Terpenoids from Abies holophylla Attenuate LPS-Induced Neuroinflammation in Microglial Cells by Suppressing the JNK-Related Signaling Pathway
Abstract
1. Introduction
2. Results
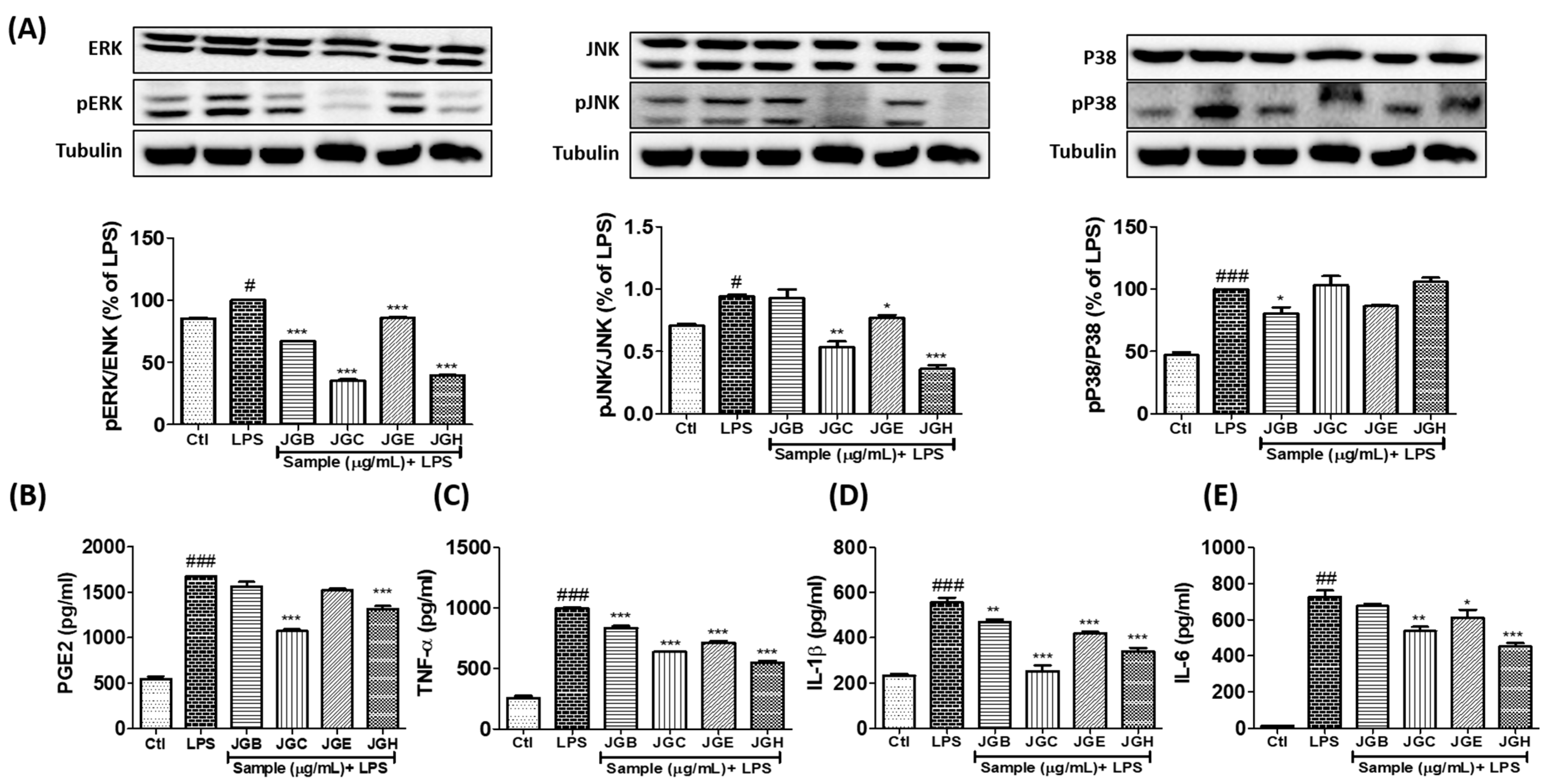
2.1. A. holophylla Subfractions Inhibited MAPK Signaling and Production of Pro-Inflammatory Cytokines in LPS-Activated Microglia
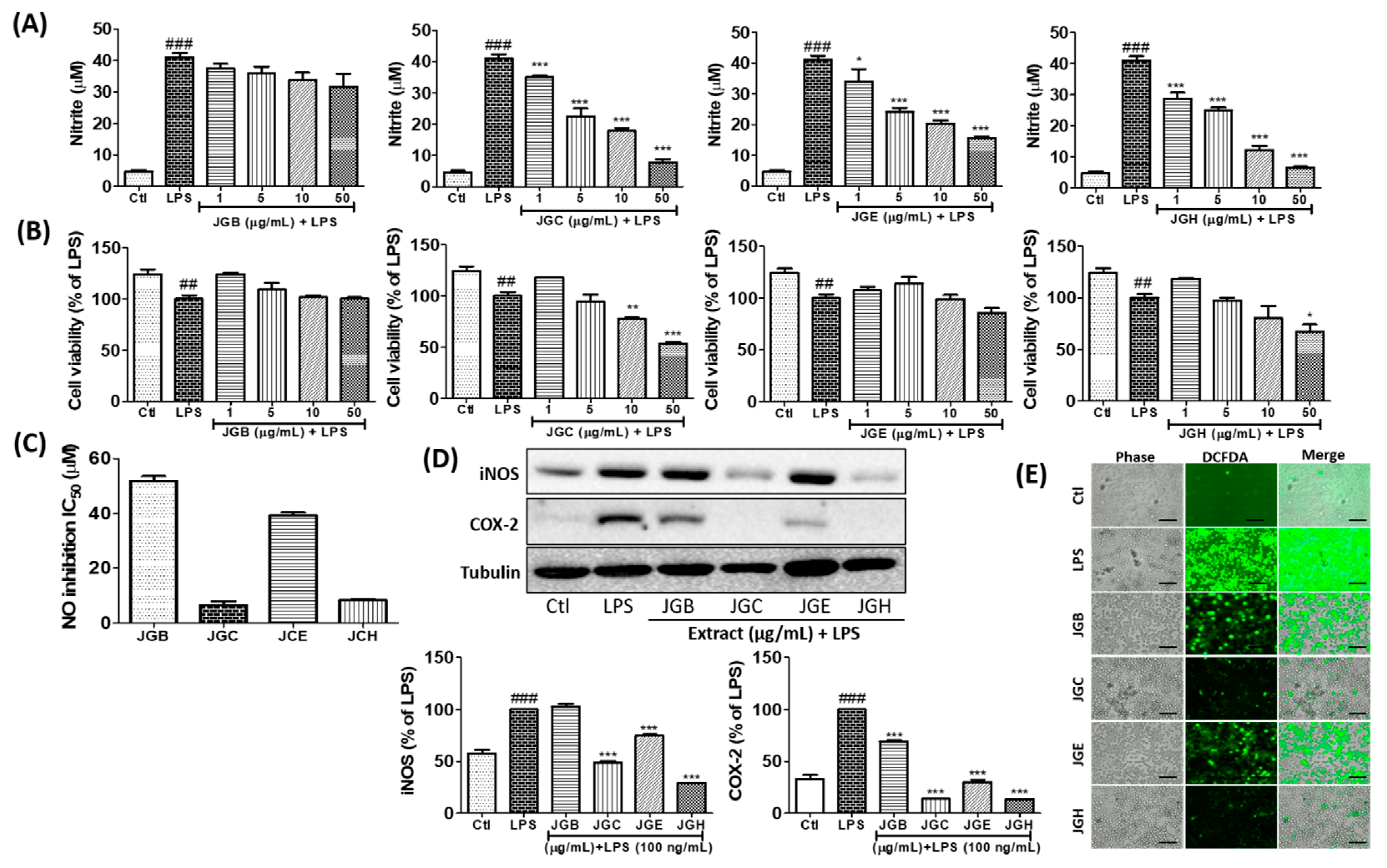
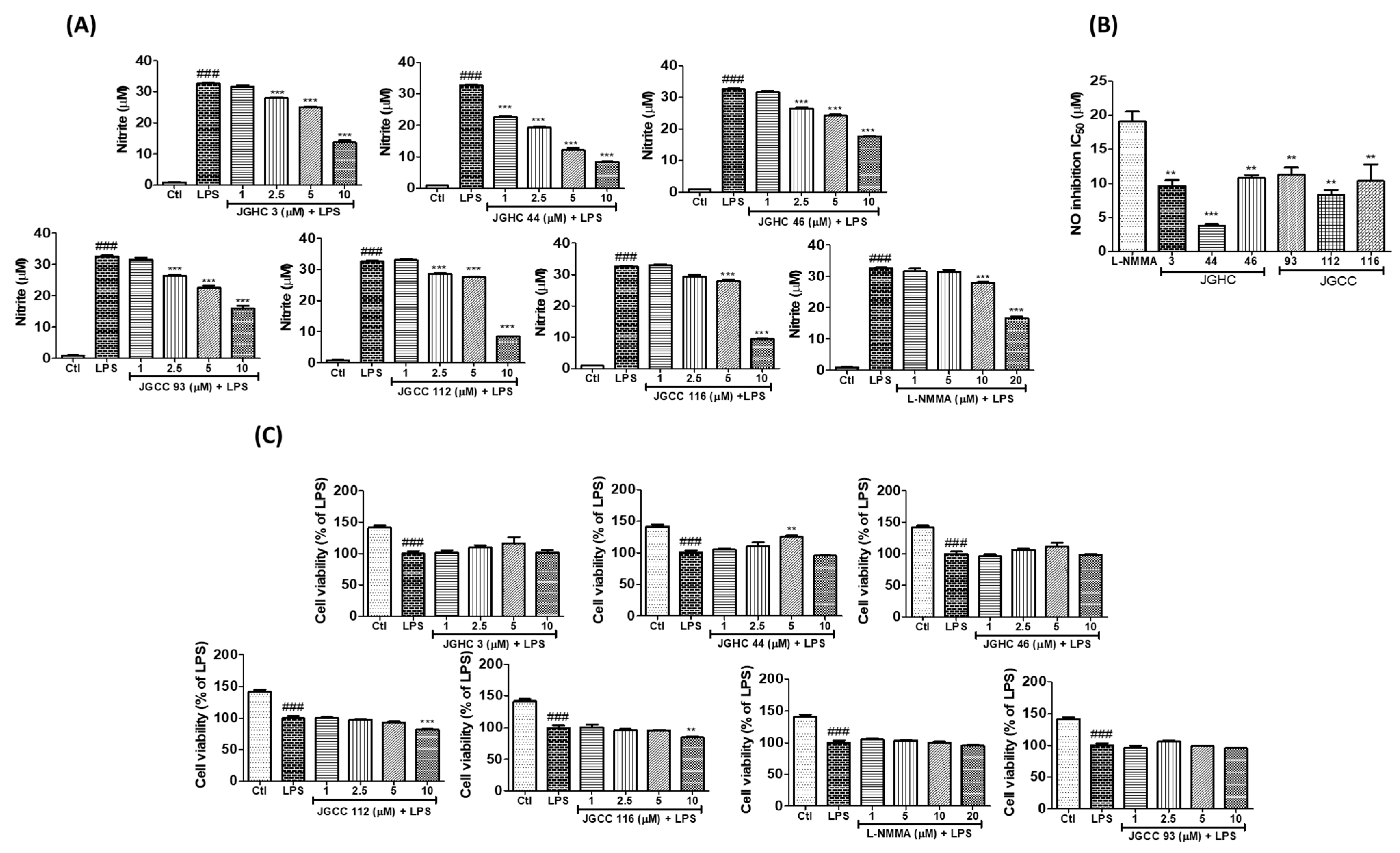
2.2. Active Components from A. holophylla Subfractions Inhibited Inflammatory NO Production in LPS-Activated Microglia
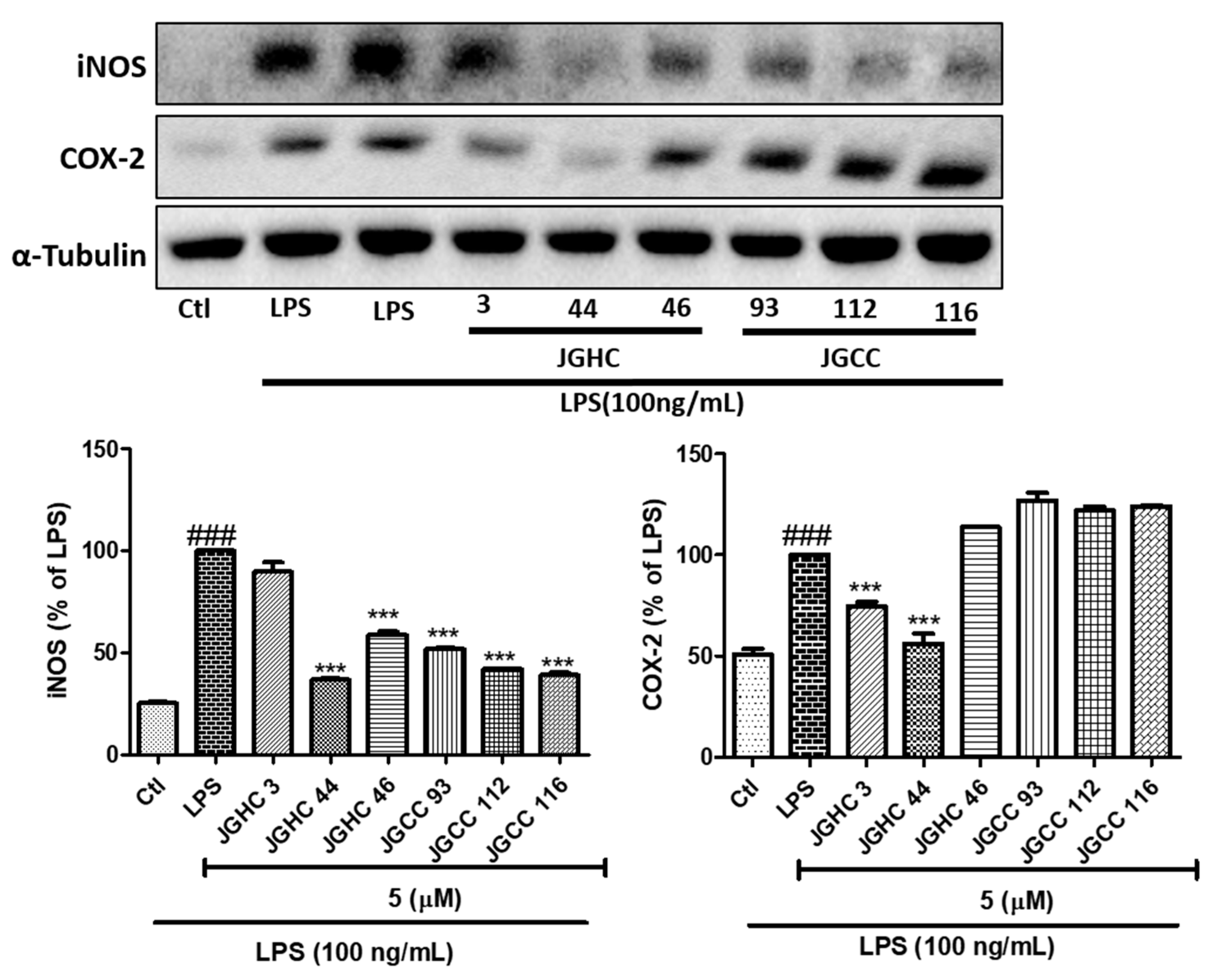
2.3. Active Components from A. holophylla Subfractions Inhibited Inflammatory Protein Expression in LPS-Activated Microglia
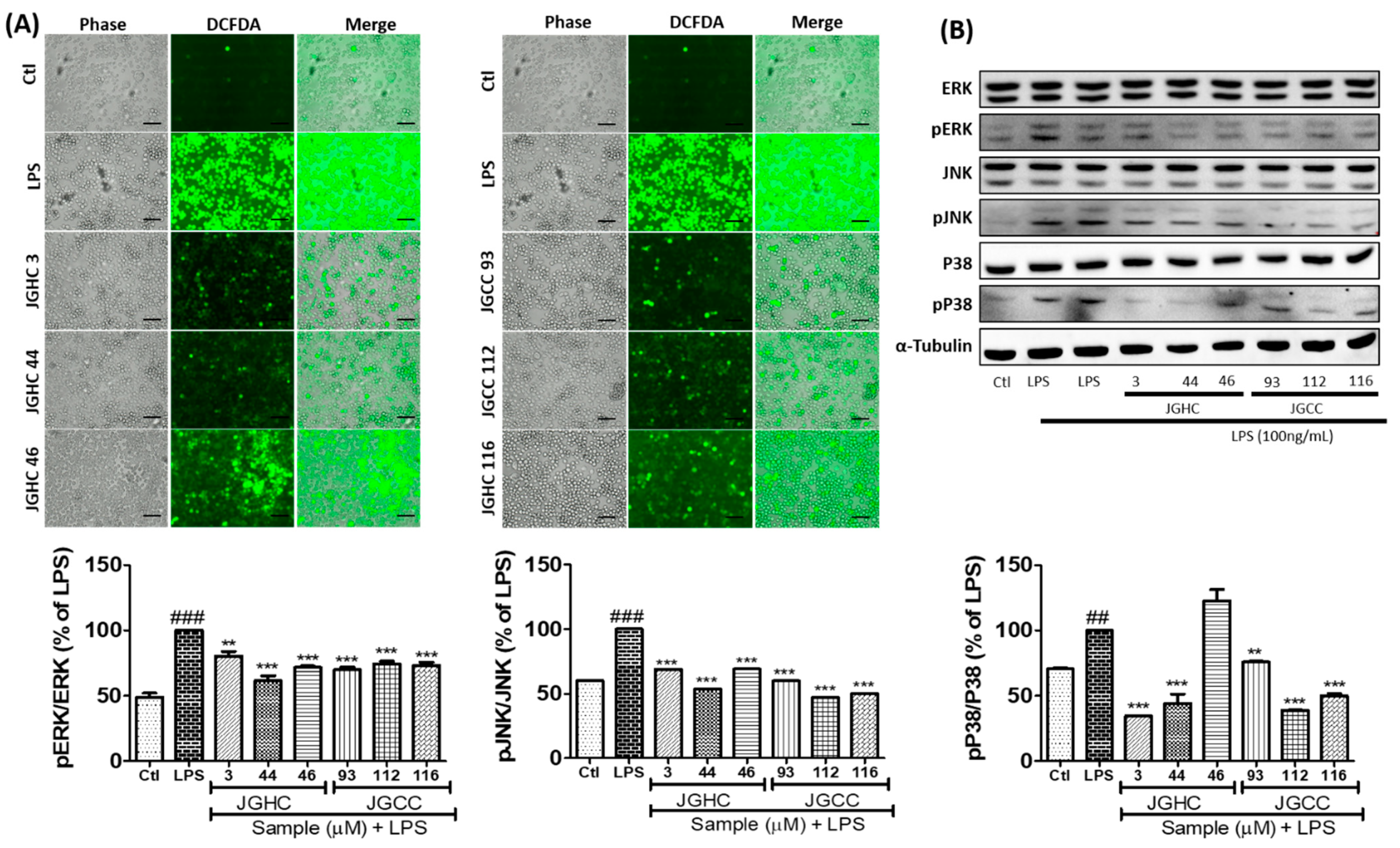
2.4. Active Components from A. holophylla Subfraction Inhibited ROS Production and MAPKs in LPS-Activated Microglia
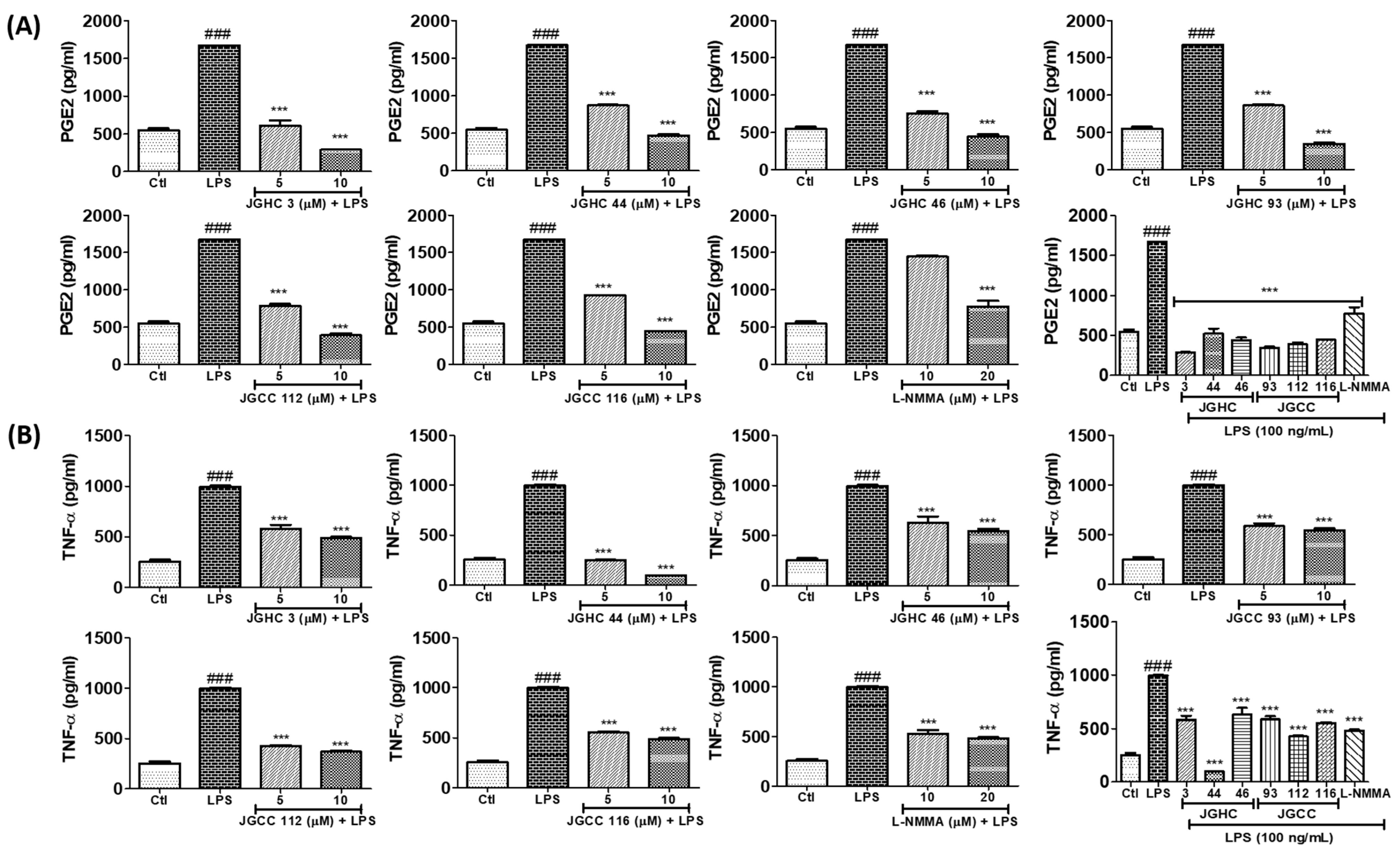
2.5. Active Components from A. holophylla Fractions Inhibited PGE2 and TNF-α Production in LPS-Activated Microglia
3. Materials and Methods
3.1. Reagents
3.2. Extraction and Isolation of A. hollophylla
3.3. Cell Treatment, Nitric Oxide Production, and Cell Viability Assay
3.4. Measurement of PGE2, TNF-α, IL-1β, and IL-6 Production
3.5. Western Blot Analysis
3.6. Measurement of ROS Production
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Suzumura, A. The Role of Microglia in Neuroinflammation. Brain Nerve 2017, 69, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, Z.; Wang, J.; Xin, Z.; Wang, J.; Li, F.; Fu, Y. Taraxasterol Inhibits LPS-Induced Inflammatory Response in BV2 Microglia Cells by Activating LXRalpha. Front. Pharmacol. 2018, 9, 278. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-kappaB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Oh, J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Holophyllane A: A Triterpenoid Possessing an Unprecedented B-nor-3,4-seco-17,14-friedo-lanostane Architecture from Abies holophylla. Sci. Rep. 2017, 7, 43646. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.H.; Zhang, S.D.; Li, Y.L.; Wu, L.; Zhu, Z.J.; Yang, X.W.; Zeng, H.W.; Li, H.L.; Wang, N.; Steinmetz, A.; et al. Sesquiterpenoids and triterpenoids from Abies holophylla and their bioactivities. Phytochemistry 2012, 74, 178–184. [Google Scholar] [CrossRef]
- Kim, C.S.; Kwon, O.W.; Kim, S.Y.; Lee, K.R. Bioactive lignans from the trunk of Abies holophylla. J. Nat. Prod. 2013, 76, 2131–2135. [Google Scholar] [CrossRef]
- Yesilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Diterpenes from the Trunk of Abies holophylla and Their Potential Neuroprotective and Anti-inflammatory Activities. J. Nat. Prod. 2016, 79, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Oh, J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Structural Characterization of Terpenoids from Abies holophylla Using Computational and Statistical Methods and Their Biological Activities. J. Nat. Prod. 2018, 81, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, J.W. Brain energy metabolism and multiple sclerosis: Progress and prospects. Arch. Pharm. Res. 2020, 43, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Frank-Cannon, T.C.; Alto, L.T.; McAlpine, F.E.; Tansey, M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68, 165–175. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Tu, C.-E.; Wang, S.-C.; Hung, Y.-L.; Su, C.-C.; Fang, S.-H.; Chen, C.-S.; Liu, P.-L.; Cheng, W.-C.; Huang, Y.-W. Corylin inhibits LPS-induced inflammatory response and attenuates the activation of NLRP3 inflammasome in microglia. BMC Complementary Altern. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and anti-inflammatory activities of allyl isothiocyanate through attenuation of JNK/NF-κB/TNF-α signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, S.E.; Madiha, S.; Gaire, B.P.; Jin, M.; Yumnam, S.; Kim, S.Y. Phytochemicals against TNFalpha-Mediated Neuroinflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 764. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009, 292, 1902–1913. [Google Scholar] [CrossRef]
- Shih, J.-H.; Tsai, Y.-F.; Li, I.; Chen, M.-H.; Huang, Y.-S. Hp-s1 Ganglioside Suppresses Proinflammatory Responses by Inhibiting MyD88-Dependent NF-κB and JNK/p38 MAPK Pathways in Lipopolysaccharide-Stimulated Microglial Cells. Mar. Drugs 2020, 18, 496. [Google Scholar] [CrossRef]
- Lee, E.J.; Choi, M.J.; Lee, G.; Gaire, B.P.; Choi, J.W.; Kim, H.S. Regulation of neuroinflammation by matrix metalloproteinase-8 inhibitor derivatives in activated microglia and astrocytes. Oncotarget 2017, 8, 78677–78690. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-W.; Tu, Y.-F.; Huang, C.-C.; Ho, C.-J. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J. Neuroinflammation 2012, 9, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, W.; Tang, M.; Zhao, Y.; Zhang, K.; Wang, X.; Li, Y. Inhibition of JNK ameliorates depressive-like behaviors and reduces the activation of pro-inflammatory cytokines and the phosphorylation of glucocorticoid receptors at serine 246 induced by neuroinflammation. Psychoneuroendocrinology 2020, 113, 104580. [Google Scholar] [CrossRef]
- Zheng, J.; Dai, Q.; Han, K.; Hong, W.; Jia, D.; Mo, Y.; Lv, Y.; Tang, H.; Fu, H.; Geng, W. JNK-IN-8, ac-Jun N-terminal kinase inhibitor, improves functional recovery through suppressing neuroinflammation in ischemic stroke. J. Cell. Physiol. 2020, 235, 2792–2799. [Google Scholar] [CrossRef]
- Schieven, G.L. The biology of p38 kinase: A central role in inflammation. Curr. Top. Med. Chem. 2005, 5, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Hammaker, D.; Topolewski, K.; Corr, M.; Boyle, D.L.; Karin, M.; Firestein, G.S. Antiinflammatory functions of p38 in mouse models of rheumatoid arthritis: Advantages of targeting upstream kinases MKK-3 or MKK-6. Arthritis Rheum 2012, 64, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-w.; Pan, Y.-q.; Tang, M.-m.; Lin, W.-j. Blocking p38 signaling reduces the activation of pro-inflammatory cytokines and the phosphorylation of p38 in the habenula and reverses depressive-like behaviors induced by neuroinflammation. Front. Pharmacol. 2018, 9, 511. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subedi, L.; Yumnam, S. Terpenoids from Abies holophylla Attenuate LPS-Induced Neuroinflammation in Microglial Cells by Suppressing the JNK-Related Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 965. https://doi.org/10.3390/ijms22020965
Subedi L, Yumnam S. Terpenoids from Abies holophylla Attenuate LPS-Induced Neuroinflammation in Microglial Cells by Suppressing the JNK-Related Signaling Pathway. International Journal of Molecular Sciences. 2021; 22(2):965. https://doi.org/10.3390/ijms22020965
Chicago/Turabian StyleSubedi, Lalita, and Silvia Yumnam. 2021. "Terpenoids from Abies holophylla Attenuate LPS-Induced Neuroinflammation in Microglial Cells by Suppressing the JNK-Related Signaling Pathway" International Journal of Molecular Sciences 22, no. 2: 965. https://doi.org/10.3390/ijms22020965
APA StyleSubedi, L., & Yumnam, S. (2021). Terpenoids from Abies holophylla Attenuate LPS-Induced Neuroinflammation in Microglial Cells by Suppressing the JNK-Related Signaling Pathway. International Journal of Molecular Sciences, 22(2), 965. https://doi.org/10.3390/ijms22020965




