Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation
Abstract
1. Introduction
2. Results
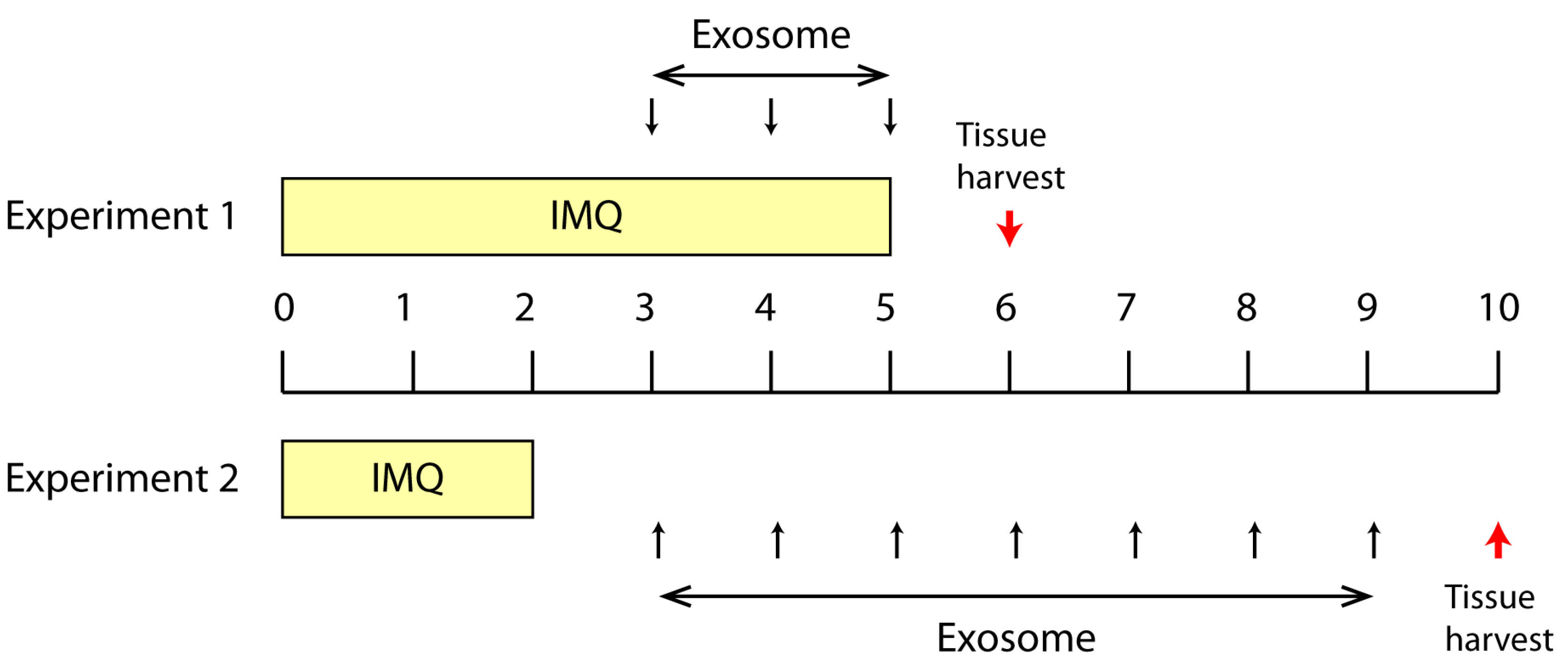
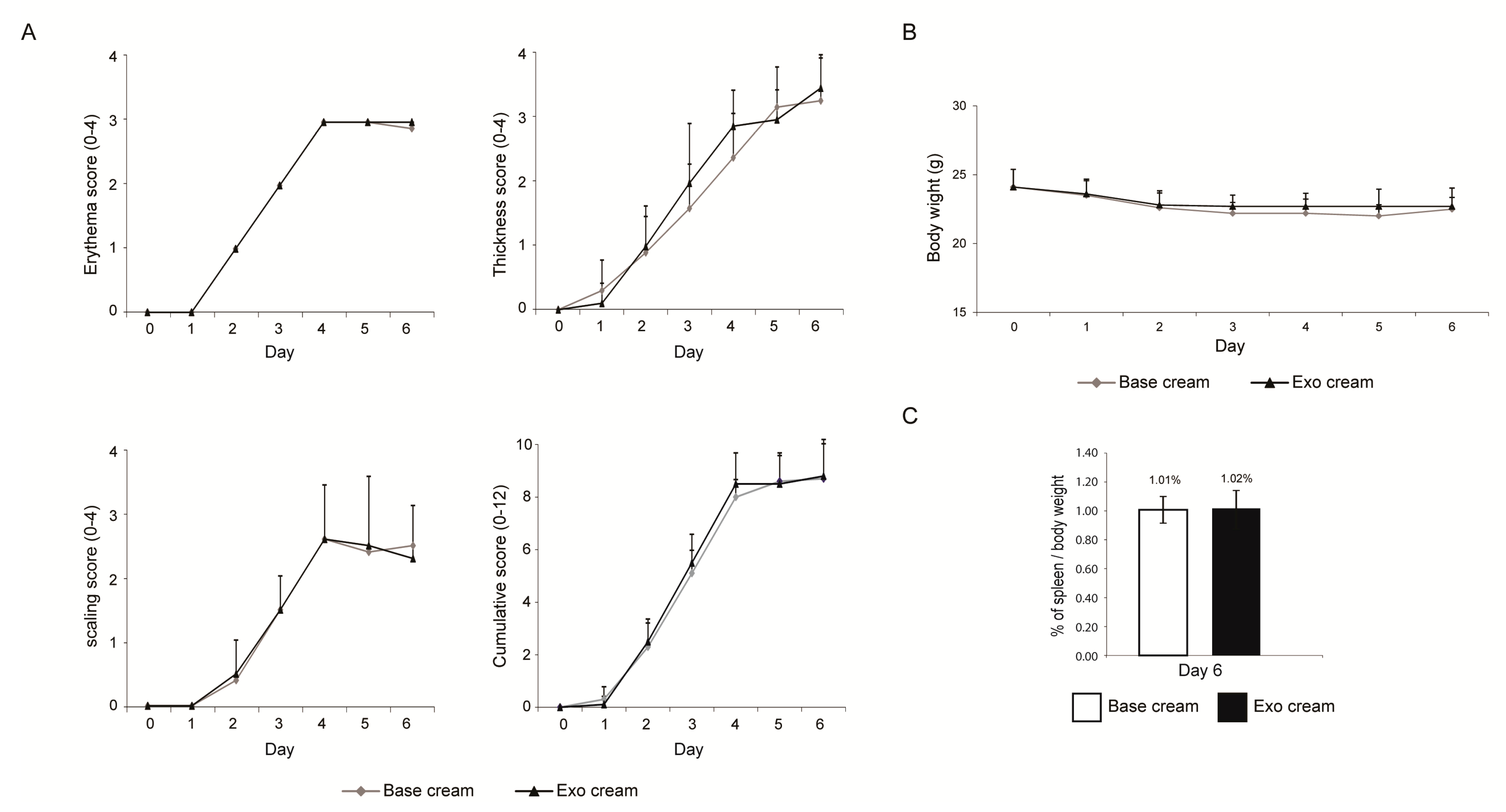
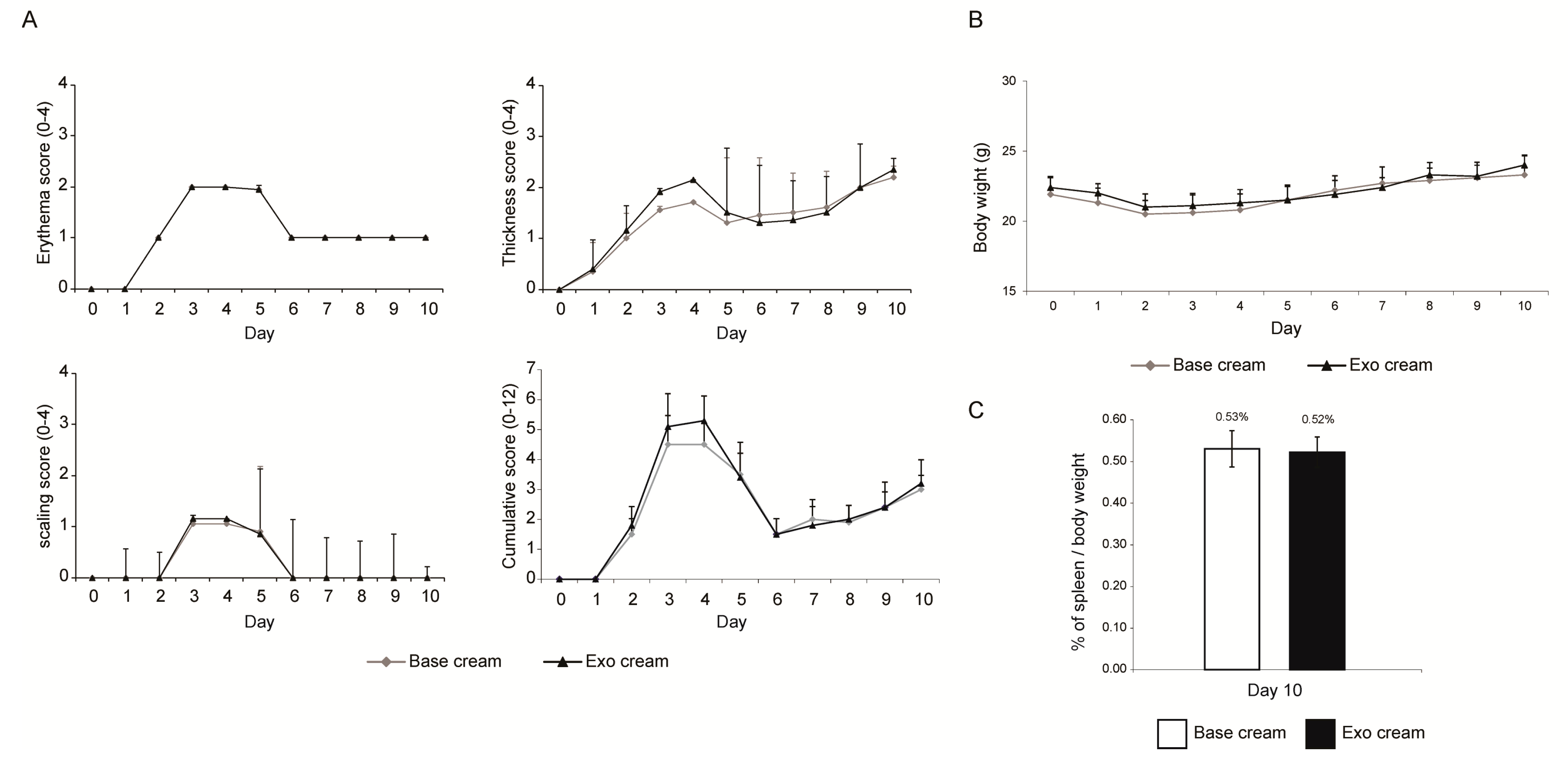
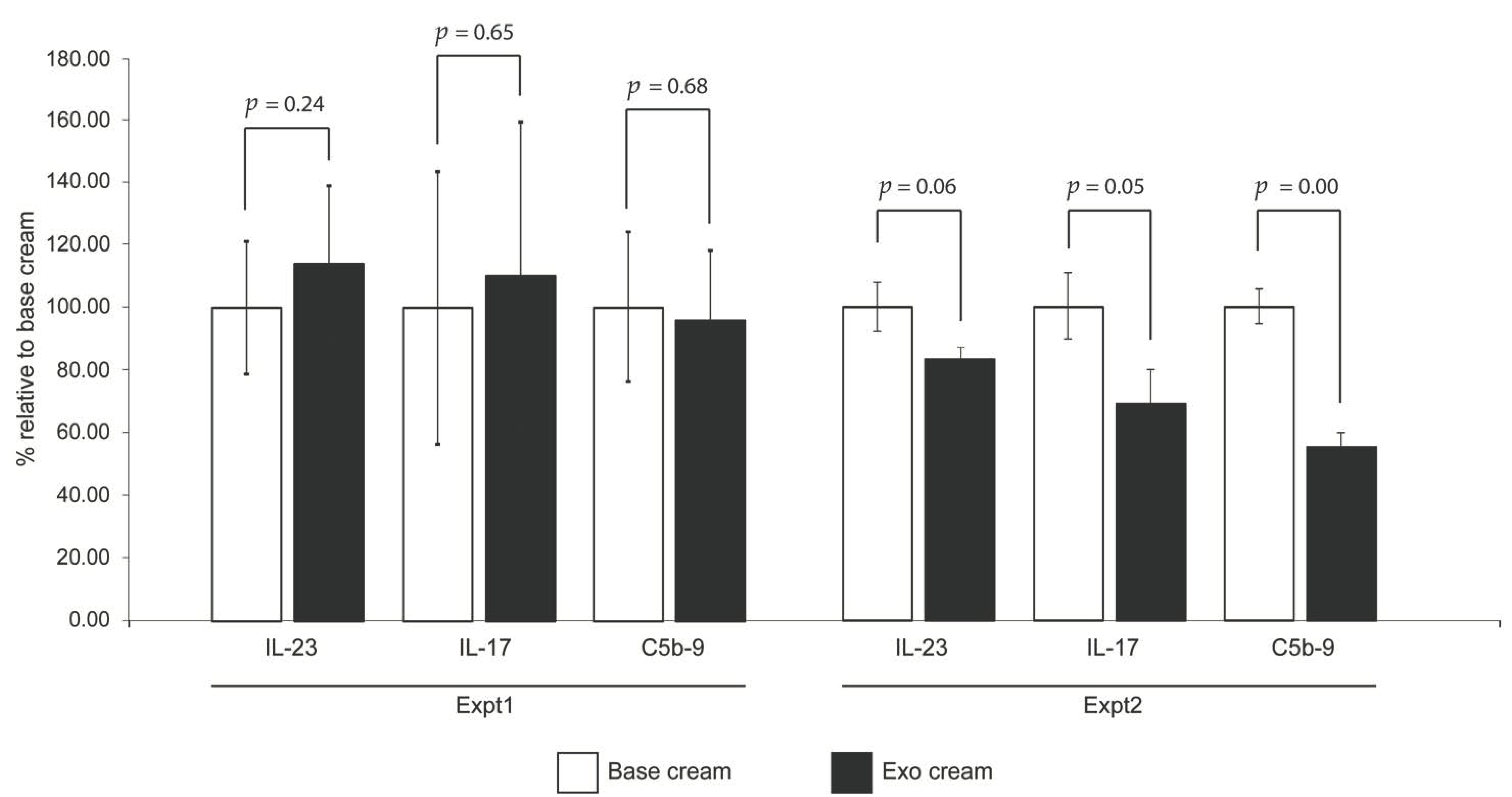
2.1. Effect of Topically Applied MSC Exosomes on the Skin Phenotype of A Mouse Model of IMQ-Induced Psoriasis Inflammation
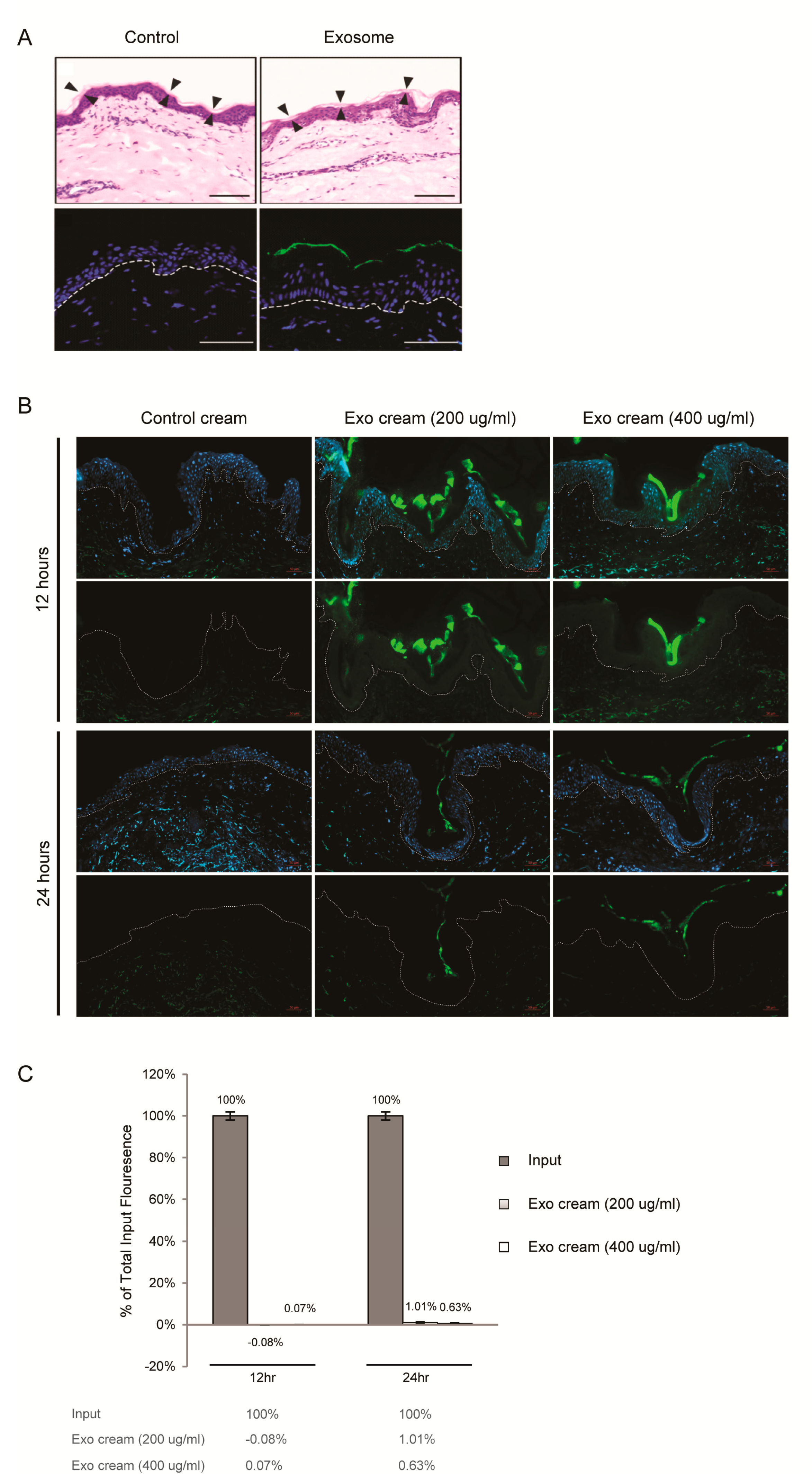
2.2. Dermal Penetrance of Topically Applied Exosomes on Human Skin
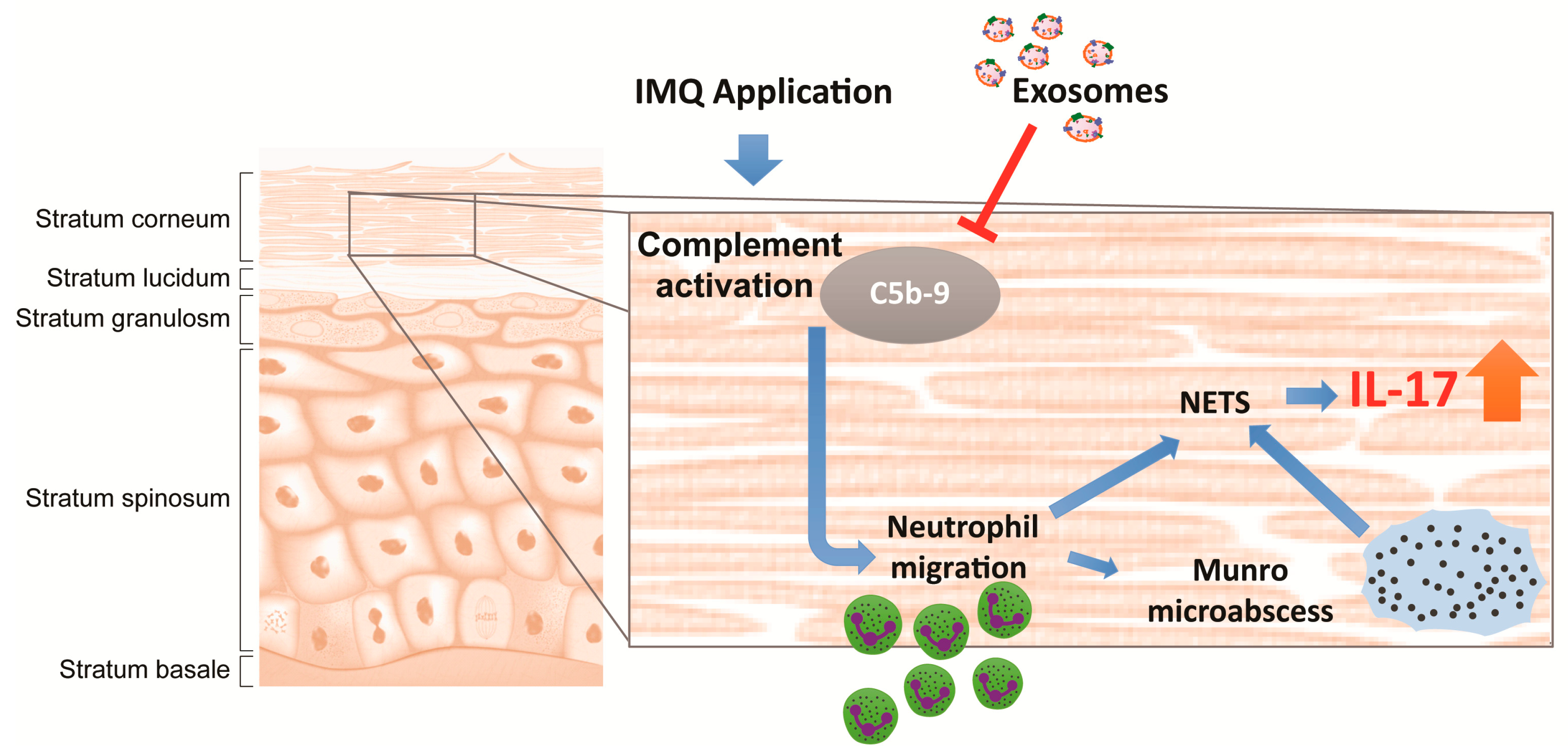
3. Discussion
4. Materials and Methods
4.1. Preparation of MSC Exosomes
4.2. Formulation of Oil-in-Water Emulsion of MSC Exosomes
4.3. Fluorescence Labeling of MSC Exosomes
4.4. IMQ Induced Psoriasis Mouse Model
4.5. IL-17, IL-23 and C5b-9 Measurement Assays
4.6. Human Skin Penetrance Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stem/stromal cell |
| IMQ | Imiquimod |
| TCC | Terminal complement complex |
| NET | Neutrophil extracellular traps |
References
- Menter, A.; Gottlieb, A.; Feldman, S.R.; Van Voorhees, A.S.; Leonardi, C.L.; Gordon, K.B.; Lebwohl, M.; Koo, J.Y.; Elmets, C.A.; Korman, N.J.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J. Am. Acad. Derm. 2008, 58, 826–850. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.; Mufti, A.; Gary Sibbald, R. Diagnosis and Management of Cutaneous Psoriasis: A Review. Adv. Ski. Wound Care 2019, 32, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Malatjalian, D.A.; Ross, J.B.; Williams, C.N.; Colwell, S.J.; Eastwood, B.J. Methotrexate hepatotoxicity in psoriatics: Report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can. J. Gastroenterol. 1996, 10, 369–375. [Google Scholar] [CrossRef]
- Mahrle, G.; Schulze, H.J.; Brautigam, M.; Mischer, P.; Schopf, R.; Jung, E.G.; Weidinger, G.; Farber, L. Anti-inflammatory efficacy of low-dose cyclosporin A in psoriatic arthritis. A prospective multicentre study. Br. J. Derm. 1996, 135, 752–757. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Efficacy and Safety of Biologics for Psoriasis and Psoriatic Arthritis and Their Impact on Comorbidities: A Literature Review. Int. J. Mol. Sci. 2020, 21, 1690. [Google Scholar] [CrossRef]
- Balsa, A.; Lula, S.; Marshall, L.; Szczypa, P.; Aikman, L. The comparative immunogenicity of biologic therapy and its clinical relevance in psoriatic arthritis: A systematic review of the literature. Expert Opin. Biol. Ther. 2018, 18, 575–584. [Google Scholar] [CrossRef]
- Jullien, D.; Prinz, J.C.; Nestle, F.O. Immunogenicity of Biotherapy Used in Psoriasis: The Science Behind the Scenes. J. Investig. Dermatol. 2015, 135, 31–38. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Haynesworth, S.E.; Baber, M.A.; Caplan, A.I. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1 alpha. J. Cell Physiol. 1996, 166, 585–592. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.Y.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef]
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.B.; Padmanabhan, J.; Lee, C.N.; de Kleijn, D.P.; Lim, S.K. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 2011, 9, 47. [Google Scholar] [CrossRef]
- Lai, R.; Yeo, R.; Tan, S.; Zhang, B.; Yin, Y.; Sze, S.; Choo, A.; Lim, S. Mesenchymal Stem Cell Exosomes: The Future MSC-Based Therapy? In Mesenchymal Stem Cell Therapy; Chase, L.G.V., Mohan, C., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 39–62. [Google Scholar]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; de Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 971907. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Tan, S.S.; Yeo, R.W.Y.; Choo, A.B.H.; Reiner, A.T.; Su, Y.; Shen, Y.; Fu, Z.; Alexander, L.; Sze, S.K.; et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J. Extracell. Vesicles 2016, 5, 29828. [Google Scholar] [CrossRef] [PubMed]
- Accarie, A.; l’Homme, B.; Benadjaoud, M.A.; Lim, S.K.; Guha, C.; Benderitter, M.; Tamarat, R.; Sémont, A. Extracellular vesicles derived from mesenchymal stromal cells mitigate intestinal toxicity in a mouse model of acute radiation syndrome. Stem Cell Res. Ther. 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yeo, R.W.Y.; Lai, R.C.; Sim, E.W.K.; Chin, K.C.; Lim, S.K. Mesenchymal stromal cell exosome–enhanced regulatory T-cell production through an antigen-presenting cell–mediated pathway. Cytotherapy 2018, 20, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.H.; Lim, S.K. Mesenchymal Stem Cells Secrete Immunologically Active Exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef]
- Leite-Silva, V.R.; de Almeida, M.M.; Fradin, A.; Grice, J.E.; Roberts, M.S. Delivery of drugs applied topically to the skin. Expert Rev. Derm. 2012, 7, 383–397. [Google Scholar] [CrossRef]
- Boutet, M.-A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef]
- Schön, M.P.; Erpenbeck, L. The Interleukin-23/Interleukin-17 Axis Links Adaptive and Innate Immunity in Psoriasis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Laboratory, T.J. Physiological Data Summary–BALB/cJ (000651) 2007. Available online: https://www.jax.org/-/media/jaxweb/files/jax-mice-and-services/physiolcal_data_000651 (accessed on 7 January 2021).
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Derm. 2012, 132, 964–975. [Google Scholar] [CrossRef]
- Brewer, J.; Bloksgaard, M.; Kubiak, J.; Sorensen, J.A.; Bagatolli, L.A. Spatially resolved two-color diffusion measurements in human skin applied to transdermal liposome penetration. J. Investig. Derm. 2013, 133, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Carubbi, F.; Cafaro, G.; Pucci, G.; Battista, F.; Bartoloni, E.; Giacomelli, R.; Schillaci, G.; Gerli, R. Targeting the IL-23/IL-17 axis for the treatment of psoriasis and psoriatic arthritis. Expert Opin. Biol. Ther. 2015, 15, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Kerdel, F.A.B.M. TNF Inhibitors in Psoriasis: A Review. Semin Cutan Med. Surg. 2015, 34, S37–S39. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Cuppari, C.; Manti, S.; Filippelli, M.; Parisi, G.F.; Borgia, F.; Briuglia, S.; Cannavo, P.; Salpietro, A.; Arrigo, T.; et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): Association with clinical severity and phenotype. Allergy Asthma Proc. 2015, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rodero, M.P.; Patel, J.; Moi, D.; Mazzieri, R.; Khosrotehrani, K. Interleukin-23 regulates interleukin-17 expression in wounds, and its inhibition accelerates diabetic wound healing through the alteration of macrophage polarization. FASEB J. 2018, 32, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal cell responses and role of TNF-alpha in impaired diabetic wound healing. BioMed. Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef] [PubMed]
- Todberg, T.; Zachariae, C.; Krustrup, D.; Skov, L. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermat. 2018, 78, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.I.; Lee, K.E.; Hong, J.Y.; Kim, M.N.; Oh, M.S.; Kim, Y.S.; Kim, K.W.; Kim, K.E.; Sohn, M.H. The role of interleukin-17 in mouse models of atopic dermatitis and contact dermatitis. Clin. Exp. Derm. 2015, 40, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N.; Kunkel, S.; Nickoloff, B.J. Modulation of leucocyte adhesion molecules, a T-cell chemotaxin (IL-8) and a regulatory cytokine (TNF-alpha) in allergic contact dermatitis (rhus dermatitis). Br. J. Derm. 1991, 124, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kelhälä, H.-L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 pathway is activated in acne lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Zhang, Q.; Yamaza, T.; Kelly, A.P.; Shi, S.; Wang, S.; Brown, J.; Wang, L.; French, S.W.; Shi, S.; Le, A.D. Tumor-Like Stem Cells Derived from Human Keloid Are Governed by the Inflammatory Niche Driven by IL-17/IL-6 Axis. PLoS ONE 2009, 4, e7798. [Google Scholar] [CrossRef]
- Yokouchi, M.; Atsugi, T.; Logtestijn, M.V.; Tanaka, R.J.; Kajimura, M.; Suematsu, M.; Furuse, M.; Amagai, M.; Kubo, A. Epidermal cell turnover across tight junctions based on Kelvin’s tetrakaidecahedron cell shape. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Sondell, B.; Thornell, L.E.; Egelrud, T. Evidence that stratum corneum chymotryptic enzyme is transported to the stratum corneum extracellular space via lamellar bodies. J. Investig. Dermatol 1995, 104, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Brattsand, M.; Stefansson, K.; Lundh, C.; Haasum, Y.; Egelrud, T. A Proteolytic Cascade of Kallikreins in the Stratum Corneum. J. Investig. Dermatol. 2005, 124, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Prévost, M.-C.; Simon, M.-F.; Chap, H.; Redoules, D.; Ceruti, I.; Tarroux, R.; Charveron, M. Identification of Two Secreted Phospholipases A2 in Human Epidermis. J. Investig. Dermatol. 2000, 114, 960–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harder, J.; Schroder, J.M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 2002, 277, 46779–46784. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Scherz, J.; Szabo, S.; Mildner, M.; Benarafa, C.; Torriglia, A.; Tschachler, E.; Eckhart, L. DNase 2 is the main DNA-degrading enzyme of the stratum corneum. PLoS ONE 2011, 6, e17581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotla, N.G.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Krishna, K.V.; Pandit, A.; Rochev, Y. Biomimetic Lipid-Based Nanosystems for Enhanced Dermal Delivery of Drugs and Bioactive Agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272. [Google Scholar] [CrossRef]
- Marinoni, B.; Ceribelli, A.; Massarotti, M.S.; Selmi, C. The Th17 axis in psoriatic disease: Pathogenetic and therapeutic implications. Auto Immun. Highlights 2014, 5, 9–19. [Google Scholar] [CrossRef]
- Dahl, M.V.; Lindroos, W.E.; Nelson, R.D. Chemokinetic and chemotactic factors in psoriasis scale extracts. J. Investig. Derm. 1978, 71, 402–406. [Google Scholar] [CrossRef]
- Tagami, H.; Ofuji, S. Leukotactic properties of soluble substances inpsoriasis scale. Br. J. Dermatol. 1976, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Terui, T.; Kato, T.; Tagami, H. Stratum corneum activation of complement through the antibody-independent alternative pathway. J. Investig. Derm. 1989, 92, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Weiss, V.C.; van Den Broek, H.; Barrett, S.; West, D.P. Immunopathology of Psoriasis: A Comparison with Other Parakeratotic Lesions. J. Investig. Dermatol. 1982, 78, 256–260. [Google Scholar] [CrossRef]
- Takematsu, H.; Tagami, H. Generation of Terminal Complement Complexes in Psoriatic Lesional Skin. Dermatology 1992, 185, 246–250. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.-S.; Yu, H.-S.; Yen, F.-L.; Lin, C.-L.; Chen, G.-S.; Lan, C.-C.E. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol 2011, 187, 490–500. [Google Scholar] [CrossRef]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell. Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Elias, P.M. The skin barrier as an innate immune element. Semin. Immunopathol. 2007, 29, 3. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Tan, S.S.; Tan, B.; Choo, A.; Lee, M.M.; Chen, T.S.; Teh, B.J.; Eng, J.K.; Sidik, H.; et al. Derivation and characterization of human fetal MSCs: An alternative cell source for large-scale production of cardioprotective microparticles. J. Mol. Cell Cardiol. 2010, 48, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.K.; de Kleijn, D.P.; Lai, R.C.; Tan, E.K.W.; Zhao, H.; Yeo, K.S.; Low, T.Y.; Lian, Q.; Lee, C.N.; Mitchell, W. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol. Cell. Proteom. 2007, 6, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
| Volume or Weight | Final Concentration | |
|---|---|---|
| Olive oil | 200 μL | 20% v/v |
| Exosomes | 200 or 400 μg | 200 or 400 μg/ml |
| 100% Seppic plus 400 1 | 40 mg | 4% w/v |
| PBS | 400 μL | 40% v/v |
| water | 400 μL | 40% v/v |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. https://doi.org/10.3390/ijms22020720
Zhang B, Lai RC, Sim WK, Choo ABH, Lane EB, Lim SK. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. International Journal of Molecular Sciences. 2021; 22(2):720. https://doi.org/10.3390/ijms22020720
Chicago/Turabian StyleZhang, Bin, Ruenn Chai Lai, Wei Kian Sim, Andre Boon Hwa Choo, Ellen Birgit Lane, and Sai Kiang Lim. 2021. "Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation" International Journal of Molecular Sciences 22, no. 2: 720. https://doi.org/10.3390/ijms22020720
APA StyleZhang, B., Lai, R. C., Sim, W. K., Choo, A. B. H., Lane, E. B., & Lim, S. K. (2021). Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. International Journal of Molecular Sciences, 22(2), 720. https://doi.org/10.3390/ijms22020720





