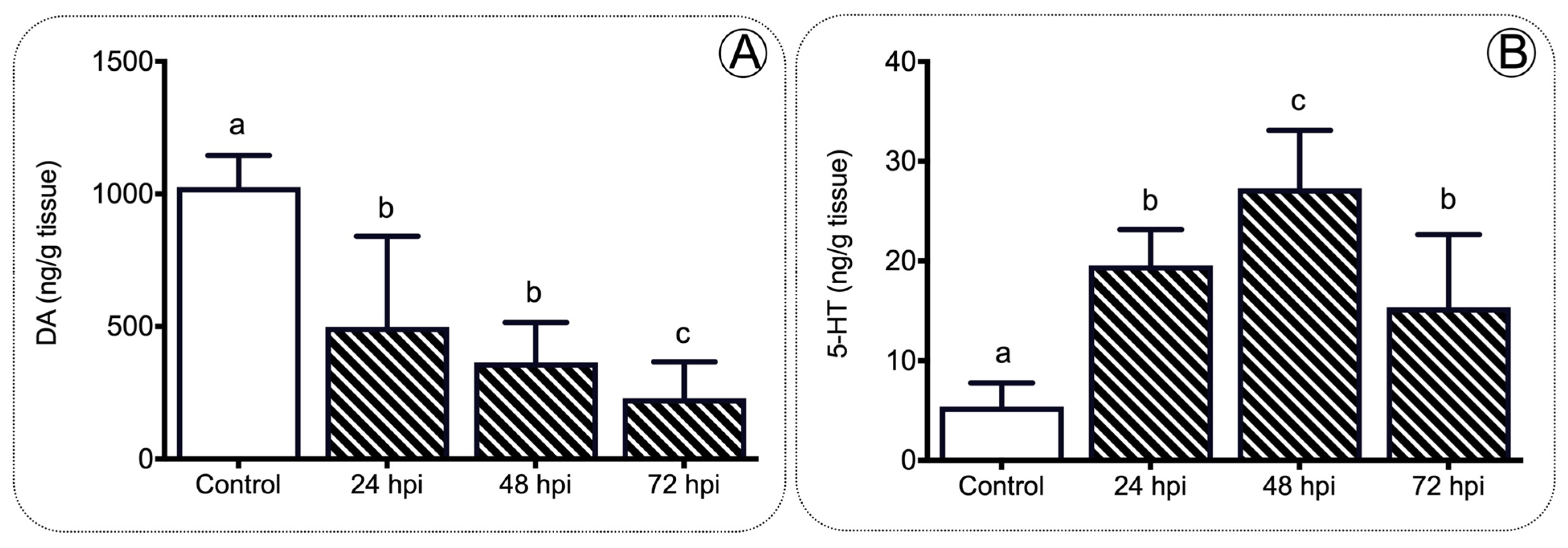
2.1. IPNv Viral Infection Increases the Levels of Cortisol
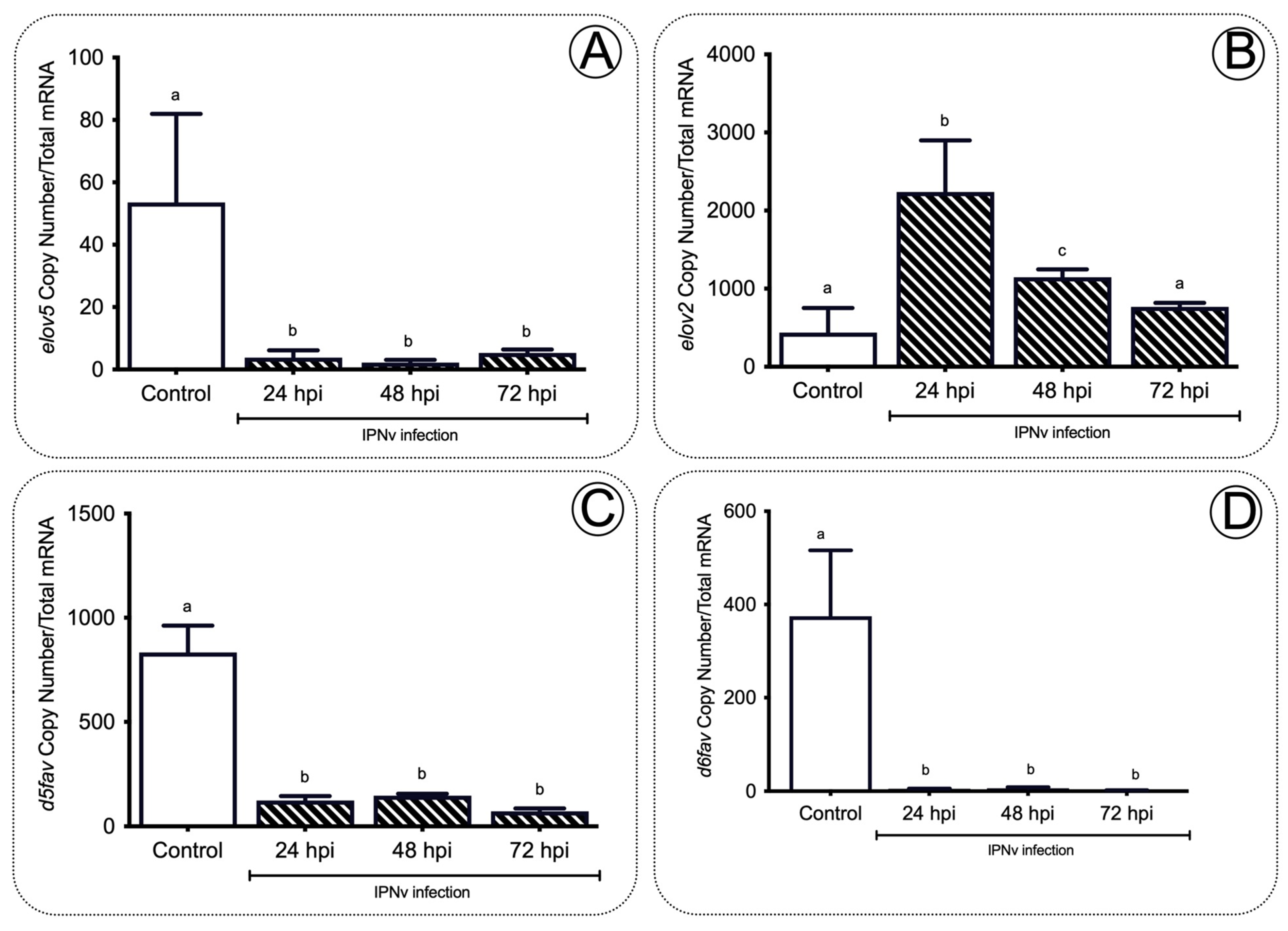
2.2. Upregulation of elovl2 in the Liver of Juvenile Salmon Is Linked with the Increases of AA and EPA during Infection with IPNv
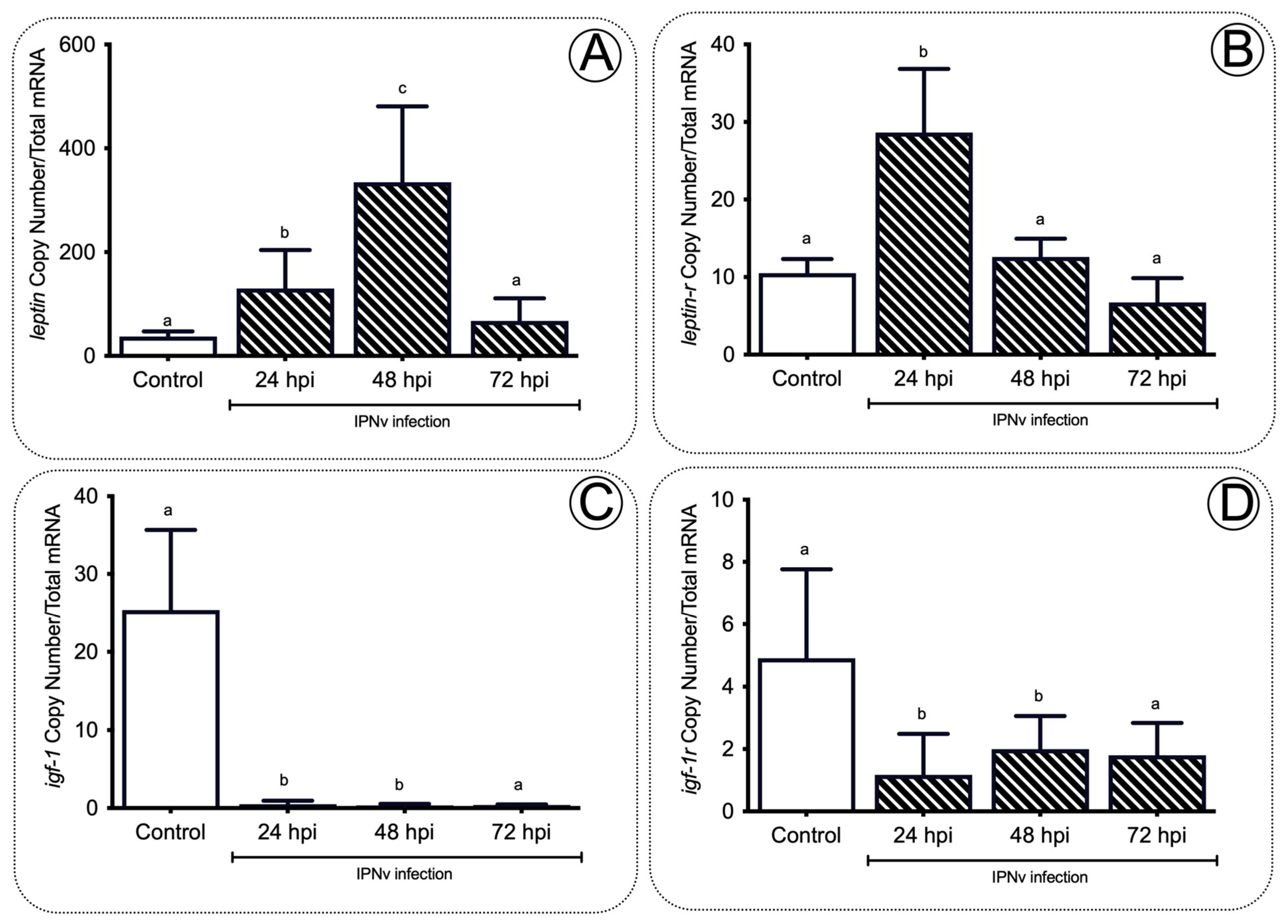
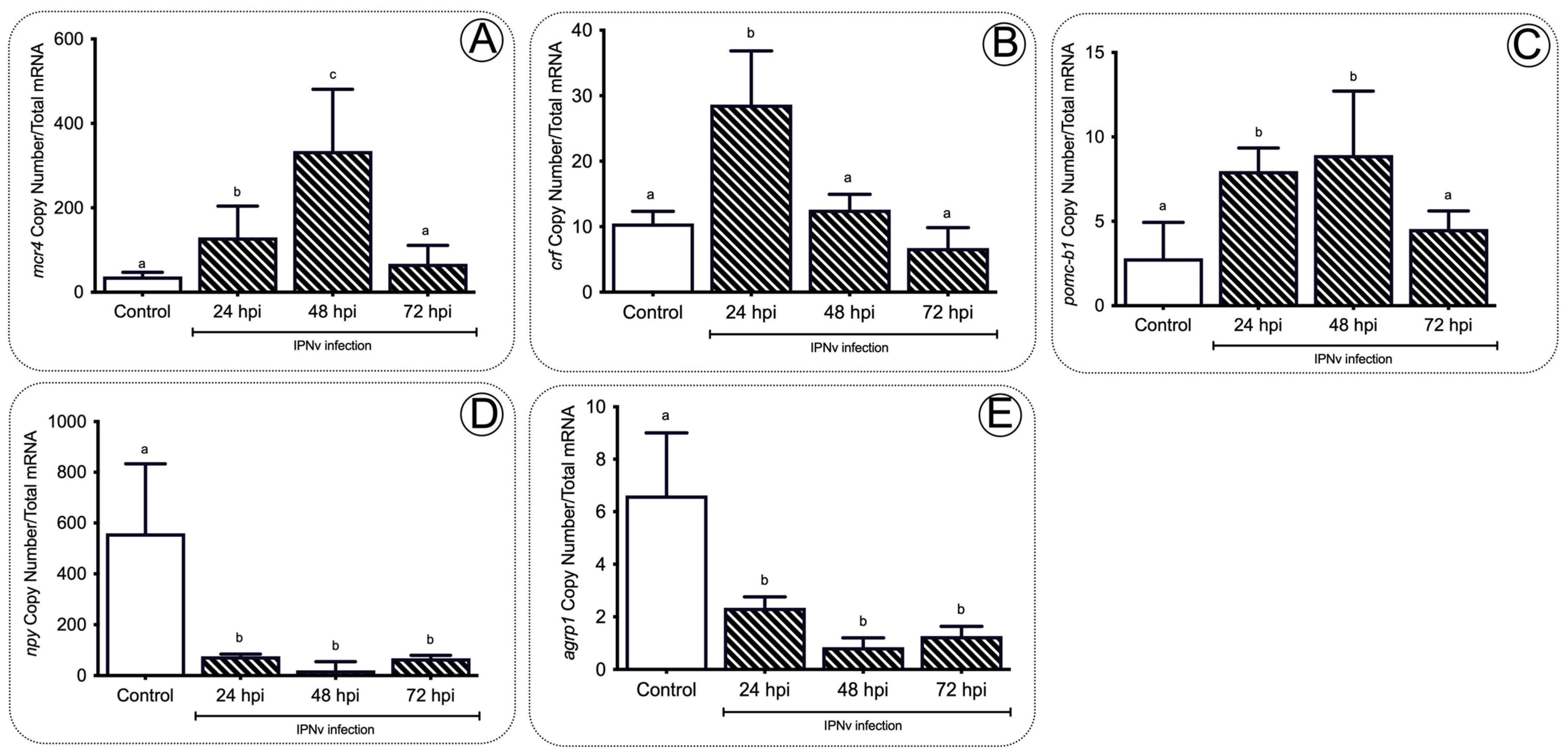
2.3. Anorexigenic Genes Are Upregulated during Infection in Juvenile Salmon
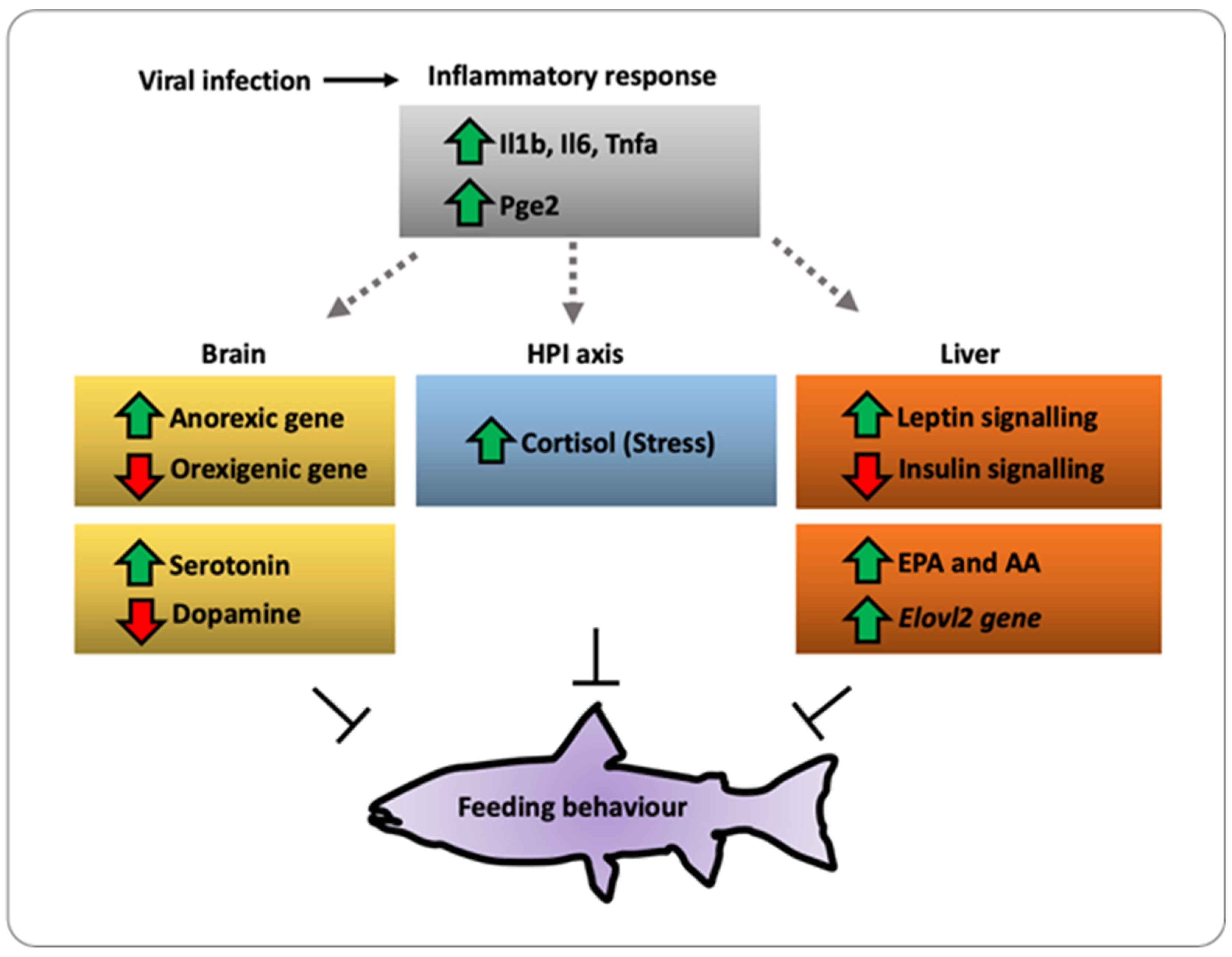
3. Discussion
3.1. Pro-Inflammatory Cytokines Regulate Feeding Behavior
3.2. The Gene elovl2 Is Upregulated during Inflammation to Promote the Accumulation of EPA in the Liver of Challenged Salmon
3.3. A Set of the Anorexic Genes Are Upregulated by Leptin Signaling during Inflammation
4. Materials and Methods
4.1. Animal and Hatchery Conditions
4.2. Infectious Pancreatic Necrosis Virus (IPNv) Challenge
4.3. RNA Extraction, cDNA Synthesis, and Transcript Quantification
4.4. Monoamine Analysis
4.5. Cortisol and Prostaglandin E2 ELISA Assays
4.6. Lipid Content
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Woods, S.C.; Begg, D.P. Regulation of the motivation to eat. Behav. Neurosci. Motiv. 2015, 27, 15–34. [Google Scholar]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-controlling endocrine systems in teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front. Neurosci. 2016, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.-W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229. [Google Scholar] [CrossRef] [PubMed]
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC neurons: From birth to death. Annu. Rev. Physiol. 2017, 79, 209–236. [Google Scholar] [CrossRef]
- Forbes, S.; Bui, S.; Robinson, B.R.; Hochgeschwender, U.; Brennan, M.B. Integrated control of appetite and fat metabolism by the leptin-proopiomelanocortin pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 4233–4237. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Rui, L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1247–E1259. [Google Scholar] [CrossRef]
- Hill, J.W. Gene expression and the control of food intake by hypothalamic POMC/CART neurons. Open Neuroendocrinol. J. 2010, 3, 21. [Google Scholar]
- D’Agostino, G.; Diano, S. Alpha-melanocyte stimulating hormone: Production and degradation. J. Mol. Med. 2010, 88, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.C.; Gueorguiev, M.; Korbonits, M. Ghrelin, the peripheral hunger hormone. Ann. Med. 2007, 39, 116–136. [Google Scholar] [CrossRef]
- Opazo, R.; Plaza-Parrochia, F.; Cardoso dos Santos, G.R.; Carneiro, G.R.; Sardela, V.F.; Romero, J.; Valladares, L. Fasting upregulates npy, agrp, and ghsr without increasing ghrelin levels in zebrafish (Danio rerio) larvae. Front. Physiol. 2019, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Chen, H.; Weingarth, D.; Trumbauer, M.E.; Novi, D.E.; Guan, X.; Yu, H.; Shen, Z.; Feng, Y.; Frazier, E.; et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002, 22, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Yasrebi, A.; Hsieh, A.; Mamounis, K.J.; Krumm, E.A.; Yang, J.A.; Magby, J.; Hu, P.; Roepke, T.A. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol. Mol. Cell. Endocrinol. 2016, 422, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Conde-Sieira, M.; Chivite, M.; Míguez, J.M.; Soengas, J.L. Stress effects on the mechanisms regulating appetite in teleost fish. Front. Endocrinol. 2018, 9, 631. [Google Scholar] [CrossRef]
- Leal, E.; Fernández-Durán, B.; Guillot, R.; Ríos, D.; Cerdá-Reverter, J.M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. J. Comp. Physiol. B 2011, 181, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At. the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Boltana, S.; Rey, S.; Roher, N.; Vargas, R.; Huerta, M.; Huntingford, F.A.; Goetz, F.W.; Moore, J.; Garcia-Valtanen, P.; Estepa, A.; et al. Behavioural fever is a synergic signal amplifying the innate immune response. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131381. [Google Scholar] [CrossRef]
- Boltana, S.; Sanhueza, N.; Donoso, A.; Aguilar, A.; Crespo, D.; Vergara, D.; Arriagada, G.; Morales-Lange, B.; Mercado, L.; Rey, S.; et al. The expression of TRPV channels, prostaglandin E2 and pro-inflammatory cytokines during behavioural fever in fish. Brain Behav. Immun. 2018, 71, 169–181. [Google Scholar] [CrossRef]
- Hao, W.; Wong, O.Y.; Liu, X.; Lee, P.; Chen, Y.; Wong, K.K. ω-3 fatty acids suppress inflammatory cytokine production by macrophages and hepatocytes. J. Pediatr. Surg. 2010, 45, 2412–2418. [Google Scholar] [CrossRef]
- Wada, M.; DeLong, C.J.; Hong, Y.H.; Rieke, C.J.; Song, I.; Sidhu, R.S.; Yuan, C.; Warnock, M.; Schmaier, A.H.; Yokoyama, C.; et al. Enzymes and Receptors of Prostaglandin Pathways with Arachidonic Acid-derived Versus Eicosapentaenoic Acid-derived Substrates and Products. J. Biol. Chem. 2007, 282, 22254–22266. [Google Scholar] [CrossRef]
- Haddad, J.J.; Saadé, N.E.; Safieh-Garabedian, B. Cytokines and neuro–immune–endocrine interactions: A role for the hypothalamic–pituitary–adrenal revolving axis. J. Neuroimmunol. 2002, 133, 1–19. [Google Scholar] [CrossRef]
- Reyes-López, F.E.; Aerts, J.; Vallejos-Vidal, E.; Ampe, B.; Dierckens, K.; Tort, L.; Bossier, P. Modulation of innate immune-related genes and glucocorticoid synthesis in gnotobiotic full-sibling European sea bass (Dicentrarchus labrax) larvae challenged with Vibrio anguillarum. Front. Immunol. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fish. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Sadoul, B.; Vijayan, M.M. Stress and growth. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–205. [Google Scholar]
- Balasch, J.C.; Tort, L. Netting the stress responses in fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Deck, C.A.; Honeycutt, J.L.; Cheung, E.; Reynolds, H.M.; Borski, R.J. Assessing the functional role of leptin in energy homeostasis and the stress response in vertebrates. Front. Endocrinol. 2017, 8, 63. [Google Scholar] [CrossRef]
- Cawley, N.X.; Li, Z.; Loh, Y.P. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol. 2016, 56, T77–T97. [Google Scholar] [CrossRef]
- Millington, G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 1–16. [Google Scholar] [CrossRef]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-pituitary-adrenal axis modulation of glucocorticoids in the cardiovascular system. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Cerdá-Reverter, J.M.; Soengas, J.L. Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: Involvement in the control of food intake. Front. Neurosci. 2017, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Comesaña, S.; Velasco, C.; Ceinos, R.M.; López-Patiño, M.A.; Míguez, J.M.; Morais, S.; Soengas, J.L. Evidence for the presence in rainbow trout brain of amino acid-sensing systems involved in the control of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R201–R215. [Google Scholar] [CrossRef]
- Otero-Rodiño, C.; Librán-Pérez, M.; Velasco, C.; López-Patiño, M.A.; Míguez, J.M.; Soengas, J.L. Evidence for the presence of glucosensor mechanisms not dependent on glucokinase in hypothalamus and hindbrain of rainbow trout (Oncorhynchus mykiss). PLoS ONE 2015, 10, e0128603. [Google Scholar] [CrossRef]
- Polakof, S.; Mommsen, T.P.; Soengas, J.L. Glucosensing and glucose homeostasis: From fish to mammals. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 160, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Taksdal, T.; Stangeland, K.; Dannevig, B. Induction of infectious pancreatic necrosis (IPN) in Atlantic salmon Salmo salar and brook trout Salvelinus fontinalis by bath challenge of fry with infectious pancreatic necrosis virus (IPNV) serotype Sp. Dis. Aquat. Org. 1997, 28, 39–44. [Google Scholar] [CrossRef]
- Wong, S.; Pinkney, J. Role of cytokines in regulating feeding behaviour. Curr. Drug Targets 2004, 5, 251–263. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, A.D.; Pacheco-López, G.; Langhans, W.; Brown, L.M. The effect of TNFα on food intake and central insulin sensitivity in rats. Physiol. Behav. 2011, 103, 17–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DeBoer, M.D.; Scarlett, J.M.; Levasseur, P.R.; Grant, W.F.; Marks, D.L. Administration of IL-1β to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase neurons in the nucleus of the solitary tract. Peptides 2009, 30, 210–218. [Google Scholar] [CrossRef][Green Version]
- Mishra, D.; Richard, J.E.; Maric, I.; Porteiro, B.; Häring, M.; Kooijman, S.; Musovic, S.; Eerola, K.; López-Ferreras, L.; Peris, E.; et al. Parabrachial interleukin-6 reduces body weight and food intake and increases thermogenesis to regulate energy metabolism. Cell Rep. 2019, 26, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Gong, D.W.; Bi, S.; Weintraub, B.D. Genomic structure and promoter analysis of the human obese gene (∗). J. Biol. Chem. 1996, 271, 3971–3974. [Google Scholar]
- Leal-Cerro, A.; Soto, A.; Martínez, M.A.; Dieguez, C.; Casanueva, F.F. Influence of cortisol status on leptin secretion. Pituitary 2001, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Saladin, R.; Auwerx, J.; Staels, B. Induction of ob Gene expression by corticosteroids is accompanied by body weight loss and reduced food intake (∗). J. Biol. Chem. 1995, 270, 15958–15961. [Google Scholar] [CrossRef] [PubMed]
- Khansari, A.R.; Balasch, J.C.; Vallejos-Vidal, E.; Parra, D.; Reyes-López, F.E.; Tort, L. Comparative immune-and stress-related transcript response induced by air exposure and Vibrio anguillarum bacterin in rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata) mucosal surfaces. Front. Immunol. 2018, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Madison, B.N.; Tavakoli, S.; Kramer, S.; Bernier, N.J. Chronic cortisol and the regulation of food intake and the endocrine growth axis in rainbow trout. J. Endocrinol. 2015, 226, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Peter, R.E. Effects of lipopolysaccharide treatment on feeding of goldfish: Role of appetite-regulating peptides. Brain Res. 2004, 998, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.; Weil, Z.M.; Nelson, R.J. Fever and sickness behaviour vary among congeneric rodents. Funct. Ecol. 2008, 22, 68–77. [Google Scholar] [CrossRef]
- Owen-Ashley, N.T.; Wingfield, J.C. Acute phase responses of passerine birds: Characterization and seasonal variation. J. Ornithol. 2007, 148, 583–591. [Google Scholar] [CrossRef]
- Uller, T.; Isaksson, C.; Olsson, M. Immune challenge reduces reproductive output and growth in a lizard. Funct. Ecol. 2006, 20, 873–879. [Google Scholar] [CrossRef]
- Wedemeyer, G.; Ross, A.; Smith, L. Some metabolic effects of bacterial endotoxins in salmonid fish. J. Fish. Board Can. 1969, 26, 115–122. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Romanovsky, A.A. Prostaglandin E2 as a mediator of fever: Synthesis and catabolism. Front. Biosci. 2004, 9, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.; Vance, J. Fatty Acid Desaturation and Chain Elongation in Eukaryotes; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Xi, Y.; Lindenmayer, L.; Kline, I.; von Einem, J.; Purdy, J.G. Human cytomegalovirus uses a host stress response to balance the elongation of saturated/monounsaturated and polyunsaturated very-long-chain fatty acids. Mbio 2021, 12, e00167-21. [Google Scholar] [CrossRef]
- Kang, J.X.; Weylandt, K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. Lipids Health Dis. 2008, 49, 133–143. [Google Scholar]
- Santos, J.L. Receptor-4 de melanocortina: Relevancia en la conducta de alimentación y en la acumulación de grasa corporal. Rev. Chil. Endocrinol. 2014, 7, 17–20. [Google Scholar]
- Bernier, N.J.; Craig, P.M. CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R982–R990. [Google Scholar] [CrossRef]
- Reyes, T.M.; Lewis, K.; Perrin, M.H.; Kunitake, K.S.; Vaughan, J.; Arias, C.A.; Hogenesch, J.B.; Gulyas, J.; Rivier, J.; Vale, W.W.; et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 2843–2848. [Google Scholar] [CrossRef]
- Boucsein, A.; Kamstra, K.; Tups, A. Central signalling cross-talk between insulin and leptin in glucose and energy homeostasis. J. Neuroendocrinol. 2021, 33, e12944. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, C.; Borgquist, A.; Nestor, C.C.; Smith, A.W.; Bosch, M.A.; Ku, S.; Wagner, E.J.; Rønnekleiv, O.K.; Kelly, M.J. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014, 19, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.; Tiganis, T. Insulin action in the brain: Roles in energy and glucose homeostasis. J. Neuroendocrinol. 2017, 29, e12513. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.M.; Schreck, C.B. Effects of acute stress on osmoregulation, feed intake, IGF-1, and cortisol in yearling steelhead trout (Oncorhynchus mykiss) during seawater adaptation. Gen. Comp. Endocrinol. 2006, 148, 195–202. [Google Scholar] [CrossRef]
- De Pedro, N.; Delgado, M.J.; Pinillos, M.L.; Alonso-Bedate, M. α1-Adrenergic and dopaminergic receptors are involved in the anoretic effect of corticotropin-releasing factor in goldfish. Life Sci. 1998, 62, 1801–1808. [Google Scholar] [CrossRef]
- Guy-Grand, B. Clinical studies with dexfenfluramine: From past to future. Obes. Res. 1995, 3, 491S–496S. [Google Scholar] [CrossRef]
- Jackson, H.C.; Needham, A.M.; Hutchins, L.J.; Mazurkiewicz, S.E.; Heal, D.J. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br. J. Pharmacol. 1997, 121, 1758–1762. [Google Scholar] [CrossRef]
- Marston, O.J.; Garfield, A.S.; Heisler, L.K. Role of central serotonin and melanocortin systems in the control of energy balance. Eur. J. Pharmacol. 2011, 660, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Simansky, K.J.; Vaidya, A. Behavioral mechanisms for the anorectic action of the serotonin (5-HT) uptake inhibitor sertraline in rats: Comparison with directly acting 5-HT agonists. Brain Res. Bull. 1990, 25, 953–960. [Google Scholar] [CrossRef]
- Zhou, Q.-Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef]
- Leal, E.; Fernández-Durán, B.; Agulleiro, M.J.; Conde-Siera, M.; Míguez, J.M.; Cerdá-Reverter, J.M. Effects of dopaminergic system activation on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): A self-feeding approach. Horm. Behav. 2013, 64, 113–121. [Google Scholar] [CrossRef]
- Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Maceira, J.J.; Otero-Rodiño, C.; Mancebo, M.J.; Soengas, J.L.; Aldegunde, M. Food intake inhibition in rainbow trout induced by activation of serotonin 5-HT2C receptors is associated with increases in POMC, CART and CRF mRNA abundance in hypothalamus. J. Comp. Physiol. B 2016, 186, 313–321. [Google Scholar] [CrossRef]
- Gesto, M.; Tintos, A.; Soengas, J.L.; Míguez, J.M. Effects of acute and prolonged naphthalene exposure on brain monoaminergic neurotransmitters in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, N.; Donoso, A.; Aguilar, A.; Farlora, R.; Carnicero, B.; Míguez, J.M.; Tort, L.; Valdes, J.A.; Boltana, S. Thermal Modulation of Monoamine Levels Influence Fish. Stress and Welfare. Front. Endocrinol. 2018, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.; Dyer, W. A rapid method of total lipid extraction and purification. Can. J. Biochem Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
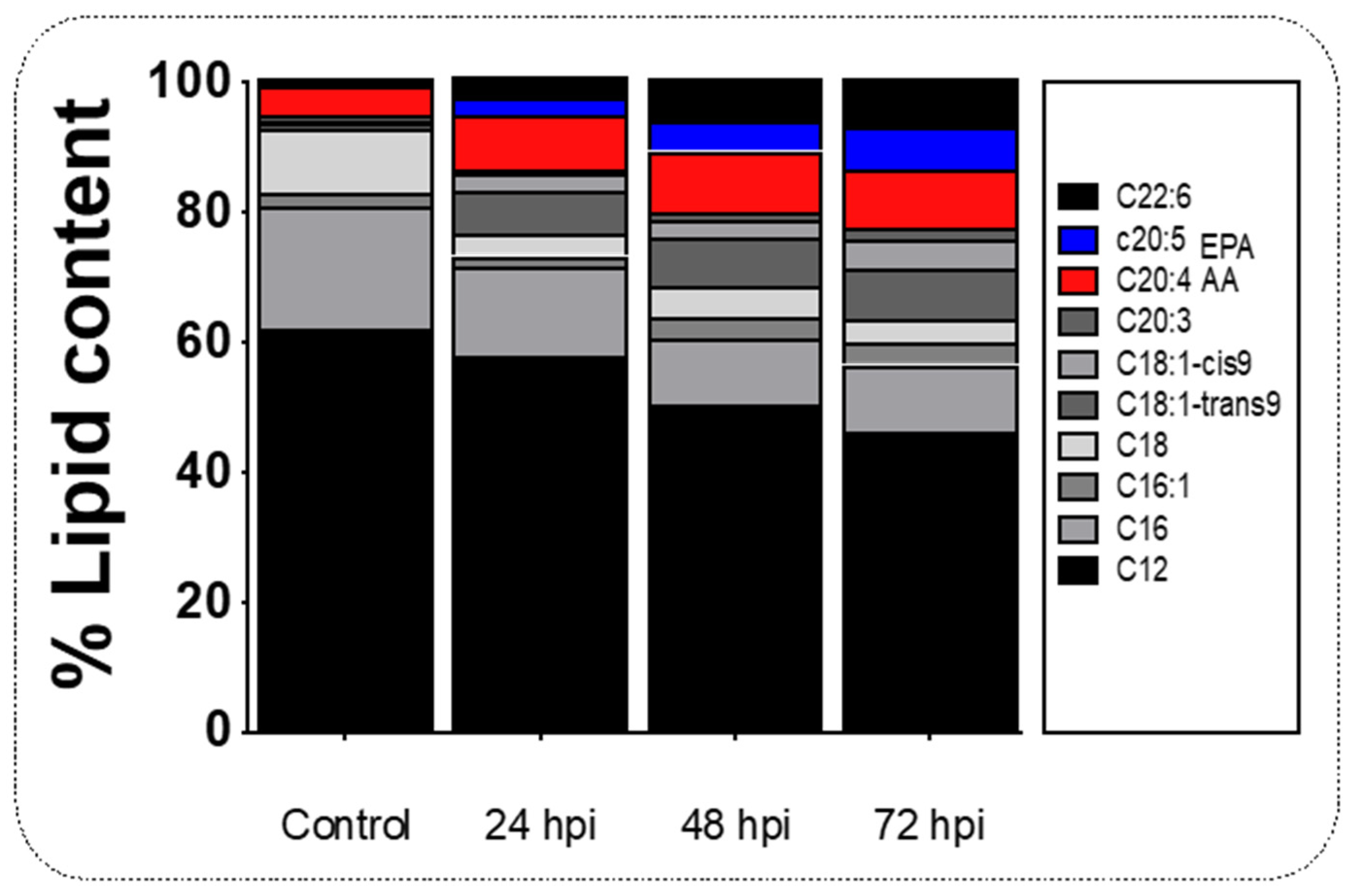
| LIPID/HPI | Control | 24 hpi | 48 hpi | 72 hpi |
|---|---|---|---|---|
| Dodecanoic acid C12:0 | 62 ± 12.12 | 50.40 ± 9.52 | 46.31 ± 10.51 | 62.96 ± 14.23 |
| Hexadecanoic acid, C16:0 | 19.1 ± 2.90 | 10.33 ± 3.80 | 10.31 ± 4.31 | 11.58 ± 4.12 |
| Palmitoleic acid, C16:1 | 1.96 ± 0.97 | 3.14 ± 1.91 | 3.55 ± 2.01 | 0.36 ± 0.03 |
| Stearic acid, C18:0 | 9.82 ± 2.13 | 5.01 ± 0.88 | 3.40 ± 1.61 | 3.55 ± 2.04 |
| Oleic acid, C18:1 trans 9 | 0.98 ± 0.31 | 7.40 ± 2.90 | 7.93 ± 2.86 | 6.19 ± 2.98 |
| cis-8,11,14-Eicosatrienoic acid methyl ester | 0.20 ± 0.01 | 2.66 ± 1.43 | 4.53 ± 1.72 | 1.23 ± 0.91 |
| Methyl eicosatrienoic C20:3 | 1.01 ± 0.31 | 1.06 ± 0.47 | 1.69 ± 0.81 | 0.11 ± 0.105 |
| Arachidonic acid (AA), C20:4 | 4.38 ± 2.01 | 9.50 ± 0.68 | 8.81 ± 3.65 | 7.98 ± 3.51 |
| Eicosapentaenoic acid (EPA), C20:5 | 0.10 ± 0.03 | 4.78 ± 2.88 | 6.77 ± 3.06 | 4.55 ± 1.27 |
| Docosahexaenoic acid (DHA), C22:6 | 4.43 ± 1.97 | 5.74 ± 1.91 | 6.70 ± 2.75 | 1.50 ± 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, D.; Fuentes, R.; Carnicero, B.; Aguilar, A.; Sanhueza, N.; San-Martin, S.; Agurto, C.; Donoso, A.; Valdivia, L.E.; Miguez, J.M.; et al. Viral Infection Drives the Regulation of Feeding Behavior Related Genes in Salmo salar. Int. J. Mol. Sci. 2021, 22, 11391. https://doi.org/10.3390/ijms222111391
Muñoz D, Fuentes R, Carnicero B, Aguilar A, Sanhueza N, San-Martin S, Agurto C, Donoso A, Valdivia LE, Miguez JM, et al. Viral Infection Drives the Regulation of Feeding Behavior Related Genes in Salmo salar. International Journal of Molecular Sciences. 2021; 22(21):11391. https://doi.org/10.3390/ijms222111391
Chicago/Turabian StyleMuñoz, David, Ricardo Fuentes, Beatriz Carnicero, Andrea Aguilar, Nataly Sanhueza, Sergio San-Martin, Cristian Agurto, Andrea Donoso, Leonardo E. Valdivia, Jesús M. Miguez, and et al. 2021. "Viral Infection Drives the Regulation of Feeding Behavior Related Genes in Salmo salar" International Journal of Molecular Sciences 22, no. 21: 11391. https://doi.org/10.3390/ijms222111391
APA StyleMuñoz, D., Fuentes, R., Carnicero, B., Aguilar, A., Sanhueza, N., San-Martin, S., Agurto, C., Donoso, A., Valdivia, L. E., Miguez, J. M., Tort, L., & Boltana, S. (2021). Viral Infection Drives the Regulation of Feeding Behavior Related Genes in Salmo salar. International Journal of Molecular Sciences, 22(21), 11391. https://doi.org/10.3390/ijms222111391







