Molecular Mechanisms of Succinimide Formation from Aspartic Acid Residues Catalyzed by Two Water Molecules in the Aqueous Phase
Abstract
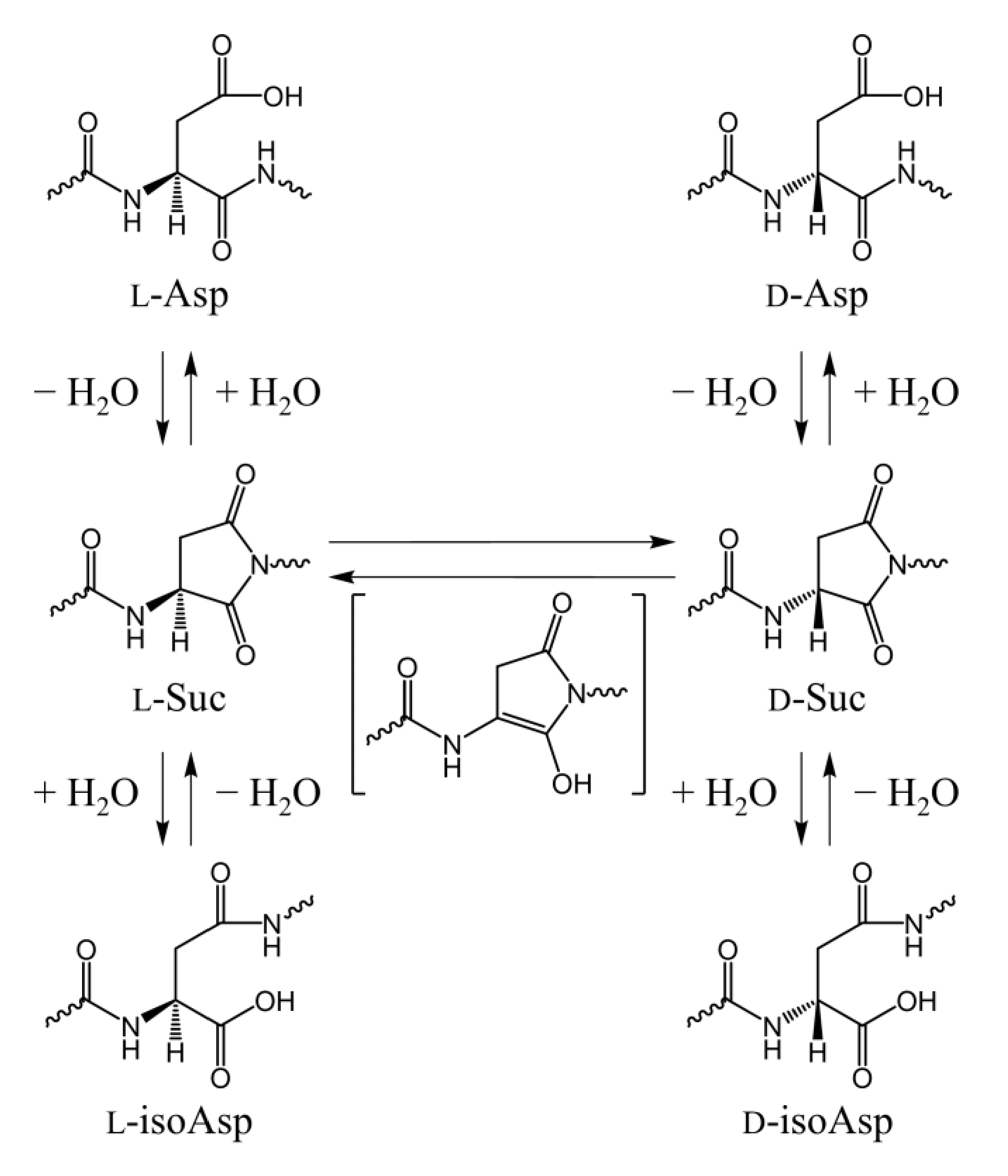
1. Introduction
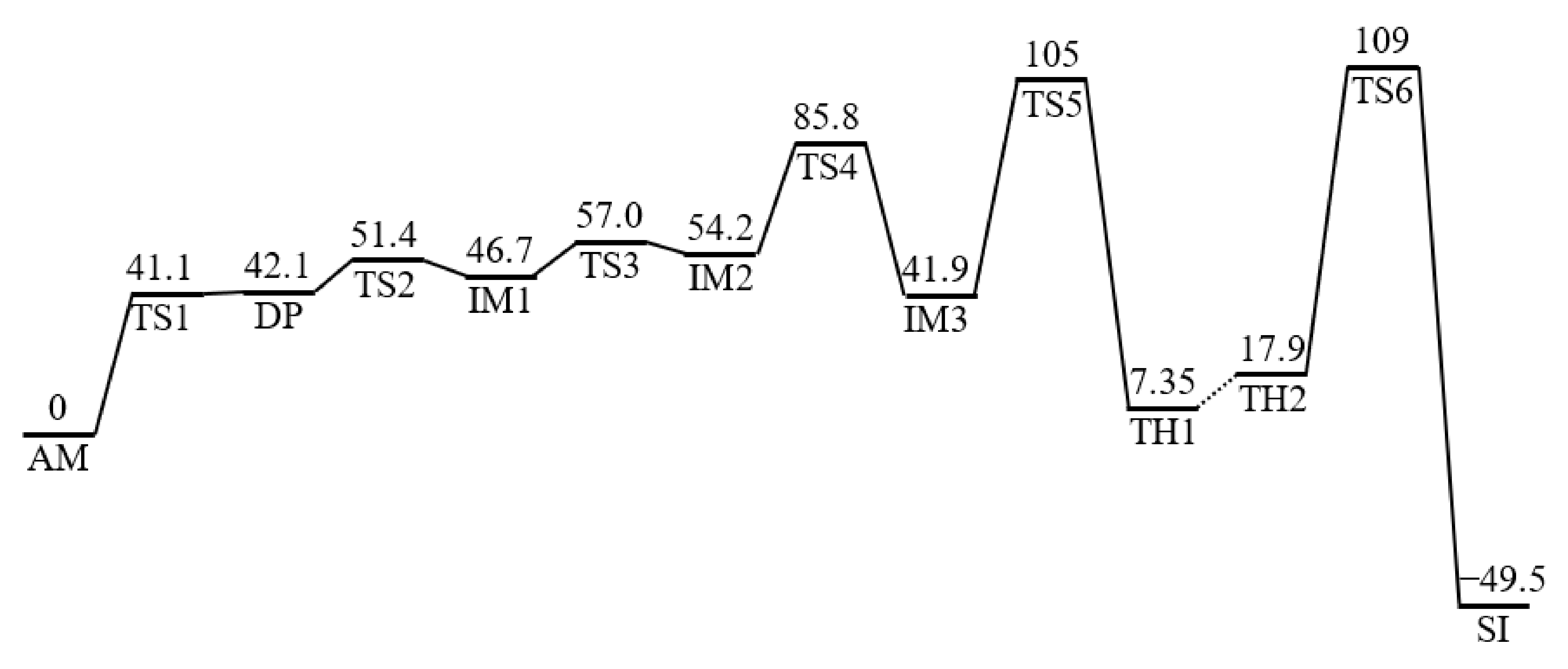
2. Results and Discussion
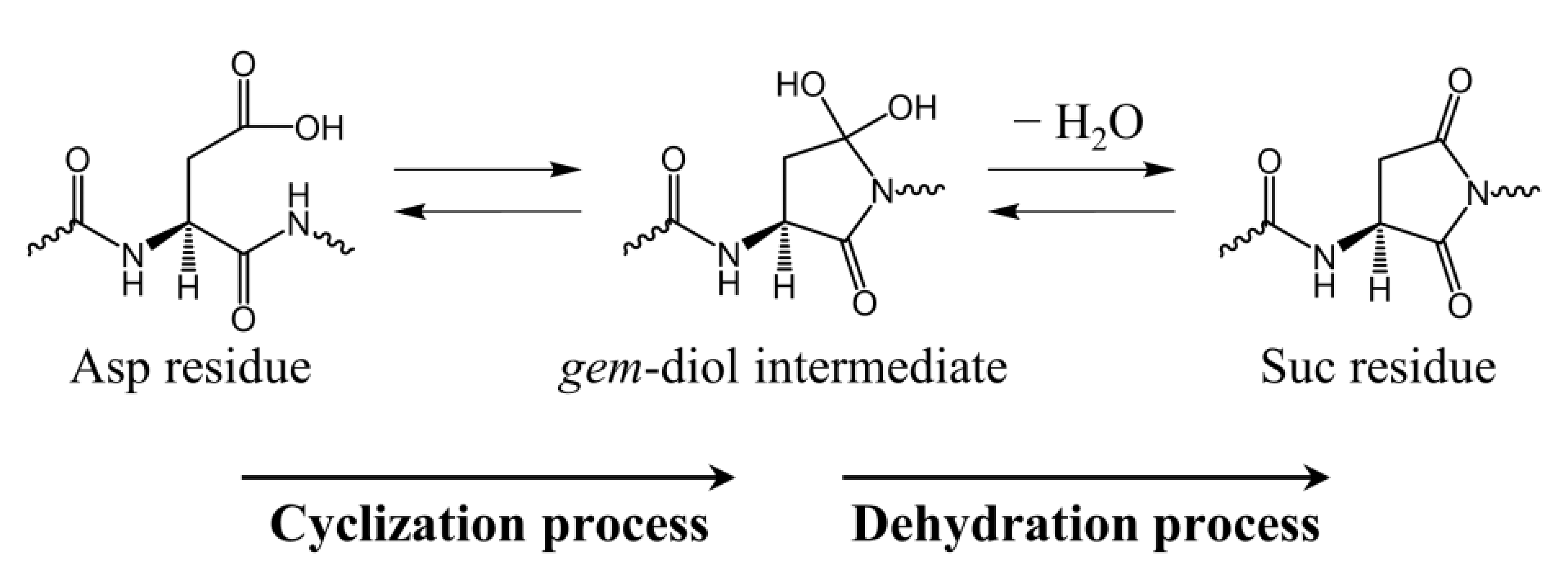
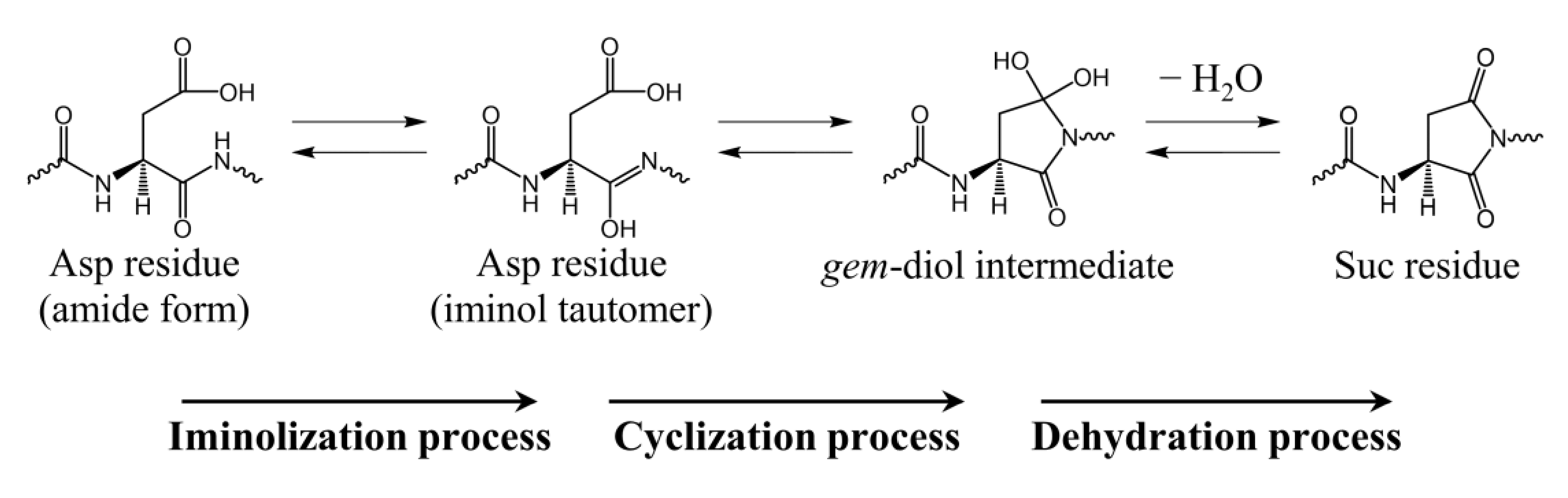
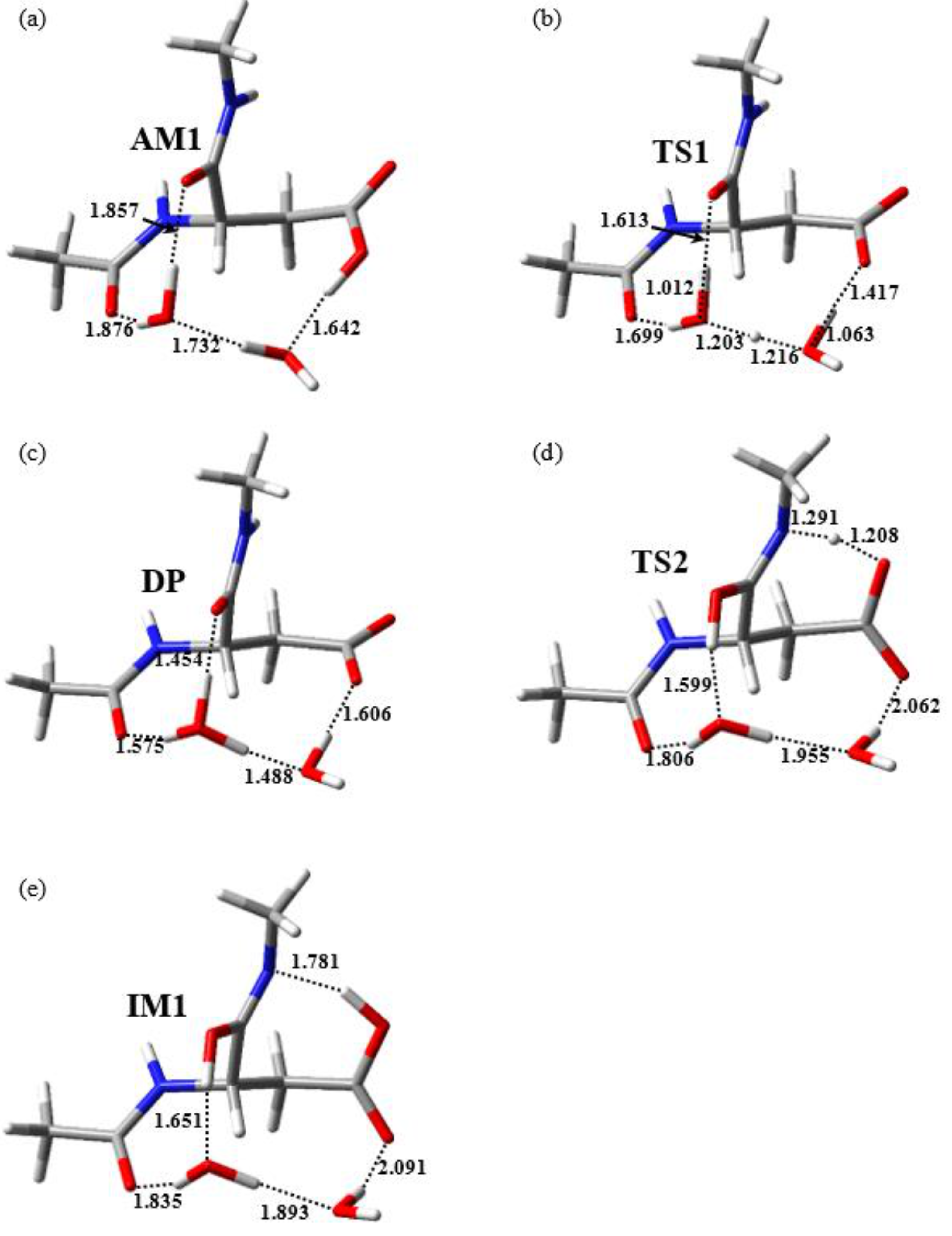
2.1. Cyclization Step
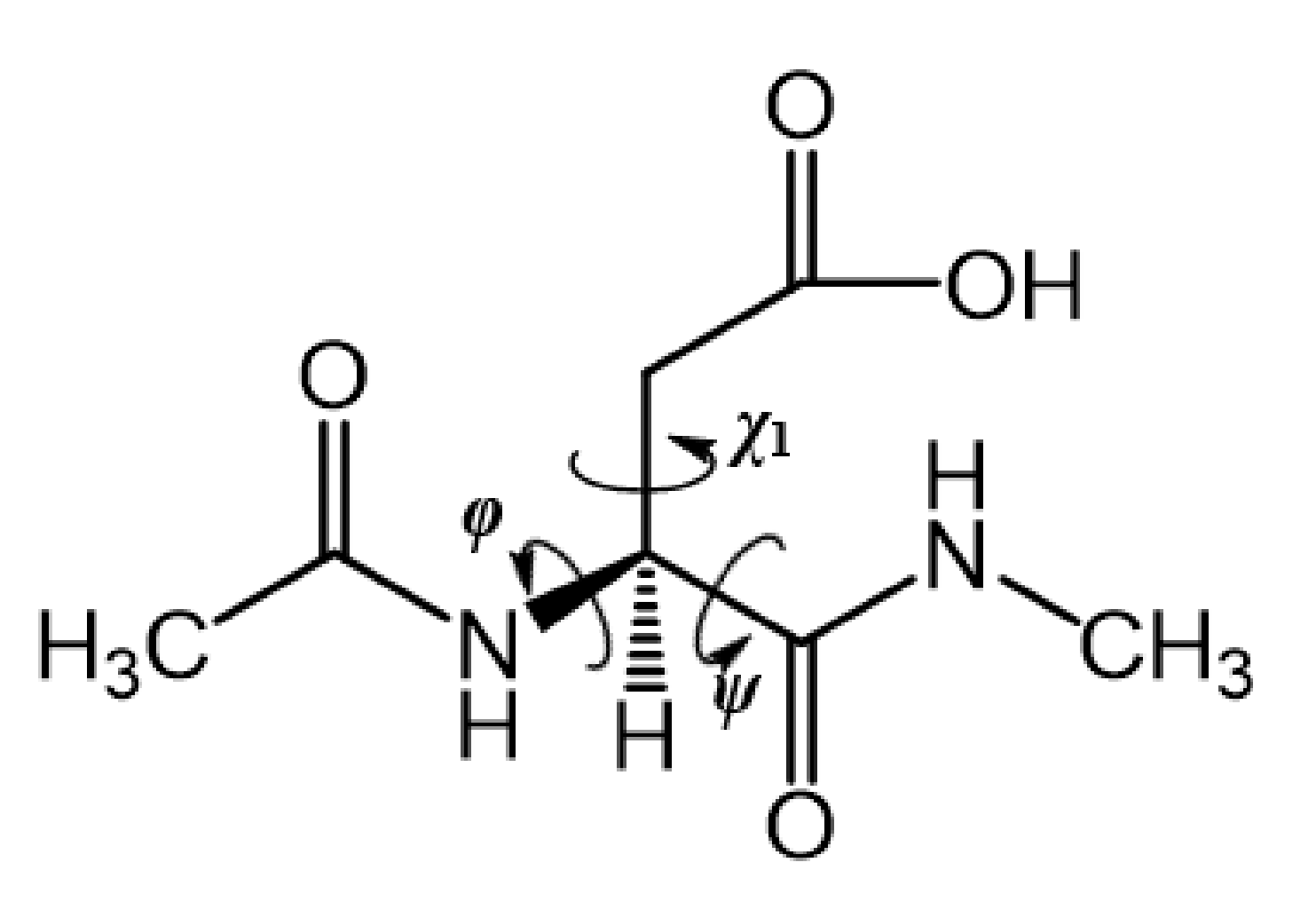
2.2. Conformational Changes of the Iminol Tautomer
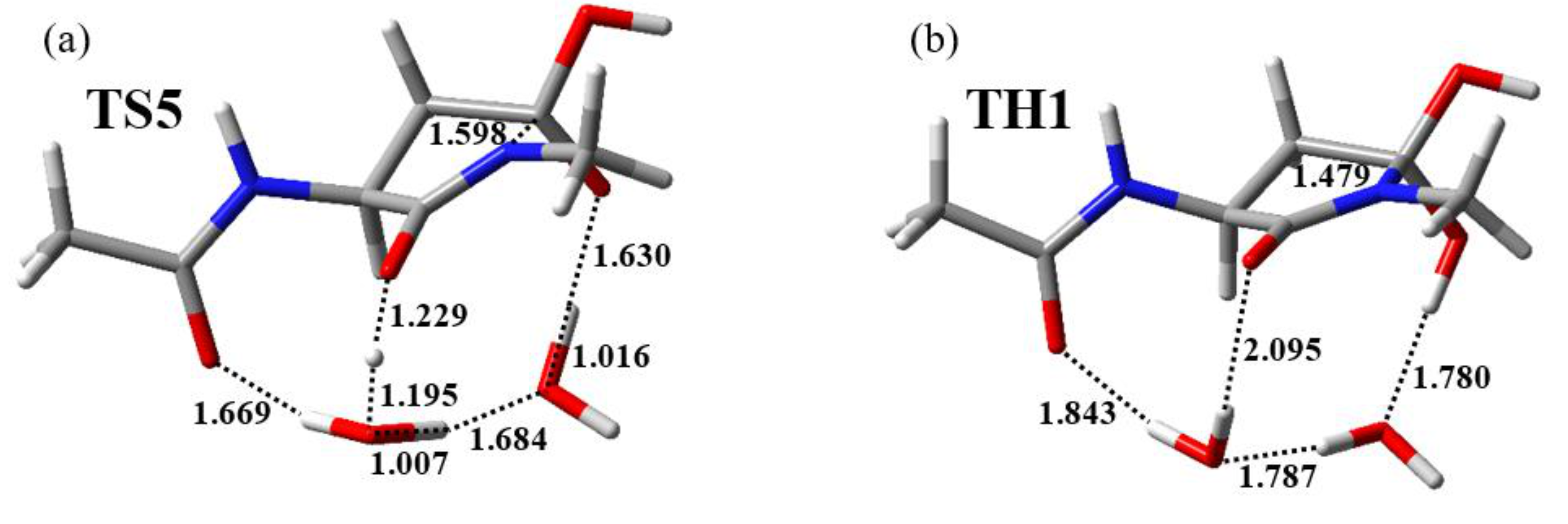
2.3. Cyclization Process
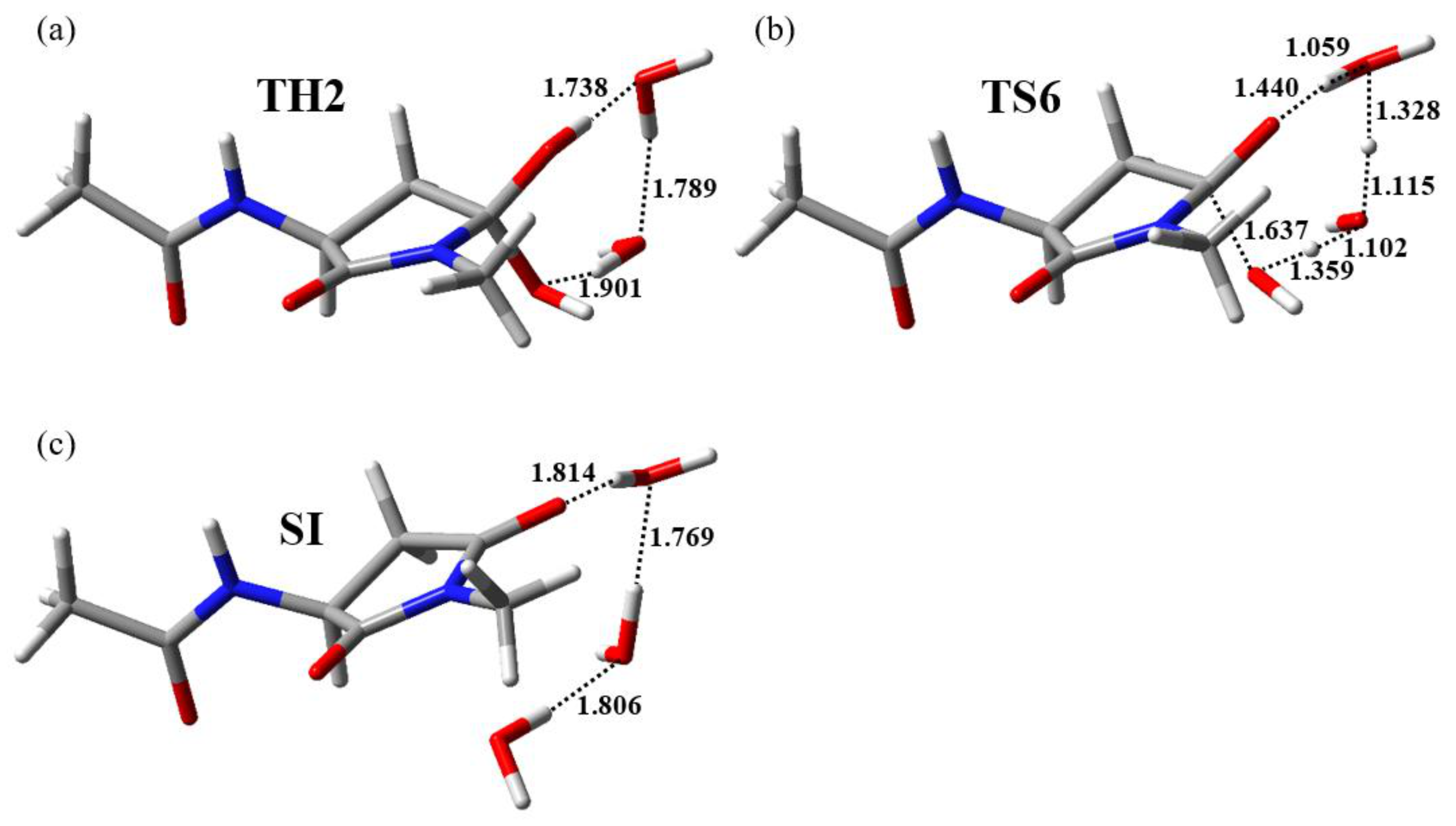
2.4. Dehydration Process
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujii, N.; Muraoka, S.; Satoh, K.; Hori, H.; Harada, K. Racemization of aspartic acids at specific sites in αA-crystallin from aged human lens. Biomed. Res. 1991, 12, 315–321. [Google Scholar] [CrossRef]
- Fujii, N.; Saito, T. Homochirality and life. Chem. Rec. 2004, 4, 267–278. [Google Scholar] [CrossRef]
- Fujii, N.; Sakaue, H.; Sasaki, H. A rapid, comprehensive liquid chromatography-mass spectrometry (LC-MS)-based survey of the Asp isomers in crystallins from human cataract lenses. J. Biol. Chem. 2012, 287, 39992–40002. [Google Scholar] [CrossRef]
- Geiger, T.; Clarke, S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar]
- Hooi, M.Y.; Raftery, M.J.; Truscott, R.J. Interconversion of the peptide isoforms of aspartate: Stability of isoaspartates. Mech. Ageing Dev. 2013, 134, 103–109. [Google Scholar] [CrossRef]
- Patel, K.; Borchardt, R.T. Chemical pathways of peptide degradation. II. Kinetics of deamidation of an asparaginyl residue in a model hexapeptide. Pharm. Res. 1990, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Tyler-Cross, R.; Schirch, V. Effects of amino acid sequence, buffers, and ionic strength on the rate and mechanism of deamidation of asparagine residues in small peptides. J. Biol. Chem. 1991, 266, 22549–22556. [Google Scholar] [CrossRef]
- Fujii, N.; Satoh, K.; Harada, K.; Ishibashi, Y. Simultaneous stereoinversion and isomerization at specific aspartic acid residues in αA-crystallin from human lens. J. Biochem. 1994, 116, 663–669. [Google Scholar] [CrossRef]
- Fujii, N.; Ishibashi, Y.; Satoh, K.; Fujino, M.; Harada, K. Simultaneous racemization and isomerization at specific aspartic acid residues in αB-crystallin from the aged human lens. Biochim. Biophys. Acta 1994, 1204, 157–163. [Google Scholar] [CrossRef]
- Kaji, Y.; Oshika, T.; Takazawa, Y.; Fukayama, M.; Takata, T.; Fujii, N. Localization of d-β-aspartic acid-containing proteins in human eyes. Investig. Opthalmol. Vis. Sci. 2007, 48, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Hooi, M.Y.S.; Truscott, R.J. Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. AGE 2010, 33, 131–141. [Google Scholar] [CrossRef]
- Shimizu, T.; Fukuda, H.; Murayama, S.; Izumiyama, N.; Shirasawa, T. Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 451–461. [Google Scholar] [CrossRef]
- Inoue, K.; Hosaka, D.; Mochizuki, N.; Akatsu, H.; Tsutsumiuchi, K.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’oka, T. Simultaneous determination of post-translational racemization and isomerization of N-terminal amyloid-β in Alzheimer’s brain tissues by covalent chiral derivatized ultraperformance liquid chromatography tandem mass spectrometry. Anal. Chem. 2013, 86, 797–804. [Google Scholar] [CrossRef]
- Ritz-Timme, S.; Laumeier, I.; Collins, M.J. Aspartic acid racemization: Evidence for marked longevity of elastin in human skin. Br. J. Dermatol. 2003, 149, 951–959. [Google Scholar] [CrossRef]
- Ritz-Timme, S.; Laumeier, I.; Collins, M. Age estimation based on aspartic acid racemization in elastin from the yellow ligaments. Int. J. Leg. Med. 2003, 117, 96–101. [Google Scholar] [CrossRef]
- Powell, J.T.; Vine, N.; Crossman, M. On the accumulation of d-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis 1992, 97, 201–208. [Google Scholar] [CrossRef]
- Ohtani, S.; Yamamoto, T. Age estimation by amino acid racemization in human teeth. J. Forensic Sci. 2010, 55, 1630–1633. [Google Scholar] [CrossRef]
- Wochna, K.; Bonikowski, R.; Śmigielski, J.; Berent, J. Aspartic acid racemization of root dentin used for dental age estimation in a Polish population sample. Forensic Sci. Med. Pathol. 2018, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Turzynski, A.; Schütz, H.; Hollmann, A.; Rochholz, G. Identification of osteocalcin as a permanent aging constituent of the bone matrix: Basis for an accurate age at death determination. Forensic Sci. Int. 1996, 77, 13–26. [Google Scholar] [CrossRef]
- Lyons, B.; Friedrich, M.G.; Raftery, M.; Truscott, R. Amyloid plaque in the human brain can decompose from Aβ(1-40/1-42) by spontaneous nonenzymatic processes. Anal. Chem. 2016, 88, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Momose, Y.; Harada, K. Kinetic study of racemization of aspartyl residues in model peptides of αA-crystallin. Int. J. Pept. Protein Res. 2009, 48, 118–122. [Google Scholar] [CrossRef]
- Takahashi, O.; Oda, A. Amide-iminol tautomerization of the C-terminal peptide groups of aspartic acid residues: Two-water-assisted mechanism, cyclization from the iminol tautomer leading to the tetrahedral intermediate of succinimide formation, and im-plication to peptide group hydrogen exchange. In Tyrosine and Aspartic Acid: Properties, Source and Health Benefits; Jones, J.E., Morano, D.M., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; p. 131147. [Google Scholar]
- Nakayoshi, T.; Kato, K.; Fukuyoshi, S.; Takahashi, H.; Takahashi, O.; Kurimoto, E.; Oda, A. Computational studies on nonenzymatic succinimide-formation mechanisms of the aspartic acid residues catalyzed by two water molecules. Biochim. Biophys. Acta 2020, 1868, 140459. [Google Scholar] [CrossRef]
- Takahashi, O.; Kobayashi, K.; Oda, A. Modeling the enolization of succinimide derivatives, a key step of racemization of aspartic acid residues: Importance of a two-H2O mechanism. Chem. Biodivers. 2010, 7, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O. Two-water-assisted racemization of the succinimide intermediate formed in proteins. A computational model study. Health 2013, 5, 2018–2021. [Google Scholar] [CrossRef]
- Takahashi, O.; Kirikoshi, R. Intramolecular cyclization of aspartic acid residues assisted by three water molecules: A density functional theory study. Comput. Sci. Discov. 2014, 7. [Google Scholar] [CrossRef]
- Takahashi, O.; Kirikoshi, R.; Manabe, N. Roles of intramolecular and intermolecular hydrogen bonding in a three-water-assisted mechanism of succinimide formation from aspartic acid residues. Molecules 2014, 19, 11440–11452. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O.; Kirikoshi, R.; Manabe, N. Acetic acid can catalyze succinimide formation from aspartic acid residues by a concerted bond reorganization mechanism: A computational study. Int. J. Mol. Sci. 2015, 16, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Nakayoshi, T.; Kato, K.; Fukuyoshi, S.; Takahashi, O.; Kurimoto, E.; Oda, A. Comparison of the activation energy barrier for succinimide formation from α- and β-aspartic acid residues obtained from density functional theory calculations. Biochim. Biophys. Acta 2018, 1866, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kirikoshi, R.; Manabe, N.; Takahashi, O. Phosphate-catalyzed succinimide formation from Asp residues: A computational study of the mechanism. Int. J. Mol. Sci. 2018, 19, 637. [Google Scholar] [CrossRef]
- Catak, S.; Monard, G.; Aviyente, V.; Ruiz-López, M.F. Deamidation of asparagine residues: Direct hydrolysis versus succin-imide-mediated deamidation mechanisms. J. Phys. Chem. A 2009, 113, 1111–1120. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Oda, A.; Nakayoshi, T.; Fukuyoshi, S.; Kurimoto, E.; Takahashi, O. Influences of conformations of peptides on stereoinversions and/or isomerizations of aspartic acid residues. Biochim. Biophys. Acta 2018, 1866, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Nakayoshi, T.; Kato, K.; Kurimoto, E.; Oda, A. Influence of the conformations of αA-crystallin peptides on the isomerization rates of aspartic acid residues. Biochim. Biophys. Acta 2020, 140480. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayoshi, T.; Kato, K.; Fukuyoshi, S.; Takahashi, O.; Kurimoto, E.; Oda, A. Molecular Mechanisms of Succinimide Formation from Aspartic Acid Residues Catalyzed by Two Water Molecules in the Aqueous Phase. Int. J. Mol. Sci. 2021, 22, 509. https://doi.org/10.3390/ijms22020509
Nakayoshi T, Kato K, Fukuyoshi S, Takahashi O, Kurimoto E, Oda A. Molecular Mechanisms of Succinimide Formation from Aspartic Acid Residues Catalyzed by Two Water Molecules in the Aqueous Phase. International Journal of Molecular Sciences. 2021; 22(2):509. https://doi.org/10.3390/ijms22020509
Chicago/Turabian StyleNakayoshi, Tomoki, Koichi Kato, Shuichi Fukuyoshi, Ohgi Takahashi, Eiji Kurimoto, and Akifumi Oda. 2021. "Molecular Mechanisms of Succinimide Formation from Aspartic Acid Residues Catalyzed by Two Water Molecules in the Aqueous Phase" International Journal of Molecular Sciences 22, no. 2: 509. https://doi.org/10.3390/ijms22020509
APA StyleNakayoshi, T., Kato, K., Fukuyoshi, S., Takahashi, O., Kurimoto, E., & Oda, A. (2021). Molecular Mechanisms of Succinimide Formation from Aspartic Acid Residues Catalyzed by Two Water Molecules in the Aqueous Phase. International Journal of Molecular Sciences, 22(2), 509. https://doi.org/10.3390/ijms22020509



