Increased Calbindin D28k Expression via Long-Term Alternate-Day Fasting Does Not Protect against Ischemia-Reperfusion Injury: A Focus on Delayed Neuronal Death, Gliosis and Immunoglobulin G Leakage
Abstract
1. Introduction
2. Results
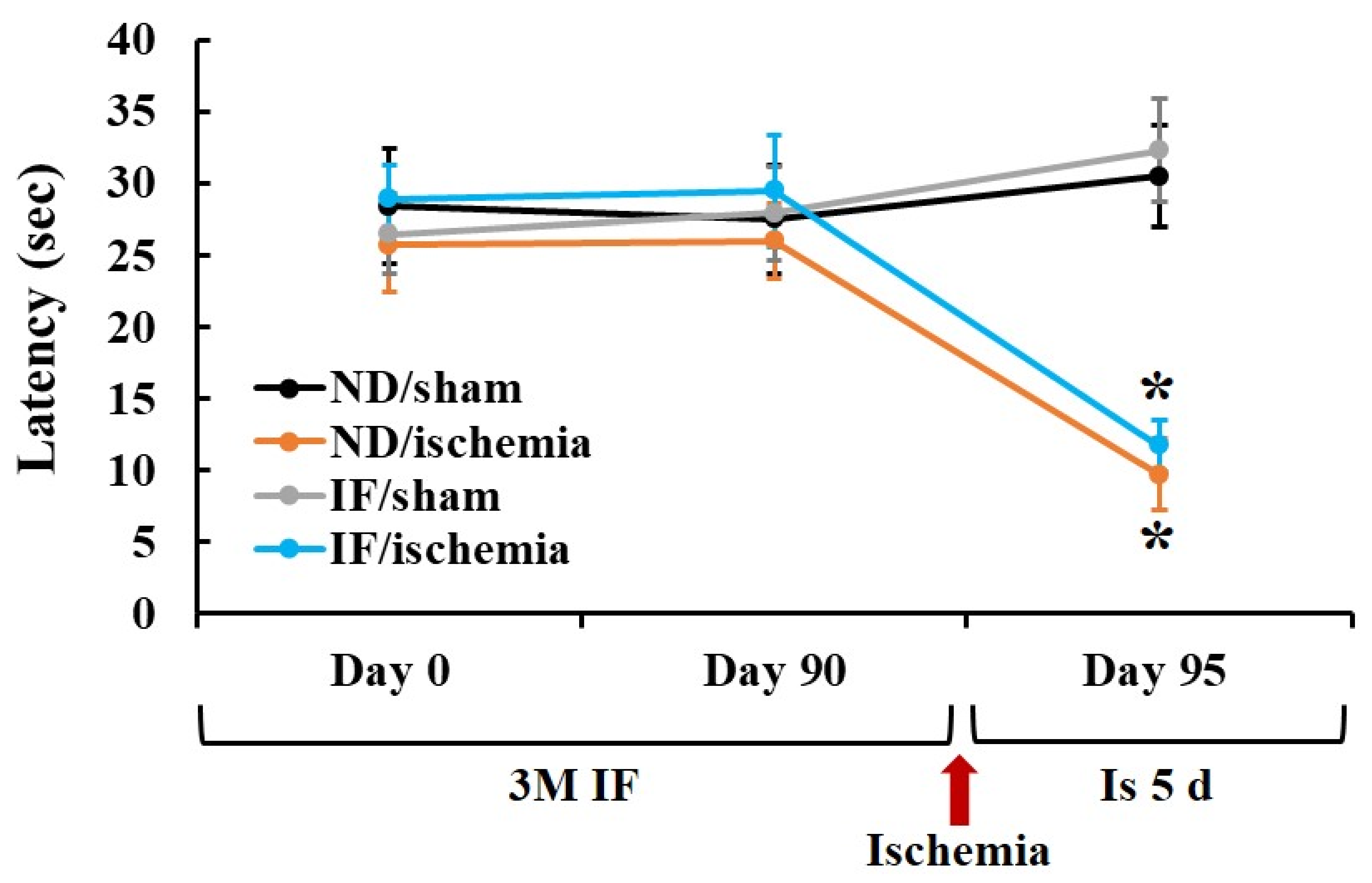
2.1. Passive Avoidance Test (PAT)
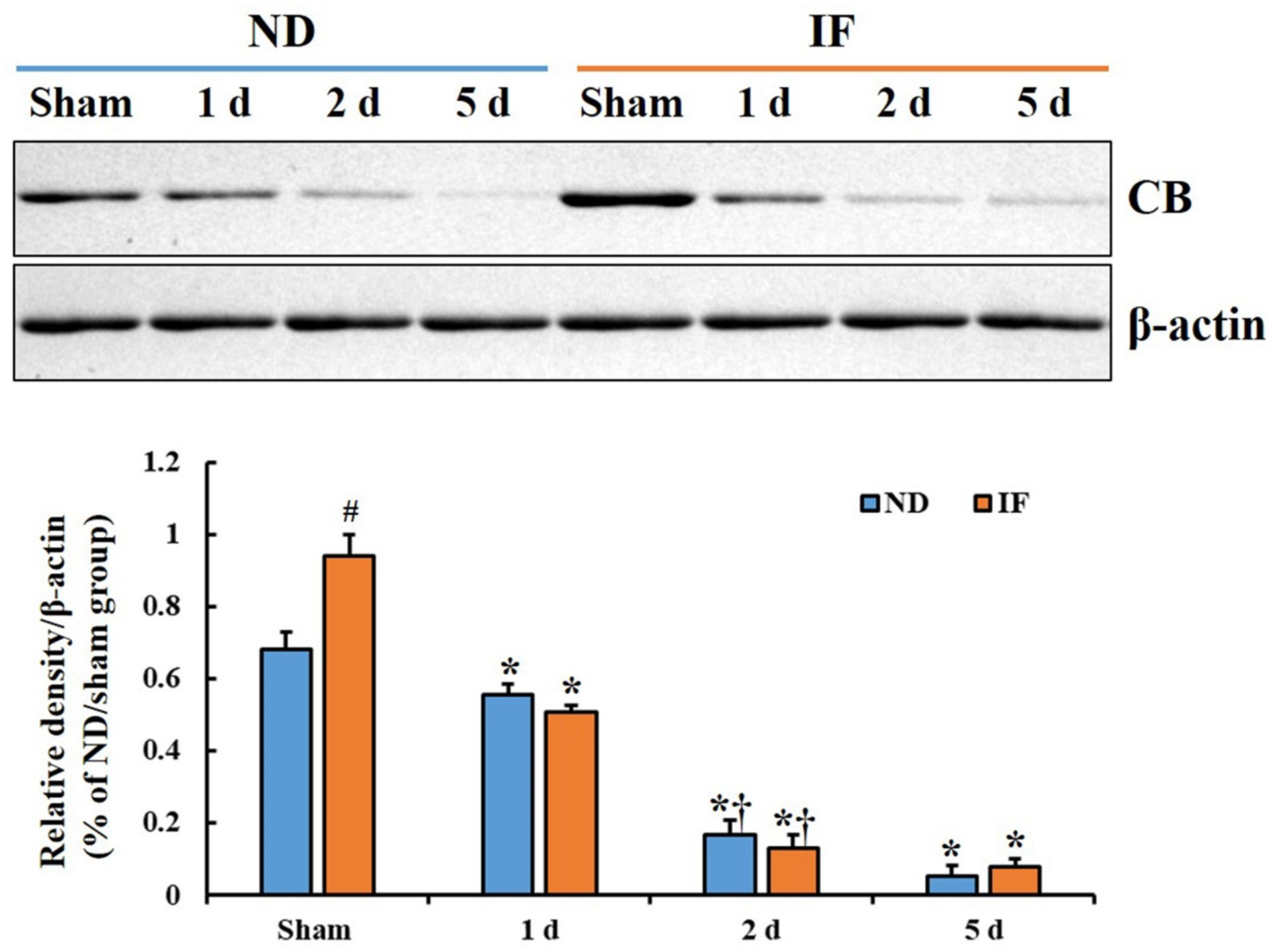
2.2. CB Protein Levels
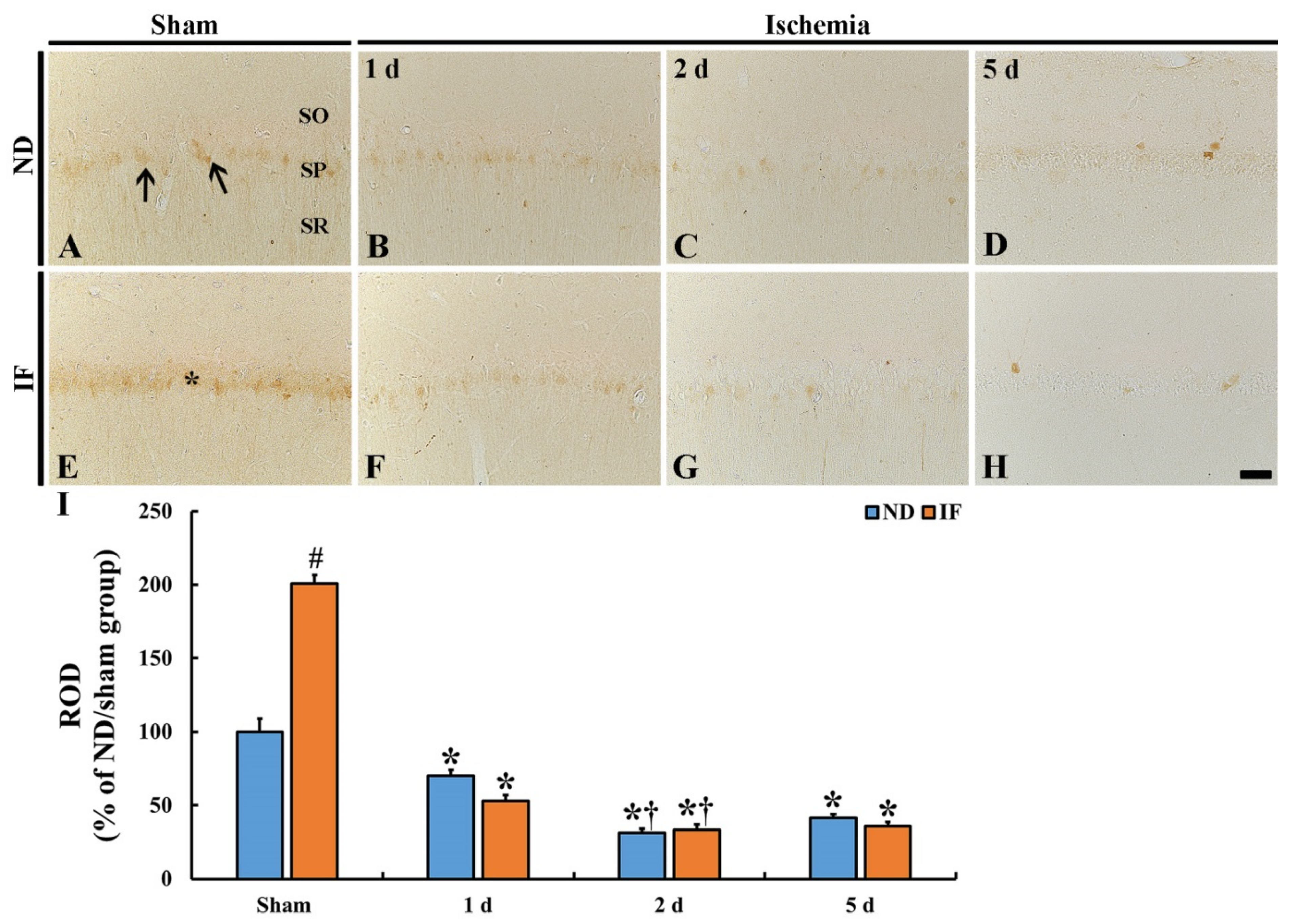
2.3. CB Immunoreactivity
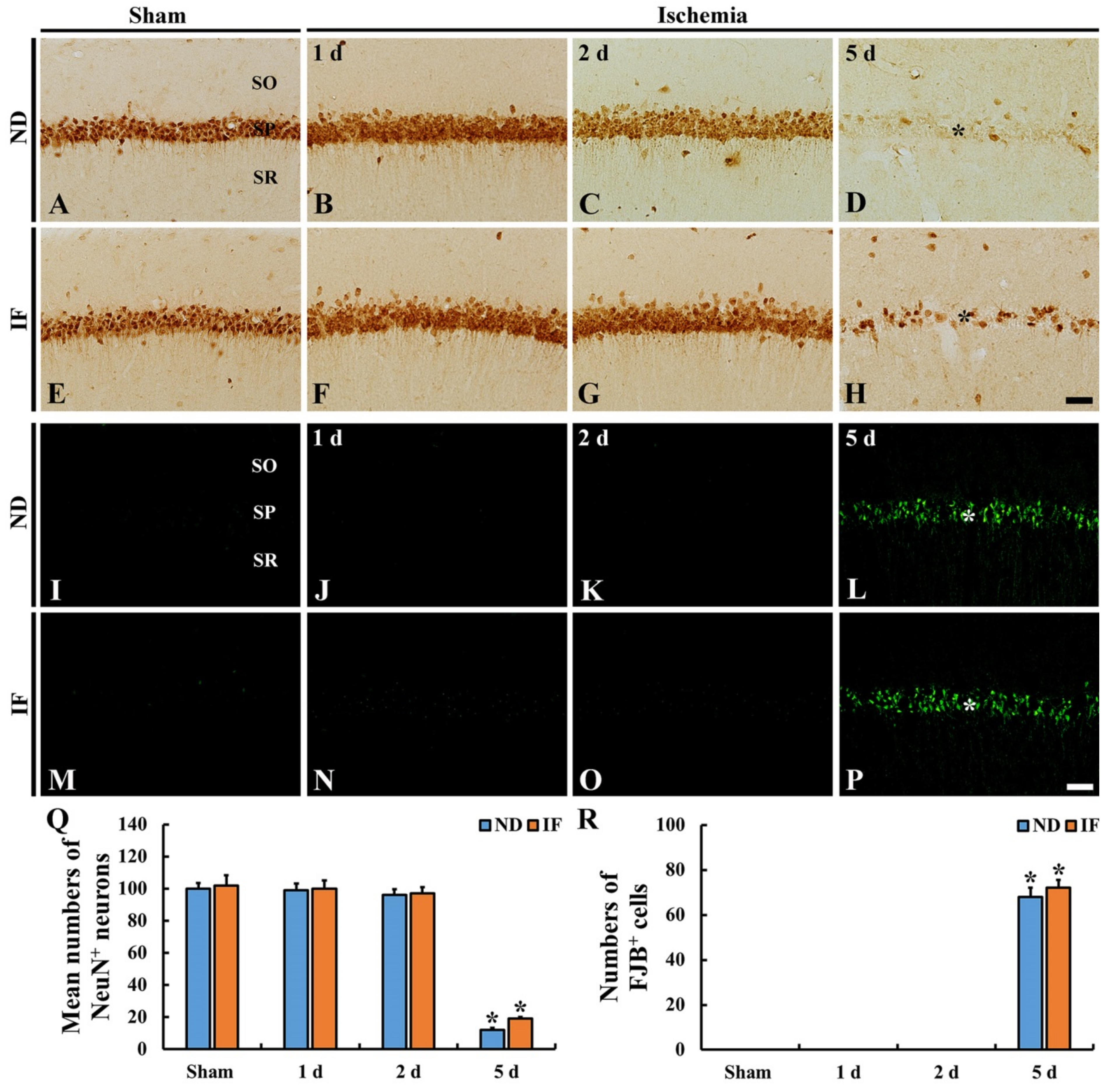
2.4. Neuroprotection
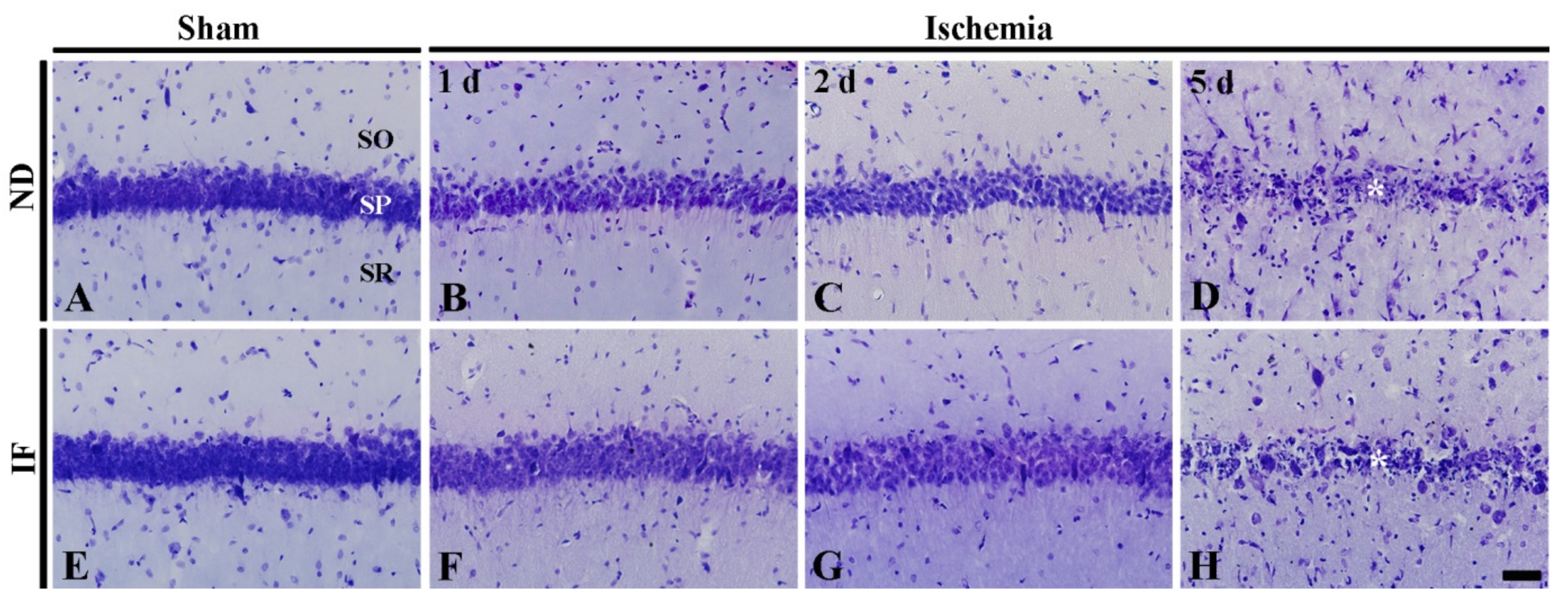
2.4.1. Cresyl Violet (CV) Positive Cells
2.4.2. Neuronal Nuclear Antigen (NeuN) Immunoreactive Neurons
2.4.3. Fluoro-Jade B (FJB)-Positive Cells
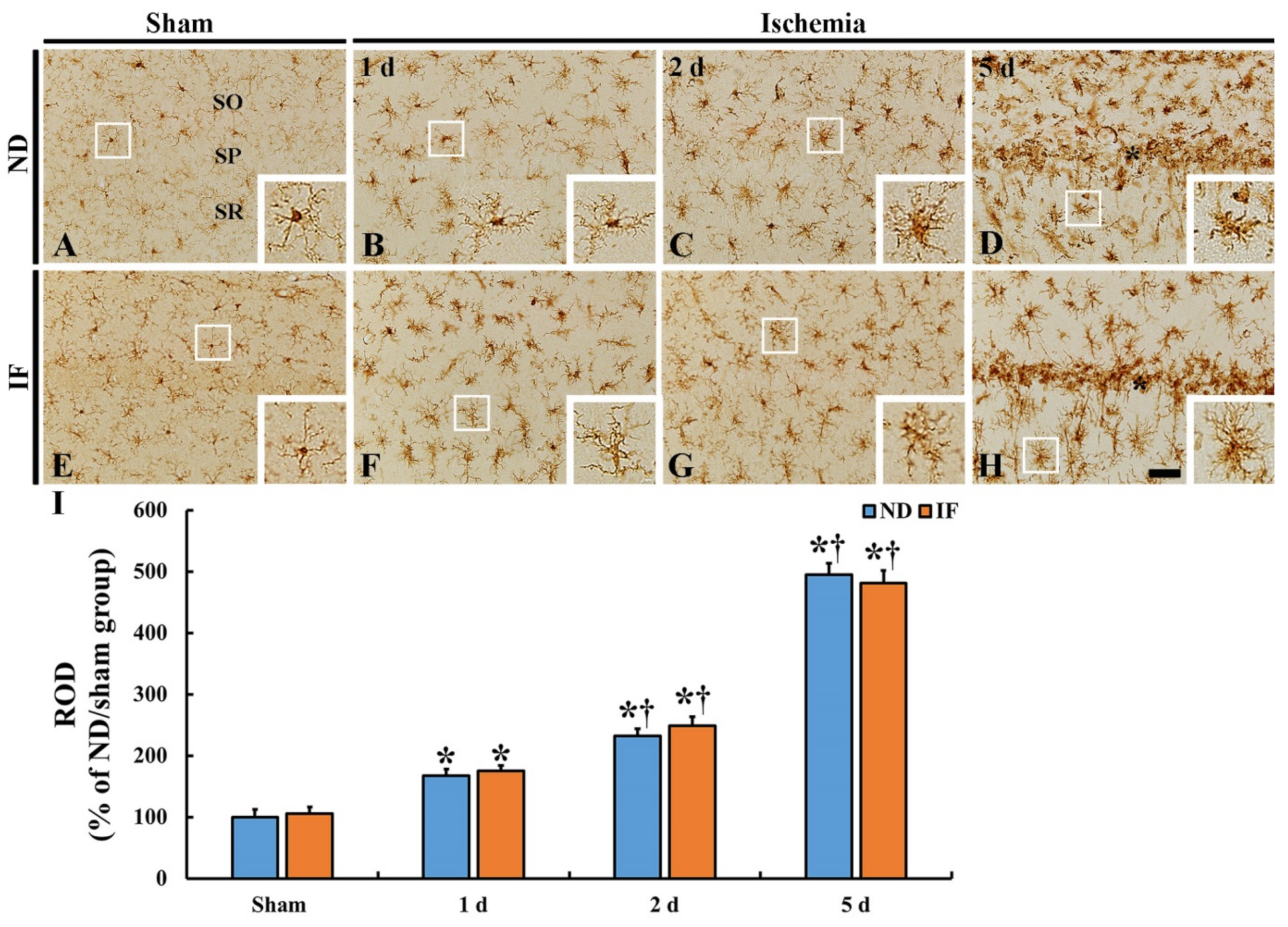
2.5. Microgliosis
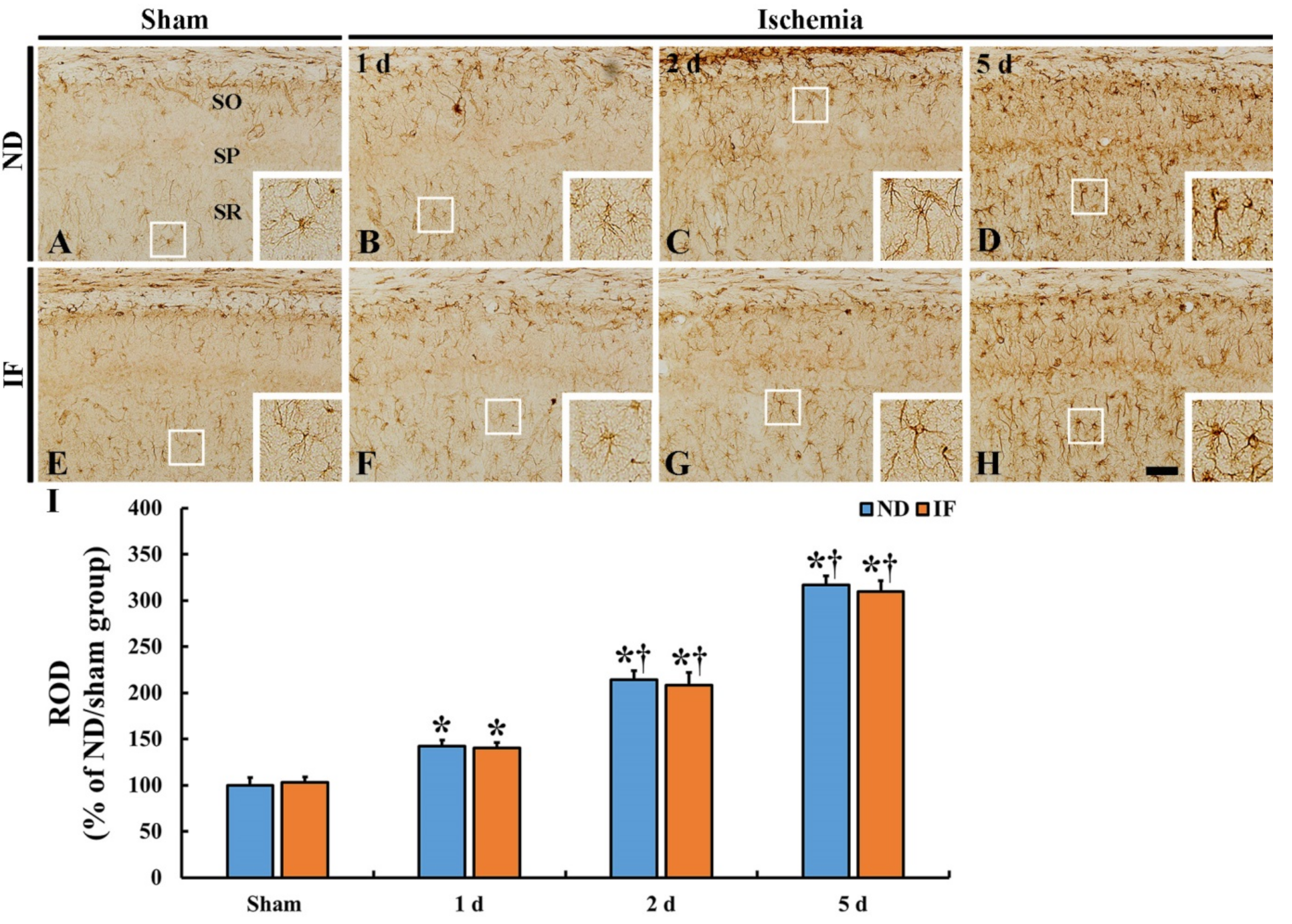
2.6. Astrogliosis
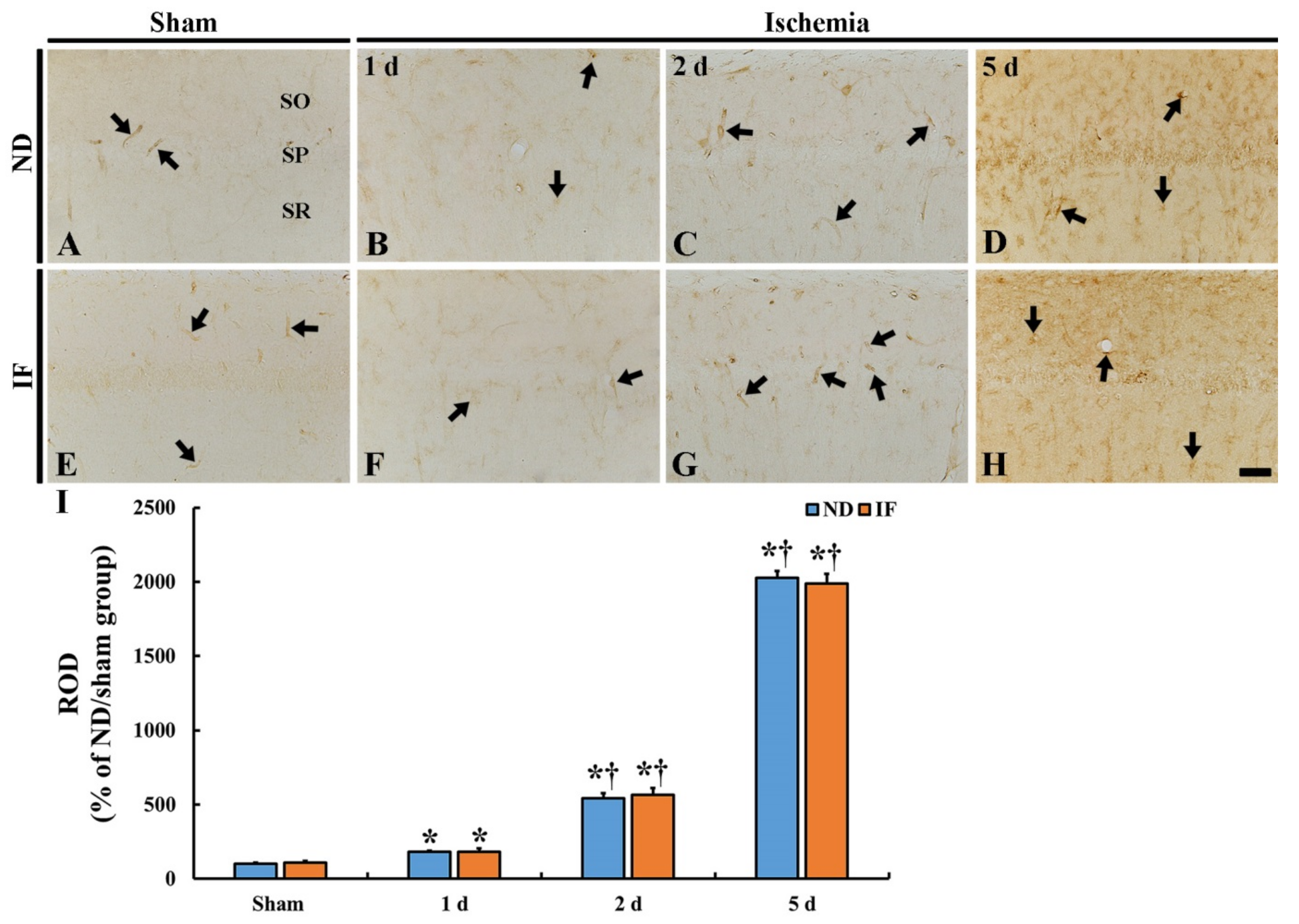
2.7. Immunoglobulin G (IgG) Immunoreactivity
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. IF and Experimental Groups
4.3. Induction of Transient Forebrain Ischemia
4.4. PAT
4.5. Western Blotting
4.6. Preparation of Histological Sections
4.7. Immunohistochemistry
4.8. CV Histochemistry
4.9. FJB Histofluorescence Staining
4.10. Data Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–Brain Barrier |
| CA | Cornu Ammonis |
| CB | Calbindin D28K |
| CV | Cresyl Violet |
| FJB | Fluoro Jade B |
| GFAP | Glial fibrillary Acidic Protein |
| Iba-1 | Ionized Calcium Binding Adapter Molecule 1 |
| IF | Intermittent Fasting |
| ND | Normal Diet |
| NeuN | Neuronal Nuclear Antigen |
| PAT | Passive Avoidance Test |
| ROD | Relative Optical Density |
| SO | Stratum Oriens |
| SP | Stratum Pyramidale |
| SR | Stratum Radiatum |
| TFI | Transient Forebrain Ischemia |
References
- Fann, D.Y.; Ng, G.Y.; Poh, L.; Arumugam, T.V. Positive effects of intermittent fasting in ischemic stroke. Exp. Gerontol. 2017, 89, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Muller, C.; de Jong, M.; et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef]
- Jeong, J.H.; Yu, K.S.; Bak, D.H.; Lee, J.H.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Kim, J.J.; Han, S.Y. Intermittent fasting is neuroprotective in focal cerebral ischemia by minimizing autophagic flux disturbance and inhibiting apoptosis. Exp. Ther. Med. 2016, 12, 3021–3028. [Google Scholar] [CrossRef] [PubMed]
- Varendi, K.; Airavaara, M.; Anttila, J.; Vose, S.; Planken, A.; Saarma, M.; Mitchell, J.R.; Andressoo, J.O. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS ONE 2014, 9, e93911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, J.H.; Noh, Y.; Shin, B.N.; Kim, S.S.; Park, J.H.; Lee, T.K.; Song, M.; Kim, H.; Lee, J.C.; Yong, J.H.; et al. Intermittent fasting increases SOD2 and catalase immunoreactivities in the hippocampus but does not protect from neuronal death following transient ischemia in gerbils. Mol. Med. Rep. 2018, 18, 4802–4812. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, B.N.; Park, J.H.; Lee, T.K.; Park, Y.E.; Lee, J.C.; Yang, G.E.; Shin, M.C.; Cho, J.H.; Lee, K.C.; et al. Pre-and post-treatment with novel antiepileptic drug oxcarbazepine exerts neuroprotective effect in the hippocampus in a gerbil model of transient global cerebral ischemia. Brain Sci. 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T.; Tamura, A.; Sano, K. Selective vulnerability of the hippocampus to ischemia—Reversible and irreversible types of ischemic cell damage. Prog. Brain Res. 1985, 63, 39–58. [Google Scholar]
- Kirino, T. Delayed neuronal death. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2000, 20, S95–S97. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to cns damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Lee, T.K.; Park, Y.; Kim, B.; Lee, J.C.; Shin, M.C.; Ohk, T.G.; Park, C.W.; Cho, J.H.; Park, J.H.; Lee, C.H.; et al. Long-term alternating fasting increases interleukin-13 in the gerbil hippocampus, but does not protect bbb and pyramidal neurons from ischemia-reperfusion injury. Neurochem. Res. 2020, 45, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Birngruber, T.; Sattler, W.; Kroath, T.; Ratzer, M.; Sinner, F.; Pieber, T.R. Assessment of blood-brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cofm). PLoS ONE 2014, 9, e98143. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, M.S.; Thoenen, H.; Meyer, M. Vulnerability of midbrain dopaminergic neurons in calbindin-d28k-deficient mice: Lack of evidence for a neuroprotective role of endogenous calbindin in mptp-treated and weaver mice. Eur. J. Neurosci. 1997, 9, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Heizmann, C.W. Calcium signaling in the brain. Acta Neurobiol. Exp. 1993, 53, 15–23. [Google Scholar]
- Nagerl, U.V.; Mody, I.; Jeub, M.; Lie, A.A.; Elger, C.E.; Beck, H. Surviving granule cells of the sclerotic human hippocampus have reduced ca(2+) influx because of a loss of calbindin-d(28k) in temporal lobe epilepsy. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 1831–1836. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Wojda, U.; Salinska, E.; Kuznicki, J. Calcium ions in neuronal degeneration. Iubmb Life 2008, 60, 575–590. [Google Scholar] [CrossRef]
- Chung, T.H.; Choi, H.S.; Lee, C.H. Change of calbindin d-28k protein expression in the mice hippocampus after lipopolysaccharide treatment. J. Vet. Med Sci. 2015, 77, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S.; Battini, R.; Ferrari, S.; Pozzi, L.; Killcross, A.S.; Robbins, T.W.; Jouvenceau, A.; Billard, J.M.; Dutar, P.; Lamour, Y.; et al. Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin d28k expression. Proc. Natl. Acad. Sci. USA 1996, 93, 8028–8033. [Google Scholar] [CrossRef] [PubMed]
- Soontornniyomkij, V.; Risbrough, V.B.; Young, J.W.; Soontornniyomkij, B.; Jeste, D.V.; Achim, C.L. Hippocampal calbindin-1 immunoreactivity correlate of recognition memory performance in aged mice. Neurosci. Lett. Shannon Clare Irel. 2012, 516, 161–165. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, B.N.; Song, M.; Kim, H.; Park, J.H.; Lee, T.K.; Park, C.W.; Park, Y.E.; Lee, J.C.; Yong, J.H.; et al. Intermittent fasting increases the expressions of sods and catalase in granule and polymorphic cells and enhances neuroblast dendrite complexity and maturation in the adult gerbil dentate gyrus. Mol. Med. Rep. 2019, 19, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Shirley, R.; Ord, E.N.; Work, L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Lee, J.C.; Won, M.H. Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat. Cell Biol. 2014, 47, 149–156. [Google Scholar] [CrossRef]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental pretreatment with chlorogenic acid prevents transient ischemia-induced cognitive decline and neuronal damage in the hippocampus through anti-oxidative and anti-inflammatory effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal ca2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Zuo, Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS ONE 2013, 8, e66069. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Klein, S. Aging, adiposity, and calorie restriction. JAMA 2007, 297, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 2012, 34, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Toescu, E.C.; Verkhratsky, A. The importance of being subtle: Small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 2007, 6, 267–273. [Google Scholar] [CrossRef]
- Villa, A.; Podini, P.; Panzeri, M.C.; Racchetti, G.; Meldolesi, J. Cytosolic ca2+ binding proteins during rat brain ageing: Loss of calbindin and calretinin in the hippocampus, with no change in the cerebellum. Eur. J. Neurosci. 1994, 6, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- De Jong, G.I.; Naber, P.A.; Van der Zee, E.A.; Thompson, L.T.; Disterhoft, J.F.; Luiten, P.G. Age-related loss of calcium binding proteins in rabbit hippocampus. Neurobiol. Aging 1996, 17, 459–465. [Google Scholar] [CrossRef]
- Bae, E.J.; Chen, B.H.; Shin, B.N.; Cho, J.H.; Kim, I.H.; Park, J.H.; Lee, J.C.; Tae, H.J.; Choi, S.Y.; Kim, J.D.; et al. Comparison of immunoreactivities of calbindin-d28k, calretinin and parvalbumin in the striatum between young, adult and aged mice, rats and gerbils. Neurochem. Res. 2015, 40, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.F.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: Evidence for a preconditioning mechanism. J. Neurosci. Res. 1999, 57, 830–839. [Google Scholar] [CrossRef]
- Ran, M.; Li, Z.; Yang, L.; Tong, L.; Zhang, L.; Dong, H. Calorie restriction attenuates cerebral ischemic injury via increasing sirt1 synthesis in the rat. Brain Res. 2015, 1610, 61–68. [Google Scholar] [CrossRef]
- Ciobanu, O.; Elena Sandu, R.; Tudor Balseanu, A.; Zavaleanu, A.; Gresita, A.; Petcu, E.B.; Uzoni, A.; Popa-Wagner, A. Caloric restriction stabilizes body weight and accelerates behavioral recovery in aged rats after focal ischemia. Aging Cell 2017, 16, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Roberge, M.C.; Hotte-Bernard, J.; Messier, C.; Plamondon, H. Food restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive ca1 ischemic injury. Behav. Brain Res. 2008, 187, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.V.; Phillips, T.M.; Cheng, A.; Morrell, C.H.; Mattson, M.P.; Wan, R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010, 67, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Klapstein, G.J.; Vietla, S.; Lieberman, D.N.; Gray, P.A.; Airaksinen, M.S.; Thoenen, H.; Meyer, M.; Mody, I. Calbindin-d28k fails to protect hippocampal neurons against ischemia in spite of its cytoplasmic calcium buffering properties: Evidence from calbindin-d28k knockout mice. Neuroscience 1998, 85, 361–373. [Google Scholar] [CrossRef]
- Cheng, B.; Christakos, S.; Mattson, M.P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 1994, 12, 139–153. [Google Scholar] [CrossRef]
- Ho, B.K.; Alexianu, M.E.; Colom, L.V.; Mohamed, A.H.; Serrano, F.; Appel, S.H. Expression of calbindin-d28k in motoneuron hybrid cells after retroviral infection with calbindin-d28k cdna prevents amyotrophic lateral sclerosis igg-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 1996, 93, 6796–6801. [Google Scholar] [CrossRef]
- Iacopino, A.; Christakos, S.; German, D.; Sonsalla, P.K.; Altar, C.A. Calbindin-d28k-containing neurons in animal models of neurodegeneration: Possible protection from excitotoxicity. Brain Res. Mol. Brain Res. 1992, 13. [Google Scholar] [CrossRef]
- Rothman, S.M.; Olney, J.W. Excitotoxicity and the nmda receptor—Still lethal after eight years. Trends Neurosci. 1995, 18, 57–58. [Google Scholar]
- Perez-Pinzon, M.A.; Yenari, M.A.; Sun, G.H.; Kunis, D.M.; Steinberg, G.K. Snx-111, a novel, presynaptic n-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J. Neurol. Sci. 1997, 153, 25–31. [Google Scholar] [CrossRef]
- Petcu, E.B.; Sfredel, V.; Platt, D.; Herndon, J.G.; Kessler, C.; Popa-Wagner, A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: A mini-review. Gerontology 2008, 54, 6–17. [Google Scholar] [CrossRef]
- Buga, A.M.; Di Napoli, M.; Popa-Wagner, A. Preclinical models of stroke in aged animals with or without comorbidities: Role of neuroinflammation. Biogerontology 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Troup, G.M.; Smith, G.S.; Walford, R.L. Life span, chronologic disease patterns, and age-related changes in relative spleen weights for the mongolian gerbil (meriones unguiculatus). Exp. Gerontol. 1969, 4, 139–143. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dinca, I.; Yalikun, S.; Walker, L.; Kroemer, H.; Kessler, C. Accelerated delimitation of the infarct zone by capillary-derived nestin-positive cells in aged rats. Curr. Neurovascular Res. 2006, 3, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Badan, I.; Platt, D.; Kessler, C.; Popa-Wagner, A. Temporal dynamics of degenerative and regenerative events associated with cerebral ischemia in aged rats. Gerontology 2003, 49, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, J.H.; Lee, T.K.; Lee, H.A.; Won, M.H.; Lee, C.H. Expression changes of CX3CL1 and CX3CR1 proteins in the hippocampal CA1 field of the gerbil following transient global cerebral ischemia. Int. J. Mol. Med. 2019, 44, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Choi, J.H.; Park, J.H.; Kim, I.H.; Cho, J.H.; Lee, J.C.; Koo, H.M.; Hwangbo, G.; Yoo, K.Y.; Lee, C.H.; et al. Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabilit. Neural Repair 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Ahn, J.H.; Hong, S.; Park, J.H.; Kim, I.H.; Cho, J.H.; Lee, T.K.; Lee, J.C.; Chen, B.H.; Shin, B.N.; Bae, E.J.; et al. Immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the somatosensory cortex of rodents during normal aging. Mol. Med. Rep. 2017, 16, 7191–7198. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, B.N.; Park, J.H.; Kim, I.H.; Cho, J.H.; Chen, B.; Lee, T.K.; Tae, H.J.; Lee, J.C.; Cho, J.H.; et al. Long-term observation of neuronal degeneration and microgliosis in the gerbil dentate gyrus after transient cerebral ischemia. J. Neurol. Sci. 2016, 363, 21–26. [Google Scholar] [CrossRef]
- Hong, S.; Ahn, J.Y.; Cho, G.S.; Kim, I.H.; Cho, J.H.; Ahn, J.H.; Park, J.H.; Won, M.H.; Chen, B.H.; Shin, B.N.; et al. Monocarboxylate transporter 4 plays a significant role in the neuroprotective mechanism of ischemic preconditioning in transient cerebral ischemia. Neural Regen. Res. 2015, 10, 1604–1611. [Google Scholar]
- Kim, M.J.; Cho, J.H.; Cho, J.H.; Park, J.H.; Ahn, J.H.; Tae, H.J.; Cho, G.S.; Yan, B.C.; Hwang, I.K.; Lee, C.H.; et al. Impact of hyperthermia before and during ischemia-reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J. Neurol. Sci. 2015, 348, 101–110. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, B.N.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Kim, D.W.; Won, M.H.; Hong, S.; Cho, J.H.; Lee, C.H. Ischemia-induced changes of PRAS40 and p-PRAS40 immunoreactivities in the gerbil hippocampal CA1 region after transient cerebral ischemia. Cell. Mol. Neurobiol. 2016, 36, 821–828. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, H.; Lee, T.-K.; Yoo, Y.H.; Ahn, J.H.; Kim, D.W.; Kim, B.; Lee, J.-C.; Park, J.H.; Lim, S.-S.; Park, J.-S.; et al. Increased Calbindin D28k Expression via Long-Term Alternate-Day Fasting Does Not Protect against Ischemia-Reperfusion Injury: A Focus on Delayed Neuronal Death, Gliosis and Immunoglobulin G Leakage. Int. J. Mol. Sci. 2021, 22, 644. https://doi.org/10.3390/ijms22020644
Sim H, Lee T-K, Yoo YH, Ahn JH, Kim DW, Kim B, Lee J-C, Park JH, Lim S-S, Park J-S, et al. Increased Calbindin D28k Expression via Long-Term Alternate-Day Fasting Does Not Protect against Ischemia-Reperfusion Injury: A Focus on Delayed Neuronal Death, Gliosis and Immunoglobulin G Leakage. International Journal of Molecular Sciences. 2021; 22(2):644. https://doi.org/10.3390/ijms22020644
Chicago/Turabian StyleSim, Hyejin, Tae-Kyeong Lee, Yeon Ho Yoo, Ji Hyeon Ahn, Dae Won Kim, Bora Kim, Jae-Chul Lee, Joon Ha Park, Soon-Sung Lim, Jung-Seok Park, and et al. 2021. "Increased Calbindin D28k Expression via Long-Term Alternate-Day Fasting Does Not Protect against Ischemia-Reperfusion Injury: A Focus on Delayed Neuronal Death, Gliosis and Immunoglobulin G Leakage" International Journal of Molecular Sciences 22, no. 2: 644. https://doi.org/10.3390/ijms22020644
APA StyleSim, H., Lee, T.-K., Yoo, Y. H., Ahn, J. H., Kim, D. W., Kim, B., Lee, J.-C., Park, J. H., Lim, S.-S., Park, J.-S., Kang, I. J., Kim, Y.-M., Shin, M. C., Cho, J. H., Park, Y., & Won, M.-H. (2021). Increased Calbindin D28k Expression via Long-Term Alternate-Day Fasting Does Not Protect against Ischemia-Reperfusion Injury: A Focus on Delayed Neuronal Death, Gliosis and Immunoglobulin G Leakage. International Journal of Molecular Sciences, 22(2), 644. https://doi.org/10.3390/ijms22020644






