Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells
Abstract
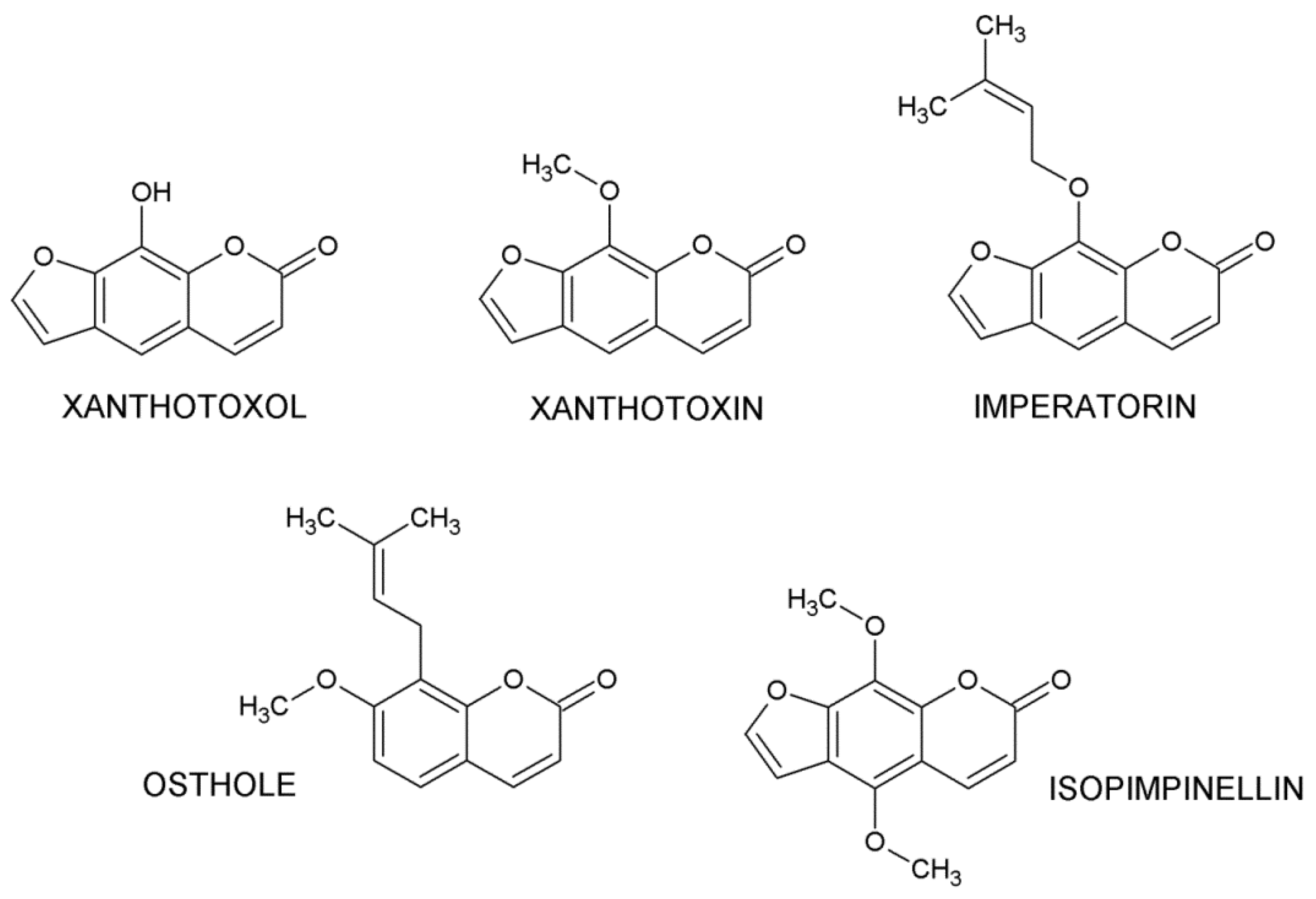
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, P.; Toloudi, M.; Chatziioannou, M.; Ioannou, E.; Knocke, D.R.; Nester, J.; Komiotis, D.; Papasotiriou, I. AnvirzelTM in combination with cisplatin in breast, colon, lung, prostate, melanoma and pancreatic cancer cell lines. BMC Pharmacol. Toxicol. 2013, 14, 18. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Kalirai, H.; Katopodis, P.; Seitz, B.; Viestenz, A.; Coupland, S.E. Electrochemotherapy with bleomycin and cisplatin enhances cytotoxicity in primary and metastatic uveal melanoma cell lines in vitro. Neoplasma 2018, 65, 210–215. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 5, 364–378. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15. [Google Scholar] [CrossRef]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Riccardi, C.; Capasso, D.; Rozza, G.M.; Platella, C.; Montesarchio, D.; Di Gaetano, S.; Marzo, T.; Pratesi, A.; Messori, L.; Roviello, G.N.; et al. Synthesis, DNA binding studies, and antiproliferative activity of novel Pt(II)-complexes with an L-alanyl-based ligand. J. Inorg. Biochem. 2020, 203, 110868. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.; Busto, N.; Fernández-Pampín, N.; Espino, G.; García, B. Appended Aromatic Moieties Determine the Cytotoxicity of Neutral Cyclometalated Platinum(II) Complexes Derived from 2-(2-Pyridyl)benzimidazole. Inorg. Chem. 2020, 59, 4961–4971. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Eigentler, T.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-kappaB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFkappaB- mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef]
- Solomon, L.A.; Ali, S.; Banerjee, S.; Munkarah, A.R.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin by genistein: The role of NF-kappaB. J. Ovarian Res. 2008, 1, 9. [Google Scholar] [CrossRef]
- Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Zhang, Q.Y.; Zheng, G.J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019, 110, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic effects of coumarins against cancer: From chemistry to medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Iqbal, P.F.; Bhat, A.R.; Azam, A. Antiamoebic coumarins from the root bark of Adina cordifolia and their new thiosemicarbazone derivatives. Eur. J. Med. Chem. 2009, 44, 2252–2259. [Google Scholar] [CrossRef]
- Tada, Y.; Shikishima, Y.; Takaishi, Y.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; et al. Coumarins and gamma-pyrone derivatives from Prangos pabularia antibacterial activity and inhibition of cytokine release. Phytochemistry 2002, 59, 649–654. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wu, X.Y.; Mi, J.; Peng, Y.J.; Wang, Z.G.; Liu, Y.; Wu, X.L.; Gao, Y. A new anti-inflammatory alkaloid from roots of Heracleum dissectum. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Andres-Mach, M.; Glensk, M.; Skalicka-Wozniak, K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: A comparative study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Ramesh, B.; Pugalendi, K.V. Impact of 7-hydroxycoumarin on hepaticmarker enzymes in streptozotocin diabetic rats marker enzymes in streptozocin diabetic rats. Indian J. Pharmacol. 2006, 38, 209–210. [Google Scholar]
- Madhavan, G.R.; Balraju, V.; Mallesham, B.; Chakrabarti, R.; Lohray, V.B. Novel coumarin derivatives of heterocyclic compounds as lipid-Lowering agents. Bioorg. Med. Chem. Lett. 2003, 13, 2547–2551. [Google Scholar] [CrossRef]
- Torres, R.; Faini, F.; Modak, B.; Urbina, F.; Labbé, C.; Guerrero, J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry 2006, 67, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.L.; Park, I.H.; Baek, S.H.; Kim, H.Y.; Park, M.K.; Park, J.H. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharmacol. 2004, 93, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Ramanithrasimbola, D.; Rakotondramanan, D.A.; Rasoanaivo, P.; Randriantsoa, A.; Ratsimamanga, S.; Palazzino, G.; Galeffi, C.; Nicoletti, M. Bronchodilator activity of Phymatodes scolopendria (Burm) Ching and its bioactive constituent. J. Ethnopharmacol. 2005, 102, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Haghighitalab, A.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Saeinasab, M.; Haghighi, F. In vitro investigation of anticancer, cell-cycle-inhibitory, and apoptosis-inducing effects of diversin, a natural prenylated coumarin, on bladder carcinoma cells. Z. Nat. C J. Biosci. 2014, 69, 99–109. [Google Scholar] [CrossRef]
- Saidu, N.E.; Valente, S.; Bana, E.; Kirsch, G.; Bagrel, D.; Montenarh, M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorg. Med. Chem. 2012, 20, 1584–1593. [Google Scholar] [CrossRef]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin derivatives as anticancer agents for lung cancer therapy: A review. Anticancer Agents Med. Chem. 2018, 18, 964–984. [Google Scholar] [CrossRef]
- Lee, B.Z.; Lee, I.S.; Pham, C.H.; Jeong, S.K.; Lee, S.; Hong, K.; Yoo, H.M. Apoptosis in leukemic cells induced by anti-proliferative coumarin isolated from the stem bark of Fraxinus rhynchophylla. J. Microbiol. Biotechnol. 2020, 30, 1214–1221. [Google Scholar] [CrossRef]
- Autore, G.; Marzocco, S.; Formisano, C.; Bruno, M.; Rosselli, S.; Jemia, M.B.; Senatore, F. Cytotoxic activity and composition of petroleum ether extract from Magydaris tomentosa (Desf.) W. D. J. Koch (Apiaceae). Molecules 2015, 20, 1571–1578. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Jafari, F.; Mohammadi, Z.; Bazvandi, L.; Hosseinzadeh, L.; Chow, N.; Bhattacharyya, P.; Farzaei, M.H.; Farooqi, A.A.; Nabavi, S.M.; et al. Potential Anticancer Properties of Osthol: A Comprehensive Mechanistic Review. Nutrients 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical Sources, Chemistry, Analysis, and Biological Activity of Furanocoumarins of Pharmaceutical Interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [PubMed]
- Venkata Sairam, K.; Gurupadayya, B.M.; Chandan, R.S.; Nagesha, D.K.; Vishwanathan, B. A review on chemical profile of coumarins and their therapeutic role in the treatment of cancer. Curr. Drug Deliv. 2016, 13, 186–201. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Zhang, Y. Osthole inhibits gastric cancer cell proliferation through regulation of PI3K/AKT. PLoS ONE 2018, 13, e0193449. [Google Scholar] [CrossRef]
- Zhu, X.; Song, X.; Xie, K.; Zhang, X.; He, W.; Liu, F. Osthole induces apoptosis and suppresses proliferation via the PI3K/Akt pathway in intrahepatic cholangiocarcinoma. Int. J. Mol. Med. 2017, 40, 1143–1151. [Google Scholar] [CrossRef]
- Liu, L.; Mao, J.; Wang, Q.; Zhang, Z.; Wu, G.; Tang, Q.; Zhao, B.; Li, L.; Li, Q. In vitro anticancer activities of osthole against renal cell carcinoma cells. Biomed. Pharmacother. 2017, 94, 1020–1027. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhang, Y.; Qu, D.; Feng, X.W.; Chen, Y.; Zhao, L. Osthole suppresses migration and invasion of A549 human lung cancer cells through inhibition of matrix metalloproteinase-2 and matrix metallopeptidase-9 in vitro. Mol. Med. Rep. 2012, 6, 1018–1022. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, J.; Xu, Y.; Huang, X.; Wang, X.; Huang, W.; Li, H. Osthole inhibits ovarian carcinoma cells through LC3-mediated autophagy and GSDME-dependent pyroptosis except for apoptosis. Eur. J. Pharmacol. 2020, 874, 172990. [Google Scholar] [CrossRef]
- Farooq, S.; Dangroo, N.A.; Priya, D.; Banday, J.A.; Sangwan, P.L.; Qurishi, M.A.; Koul, S.; Saxena, A.K. Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Prangos pabularia. PLoS ONE 2014, 9, e108713. [Google Scholar] [CrossRef]
- Abdel Hafez, O.M.; Amin, K.M.; Abdel-Latif, N.A.; Mohamed, T.K.; Ahmed, E.Y.; Maher, T. Synthesis and antitumor activity of some new xanthotoxin derivatives. Eur. J. Med. Chem. 2009, 44, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, W.; Prawat, U.; Saewan, N.; Laphookhieo, S. New coumarins from Clausena lansium twigs. J. Braz. Chem. Soc. 2010, 21, 665–668. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Ryu, S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res. 2007, 21, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Jarząb, A.; Luszczki, J.J.; Guz, M.; Skalicka-Woźniak, K.; Hałasa, M.; Smok-Kalwat, J.; Polberg, K.; Stepulak, A. Combination of osthole and cisplatin against rhabdomyosarcoma TE671 cells yielded additive pharmacologic interaction by means of isobolographic analysis. Anticancer Res. 2018, 38, 205–210. [Google Scholar]
- Grabarska, A.; Luszczki, J.J.; Nowosadzka, E.; Gumbarewicz, E.; Jeleniewicz, W.; Dmoszyńska-Graniczka, M.; Kowalczuk, K.; Kupisz, K.; Polberg, K.; Stepulak, A. Histone deacetylase inhibitor SAHA as potential targeted therapy agent for larynx cancer cells. J. Cancer 2017, 8, 19–28. [Google Scholar] [CrossRef]
- Bobiński, M.; Okła, K.; Luszczki, J.J.; Bednarek, W.; Wawruszak, A.; Moreno-Bueno, G.; Dmoszyńska-Graniczka, M.; Tarkowski, R.; Kotarski, J. Isobolographic analysis demonstrates the additive and synergistic effects of gemcitabine combined with fucoidan in uterine sarcomas and carcinosarcoma cells. Cancers 2019, 12, 107. [Google Scholar] [CrossRef]
- Wawruszak, A.; Luszczki, J.J.; Kalafut, J.; Okla, K.; Halasa, M.; Rivero-Muller, A.; Stepulak, A. Additive Pharmacological Interaction between Cisplatin (CDDP) and Histone Deacetylase Inhibitors (HDIs) in MDA-MB-231 Triple Negative Breast Cancer (TNBC) Cells with Altered Notch1 Activity—An Isobolographic Analysis. Int. J. Mol. Sci. 2019, 20, 3663. [Google Scholar] [CrossRef]
- Grabarska, A.; Skalicka-Woźniak, K.; Kiełbus, M.; Dmoszyńska-Graniczka, M.; Miziak, P.; Szumiło, J.; Nowosadzka, E.; Kowalczuk, K.; Khalifa, S.; Smok-Kalwat, J.; et al. Imperatorin as a Promising Chemotherapeutic Agent Against Human Larynx Cancer and Rhabdomyosarcoma Cells. Molecules 2020, 25, 2046. [Google Scholar] [CrossRef]
- Jarząb, A.; Grabarska, A.; Kiełbus, M.; Jeleniewicz, W.; Dmoszyńska-Graniczka, M.; Skalicka-Woźniak, K.; Sieniawska, E.; Polberg, K.; Stepulak, A. Osthole induces apoptosis, suppresses cell-cycle progression and proliferation of cancer cells. Anticancer Res. 2014, 34, 6473–6480. [Google Scholar]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.A.; Gholamian Dehkordi, N.; Ghamghami, M.; Amiri, A.H.; Dalir Abdolahinia, E.; Elahian, F. ABC-transporter blockage mediated by xanthotoxin and bergapten is the major pathway for chemosensitization of multidrug-resistant cancer cells. Toxicol. Appl. Pharmacol. 2017, 337, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, F.; Li, X.; Liu, Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019, 514, 510–517. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, X.; Qiu, J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. Pharmacother. 2020, 131, 110676. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: A practical application. Naunyn Schmiedeberg’s Arch. Pharmacol. 2007, 375, 105–114. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Czuczwar, S.J. Biphasic characteristic of interactions between stiripentol and carbamazepine in the mouse maximal electroshock-induced seizure model: A three-dimensional isobolographic analysis. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 374, 51–64. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Filip, D.; Czuczwar, S.J. Additive interactions of pregabalin with lamotrigine, oxcarbazepine and topiramate in the mouse maximal electroshock-induced seizure model: A type I isobolographic analysis for non-parallel dose-response relationship curves. Epilepsy Res. 2010, 9, 166–175. [Google Scholar] [CrossRef]
- Litchfield, J.T.J.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]
| Drug | FM55P IC50 (µM ± S.E.) | FM55M2 IC50 (µM ± S.E.) |
|---|---|---|
| CDDP | 1.49 ± 0.30 | 1.70 ± 0.35 |
| OST | 67.26 ± 16.35 2 | 89.58 ± 16.30 1 |
| XIN | 182.64 ± 30.59 1 | 179.74 ± 19.19 2 |
| XOL | 93.09 ± 10.68 2 | 66.27 ± 12.57 1 |
| ISO | 156.81 ± 19.08 1 | 129.36 ± 19.05 2 |
| IMP | 151.58 ± 9.85 2 | 180.53 ± 7.56 2 |
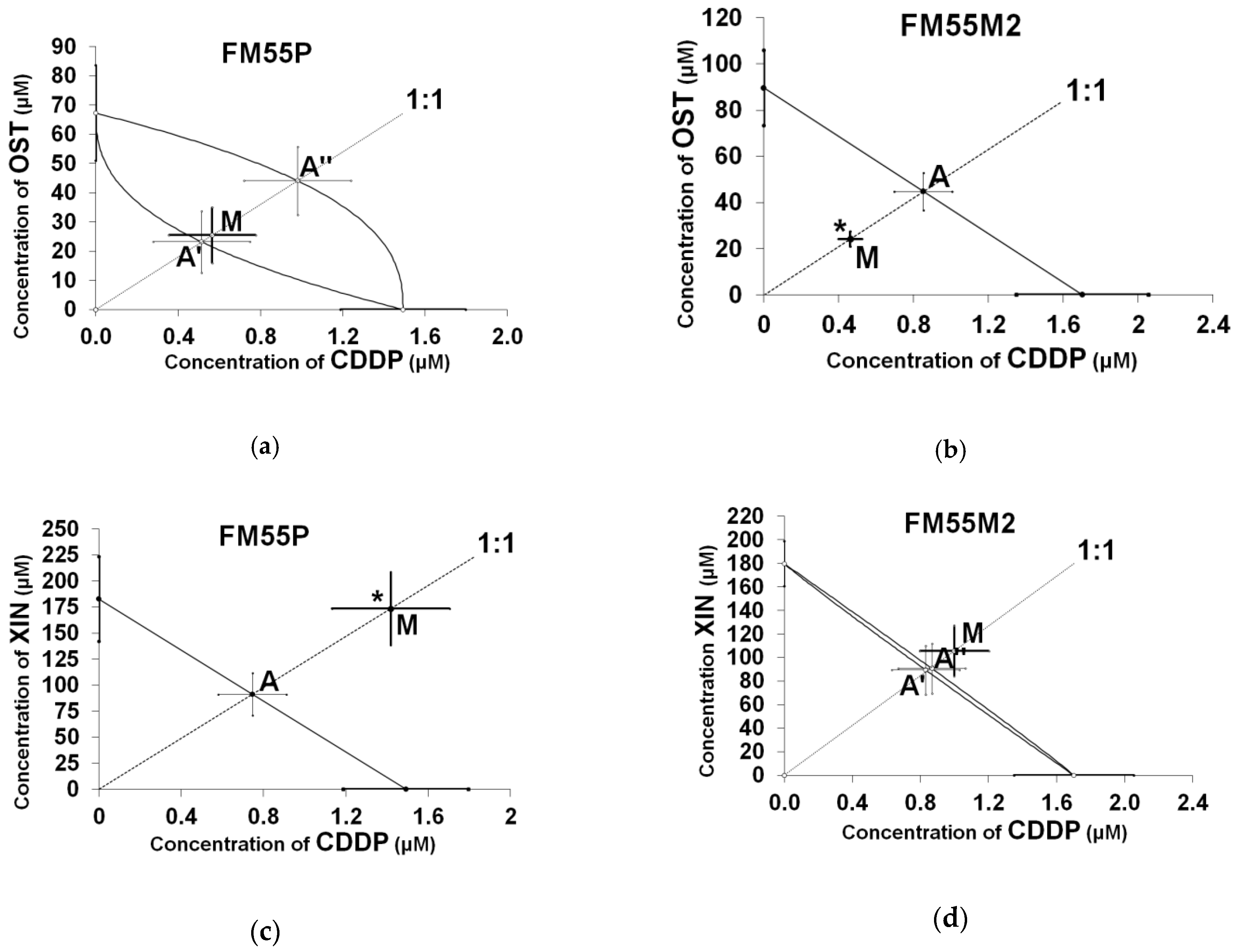
| Cell Line | Drug Combination | IC50 exp (µM ± S.E.) | nexp | IC50 add (µM ± S.E.) | nadd | Interaction |
|---|---|---|---|---|---|---|
| FM55P | CDDP + ISO | 190.65 ± 34.95 *** | 96 | 79.15 ± 9.69 | 140 | Antagonistic |
| FM55M2 | CDDP + XOL | 32.24 ± 3.64 | 96 | 33.99 ± 6.46 | 140 | Additive |
| FM55P | CDDP + XIN | 174.95 ± 35.28 * | 96 | 92.07 ± 20.45 | 140 | Antagonistic |
| FM55M2 | CDDP + OST | 24.73 ± 3.34 * | 96 | 45.64 ± 8.32 | 164 | Synergistic |
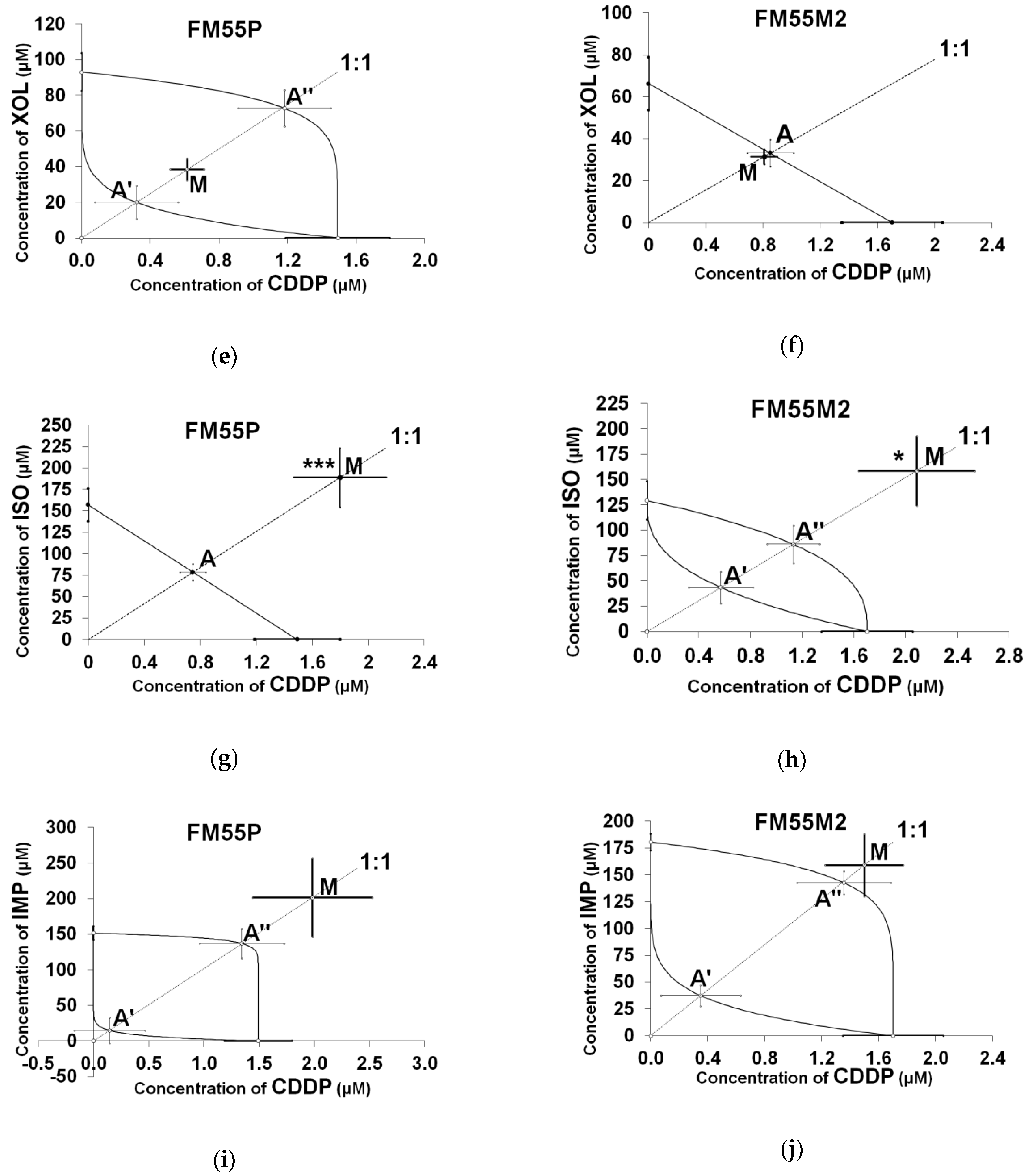
| Cell Line | Drug Combination | IC50 exp (µM ± S.E.) | nexp | L-IC50 add (µM ± S.E.) | nadd | U-IC50 add (µM ± S.E.) | Interaction |
|---|---|---|---|---|---|---|---|
| FM55P | CDDP + IMP | 203.35 ± 55.54 | 96 | 14.83 ± 18.64 | 116 | 138.36 ± 20.99 | Additive |
| FM55M2 | CDDP + IMP | 160.55 ± 29.29 | 96 | 37.86 ± 10.04 | 140 | 143.99 ± 11.10 | Additive |
| FM55M2 | CDDP + ISO | 160.53 ± 34.71 * | 96 | 43.96 ± 15.76 | 140 | 87.10 ± 19.07 | Antagonistic |
| FM55P | CDDP + XOL | 39.00 ± 6.20 | 72 | 20.29 ± 9.57 | 116 | 73.96 ± 10.46 | Additive |
| FM55M2 | CDDP + XIN | 106.52 ± 21.62 | 96 | 90.30 ± 21.05 | 140 | 91.57 ± 21.05 | Additive |
| FM55P | CDDP + OST | 26.03 ± 9.71 | 96 | 23.66 ± 10.85 | 164 | 45.09 ± 11.98 | Additive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska-Łuczka, P.; Grabarska, A.; Florek-Łuszczki, M.; Plewa, Z.; Łuszczki, J.J. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 537. https://doi.org/10.3390/ijms22020537
Wróblewska-Łuczka P, Grabarska A, Florek-Łuszczki M, Plewa Z, Łuszczki JJ. Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. International Journal of Molecular Sciences. 2021; 22(2):537. https://doi.org/10.3390/ijms22020537
Chicago/Turabian StyleWróblewska-Łuczka, Paula, Aneta Grabarska, Magdalena Florek-Łuszczki, Zbigniew Plewa, and Jarogniew J. Łuszczki. 2021. "Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells" International Journal of Molecular Sciences 22, no. 2: 537. https://doi.org/10.3390/ijms22020537
APA StyleWróblewska-Łuczka, P., Grabarska, A., Florek-Łuszczki, M., Plewa, Z., & Łuszczki, J. J. (2021). Synergy, Additivity, and Antagonism between Cisplatin and Selected Coumarins in Human Melanoma Cells. International Journal of Molecular Sciences, 22(2), 537. https://doi.org/10.3390/ijms22020537





