Evaluation of the Multimycotoxin-Degrading Efficiency of Rhodococcus erythropolis NI1 Strain with the Three-Step Zebrafish Microinjection Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Toxicity Effects of NI1 Bacterial Metabolites
2.2. Toxicity Effects of (AFB1, ZEN, T-2 in Individual and in Combination, and Their NI1 Degradation Products on Zebrafish Embryos
2.2.1. AFB1 Treatment
2.2.2. ZEN Treatment
2.2.3. T-2 Treatment
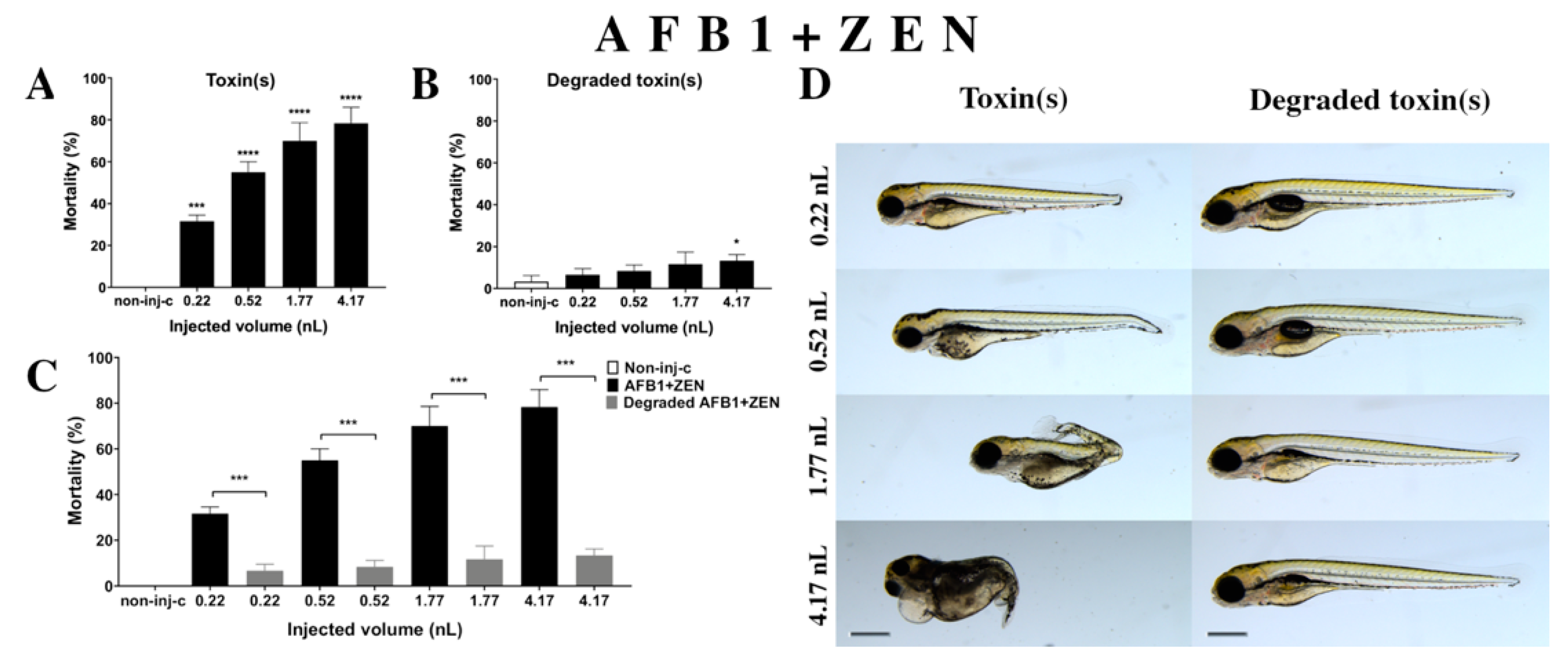
2.2.4. AFB1 and ZEN (AFB1+ZEN) Mixture Treatment
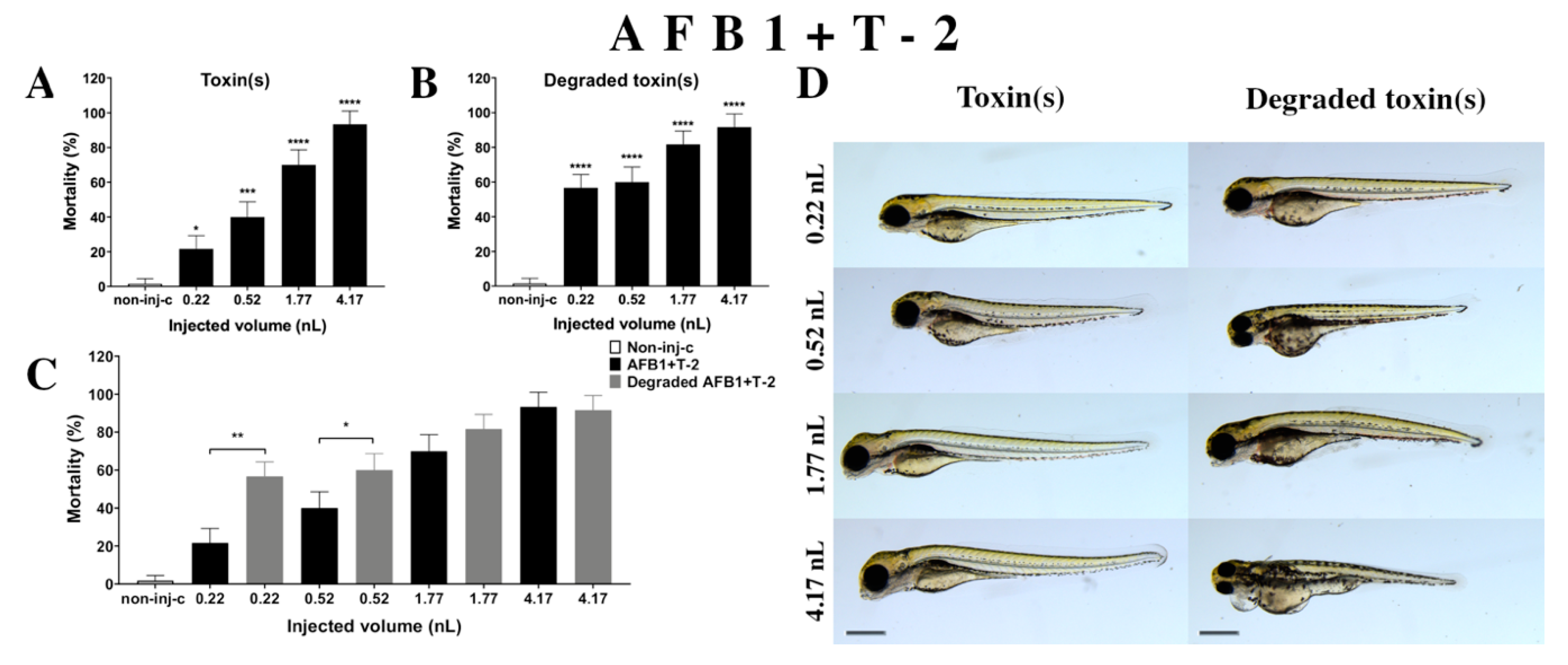
2.2.5. AFB1 and T-2 (AFB1+T-2) Mixture Treatment
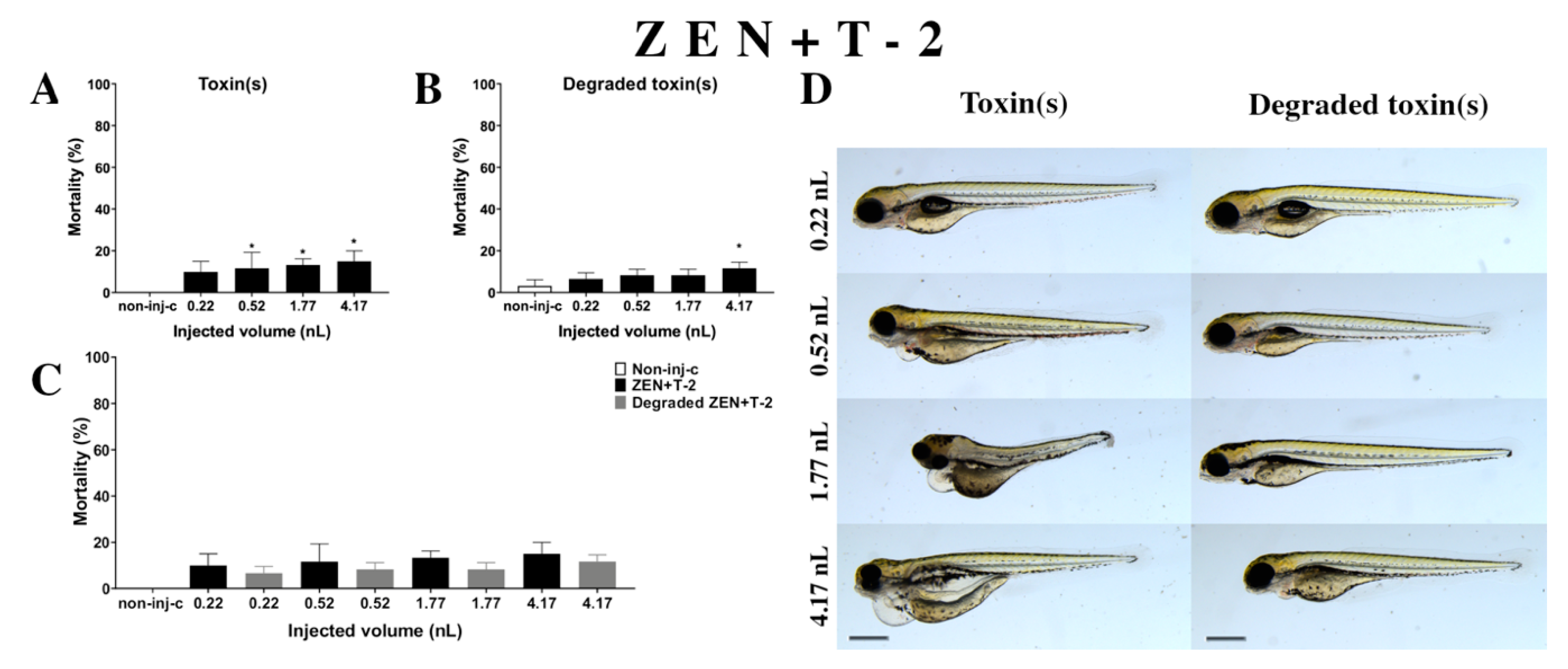
2.2.6. ZEN and T-2 (ZEN+T-2) Mixture Treatment
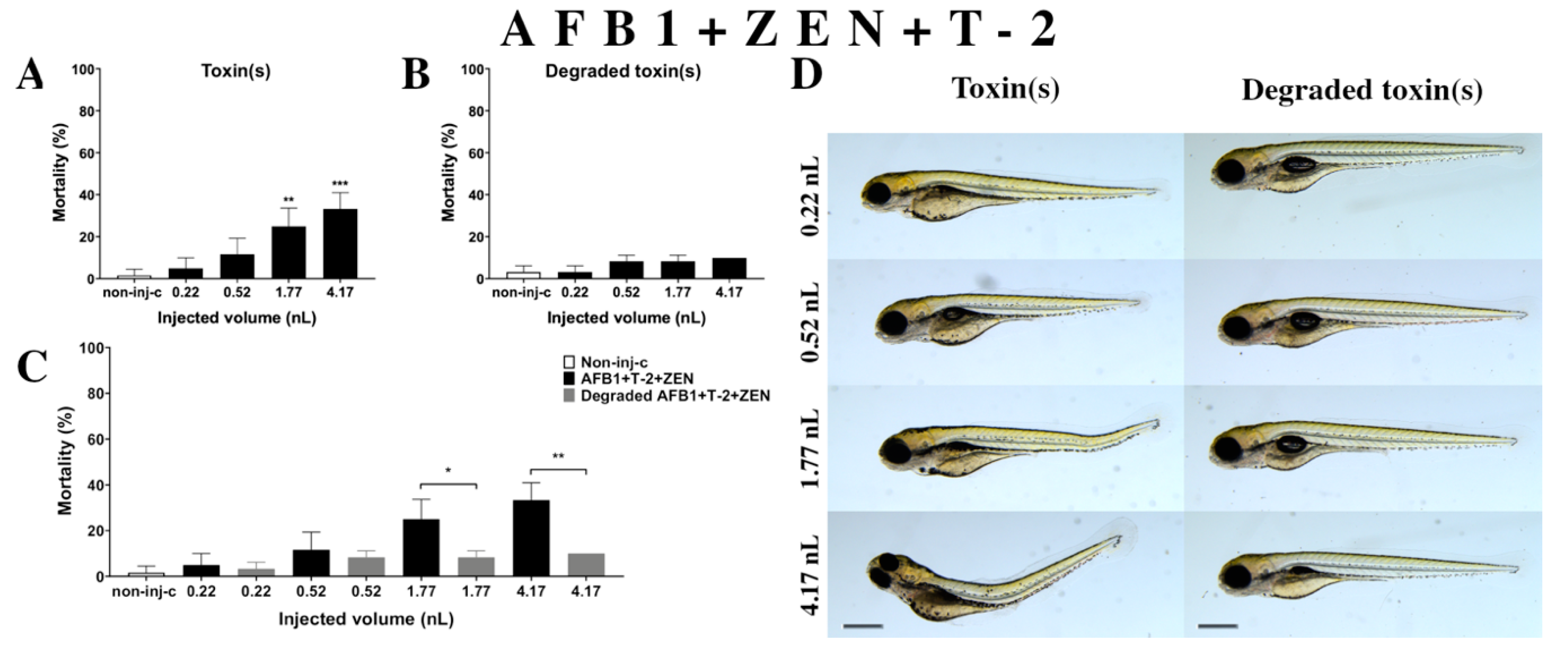
2.2.7. AFB1 and ZEN and T-2 (AFB1+ZEN+T-2) Mixture Treatment
2.3. Analytical Results
2.4. Evaluation of Interactions for Combined Mycotoxins
3. Discussion
4. Materials and Methods
4.1. Animal Protection
4.2. Mycotoxin and Mixture Degradation Experiments
4.3. Measurement of Mycotoxin Concentrations
4.4. Zebrafish Maintenance and Egg Collection
4.5. Microinjection
4.6. Toxicological Endpoints
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marroquín-Cardona, A.; Johnson, N.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.D.; Marin, D.E.; Taranu, I.; Tabuc, C.; Nicolau, A.I.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- CAST. Mycotoxins: Risks in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2003; ISBN 1-887383-22-0. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Toxicology of mycotoxins. EXS 2010, 100, 31–63. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies, critical reviews. Crit. Rev. Food Sci. Nutr. 2016, 57, 3489–3507. [Google Scholar] [CrossRef]
- Aksoy, A.; Yavuz, O.; Kursad Das, Y.; Guvenc, D.; Muglali, O.H. Occurrence of aflatoxin B1, T-2 toxin and zearalenone in compound animal feed. J. Anim. Vet. Adv. 2009, 8, 403–407. [Google Scholar]
- Chlebicz, A.; Śliżewska, K. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 toxin and zearalenone by probiotic bacteria from genus Lactobacillus and Saccharomyces cerevisiae yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S. Aflatoxin. J. Am. Oil Chem. Soc. 1989, 66, 1398–1413. [Google Scholar]
- Hoerr, F.J.; D’Andrea, G.H. Biological effects of aflatoxin in swine. In Aflatoxin and Aspergillus flavus in Corn; Diener, U.L., Asquith, R.L., Dickens, J.W., Eds.; Southern Cooperative Series Bulletin 279; Auburn University: Auburn, AL, USA, 1983; pp. 51–55. [Google Scholar]
- Miller, D.M.; Stuart, B.P.; Crowell, W.A. Experimental aflatoxicosis in swine: Morphological and clinical pathological results. Can. J. Comp. Med. 1981, 45, 343–351. [Google Scholar]
- Bodine, A.B.; Mertens, D.R. Toxicology, metabolism, and physiological effects of aflatoxin in the bovine. In Aflatoxin and As-pergillus flavus in Corn; Diener, U.L., Asquith, R.L., Dickens, J.W., Eds.; Southern Cooperative Series Bulletin 279; Auburn Universi-ty: Auburn, AL, USA, 1983; pp. 46–50. [Google Scholar]
- Edds, G.T.; Bortell, R.A. Biological effects of aflatoxin: Poultry. In Aflatoxin and Aspergillus flavus in Corn; Diener, U.L., Asquith, R.L., Dickens, J.W., Eds.; Southern Cooperative Series Bulletin 279; Auburn University: Auburn, AL, USA, 1983; pp. 56–61. [Google Scholar]
- Dvorak, R.; Jagos, J.; Bouda, J.; Piskac, A.; Zapletal, O. Changes in the clinico-biochemical indices in the rumirid juice and urine in cases of experimental aflatoxicosis in dairy cows. Vet. Med. 1977, 22, 161–169. (In Czech) [Google Scholar]
- Ngindu, A.; Kenya, P.; Ocheng, D.; Omondi, T.; Ngare, W.; Gatei, D.; Johnson, B.; Ngira, J.; Nandwa, H.; Jansen, A.; et al. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet 1982, 319, 1346–1348. [Google Scholar] [CrossRef]
- Kurtz, H.J.; Mirocha, C.J. Zearalenone (F2) induced estrogenic syndrome in swine. In Mycotoxic Fungi, Mycotoxins, Mycotoxi-cosis; Wyllie, T.D., Morehouse, L.G., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1978; Volume 2, pp. 369–393. [Google Scholar]
- Saenz de Rodriguez, C.A.; Bongiovanni, A.M.; Conde de Borrego, L. An epidemic of precocious development in Puerto Rican children. J. Pediatr. 1985, 107, 393–396. [Google Scholar] [CrossRef]
- Hsieh, D.P.H. Potential human health hazards of mycotoxins. In Mycotoxins and Phytotoxins; Natori, S., Hashimoto, K., Ueno, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 69–80. [Google Scholar]
- Szuets, P.; Mesterhazy, A.; Falkay, G.; Bartok, T. Early thelarche symptoms in children and their relations to zearalenone contamination in foodstuffs. Cereal Res. Comm. 1997, 25, 429–436. [Google Scholar] [CrossRef]
- Hsu, I.-C.; Smalley, E.B.; Strong, F.M.; Ribelin, W.E. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl. Microbiol. 1972, 24, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Moosavi, M. Review on T-2 toxin. Jundishapur J. Natl. Pharm. Prod. 2010, 5, 26–38. [Google Scholar]
- European Commision. Opinion of the Scientific Committee on Food on Fusarium Toxins Part 5: T-2 Toxin and HT-2 Toxin; European Commission: Brussels, Belgium, 2001. [Google Scholar]
- International Programme of Chemical Safety. Selected Mycotoxins: Ochratoxins, Trichothecenes, Ergot; World Health Organization: Geneva, Switzerland, 1990; ISBN 92-4-157105-5. [Google Scholar]
- Yuan, G.; Wang, Y.-M.; Yuan, X.; Zhang, T.; Zhao, J.; Huang, L.; Peng, S. T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J. Environ. Sci. 2014, 26, 917–925. [Google Scholar] [CrossRef]
- Boonchuvit, B.; Hamilton, P.B.; Burmeister, H.R. Interaction of T-2 Toxin with Salmonella infections of chickens. Poult. Sci. 1975, 54, 1693–1696. [Google Scholar] [CrossRef]
- Kanai, K.; Kondo, E. Decreased resistance to mycobacterial infection in mice fed a trichothecene compound (T-2 toxin). Jpn. J. Med. Sci. Biol. 1984, 37, 97–104. [Google Scholar] [CrossRef]
- Yarom, R.; Sherman, Y.; More, R.; Ginsburg, I.; Borinski, R.; Yagen, B. T-2 toxin effect on bacterial infection and leukocyte functions. Toxicol. Appl. Pharmacol. 1984, 75, 60–68. [Google Scholar] [CrossRef]
- Jagadeesan, V.; Rukmini, C.; Vijayaraghavan, M.; Tulpule, P. Immune studies with T-2 toxin: Effect of feeding and withdrawal in monkeys. Food Chem. Toxicol. 1982, 20, 83–87. [Google Scholar] [CrossRef]
- Beardall, J.M.; Miller, J.D. Diseases in humans with mycotoxins as possible causes. In Mycotoxins in Grain: Compounds Other than Aflatoxin; Miller, J.D., Trenholm, H.L., Eds.; Eagan Press: St. Paul, MN, USA, 1994; p. 552. ISBN 0962440752. [Google Scholar]
- Bhat, R.; Ramakrishna, Y.; Beedu, S.; Munshi, K. Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat products in Kashmir Valley, India. Lancet 1989, 333, 35–37. [Google Scholar] [CrossRef]
- Joffe, A.Z. Toxicity of Fusarium Poae and F. sporotrichioides and its relation to alimentary toxic aleukia. In Mycotoxins; Purchase, I.F.H., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 229–262. [Google Scholar]
- Assunção, R.; Silva, M.J.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef]
- Mckean, C.; Tang, L.; Billam, M.; Tang, M.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.-S. Comparative acute and combinative toxicity of aflatoxin B1 and T-2 toxin in animals and immortalized human cell lines. J. Appl. Toxicol. 2006, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Oswald, I.P. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Binder, E.M. Managing the risk of mycotoxins in modern feed production. Anim. Feed Sci. Technol. 2007, 133, 149–166. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Bata, Á.; Lásztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar] [CrossRef]
- Clements, M.J.; White, D.G. Identifying sources of resistance to aflatoxin and fumonisin contamination in corn grain. J. Toxicol. Toxin Rev. 2004, 23, 381–396. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.-F.; Doré, T.; Rossignol, L. Influence of cropping system on Fusarium head blight and mycotoxin levels in winter wheat. Crop. Prot. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Bhattacharya, S.; Palaniswamy, M.; Angayarkanni, J. Biodegradation of aflatoxin B1 in contaminated rice straw by Pleurotus ostreatus MTCC 142 and Pleurotus ostreatus GHBBF10 in the presence of metal salts and surfactants. World J. Microbiol. Biotechnol. 2014, 30, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.; Gelderblom, W.; Botha, A.; Van Zyl, W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Matthies, I.; Woerfel, G.; Karlovsky, P. Induction of a zearalenone degrading enzyme caused by the substrate and its derivatives. Mycotoxin Res. 2001, 17, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Kakeya, H.; Takahashi-Ando, N.; Kimura, M.; Onose, R.; Yamaguchi, I.; Osada, H. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 2002, 66, 2723–2726. [Google Scholar] [CrossRef]
- Ueno, Y.; Nakayama, K.; Ishii, K.; Tashiro, F.; Minoda, Y.; Omori, T.; Komagata, K. Metabolism of T-2 toxin in Curtobacterium sp. strain 114-2. Appl. Environ. Microbiol. 1983, 46, 120–127. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Cimmarusti, M.T.; Mirabelli, V.; Haidukowski, M.; Logrieco, A.; Caliandro, R.; Mulè, G. In vitro single and combined mycotoxins degradation by Ery4 laccase from Pleurotus eryngii and redox mediators. Food Control 2018, 90, 401–406. [Google Scholar] [CrossRef]
- Kiessling, K.H.; Pettersson, H.; Sandholm, K.; Olsen, M. Metabolism of aflatoxin, ochratoxin, zearalenone, and trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984, 47, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Cserháti, M.; Kriszt, B.; Krifaton, C.; Szoboszlay, S.; Háhn, J.; Tóth, S.; Nagy, I.; Kukolya, J. Mycotoxin-degradation profile of Rhodococcus strains. Int. J. Food Microbiol. 2013, 166, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Garai, E.; Risa, A.; Varga, E.; Cserháti, M.; Kriszt, B.; Urbányi, B.; Csenki-Bakos, Z. Qualifying the T-2 toxin-degrading properties of seven microbes with zebrafish embryo microinjection method. Toxins 2020, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.; Fremy, J.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef]
- European Food Safety Authority. Statement on the establishment of guidelines for the assessment of additives from the functional group’ substances for reduction of the contamination of feed by mycotoxins. EFSA J. 2010, 8, 1693. [Google Scholar] [CrossRef]
- Csenki-Bakos, Z.; Garai, E.; Risa, A.; Cserháti, M.; Bakos, K.; Márton, D.; Bokor, Z.; Kriszt, B.; Urbányi, B. Biological evaluation of microbial toxin degradation by microinjected zebrafish (Danio rerio) embryos. Chemosphere 2019, 227, 151–161. [Google Scholar] [CrossRef]
- Zuberi, Z.; Eeza, M.N.H.; Matysik, J.; Berry, J.P.; Alia, A. NMR-based metabolic profiles of intact zebrafish embryos exposed to aflatoxin B1 recapitulates hepatotoxicity and supports possible neurotoxicity. Toxins 2019, 11, 258. [Google Scholar] [CrossRef]
- Juan-García, A.; Bind, M.-A.; Engert, F. Larval zebrafish as an in vitro model for evaluating toxicological effects of mycotoxins. Ecotoxicol. Environ. Saf. 2020, 202, 110909. [Google Scholar] [CrossRef]
- Wu, T.-S.; Cheng, Y.-C.; Chen, P.-J.; Huang, Y.-T.; Yu, F.-Y.; Liu, B.-H. Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere 2019, 217, 905–913. [Google Scholar] [CrossRef]
- Bakos, K.; Kovács, R.; Staszny, Á.; Kánainé Sipos, D.; Urbányi, B.; Müller, F.; Csenki, Z.; Kovács, B. Developmental toxicity and estrogenic potency of zearalenone in zebrafish (Danio rerio). Aquat. Toxicol. 2013, 136, 13–21. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neuro-toxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; George, S.; Li, C.; Gurusamy, S.; Sun, X.; Gong, Z.; Qian, H. Combined toxicity of prevalent mycotoxins studied in fish cell line and zebrafish larvae revealed that type of interactions is dose-dependent. Aquat. Toxicol. 2017, 193, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Kipper, M.; Hauschild, L.; Lehnen, C.R.; Remus, A.; Melchior, R. Meta-analysis of individual and combined effects of mycotoxins on growing pigs. Sci. Agricola 2016, 73, 328–331. [Google Scholar] [CrossRef]
- Jia, R.; Ma, Q.; Fan, Y.; Ji, C.; Zhang, J.; Liu, T.; Zhao, L. The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality and mycotoxins residues in eggs of layers and the protective effect of Bacillus subtilis biodegradation product. Food Chem. Toxicol. 2016, 90, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zheng, N.; Fan, C.; Cheng, J.; Wang, S.; Jabar, A.; Wang, J.; Cheng, J. Effects of aflatoxin B1 combined with ochratoxin A and/or zearalenone on metabolism, immune function, and antioxidant status in lactating dairy goats. Asian Australas. J. Anim. Sci. 2017, 31, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Gao, X.; Li, C.; Krumm, C.S.; Qi, D.S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon 2015, 95, 6–12. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, N.; Qi, D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013, 65, 1149–1157. [Google Scholar] [CrossRef]
- Signorini, M.L.; Gaggiotti, M.; Molineri, A.; Chiericatti, C.A.; Zapata de Basĺlico, M.L.; Basílico, J.C.; Pisani, M. Exposure as-sessment of mycotoxins in cow’s milk in Argentina. Food Chem. Toxicol. 2012, 50, 250–257. [Google Scholar] [CrossRef]
- Sirot, V.; Fremy, J.-M.; Leblanc, J.-C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Sprong, C.R.; De Wit-Bos, L.; Zeilmaker, M.; Alewijn, M.; Castenmiller, J.; Mengelers, M. A mycotoxin-dedicated total diet study in the Netherlands in 2013: Part I—Design. World Mycotoxin J. 2016, 9, 73–88. [Google Scholar] [CrossRef]
- Vargas, E.A.; Preis, R.A.; Castro, L.; Silva, C.M.G. Co-occurrence of aflatoxins B1, B2, G1, G2, zearalenone and fumonisin B1 in Brazilian corn. Food Addit. Contam. 2001, 18, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Scudamore, K.A.; Hetmanski, M.T.; Chan, H.K.; Collins, S. Occurrence of mycotoxins in raw ingredients used for animal feeding stuffs in the United Kingdom in 1992. Food Addit. Contam. 1997, 14, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; Vries, I.; Oerke, E.C. Fusarium species and my-cotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Griessler, K.; Rodrigues, I.; Handl, J.; Hofstetter, U. Occurrence of mycotoxins in Southern Europe. World Mycotoxin J. 2010, 3, 301–309. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E.; López de Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in barley from a northern region of Spain. Food Chem. 2012, 132, 35–42. [Google Scholar]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef]
- Njumbe Ediage, E.; Diana Di Mavungu, J.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.A.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Rubert, J.; Soriano, J.M.; Mañes, J.; Soler, C. Rapid mycotoxin analysis in human urine: A pilot study. Food Chem. Toxicol. 2011, 49, 2299–2304. [Google Scholar] [CrossRef]
- Girish, C.K.; Degewoda, C. Efficacy of glucomannan-containing yeast product (Mycosorb®) and hydrated sodium calcium aluminosilicate in preventing the individual and combined toxicity of aflatoxin and T-2 toxin in commercial broilers. Asian Australas. J. Anim. Sci. 2006, 19, 877–883. [Google Scholar]
- Harvey, R.; Kubena, L.F.; Huff, W.E.; Corrier, D.E.; Rottinghaus, G.E.; Phillips, T.D. Effects of treatment of growing swine with aflatoxin and T-2 toxin. Am. J. Vet. Res. 1990, 51, 1688–1693. [Google Scholar] [PubMed]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Doerr, J.A. Mycotoxin interactions in poultry and swine. J. Anim. Sci. 1987, 66, 2351–2355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubena, L.; Harvey, R.; Huff, W.E.; Corrier, D.E.; Phillips, T.; Rottinghaus, G.E. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T-2 toxin. Poult. Sci. 1990, 69, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, R.; Balachandran, C.; Manohar, B.M. Effect of feeding aflatoxin and T-2 toxin on the growth rate and haematology of Japanese quail. Indian Vet. J. 2005, 82, 597–600. [Google Scholar]
- Raju, M.; Devegowda, G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br. Poult. Sci. 2000, 41, 640–650. [Google Scholar] [PubMed]
- Tamimi, S.O.; Natour, R.M.; Halabi, K.S. Individual and combined effects of chronic T-2 toxin and aflatoxin B1 mycotoxins on rat lives and kidney. Arab Gulf J. Sci. Res. 1997, 15, 717–732. [Google Scholar]
- Rajom, R.; Sedmíková, M.; Jílek, M.; Koubková, M.; Hartlová, H.; Bárta, I.; Śmerák, P. Combined effects of repeated low doses of aflatoxin B1 and T-2 toxin on the Chinese hamster. Vet. Med. 2001, 46, 301–307. [Google Scholar]
- García-Moraleja, A.; Font, G.; Mańes, J.; Ferrer, E. Analysis of mycotoxins in coffee and risk assessment in Spanish adolescents and adults. Food Chem. Toxicol. 2015, 86, 225–233. [Google Scholar]
- Monbaliu, S.; van Poucke, C.; Detavernier, C.; Dumoulin, F.; Van De Velde, M.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of mycotoxins in feed as analyzed by a multi-mycotoxin LC-MS/MS method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef]
- Grajewski, J.; Błajet-Kosicka, A.; Twarużek, M.; Kosicki, R. Occurrence of mycotoxins in Polish animal feed in years 2006–2009. J. Anim. Physiol. Anim. Nutr. 2012, 96, 870–877. [Google Scholar] [CrossRef]
- Bouaziz, C.; Bouslimi, A.; Kadri, R.; Zaied, C.; Bacha, H.; Abid-Essefi, S. The in vitro effects of zearalenone and T-2 toxins on Vero cells. Exp. Toxicol. Pathol. 2013, 65, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Ficheux, A.; Sibiril, Y.; Parent-Massin, D. Co-exposure of Fusarium mycotoxins: In vitro myelotoxicity assessment on human hematopoietic progenitors. Toxicon 2012, 60, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Tajima, O.; Schoen, E.D.; Feron, V.; Groten, J. Statistically designed experiments in a tiered approach to screen mixtures of Fusarium mycotoxins for possible interactions. Food Chem. Toxicol. 2002, 40, 685–695. [Google Scholar] [CrossRef]
- De Boevre, M.; Jacxsens, L.; Lachat, C.; Eeckhout, M.; Di Mavungu, J.D.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; De Meulenaer, B.; et al. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292. [Google Scholar] [CrossRef]
- Labuda, R.; Parich, A.; Berthiller, F.; Tancinová, D. Incidence of trichothecenes and zearalenone in poultry feed mixtures from Slovakia. Int. J. Food Microbiol. 2005, 105, 19–25. [Google Scholar] [CrossRef]
- Rafai, P.; Bata, A.; Vanyi, A.; Papp, Z.; Brydl, E.; Jakab, L.; Tuboly, S.; Tury, E. Effect of various levels of T-2 toxin on the clinical status, performance and metabolism of growing pigs. Vet. Rec. 1995, 136, 485–489. [Google Scholar] [CrossRef]
- Abia, W.A.; Warth, B.; Michael, S.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.-M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Speijers, G.; Speijers, M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- Šegvić Klarić, M. Štetni učinci kombiniranih mikotoksina [Adverse effects of combined mycotoxins]. Arch. Hig. Toksicol. 2012, 63, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. The mass-action law based algorithms for quantitative econo-green bio-research. Integr. Biol. 2011, 3, 548–559. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug com-bination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Khezri, A.; Herranz-Jusdado, J.; Ropstad, E.; Fraser, T. Mycotoxins induce developmental toxicity and behavioral aberrations in zebrafish larvae. Environ. Pollut. 2018, 242, 500–506. [Google Scholar] [CrossRef]
- Biomin. BIOMIN Mycotoxin Survey Q3 2020 Results. Available online: https://www.biomin.net/science-hub/biomin-mycotoxin-survey-q3-2020-results/ (accessed on 23 December 2019).
- Lyagin, I.; Efremenko, E. Enzymes for detoxification of various mycotoxins: Origins and mechanisms of catalytic action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef]
- Risa, A.; Divinyi, D.M.; Baka, E.; Krifaton, C. Aflatoxin B1 detoxification by cell-free extracts of Rhodococcus strains. Acta Microbiol. Immunol. Hung. 2017, 64, 423–438. [Google Scholar] [CrossRef]
- Risa, A. Bacterial Detoxification of Aflatoxin B1 and Zearalenon with Living Cells and Cell-Free Extracts. Ph.D. Dissertation, Szent István University, Gödöllő, Hungary, 2019. [Google Scholar]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations and for General Dose-Effect Analysis, and User’s Guide a Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and The Determination of IC50, ED50, and LD50 Values; CompuSyn, Inc.: Paramus, NJ, USA, 2005; Available online: http://www.combosyn.com/feature.html (accessed on 7 August 2019).
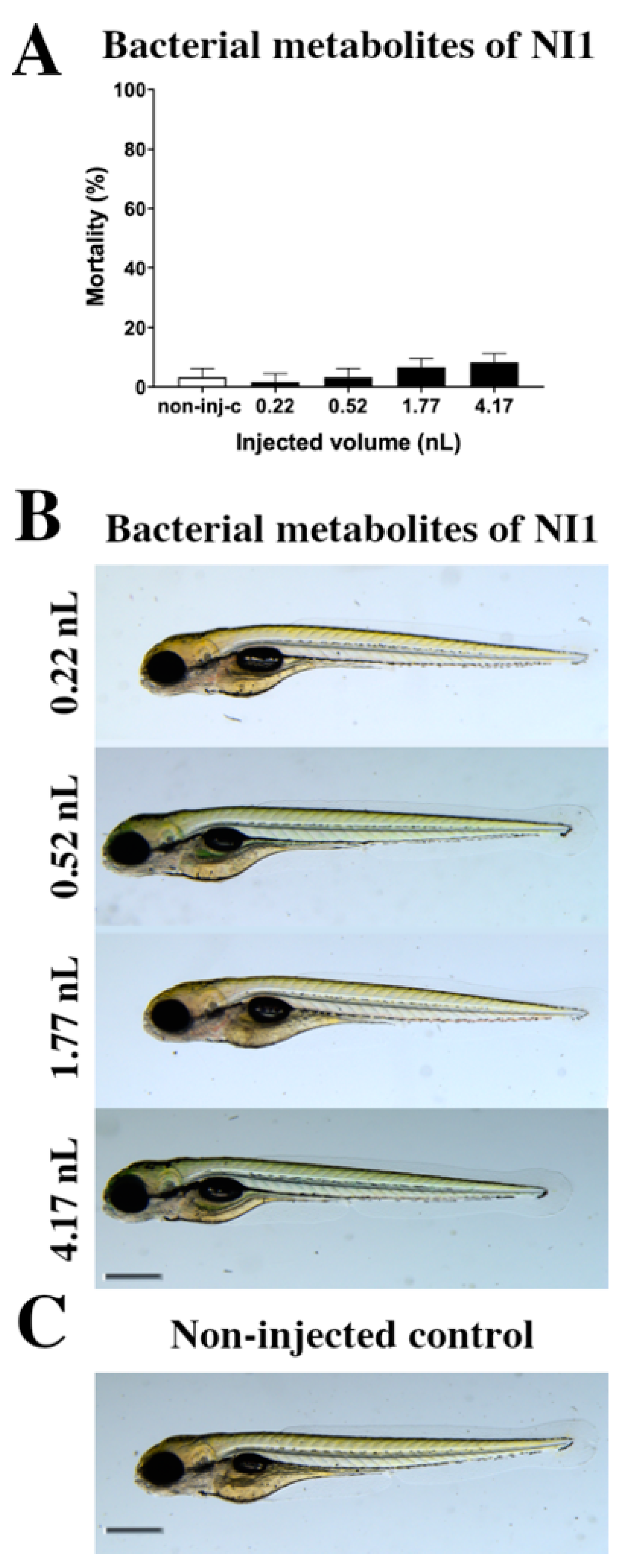
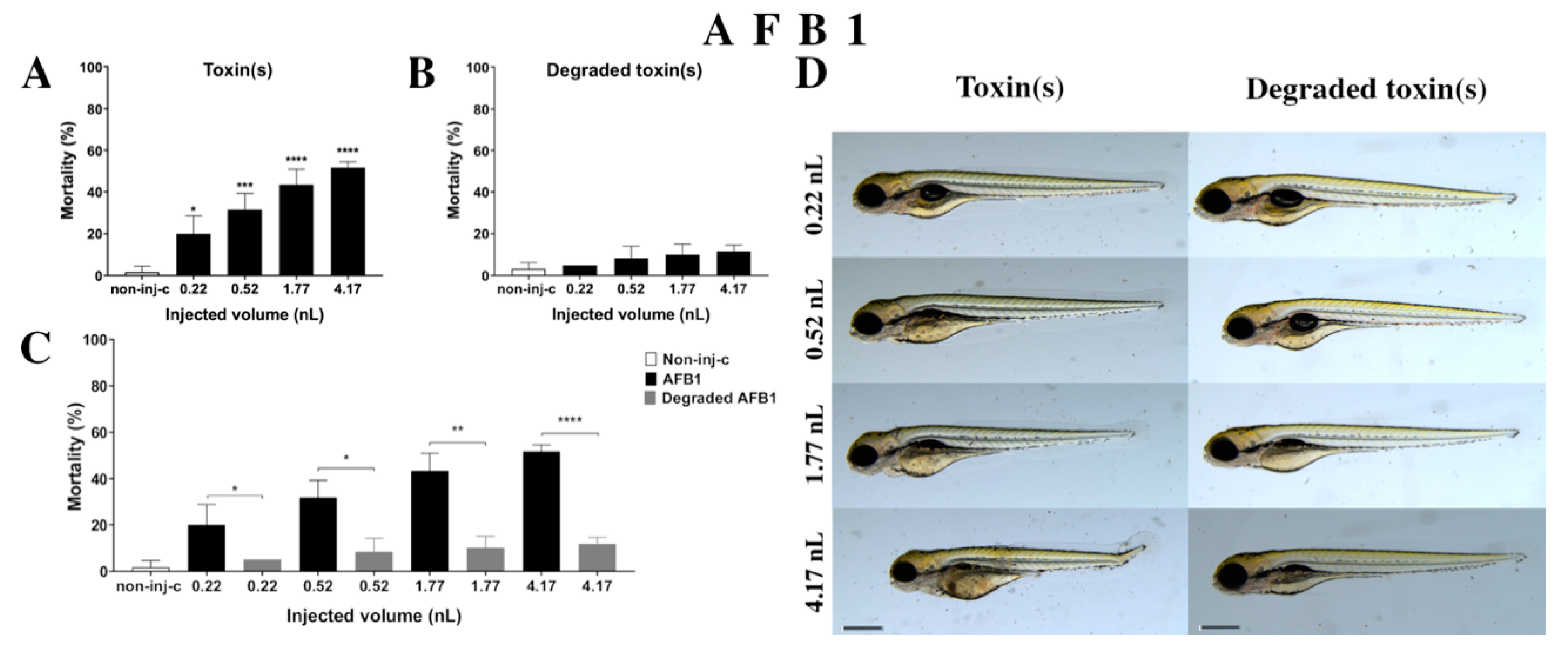
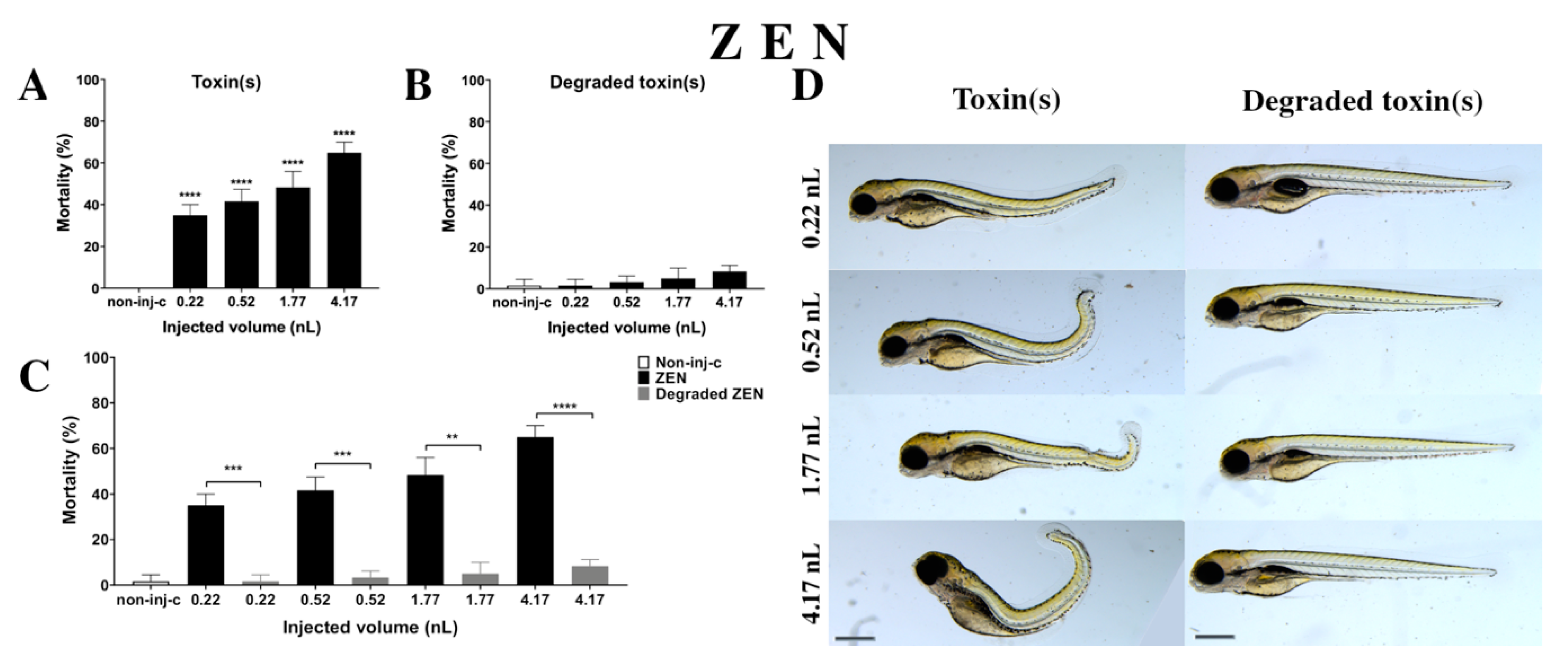
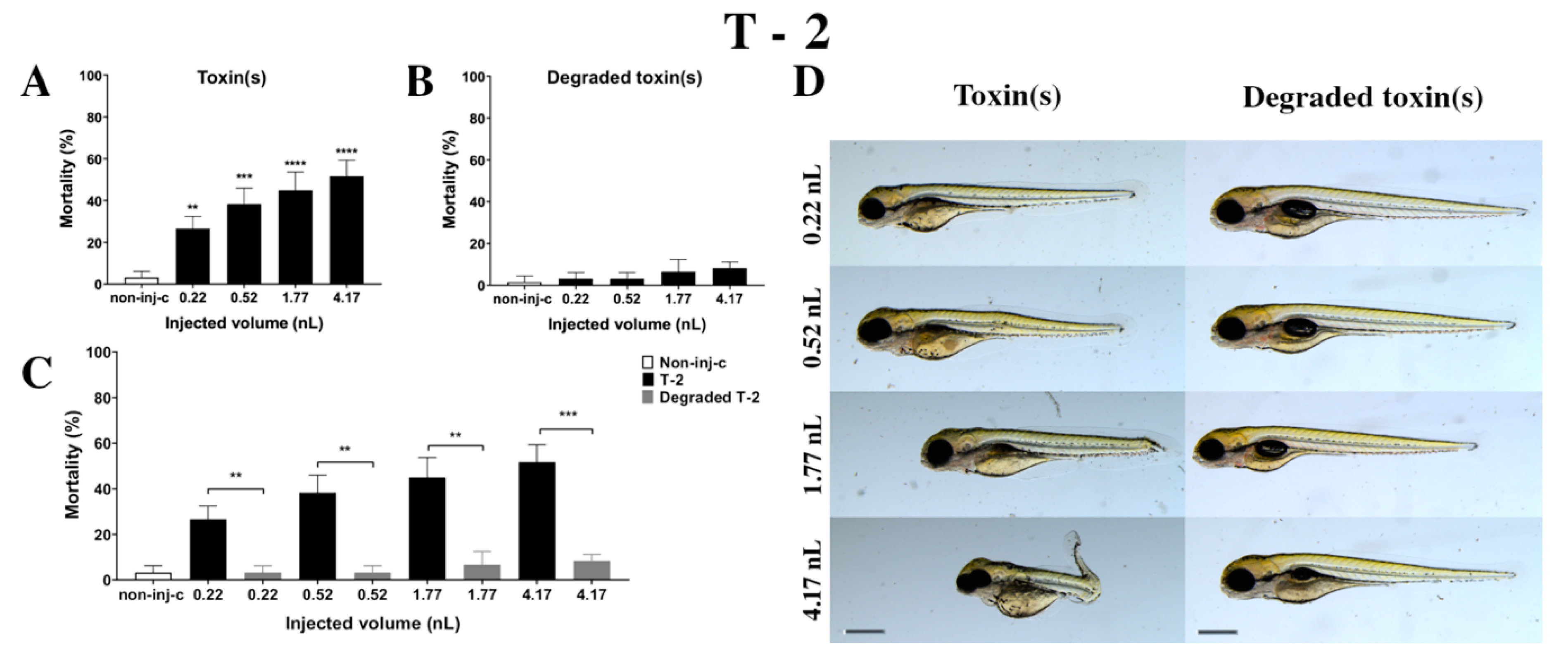

| Samples | Before Degradation (ng ± SD) | After Degradation (ng ± SD) | Degradation Efficiency (%) | |
|---|---|---|---|---|
| Supernatants | Pellet | |||
| AFB1 | 52,735 ± 4424 | 136.4 ± 38.2 | 15.07 ± 2.55 | 99.69 |
| ZEN | 24,040 ± 1164 | 3154 ± 105 | 425.7 ± 130.3 | 84.76 |
| T-2 | 70,123 ± 4291 | <LOD | <LOD | 100.00 |
| AFB1+ZEN | 61,684 ± 1819 | 71.30 ± 4.42 | 13.77 ± 4.29 | 99.82 |
| 29,273 ± 3301 | 1566 ± 99 | 244.7 ± 40.0 | 94.01 | |
| AFB1+T-2 | 52,735 ± 6326 | 56.58 ± 2.84 | 10.51 ± 2.27 | 99.84 |
| 55,239 ± 6326 | <LOD | <LOD | 100.00 | |
| ZEN+T-2 | 21,367 ± 4699 | 997.3 ± 97.6 | 76.02 ± 64.33 | 91.36 |
| 55,239 ± 3634 | <LOD | <LOD | 100.00 | |
| AFB1+ZEN+T-2 | 47,684 ± 2184 | 67.46 ± 10.55 | <LOD | 99.84 |
| 23,493 ± 1938 | 673.5 ± 280.6 | <LOD | 95.69 | |
| 65,446 ± 1178 | <LOD | <LOD | 100.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garai, E.; Risa, A.; Varga, E.; Cserháti, M.; Kriszt, B.; Urbányi, B.; Csenki, Z. Evaluation of the Multimycotoxin-Degrading Efficiency of Rhodococcus erythropolis NI1 Strain with the Three-Step Zebrafish Microinjection Method. Int. J. Mol. Sci. 2021, 22, 724. https://doi.org/10.3390/ijms22020724
Garai E, Risa A, Varga E, Cserháti M, Kriszt B, Urbányi B, Csenki Z. Evaluation of the Multimycotoxin-Degrading Efficiency of Rhodococcus erythropolis NI1 Strain with the Three-Step Zebrafish Microinjection Method. International Journal of Molecular Sciences. 2021; 22(2):724. https://doi.org/10.3390/ijms22020724
Chicago/Turabian StyleGarai, Edina, Anita Risa, Emese Varga, Mátyás Cserháti, Balázs Kriszt, Béla Urbányi, and Zsolt Csenki. 2021. "Evaluation of the Multimycotoxin-Degrading Efficiency of Rhodococcus erythropolis NI1 Strain with the Three-Step Zebrafish Microinjection Method" International Journal of Molecular Sciences 22, no. 2: 724. https://doi.org/10.3390/ijms22020724
APA StyleGarai, E., Risa, A., Varga, E., Cserháti, M., Kriszt, B., Urbányi, B., & Csenki, Z. (2021). Evaluation of the Multimycotoxin-Degrading Efficiency of Rhodococcus erythropolis NI1 Strain with the Three-Step Zebrafish Microinjection Method. International Journal of Molecular Sciences, 22(2), 724. https://doi.org/10.3390/ijms22020724




