The Interplay between HGF/c-met Axis and Nox4 in BRAF Mutated Melanoma
Abstract
1. Introduction
2. Results
2.1. Study Population and Clinical Follow-Up
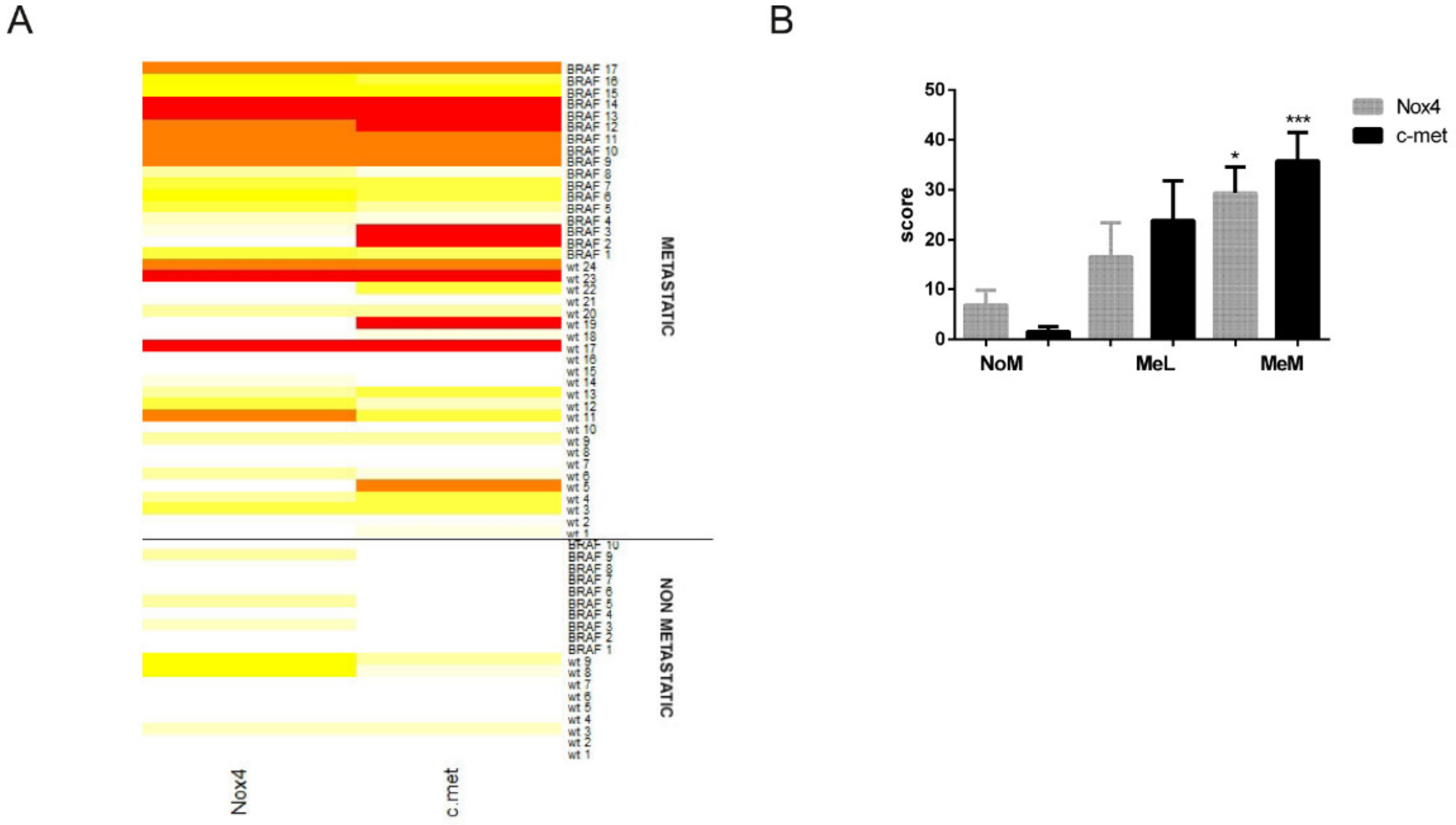
2.2. Immunohistochemical Analyses Correlated with Malignant Melanoma Type
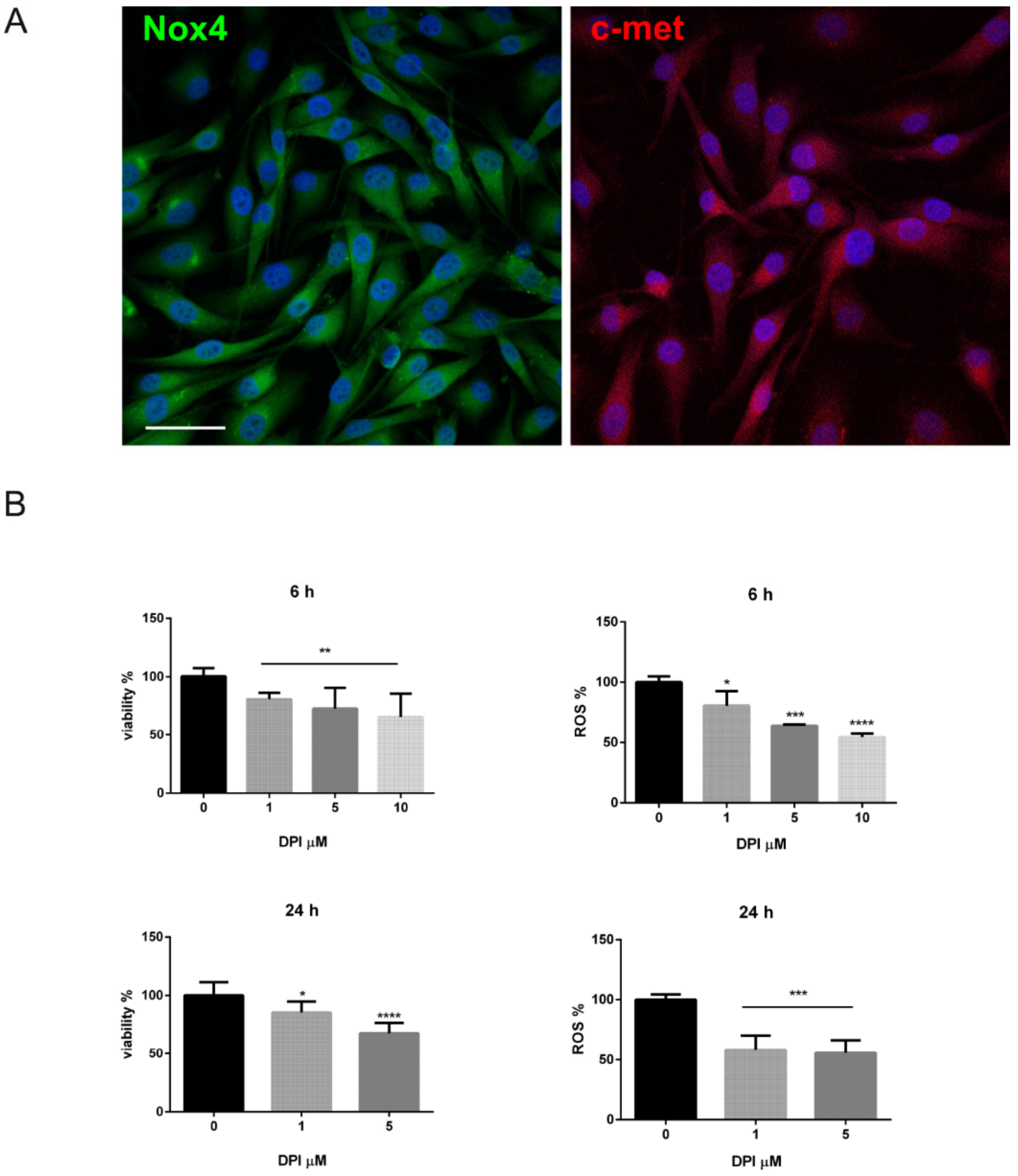
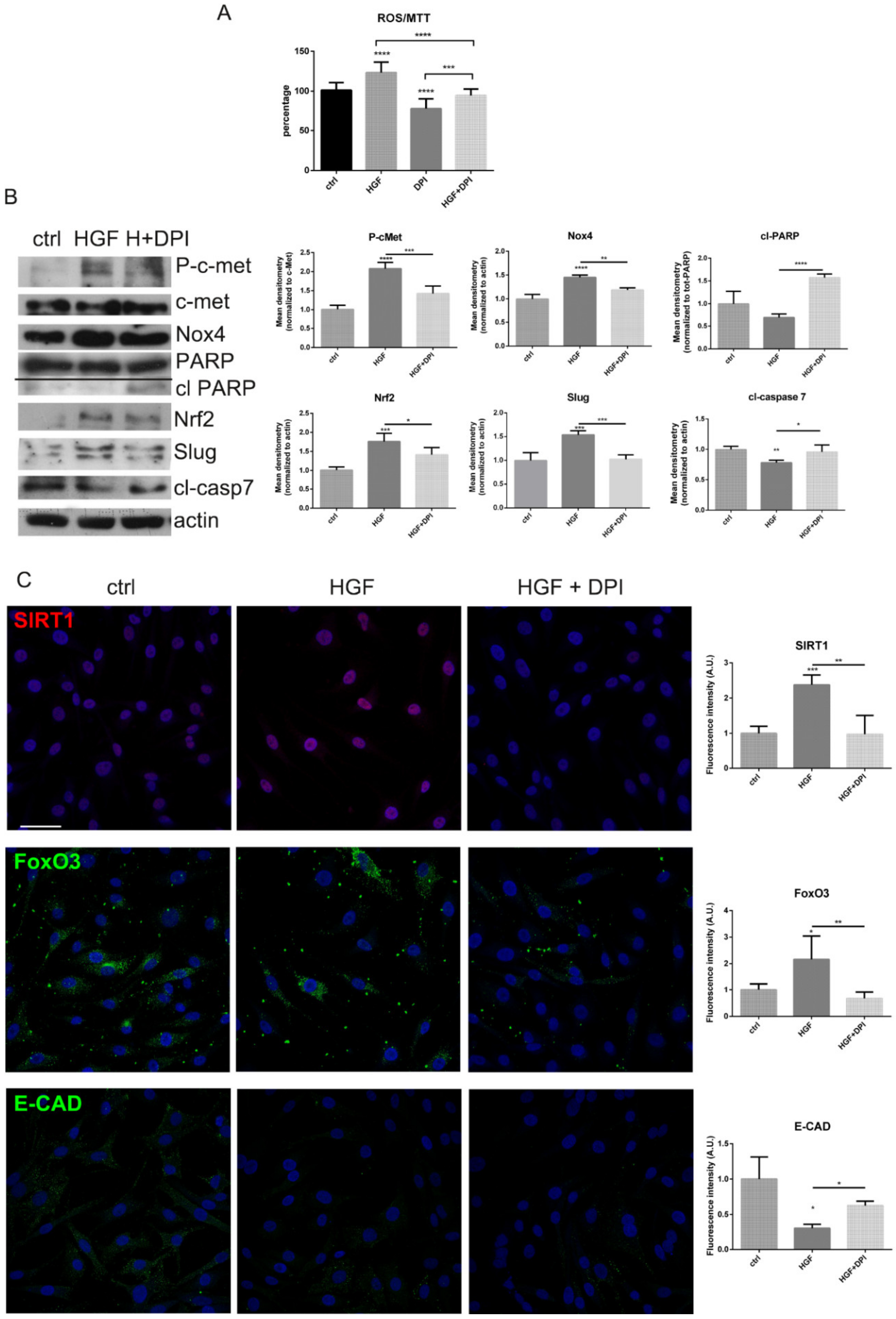
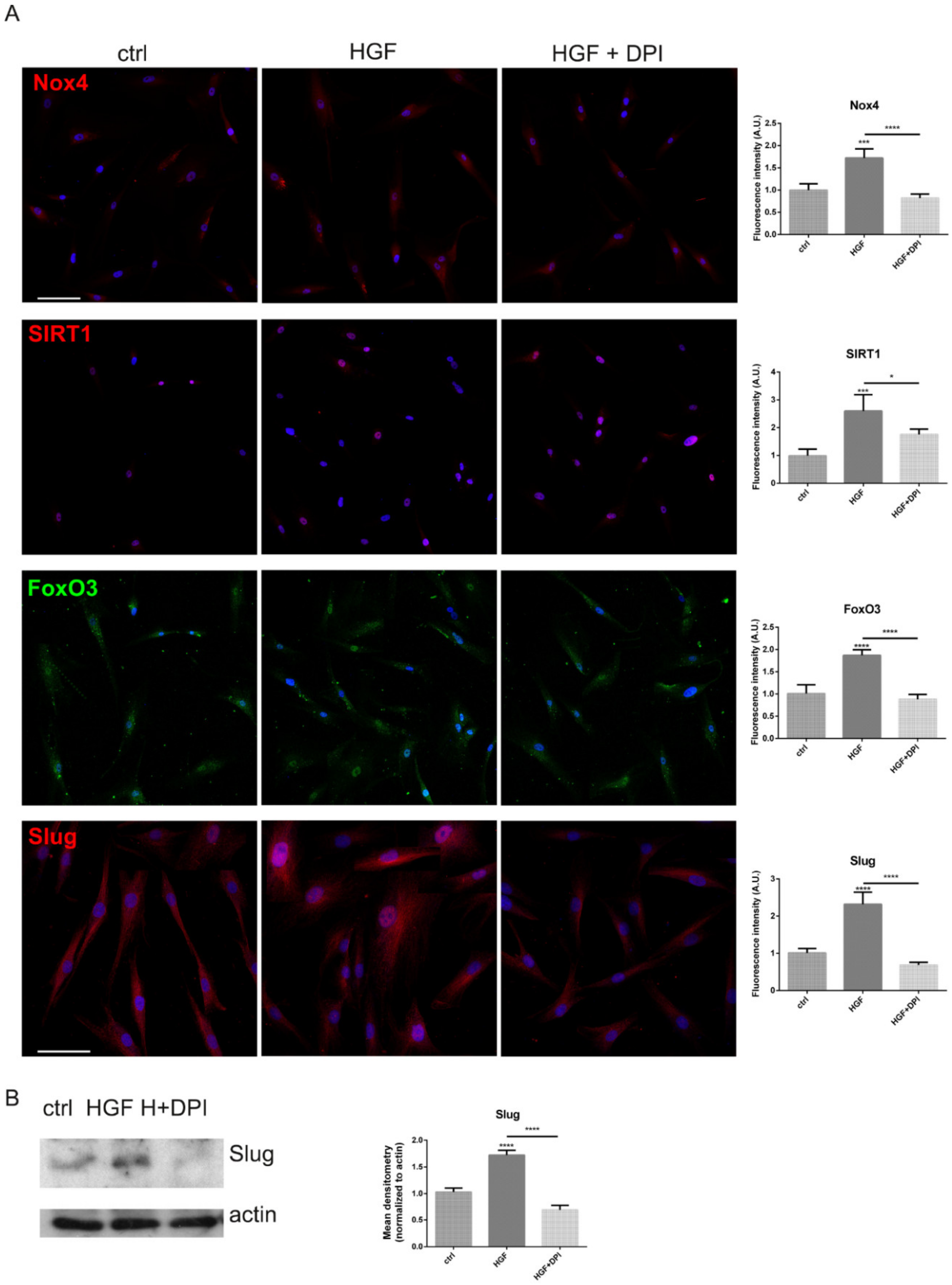
2.3. Is the Expression of Nox4 Related to c-met Activation?
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Histological Study
4.3. Cell Culture and Treatments
4.4. MTT Assay
4.5. ROS Detection
4.6. Cellular Extracts Preparation
4.7. SDS PAGE and Western Blot
4.8. Immunofluorescence and Confocal Microscopy
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| DCFH-DA | dichlorodihydrofluorescein diacetate |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DPI | diphenyleneiodonium |
| EDTA | ethylenediaminetetraacetic acid |
| EMT | epithelial-mesenchymal transition |
| HGF | hepatocyte growth factor |
| IHC | immunohistochemical |
| MeM | metastasizing melanoma |
| MeL | melanoma with positive lymph nodes |
| Nox | NADPH oxidase |
| NoM | non-metastatic melanoma |
| PBS | phosphate buffered saline |
| ROS | reactive oxygen species |
| SDS | sodium dodecyl sulphate |
| WB | western blot |
References
- Czyz, M. HGF/c-MET signaling in melanocytes and melanoma. Int. J. Mol. Sci. 2018, 19, 3844. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Mishra, R.; Patel, H.; Abdulsalam, S.; Greis, K.D.; Kadekaro, A.L.; Merino, E.J.; Garrett, J.T. Utilization of reactive oxygen species targeted therapy to prolong the efficacy of braf inhibitors in melanoma. J. Cancer 2018, 9, 4665–4676. [Google Scholar] [CrossRef] [PubMed]
- Khamari, R.; Trinh, A.; Gabert, P.E.; Corazao-Rozas, P.; Riveros-Cruz, S.; Balayssac, S.; Malet-Martino, M.; Dekiouk, S.; Curt, M.J.C.; Maboudou, P.; et al. Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Dellinger, R.; Meyskens, F.L. Updates of reactive oxygen species in melanoma etiology and progression. Arch. Biochem. Biophys. 2014, 563, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kappes, J.C.; Katiyar, S.K. Inhibition of NADPH oxidase 1 activity and blocking the binding of cytosolic and membrane-bound proteins by honokiol inhibit migratory potential of melanoma cells. Oncotarget 2016, 7, 7899–7912. [Google Scholar] [CrossRef]
- Liu, F.; Garcia, A.M.G.; Meyskens, F.L. NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. J. Investig. Dermatol. 2012, 132, 2033–2041. [Google Scholar] [CrossRef]
- Komatsu, D.; Kato, M.; Nakayama, J.; Miyagawa, S.; Kamata, T. NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression. Oncogene 2008, 27, 4724–4732. [Google Scholar] [CrossRef]
- Kim, E.Y.; Seo, J.M.; Cho, K.J.; Kim, J.H. Ras-induced invasion and metastasis are regulated by a leukotriene B 4 receptor BLT2-linked pathway. Oncogene 2010, 29, 1167–1178. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Dürr, P.; et al. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef]
- Yamaura, M.; Mitsushita, J.; Furuta, S.; Kiniwa, Y.; Ashida, A.; Goto, Y.; Shang, W.H.; Kubodera, M.; Kato, M.; Takata, M.; et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009, 69, 2647–2654. [Google Scholar] [CrossRef]
- Maraldi, T. Natural Compounds as Modulators of NADPH Oxidases. Oxidative Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Nam, L.B.; Keum, Y.S. Binding partners of NRF2: Functions and regulatory mechanisms. Arch. Biochem. Biophys. 2019, 678, 108184. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsy, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Pearlman, R.L.; de Oca, M.K.M.; Pal, H.C.; Afaq, F. Potential therapeutic targets of epithelial–mesenchymal transition in melanoma. Cancer Lett. 2017, 391, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Hodorogea, A.; Calinescu, A.; Antohe, M.; Balaban, M.; Nedelcu, R.I.; Turcu, G.; Ion, D.A.; Badarau, I.A.; Popescu, C.M.; Popescu, R.; et al. Epithelial-Mesenchymal Transition in Skin Cancers: A Review. Anal. Cell. Pathol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Usatyuk, P.V.; Fu, P.; Mohan, V.; Epshtein, Y.; Jacobson, J.R.; Gomez-Cambronero, J.; Wary, K.K.; Bindokas, V.; Dudek, S.M.; Salgia, R.; et al. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in Hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J. Biol. Chem. 2014, 289, 13476–13491. [Google Scholar] [CrossRef]
- Ribeiro-Pereira, C.; Moraes, J.A.; Souza, M.D.J.; Laurindo, F.R.; Arruda, M.A.; Barja-Fidalgo, C. Redox modulation of FAK controls melanoma survival—Role of NOX4. PLoS ONE 2014, 9, e99481. [Google Scholar] [CrossRef]
- Dustin, C.M.; Heppner, D.E.; Lin, M.C.J.; van der Vliet, A. Redox regulation of tyrosine kinase signalling: More than meets the eye. J. Biochem. 2020, 167, 151–163. [Google Scholar] [CrossRef]
- Sun, T.; Jiao, L.; Wang, Y.; Yu, Y.; Ming, L. SIRT1 induces epithelial-mesenchymal transition by promoting autophagic degradation of E-cadherin in melanoma cells article. Cell Death Dis. 2018, 9, 1–10. [Google Scholar]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; Van Den Broek, M.; et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Beretti, F.; Anselmi, L.; Franchin, C.; Arrigoni, G.; Braglia, L.; Mandrioli, J.; Vinceti, M.; Marmiroli, S. Influence of selenium on the emergence of neuro tubule defects in a neuron-like cell line and its implications for amyotrophic lateral sclerosis. NeuroToxicology 2019, 75, 209–220. [Google Scholar] [CrossRef]
- Casciaro, F.; Beretti, F.; Zavatti, M.; McCubrey, J.A.; Ratti, S.; Marmiroli, S.; Follo, M.Y.; Maraldi, T. Nuclear Nox4 interaction with prelamin A is associated with nuclear redox control of stem cell aging. Aging 2018, 10, 2911–2934. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Guida, M.; Zavatti, M.; Resca, E.; Bertoni, L.; La Sala, G.B.; De Pol, A. Nuclear Nox4 Role in Stemness Power of Human Amniotic Fluid Stem Cells. Oxidative Med. Cell. Longev. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Guida, M.; Maraldi, T.; Resca, E.; Beretti, F.; Zavatti, M.; Bertoni, L.; La Sala, G.B.; De Pol, A. Inhibition of nuclear Nox4 activity by plumbagin: Effect on proliferative capacity in human amniotic stem cells. Oxidative Med. Cell. Longev. 2013, 2013, 680816. [Google Scholar] [CrossRef]

| M Type | Age (Years) | Breslow (mm) | Anatomic Site |
|---|---|---|---|
| NoM | 53.2 ± 13.2 | 1.1 (range 0.0 ≥ 4.0) | 10T, 6HN, 1UL, 2LL |
| MeL | 65.8 ± 12.4 | 3.2 (range 1.1 ≥ 8.0) | 4T, 2HN,1LL |
| MeM | 60.1 ± 16.3 | 3.6 (range 1.0 ≥ 50.0) | 20T, 4HN, 3UL, 5LL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretti, F.; Farnetani, F.; Reggiani Bonetti, L.; Fabbiani, L.; Zavatti, M.; Maiorana, A.; Pellacani, G.; Maraldi, T. The Interplay between HGF/c-met Axis and Nox4 in BRAF Mutated Melanoma. Int. J. Mol. Sci. 2021, 22, 761. https://doi.org/10.3390/ijms22020761
Beretti F, Farnetani F, Reggiani Bonetti L, Fabbiani L, Zavatti M, Maiorana A, Pellacani G, Maraldi T. The Interplay between HGF/c-met Axis and Nox4 in BRAF Mutated Melanoma. International Journal of Molecular Sciences. 2021; 22(2):761. https://doi.org/10.3390/ijms22020761
Chicago/Turabian StyleBeretti, Francesca, Francesca Farnetani, Luca Reggiani Bonetti, Luca Fabbiani, Manuela Zavatti, Antonino Maiorana, Giovanni Pellacani, and Tullia Maraldi. 2021. "The Interplay between HGF/c-met Axis and Nox4 in BRAF Mutated Melanoma" International Journal of Molecular Sciences 22, no. 2: 761. https://doi.org/10.3390/ijms22020761
APA StyleBeretti, F., Farnetani, F., Reggiani Bonetti, L., Fabbiani, L., Zavatti, M., Maiorana, A., Pellacani, G., & Maraldi, T. (2021). The Interplay between HGF/c-met Axis and Nox4 in BRAF Mutated Melanoma. International Journal of Molecular Sciences, 22(2), 761. https://doi.org/10.3390/ijms22020761









