Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells
Abstract
1. Introduction
2. Results
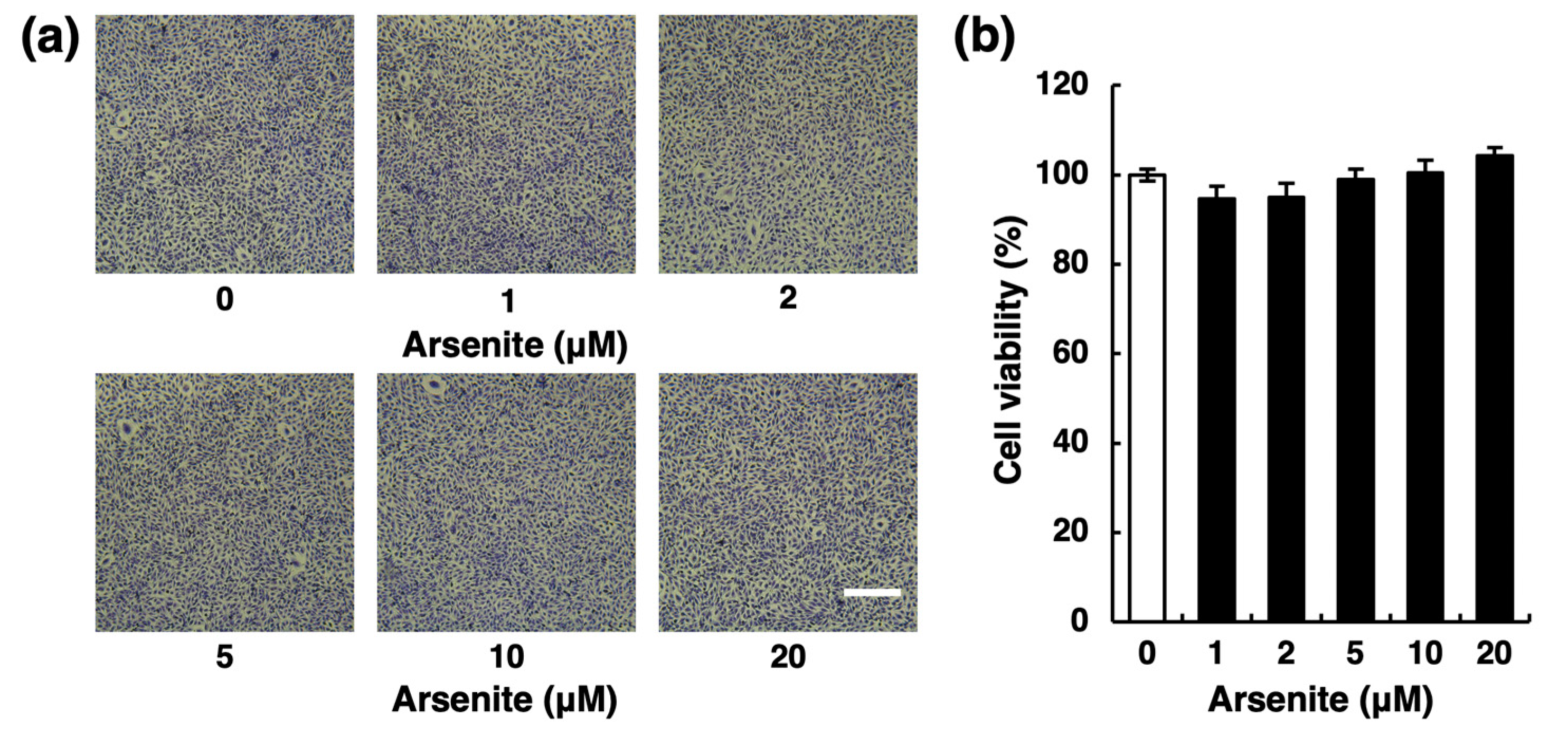
2.1. Arsenite Inhibits Fibrinolytic Activity in Endothelial EA.hy926 Cells without Inducing Nonspecific Cell Damage
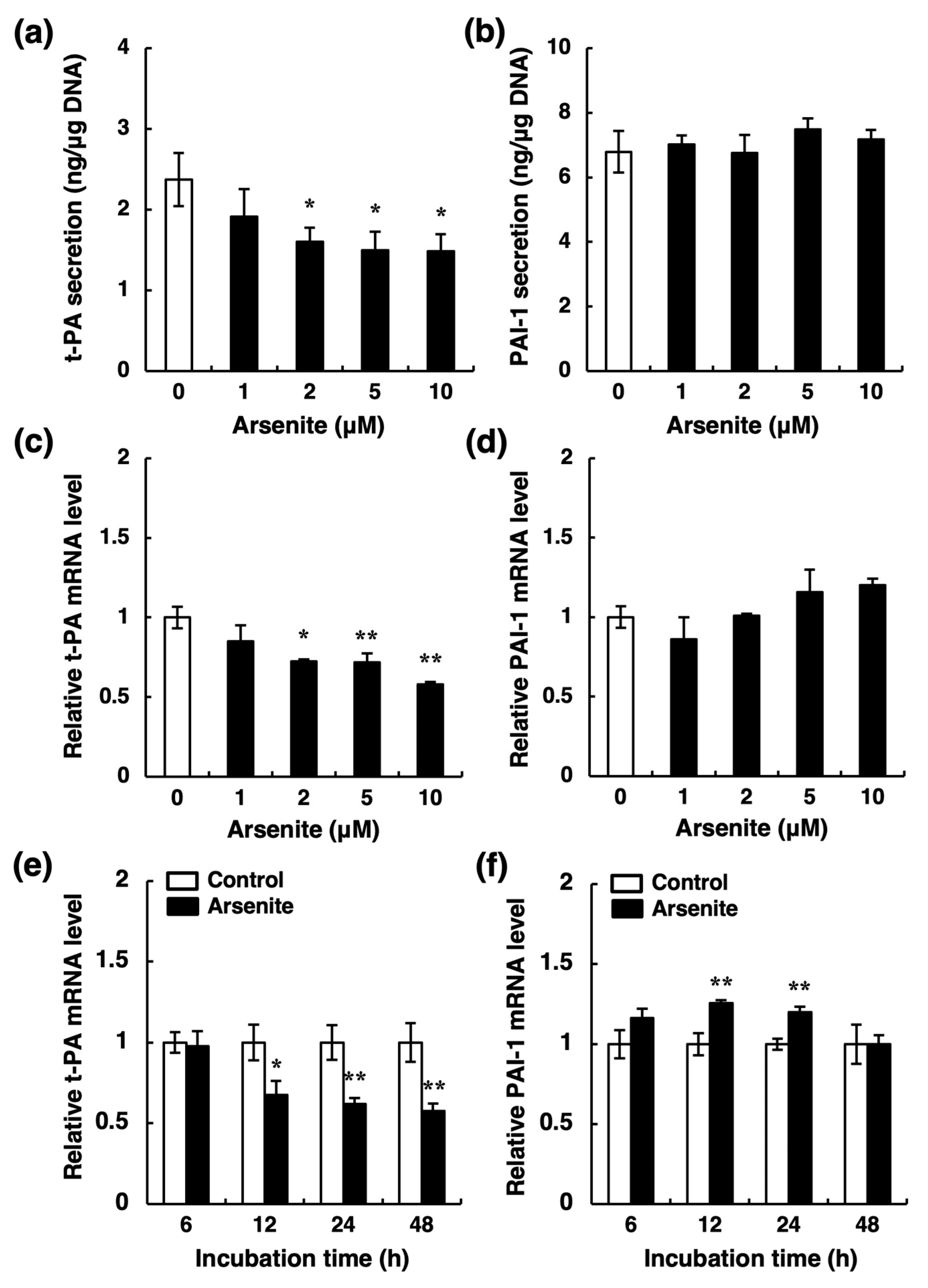
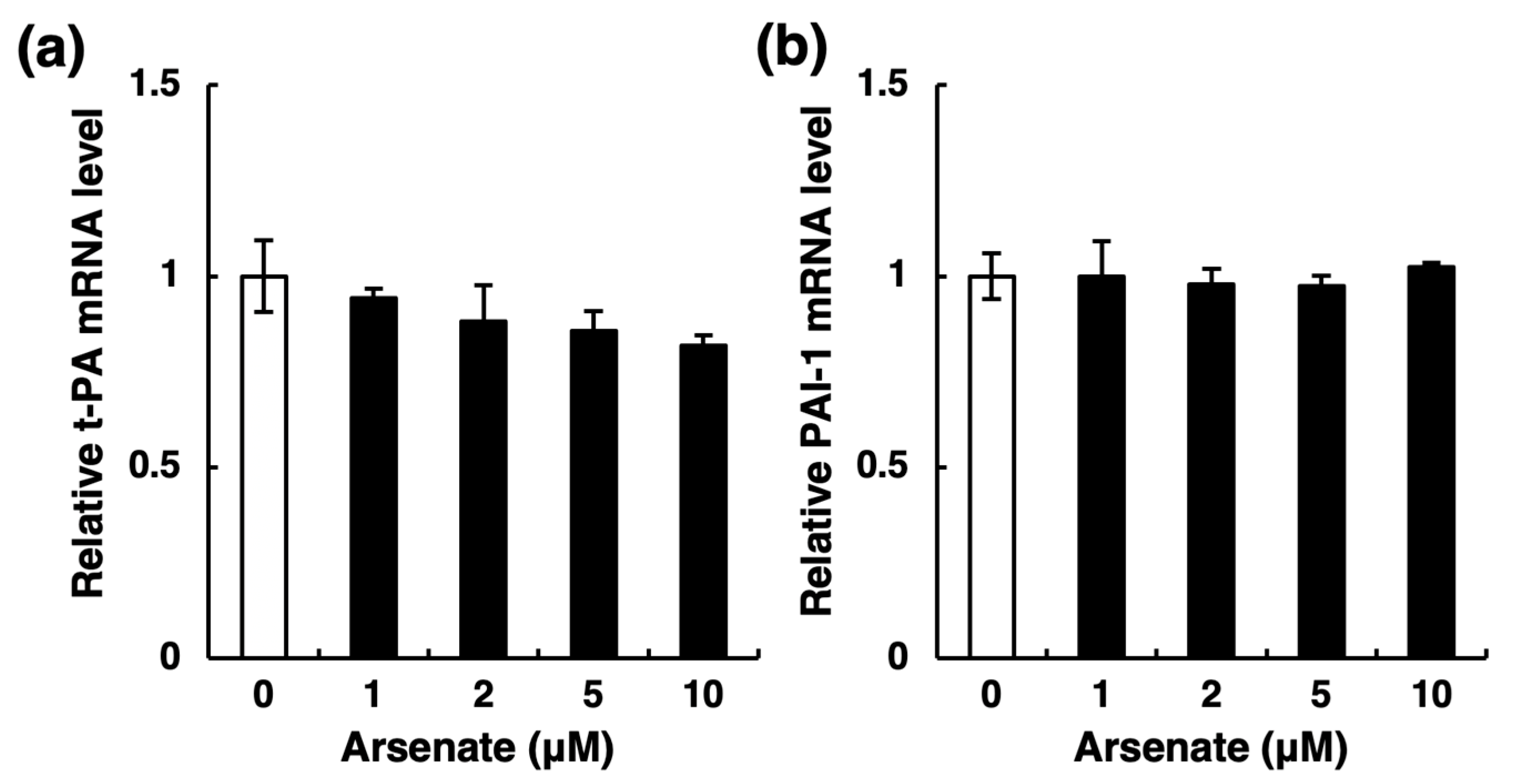
2.2. Arsenite, but Not Arsenate, Selectively Inhibits Endothelial t-PA Synthesis
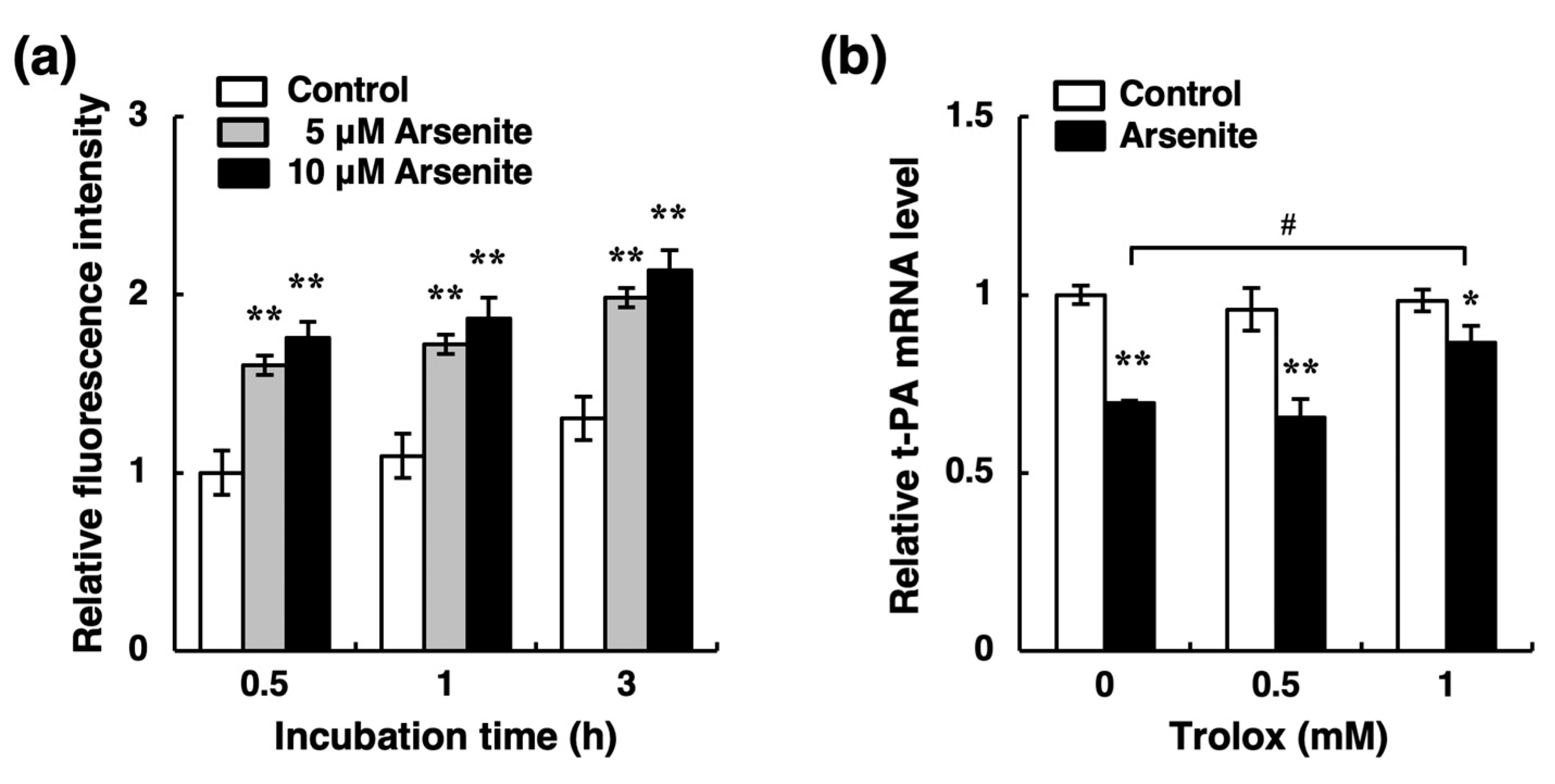
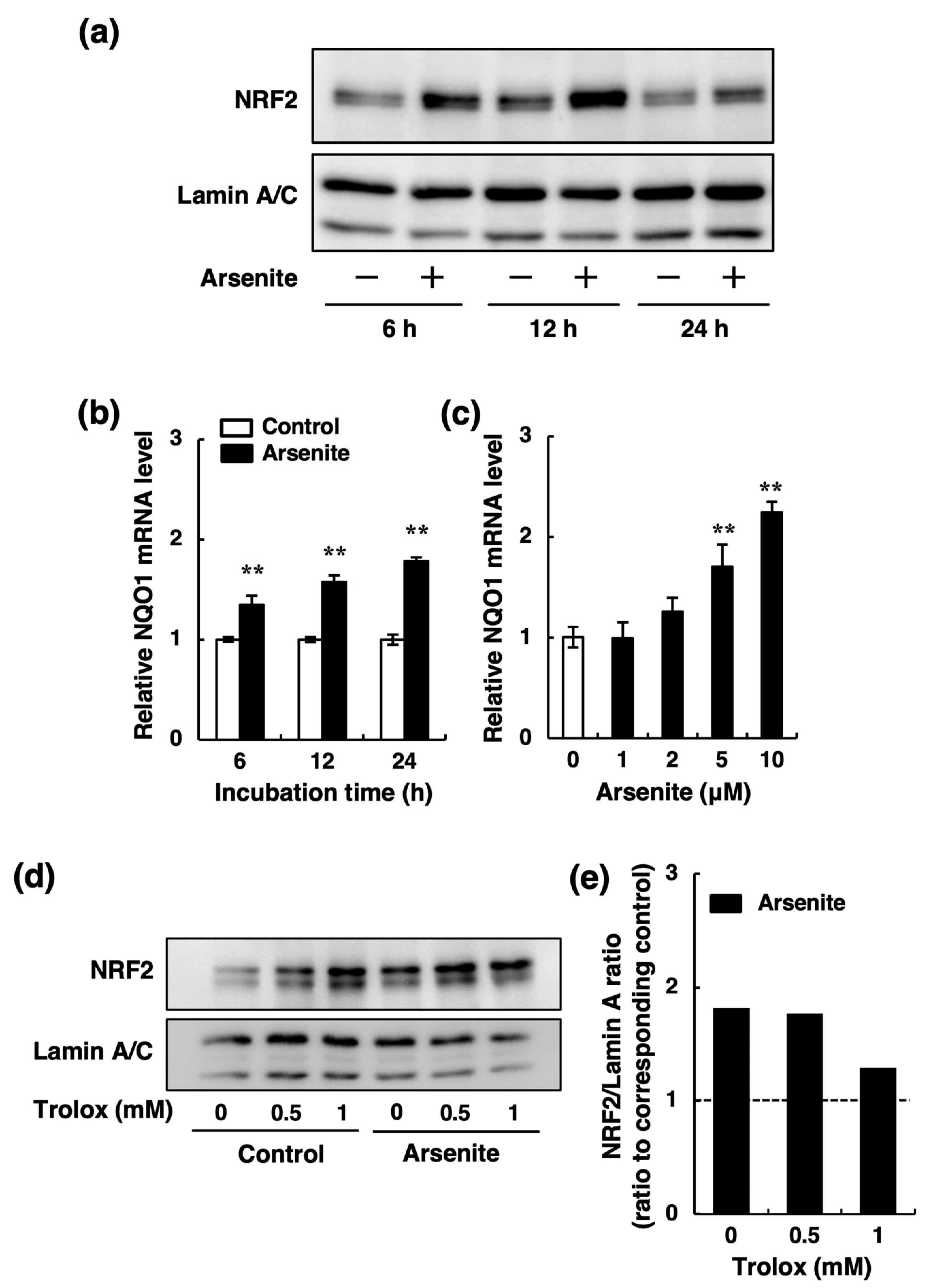
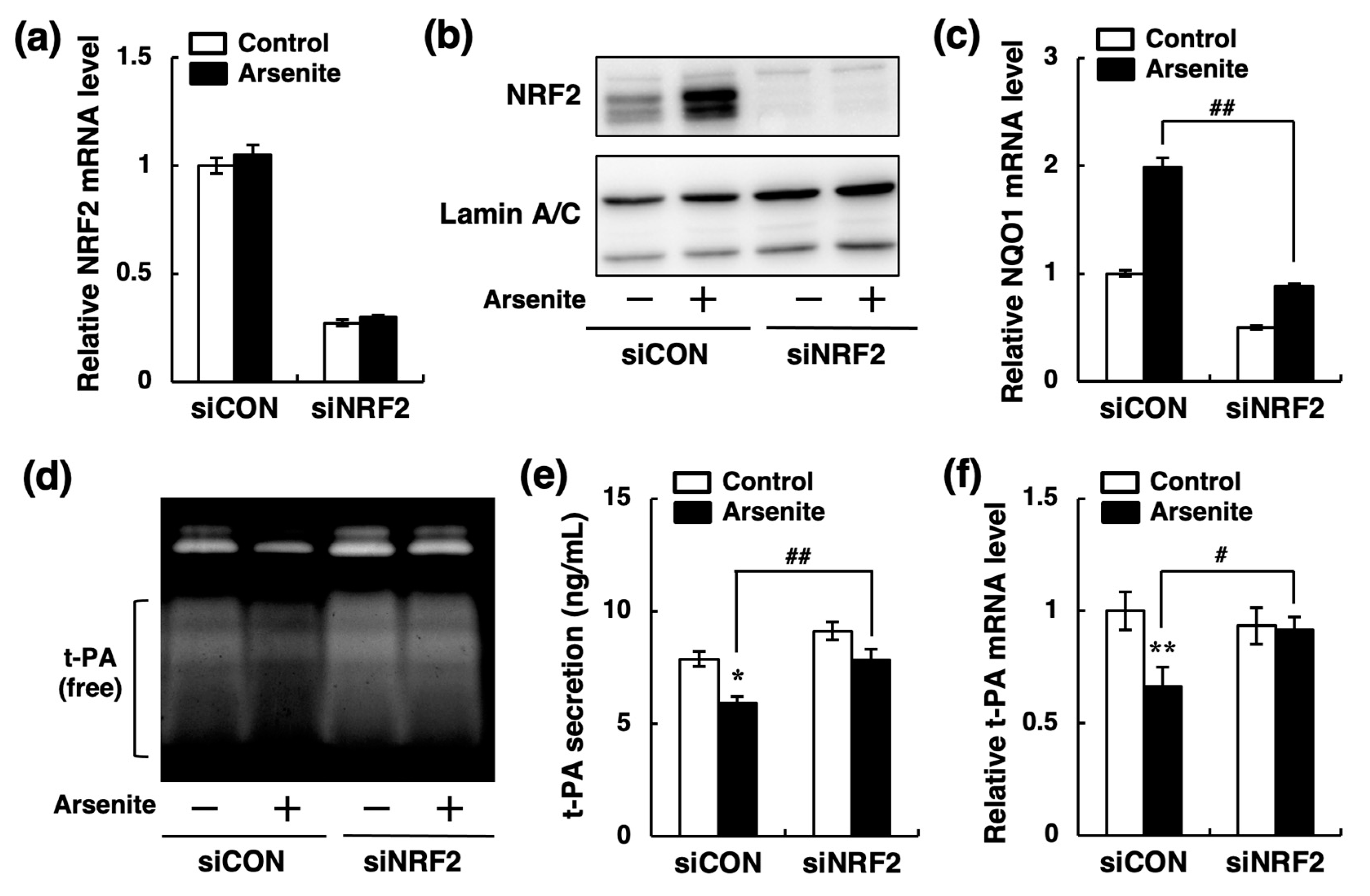
2.3. Arsenite Inhibits t-PA Synthesis via the NRF2 Pathway
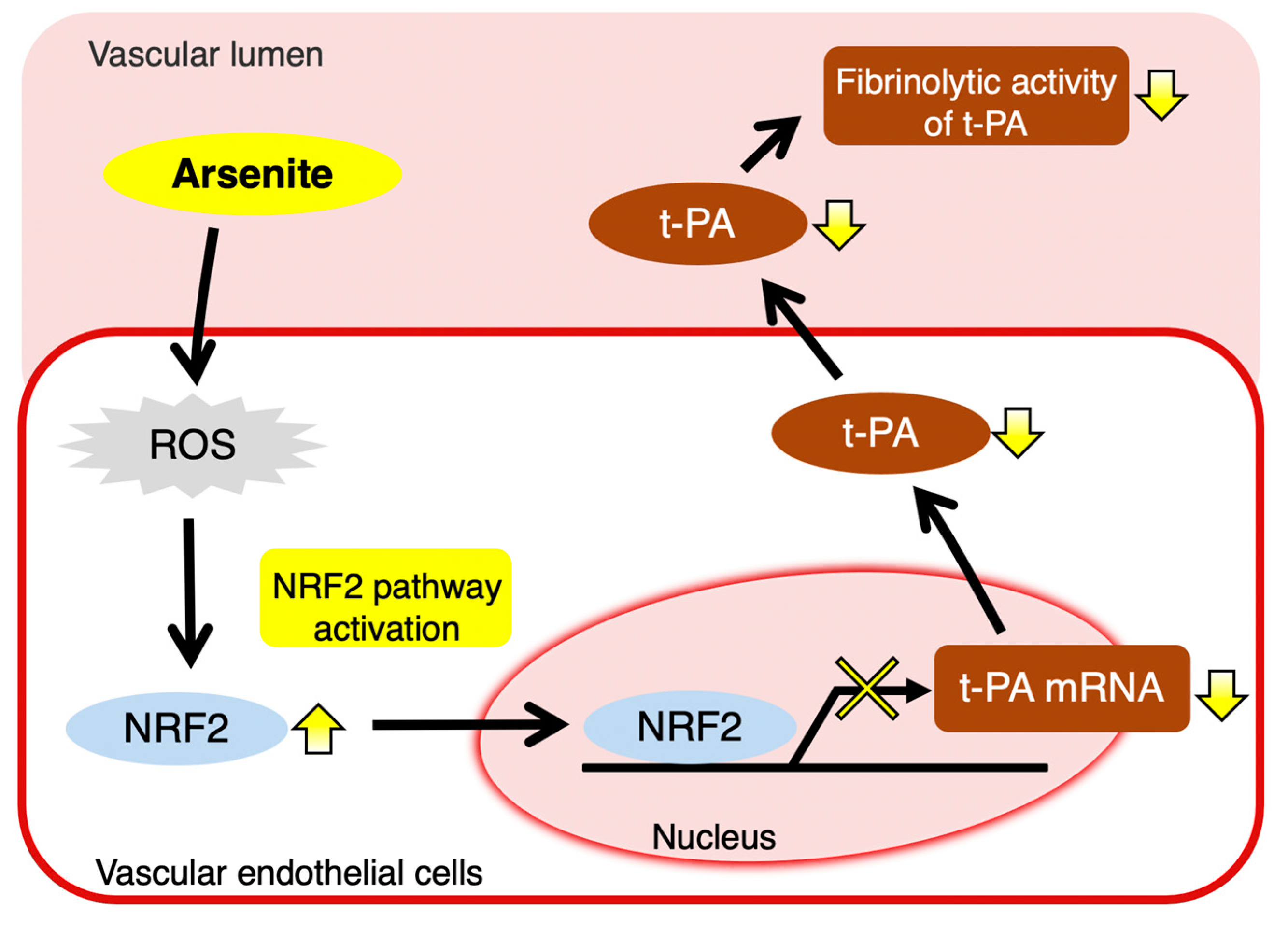
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Morphological Observation and Cell Viability Assay
4.3. Fibrin Zymography
4.4. Measurement of t-PA, PAI-1, and PGI2 Secretion
4.5. siRNA Transfection
4.6. Total RNA Isolation
4.7. Reverse Transcription (RT) and Real-Time RT-qPCR
4.8. Cellular ROS Assay
4.9. Western Blotting
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| ANOVA | analysis of variance |
| DMEM | Dulbecco’s modified Eagle’s medium |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HMECs | human microvascular endothelial cells |
| HRP | horseradish peroxidase |
| HUVECs | human umbilical vein endothelial cells |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| Nox | NADPH oxidase |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| NRF2 | nuclear factor E2 related factor 2 |
| P38 MAPK | p38 mitogen-activated protein kinase |
| PAI-1 | plasminogen activator inhibitor-1 |
| PGI2 | prostacyclin |
| ROS | reactive oxygen species |
| RT | reverse transcription |
| S.D. | standard deviation |
| SDS | sodium dodecyl sulfate |
| siRNA | small interfering RNA |
| t-PA | tissue-type plasminogen activator |
| u-PA | urokinase-type plasminogen activator |
References
- James, K.A.; Byers, T.; Hokanson, J.E.; Meliker, J.R.; Zerbe, G.O.; Marshall, J.A. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ. Health Perspect. 2015, 123, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.S.; Tingle, T.N.; O’Day, P.A.; Waychunas, G.A.; Bird, D.K. Arsenic speciation in pyrite and secondary weathering phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem. 2000, 15, 1219–1244. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.P.; Chu, H.M.; How, S.W.; Fong, J.M.; Lin, C.S.; Yeh, S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968, 40, 453–463. [Google Scholar]
- Engel, R.R.; Hopenhayn-Rich, C.; Receveur, O.; Smith, A.H. Vascular effects of chronic arsenic exposure: A review. Epidemiol. Rev. 1994, 16, 184–209. [Google Scholar] [CrossRef]
- Chen, C.J.; Hsueh, Y.M.; Lai, M.S.; Shyu, M.P.; Chen, S.Y.; Wu, M.M.; Kuo, T.L.; Tai, T.Y. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension 1995, 25, 53–60. [Google Scholar] [CrossRef]
- Kadono, T.; Inaoka, T.; Murayama, N.; Ushijima, K.; Nagano, M.; Nakamura, S.; Watanabe, C.; Tamaki, K.; Ohtsuka, R. Skin manifestations of arsenicosis in two villages in Bangladesh. Int. J. Dermatol. 2002, 41, 841–846. [Google Scholar] [CrossRef]
- Sobel, M.H.; Sanchez, T.R.; Jones, M.R.; Kaufman, J.D.; Francesconi, K.A.; Blaha, M.J.; Vaidya, D.; Shimbo, D.; Gossler, W.; Gamble, M.V.; et al. Rice Intake, Arsenic Exposure, and Subclinical Cardiovascular Disease Among US Adults in MESA. J. Am. Heart Assoc. 2020, 9, e015658. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar]
- Balakumar, P.; Kaur, J. Arsenic exposure and cardiovascular disorders: An overview. Cardiovasc. Toxicol. 2009, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Hulderman, T.; Harki, D.; Luster, M.I. Arsenic exposure accelerates atherogenesis in apolipoprotein E(-/-) mice. Environ. Health Perspect. 2003, 111, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Vladykovskaya, E.N.; Haberzettl, P.; Sithu, S.D.; D’Souza, S.E.; States, J.C. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE-/- mice. Toxicol. Appl. Pharmacol. 2009, 241, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Negro Silva, L.F.; Lemaire, M.; Lemarie, C.A.; Plourde, D.; Bolt, A.M.; Chiavatti, C.; Bohle, D.S.; Slavkovich, V.; Graziano, J.H.; Lehoux, S.; et al. Effects of Inorganic Arsenic, Methylated Arsenicals, and Arsenobetaine on Atherosclerosis in the Mouse Model and the Role of As3mt-Mediated Methylation. Environ. Health Perspect. 2017, 125, 077001. [Google Scholar] [CrossRef]
- Makhani, K.; Chiavatti, C.; Plourde, D.; Negro Silva, L.F.; Lemaire, M.; Lemarie, C.A.; Lehoux, S.; Mann, K.K. Using the Apolipoprotein E Knock-Out Mouse Model to Define Atherosclerotic Plaque Changes Induced by Low Dose Arsenic. Toxicol. Sci. 2018, 166, 213–218. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sueishi, K. The coagulation and fibrinolysis systems and atherosclerosis. Lab. Investig. 1993, 69, 5–18. [Google Scholar]
- Sueishi, K.; Ichikawa, K.; Kato, K.; Nakagawa, K.; Chen, Y.X. Atherosclerosis: Coagulation and fibrinolysis. Semin. Thromb. Hemost. 1998, 24, 255–260. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Collen, D. On the role of coagulation and fibrinolysis in atherosclerosis. Ann. Epidemiol. 1992, 2, 427–438. [Google Scholar] [CrossRef]
- Flute, P.T. Coagulation and fibrinolysis after injury. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 1970, 4, 102–109. [Google Scholar] [CrossRef][Green Version]
- Levin, E.G.; Loskutoff, D.J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J. Cell Biol. 1982, 94, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Collen, D. Ham-Wasserman lecture: Role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematol. Am. Soc. Hematol. Educ. Program 2001, 1–9. [Google Scholar] [CrossRef]
- van Mourik, J.A.; Lawrence, D.A.; Loskutoff, D.J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J. Biol. Chem. 1984, 259, 14914–14921. [Google Scholar] [CrossRef]
- Gelehrter, T.D.; Sznycer-Laszuk, R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J. Clin. Investig. 1986, 77, 165–169. [Google Scholar] [CrossRef]
- Heaton, J.H.; Dame, M.K.; Gelehrter, T.D. Thrombin induction of plasminogen activator inhibitor mRNA in human umbilical vein endothelial cells in culture. J. Lab. Clin. Med. 1992, 120, 222–228. [Google Scholar] [PubMed]
- Wu, H.L.; Yang, W.H.; Wang, M.Y.; Shi, G.Y. Impaired fibrinolysis in patients with Blackfoot disease. Thromb. Res. 1993, 72, 211–218. [Google Scholar] [CrossRef]
- Jiang, S.J.; Lin, T.M.; Wu, H.L.; Han, H.S.; Shi, G.Y. Decrease of fibrinolytic activity in human endothelial cells by arsenite. Thromb. Res. 2002, 105, 55–62. [Google Scholar] [CrossRef]
- Francis, R.B., Jr.; Neely, S. Inhibition of endothelial secretion of tissue-type plasminogen activator and its rapid inhibitor by agents which increase intracellular cyclic AMP. Biochim. Biophys. Acta 1989, 1012, 207–213. [Google Scholar] [CrossRef]
- Kooistra, T.; Bosma, P.J.; Toet, K.; Cohen, L.H.; Griffioen, M.; van den Berg, E.; le Clercq, L.; van Hinsbergh, V.W. Role of protein kinase C and cyclic adenosine monophosphate in the regulation of tissue-type plasminogen activator, plasminogen activator inhibitor-1, and platelet-derived growth factor mRNA levels in human endothelial cells. Possible involvement of proto-oncogenes c-jun and c-fos. Arterioscler. Thromb. 1991, 11, 1042–1052. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef]
- Cui, Y.; Han, Z.; Hu, Y.; Song, G.; Hao, C.; Xia, H.; Ma, X. MicroRNA-181b and microRNA-9 mediate arsenic-induced angiogenesis via NRP1. J. Cell. Physiol. 2012, 227, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Wang, X.; Chang, Q.; Hitron, A.; Zhang, Z.; Xu, M.; Chen, G.; Luo, J.; Jiang, B.; Fang, J.; et al. Arsenic promotes angiogenesis in vitro via a heme oxygenase-1-dependent mechanism. Toxicol. Appl. Pharmacol. 2010, 244, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nakano, T.; Katoh, G.; Shinoda, Y.; Yamamoto, C.; Yoshida, E.; Kaji, T.; Fujiwara, Y. Nuclear factor erythroid 2-related factor 2 (NRF2) is a negative regulator of tissue plasminogen activator synthesis in cultured human vascular endothelial EA.hy926 cells. J. Toxicol. Sci. 2020, 45, 237–243. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Sumi, D. Arsenic: Signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Aono, J.; Yanagawa, T.; Itoh, K.; Li, B.; Yoshida, H.; Kumagai, Y.; Yamamoto, M.; Ishii, T. Activation of Nrf2 and accumulation of ubiquitinated A170 by arsenic in osteoblasts. Biochem. Biophys. Res. Commun. 2003, 305, 271–277. [Google Scholar] [CrossRef]
- Pi, J.; Qu, W.; Reece, J.M.; Kumagai, Y.; Waalkes, M.P. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: Involvement of hydrogen peroxide. Exp. Cell Res. 2003, 290, 234–245. [Google Scholar] [CrossRef]
- Messier, E.M.; Bahmed, K.; Tuder, R.M.; Chu, H.W.; Bowler, R.P.; Kosmider, B. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type II cells from injury by cigarette smoke. Cell Death Dis. 2013, 4, e573. [Google Scholar] [CrossRef]
- Fu, X.; Chen, D.; Ma, Y.; Yuan, W.; Zhu, L. Bovine Herpesvirus 1 Productive Infection Led to Inactivation of Nrf2 Signaling through Diverse Approaches. Oxid. Med. Cell. Longev. 2019, 2019, 4957878. [Google Scholar] [CrossRef]
- Ellinsworth, D.C. Arsenic, reactive oxygen, and endothelial dysfunction. J. Pharmacol. Exp. Ther. 2015, 353, 458–464. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Miller, D.S.; Saavedra, J.E.; Keefer, L.K.; Johnson, D.R.; Klaassen, C.D.; Waalkes, M.P. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol. Pharmacol. 2001, 60, 302–309. [Google Scholar] [CrossRef]
- Shinkai, Y.; Sumi, D.; Fukami, I.; Ishii, T.; Kumagai, Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006, 580, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Jun, J.; Thimmulappa, R.; Bedja, D.; Antero, M.; Gabrielson, K.L.; Polotsky, V.Y.; Biswal, S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE 2008, 3, e3791. [Google Scholar] [CrossRef] [PubMed]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Wang, X.; Castellani, L.W.; Reue, K.; Lusis, A.J.; et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, G.; Shi, X.; Zhou, H.; Liu, M.; Chen, Y.; Feng, D.; Zhang, P.; Wu, L.; Lv, X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018, 500, 790–796. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008, 102, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Thurston, G.; Hochman, J.S.; Lamas, G.A. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Navas-Acien, A.; Mark, D.B.; Lee, K.L. Heavy Metals, Cardiovascular Disease, and the Unexpected Benefits of Chelation Therapy. J. Am. Coll. Cardiol. 2016, 67, 2411–2418. [Google Scholar] [CrossRef]
- Kaji, T.; Yamamoto, C.; Sakamoto, M.; Kozuka, H. Inhibitory effect of lead on the release of tissue plasminogen activator from human vascular endothelial cells in culture. Toxicology 1992, 73, 219–227. [Google Scholar] [CrossRef]
- Yamamoto, C.; Kaji, T.; Sakamoto, M.; Kozuka, H. Cadmium stimulation of plasminogen activator inhibitor-1 release from human vascular endothelial cells in culture. Toxicology 1993, 83, 215–223. [Google Scholar] [CrossRef]
- Shinkai, Y.; Kaji, T. Cellular defense mechanisms against lead toxicity in the vascular system. Biol. Pharm. Bull. 2012, 35, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Saku, T.; Furthmayr, H. Characterization of the major heparan sulfate proteoglycan secreted by bovine aortic endothelial cells in culture. Homology to the large molecular weight molecule of basement membranes. J. Biol. Chem. 1989, 264, 3514–3523. [Google Scholar] [CrossRef]
- Kaji, T.; Yamada, A.; Miyajima, S.; Yamamoto, C.; Fujiwara, Y.; Wight, T.N.; Kinsella, M.G. Cell density-dependent regulation of proteoglycan synthesis by transforming growth factor-beta(1) in cultured bovine aortic endothelial cells. J. Biol. Chem. 2000, 275, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; Cassiman, J.J.; Van den Berghe, H.; Vermylen, J.; David, G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 1992, 267, 20435–20443. [Google Scholar] [CrossRef]
- Whinna, H.C.; Choi, H.U.; Rosenberg, L.C.; Church, F.C. Interaction of heparin cofactor II with biglycan and decorin. J. Biol. Chem. 1993, 268, 3920–3924. [Google Scholar] [CrossRef]
- Wu, D.P.; Nakano, T.; Tsuneoka, Y.; Takahashi, T.; Shinoda, Y.; Kaji, T.; Fujiwara, Y. Arsenite inhibits gene expression of perlecan, syndecan-1, -2, -3 and biglycan in cultured vascular endothelial cells. Fundam. Toxicol. Sci. 2020, 7, 77–83. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, Y.; Yamamoto, C.; Hirooka, T.; Terada, N.; Satoh, M.; Kaji, T. Arsenite but not arsenate inhibits general proteoglycan synthesis in cultured arterial smooth muscle cells. J. Toxicol. Sci. 2008, 33, 487–492. [Google Scholar] [CrossRef]
- Kissane, J.M.; Robins, E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J. Biol. Chem. 1958, 233, 184–188. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, S.; Misawa, S.; Akimoto, J.; Shinoda, Y.; Fujiwara, Y. Photodynamic therapy using talaporfin sodium induces heme oxygenase-1 expression in rat malignant meningioma KMY-J cells. J. Toxicol. Sci. 2018, 43, 353–358. [Google Scholar] [CrossRef]

| Gene Name (Protein Name) | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH (GAPDH) | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA | 138 |
| NFE2L2 (NRF2) | GGTTCCAAGTCCAGAAGCCA | GGTTGGGGTCTTCTGTGGAG | 158 |
| NQO1 (NQO1) | TCGTCTGTATTCCCACTTCCTTC | AGCATCTACTTCATCAGCACCATC | 109 |
| PLAT (t-PA) | AGCGAGCCAAGGTGTTTCAA | CTTCCCAGCAAATCCTTCGGG | 93 |
| PLAU (u-PA) | CCAAAATGCTGTGTGCTGCT | CCCCAGCTCACAATTCCAGT | 121 |
| SERPINE1 (PAI-1) | CTGGCCCTTGTCTTTGGTGA | GGGTGAGAAAACCACGTTGC | 138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, T.; Takahashi, T.; Yamamoto, C.; Yoshida, E.; Kaji, T.; Fujiwara, Y. Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells. Int. J. Mol. Sci. 2021, 22, 739. https://doi.org/10.3390/ijms22020739
Nakano T, Takahashi T, Yamamoto C, Yoshida E, Kaji T, Fujiwara Y. Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells. International Journal of Molecular Sciences. 2021; 22(2):739. https://doi.org/10.3390/ijms22020739
Chicago/Turabian StyleNakano, Tsuyoshi, Tsutomu Takahashi, Chika Yamamoto, Eiko Yoshida, Toshiyuki Kaji, and Yasuyuki Fujiwara. 2021. "Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells" International Journal of Molecular Sciences 22, no. 2: 739. https://doi.org/10.3390/ijms22020739
APA StyleNakano, T., Takahashi, T., Yamamoto, C., Yoshida, E., Kaji, T., & Fujiwara, Y. (2021). Arsenite Inhibits Tissue-Type Plasminogen Activator Synthesis through NRF2 Activation in Cultured Human Vascular Endothelial EA.hy926 Cells. International Journal of Molecular Sciences, 22(2), 739. https://doi.org/10.3390/ijms22020739







