Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma
Abstract
1. Introduction
2. Results
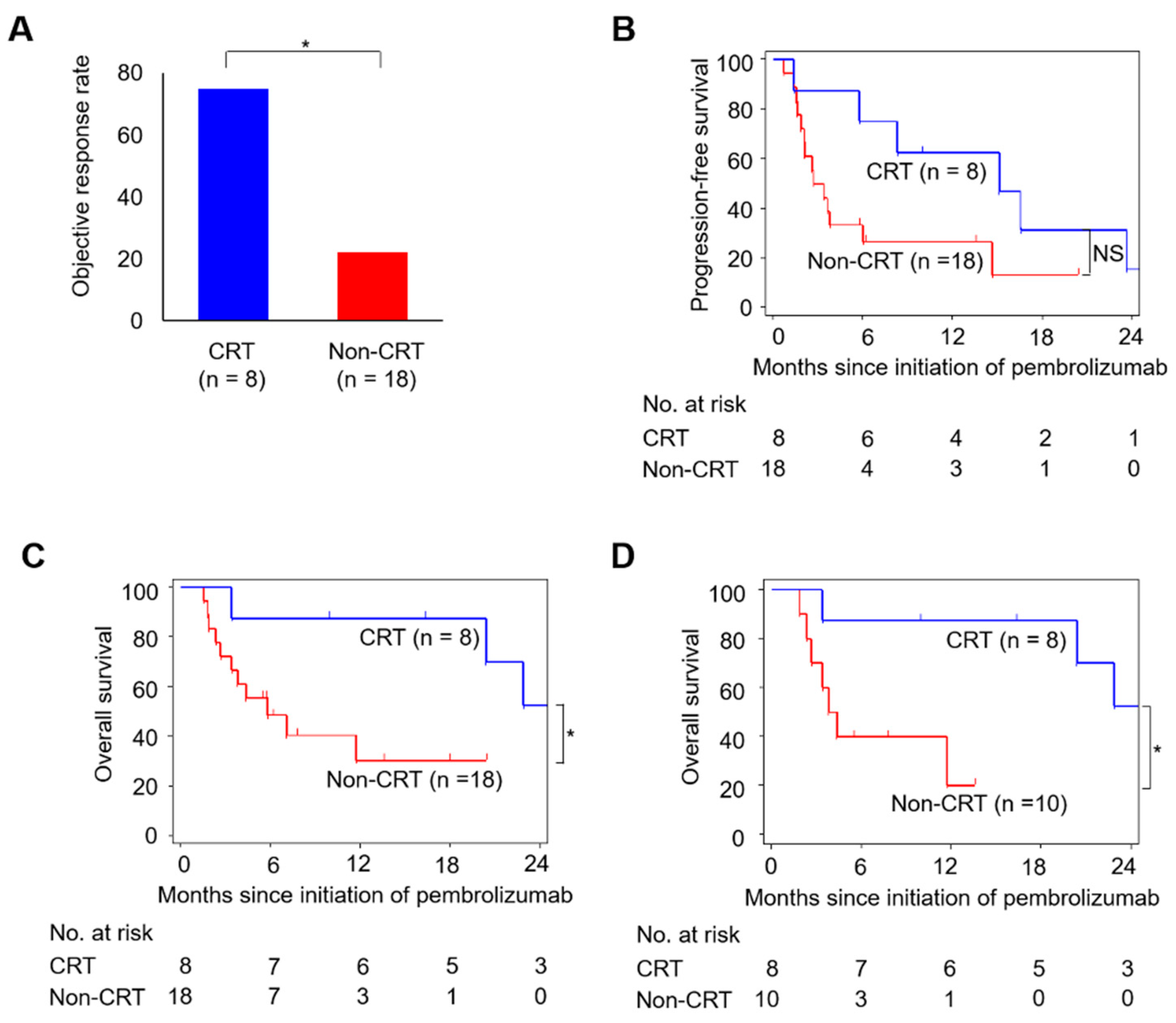
2.1. Cisplatin-Based CRT Improved the Efficacy of Later Pembrolizumab Therapy in Patients with Advanced UC
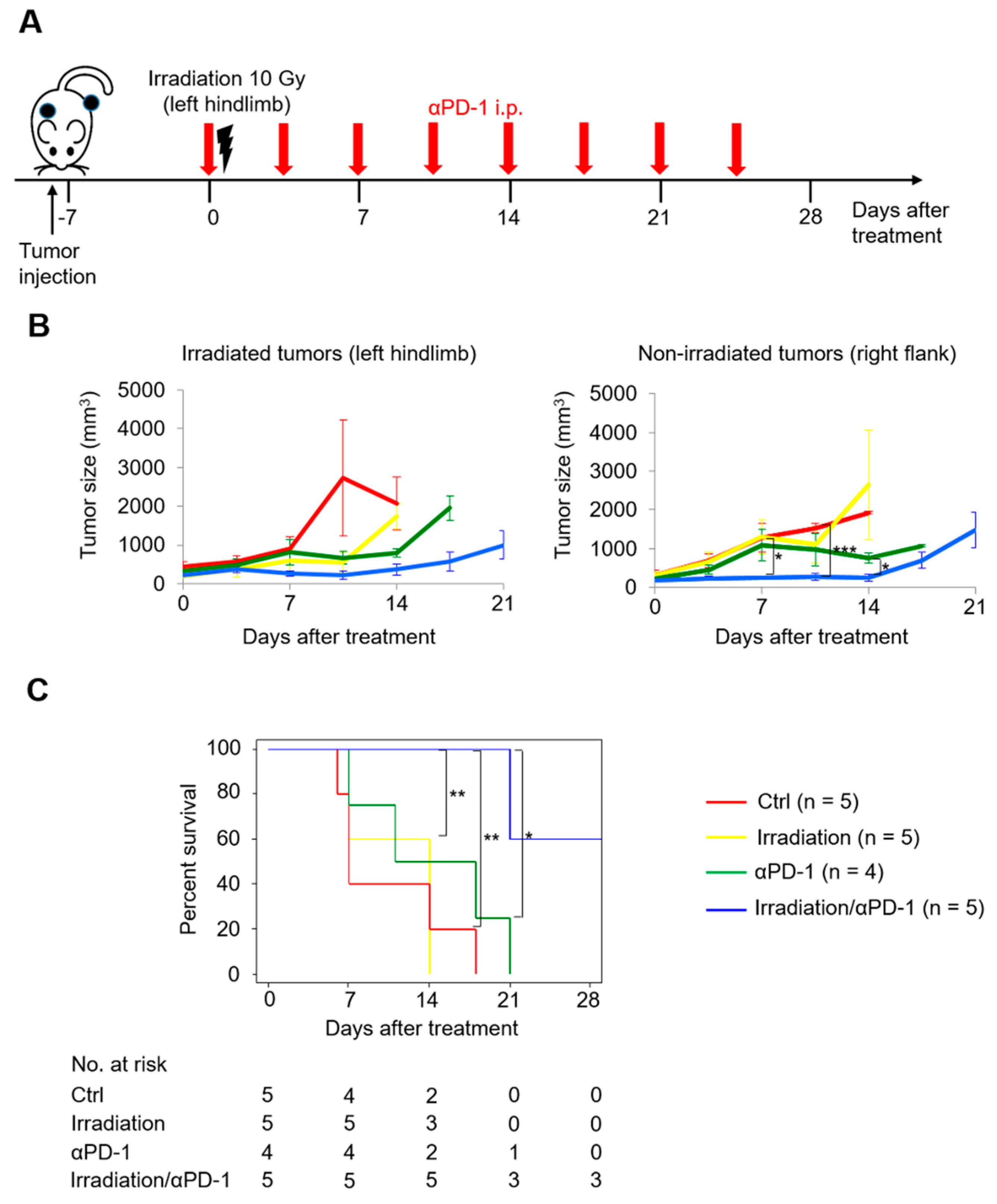
2.2. Abscopal Effects in a UC Mouse Model
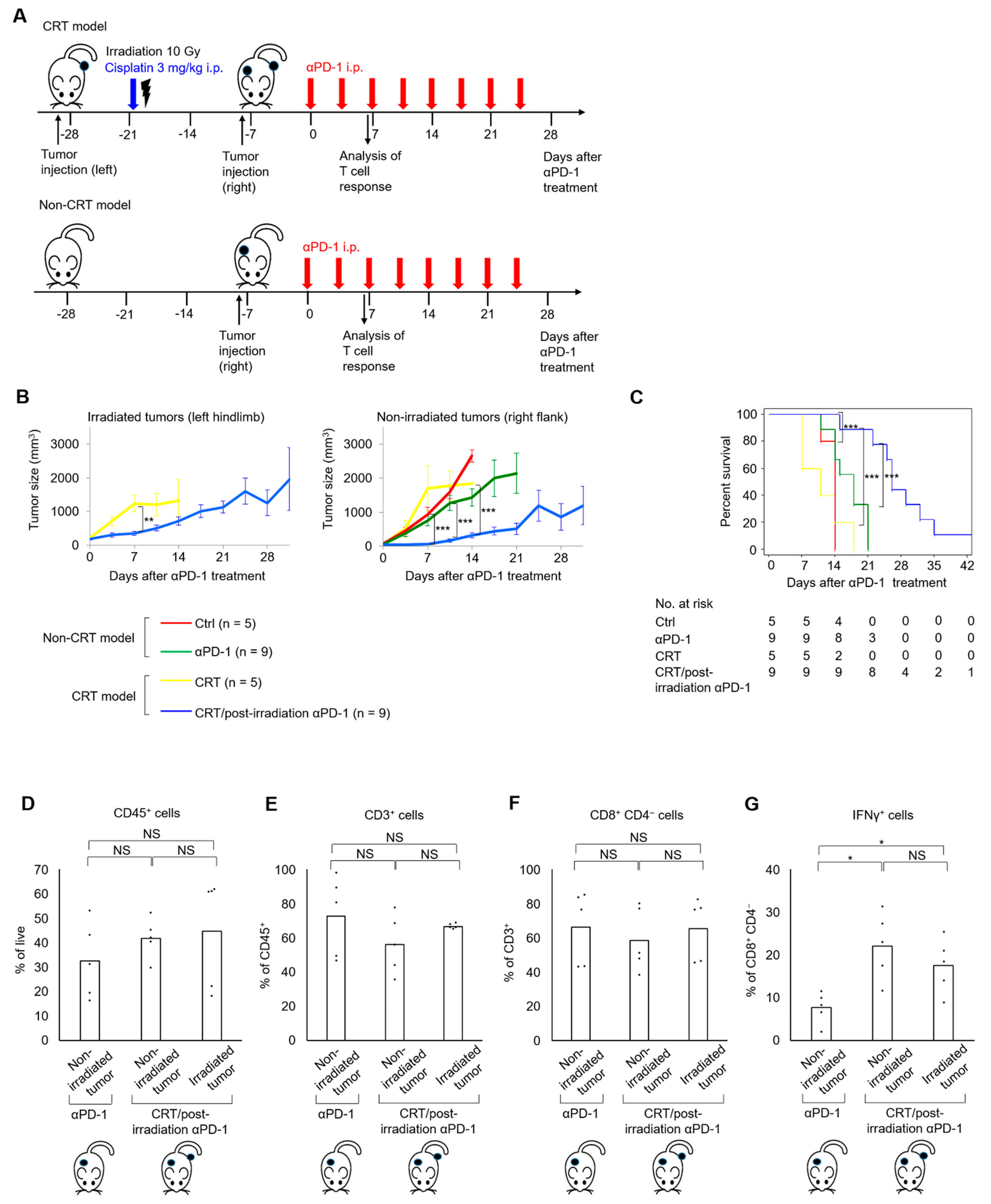
2.3. Combination of Cisplatin and Irradiation Promoted Antitumor Effects of Later Anti–PD-1 Treatment in UC
2.4. Combination of Cisplatin and Irradiation Strongly Exerted Direct Cytotoxic Effects on UC
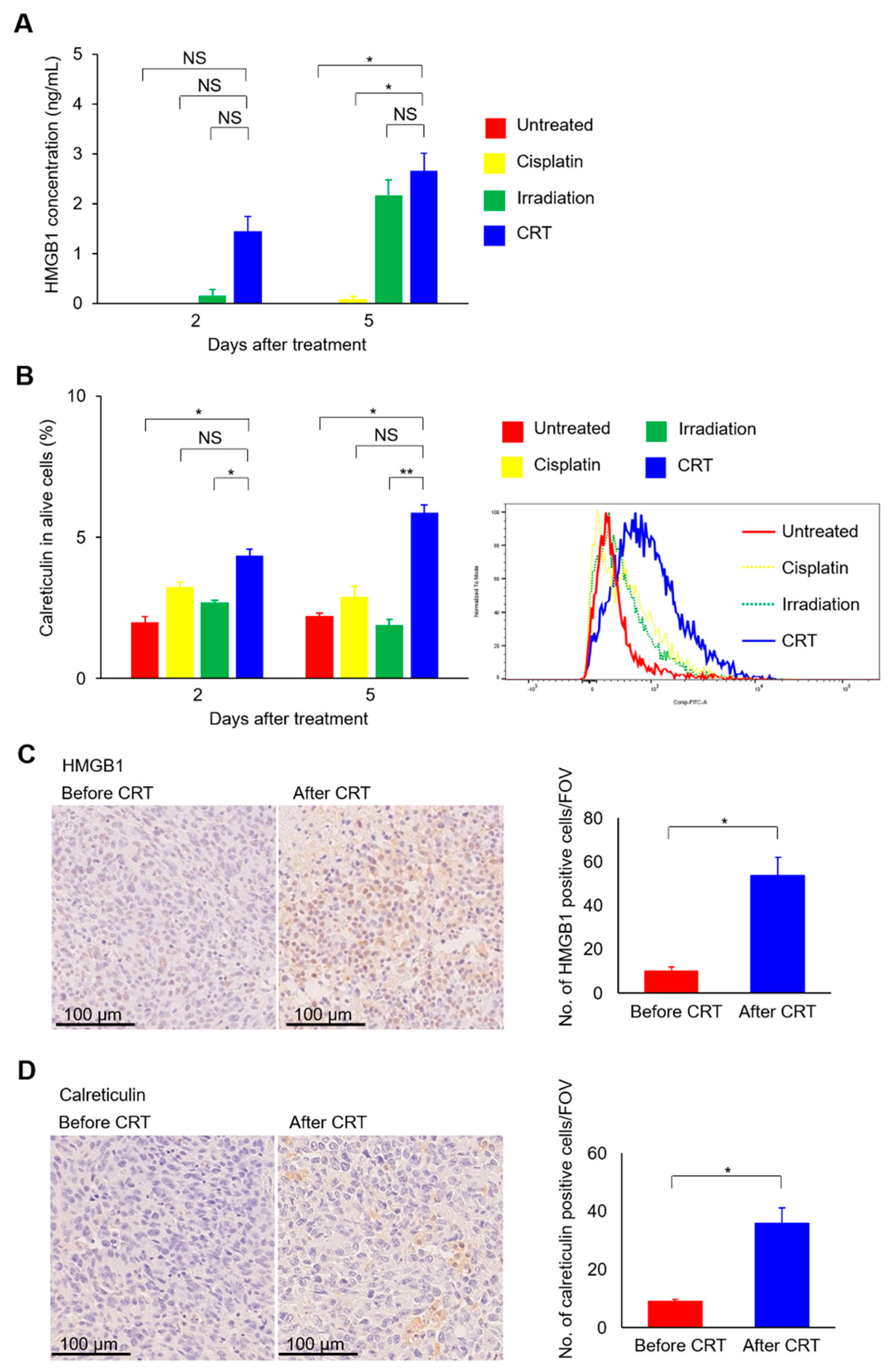
2.5. Combination of Cisplatin and Irradiation Strongly Induced Immunogenic Cell Death in UC
3. Discussion
4. Materials and Methods
4.1. Clinical Investigations in an Advanced UC Patient Cohort
4.2. Mice and Cell Line
4.3. In Vivo Tumor Inoculation and Treatment
4.4. Tumor Irradiation
4.5. Flow Cytometric Analysis
4.6. Enzyme-Linked Immunoassay (ELISA)
4.7. Cytotoxicity Assay
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellmunt, J.; von der Maase, H.; Mead, G.M.; Skoneczna, I.; De Santis, M.; Daugaard, G.; Boehle, A.; Chevreau, C.; Paz-Ares, L.; Laufman, L.R.; et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J. Clin. Oncol. 2012, 30, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Necchi, A.; Rosen, G.; Hariharan, S.; Apolo, A.B. Anti-Programmed Cell Death 1/Ligand 1 (PD-1/PD-L1) Antibodies for the Treatment of Urothelial Carcinoma: State of the Art and Future Development. Clin. Genitourin. Cancer 2018, 16, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Tamura, D.; Jinnouchi, N.; Abe, M.; Ikarashi, D.; Matsuura, T.; Kato, R.; Maekawa, S.; Kato, Y.; Kanehira, M.; Takata, R.; et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: Experience in real-world clinical practice. Int. J. Clin. Oncol. 2020, 25, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Obara, W.; Kato, R.; Kato, Y.; Kanehira, M.; Takata, R. Recent progress in immunotherapy for urological cancer. Int. J. Urol. 2017, 24, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Miyake, M.; Hori, S.; Ohnishi, S.; Toritsuka, M.; Fujii, T.; Shimizu, T.; Owari, T.; Morizawa, Y.; Gotoh, D.; Itami, Y.; et al. Supplementary granulocyte macrophage colony-stimulating factor to chemotherapy and programmed death-ligand 1 blockade decreases local recurrence after surgery in bladder cancer. Cancer Sci. 2019, 110, 3315–3327. [Google Scholar] [CrossRef]
- Takeyama, Y.; Kato, M.; Tamada, S.; Azuma, Y.; Shimizu, Y.; Iguchi, T.; Yamasaki, T.; Gi, M.; Wanibuchi, H.; Nakatani, T. Myeloid-derived suppressor cells are essential partners for immune checkpoint inhibitors in the treatment of cisplatin-resistant bladder cancer. Cancer Lett. 2020, 479, 89–99. [Google Scholar] [CrossRef]
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef]
- Chen, R.C.; Shipley, W.U.; Efstathiou, J.A.; Zietman, A.L. Trimodality bladder preservation therapy for muscle-invasive bladder cancer. J. Natl. Compr. Canc. Netw. 2013, 11, 952–960. [Google Scholar] [CrossRef]
- Kijima, T.; Tanaka, H.; Koga, F.; Masuda, H.; Yoshida, S.; Yokoyama, M.; Ishioka, J.; Matsuoka, Y.; Saito, K.; Kihara, K.; et al. Selective tetramodal bladder-preservation therapy, incorporating induction chemoradiotherapy and consolidative partial cystectomy with pelvic lymph node dissection for muscle-invasive bladder cancer: Oncological and functional outcomes of 107 patients. BJU Int. 2019, 124, 242–250. [Google Scholar] [CrossRef]
- Koga, F.; Kihara, K.; Yoshida, S.; Yokoyama, M.; Saito, K.; Masuda, H.; Fujii, Y.; Kawakami, S. Selective bladder-sparing protocol consisting of induction low-dose chemoradiotherapy plus partial cystectomy with pelvic lymph node dissection against muscle-invasive bladder cancer: Oncological outcomes of the initial 46 patients. BJU Int. 2012, 109, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rompré-Brodeur, A.; Shinde-Jadhav, S.; Ayoub, M.; Piccirillo, C.A.; Seuntjens, J.; Brimo, F.; Mansure, J.J.; Kassouf, W. PD-1/PD-L1 Immune Checkpoint Inhibition with Radiation in Bladder Cancer: In Situ and Abscopal Effects. Mol. Cancer Ther. 2020, 19, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Garasa, S.; Barbes, B.; Solorzano, J.L.; Perez-Gracia, J.L.; Labiano, S.; Sanmamed, M.F.; Azpilikueta, A.; Bolaños, E.; et al. Abscopal Effects of Radiotherapy Are Enhanced by Combined Immunostimulatory mAbs and Are Dependent on CD8 T Cells and Crosspriming. Cancer Res. 2016, 76, 5994–6005. [Google Scholar] [CrossRef]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef]
- Kroon, P.; Frijlink, E.; Iglesias-Guimarais, V.; Volkov, A.; van Buuren, M.M.; Schumacher, T.N.; Verheij, M.; Borst, J.; Verbrugge, I. Radiotherapy and Cisplatin Increase Immunotherapy Efficacy by Enabling Local and Systemic Intratumoral T-cell Activity. Cancer Immunol. Res. 2019, 7, 670–682. [Google Scholar] [CrossRef]
- Luo, R.; Firat, E.; Gaedicke, S.; Guffart, E.; Watanabe, T.; Niedermann, G. Cisplatin Facilitates Radiation-Induced Abscopal Effects in Conjunction with PD-1 Checkpoint Blockade Through CXCR3/CXCL10-Mediated T-cell Recruitment. Clin. Cancer Res. 2019, 25, 7243–7255. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, 1. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef]
- Li, D.; Chen, R.; Wang, Y.W.; Fornace, A.J., Jr.; Li, H.H. Prior irradiation results in elevated programmed cell death protein 1 (PD-1) in T cells. Int. J. Radiat. Biol. 2018, 94, 488–494. [Google Scholar] [CrossRef]
- Gong, J.; Le, T.Q.; Massarelli, E.; Hendifar, A.E.; Tuli, R. Radiation therapy and PD-1/PD-L1 blockade: The clinical development of an evolving anticancer combination. J. Immunother. Cancer 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ye, W.; Xiao, R.; Silvin, C.; Padget, M.; Hodge, J.W.; Van Waes, C.; Schmitt, N.C. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Tree, A.C.; Jones, K.; Hafeez, S.; Sharabiani, M.T.A.; Harrington, K.J.; Lalondrelle, S.; Ahmed, M.; Huddart, R.A. Dose-limiting Urinary Toxicity With Pembrolizumab Combined With Weekly Hypofractionated Radiation Therapy in Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1168–1171. [Google Scholar] [CrossRef]
- Daro-Faye, M.; Kassouf, W.; Souhami, L.; Marcq, G.; Cury, F.; Niazi, T.; Sargos, P. Combined radiotherapy and immunotherapy in urothelial bladder cancer: Harnessing the full potential of the anti-tumor immune response. World J. Urol. 2020. [Google Scholar] [CrossRef]
- Fukushima, H.; Kijima, T.; Fukuda, S.; Moriyama, S.; Uehara, S.; Yasuda, Y.; Tanaka, H.; Yoshida, S.; Yokoyama, M.; Matsuoka, Y.; et al. Impact of radiotherapy to the primary tumor on the efficacy of pembrolizumab for patients with advanced urothelial cancer: A preliminary study. Cancer Med. 2020, 9, 8355–8363. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall, n (%) | CRT Group, n (%) | Non-CRT Group, n (%) | p-Value | |
|---|---|---|---|---|---|
| No. of patients | 26 (100) | 8 (31) | 18 (69) | ||
| Age (years) | 74 (36–87) | 74 (46–86) | 76 (36–87) | 0.96 | |
| Sex | Male | 19 (73) | 8 (100) | 11 (61) | 0.039 |
| Female | 7 (27) | 0 (0) | 7 (39) | ||
| ECOG PS | 0–1 | 23 (88) | 7 (88) | 16 (89) | 0.92 |
| ≥2 | 3 (12) | 1 (13) | 2 (11) | ||
| Primary site | Bladder | 18 (69) | 8 (100) | 10 (56) | 0.023 |
| UUT | 8 (31) | 0 (0) | 8 (44) | ||
| Lymph node metastasis | No | 7 (21) | 1 (13) | 6 (33) | 0.27 |
| Yes | 19 (73) | 7 (88) | 12 (67) | ||
| Visceral metastasis | No | 11 (42) | 2 (25) | 9 (50) | 0.23 |
| Yes | 15 (58) | 6 (75) | 9 (50) | ||
| Previous curative surgery | No | 15 (42) | 5 (63) | 10 (56) | 0.74 |
| Yes | 11 (58) | 3 (38) | 8 (44) | ||
| Line of pembrolizumab | 2nd | 23 (88) | 7 (88) | 16 (89) | 0.92 |
| 3rd or later | 3 (12) | 1 (13) | 2 (11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, H.; Yoshida, S.; Kijima, T.; Nakamura, Y.; Fukuda, S.; Uehara, S.; Yasuda, Y.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; et al. Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma. Int. J. Mol. Sci. 2021, 22, 535. https://doi.org/10.3390/ijms22020535
Fukushima H, Yoshida S, Kijima T, Nakamura Y, Fukuda S, Uehara S, Yasuda Y, Tanaka H, Yokoyama M, Matsuoka Y, et al. Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma. International Journal of Molecular Sciences. 2021; 22(2):535. https://doi.org/10.3390/ijms22020535
Chicago/Turabian StyleFukushima, Hiroshi, Soichiro Yoshida, Toshiki Kijima, Yuki Nakamura, Shohei Fukuda, Sho Uehara, Yosuke Yasuda, Hajime Tanaka, Minato Yokoyama, Yoh Matsuoka, and et al. 2021. "Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma" International Journal of Molecular Sciences 22, no. 2: 535. https://doi.org/10.3390/ijms22020535
APA StyleFukushima, H., Yoshida, S., Kijima, T., Nakamura, Y., Fukuda, S., Uehara, S., Yasuda, Y., Tanaka, H., Yokoyama, M., Matsuoka, Y., & Fujii, Y. (2021). Combination of Cisplatin and Irradiation Induces Immunogenic Cell Death and Potentiates Postirradiation Anti–PD-1 Treatment Efficacy in Urothelial Carcinoma. International Journal of Molecular Sciences, 22(2), 535. https://doi.org/10.3390/ijms22020535








