Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells
Abstract
1. Introduction
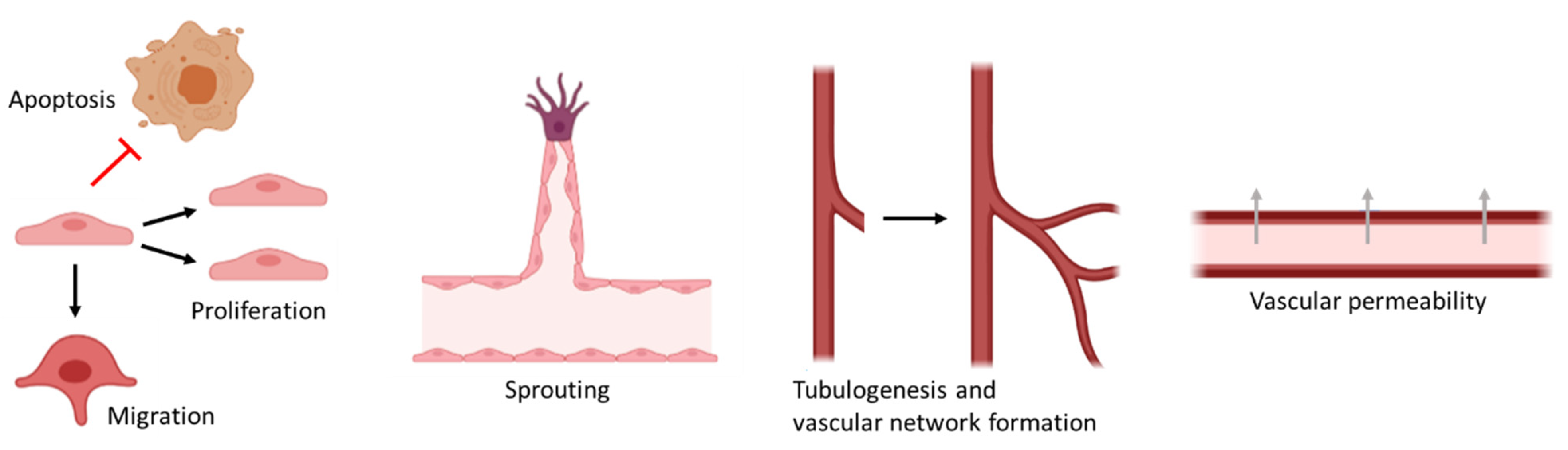
2. Endothelial Cells
3. Adipose-Derived Mesenchymal Stem/Stromal Cells
3.1. Basic Characteristics of ASCs
3.2. Angiogenic Properties of ASCs
3.3. ASC Differentiation into Endothelial Cells
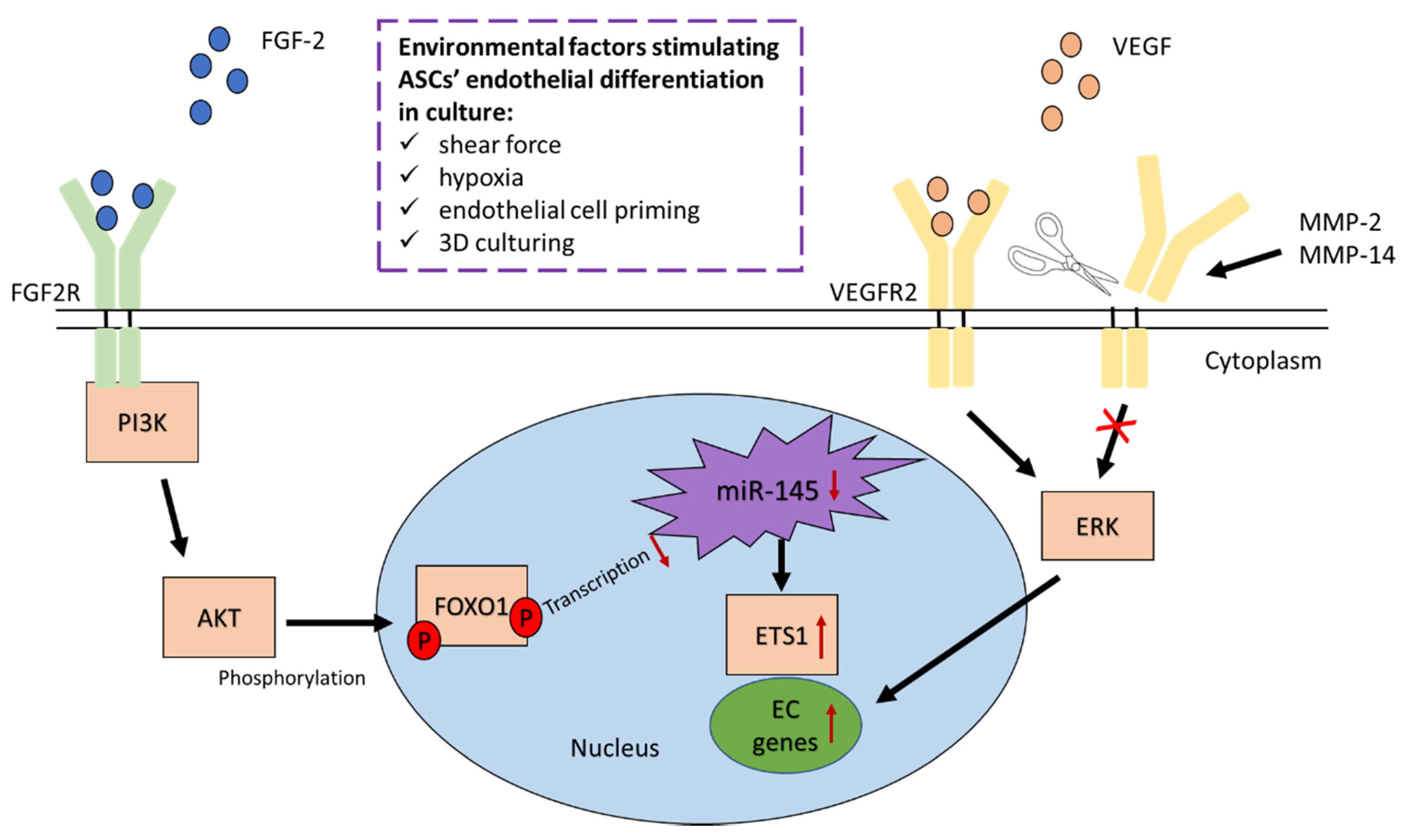
3.3.1. Fibroblast Growth Factor 2 Regulates Endothelial Differentiation of ASCs
3.3.2. VEGFR2 and VEGFR3 Activation Induces Endothelial Differentiation of ASCs
4. ASC—Endothelium Crosstalk
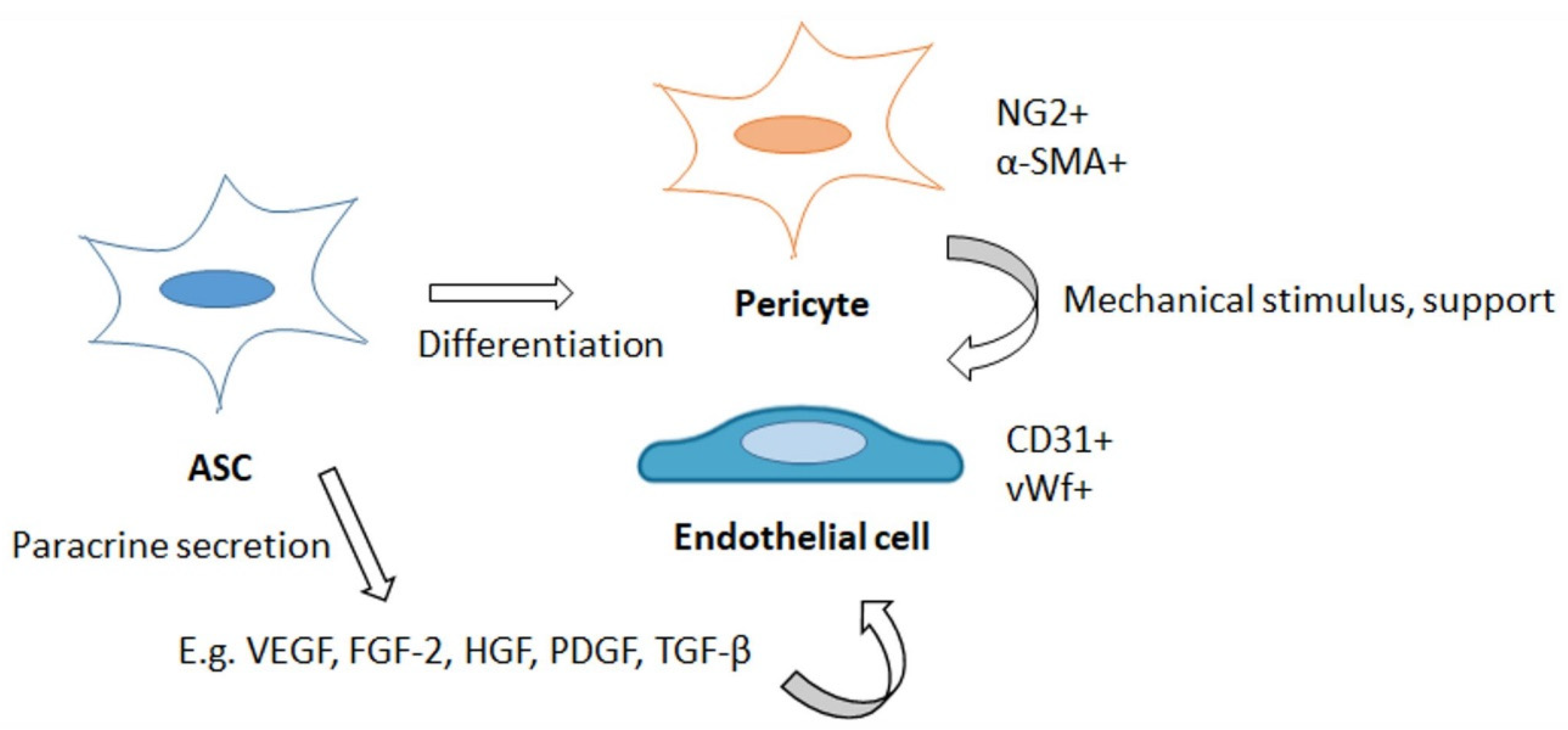
4.1. ASCs as Pericytes
4.2. Co-Cultures of ASCs and Endothelial Cells
4.2.1. Human ASCs Promote Tube Formation in Co-Culture Systems and Matrices
4.2.2. Direct Contact between the Cell Types Is Essential for Vascular Network Formation
4.2.3. Comparison of Different Cell Types in Co-Culture Settings
4.2.4. Special Characteristics of the Co-Culture Secretome
4.2.5. Tubulogenesis-Influencing Factors in Co-Cultures
4.2.6. Endothelial Cells Affect the Angiogenic Potential of hASCs
4.3. Co-Transplantation of ASCs and Endothelial Cells In Vivo
The Effect of EC Origin on the Formation of Vascular Structures In Vivo
5. Extracellular Vesicle-Facilitated Crosstalk between ASCs and Endothelial Cells
5.1. Advantages of EVs
5.2. EVs Derived from Adipose Tissue Mesenchymal Stem/Stromal Cells
5.3. PDGF Enhances the Pro-Angiogenic Potential of ASC EVs
5.4. The Effect of Human ASC-EVs on Endothelial Cells
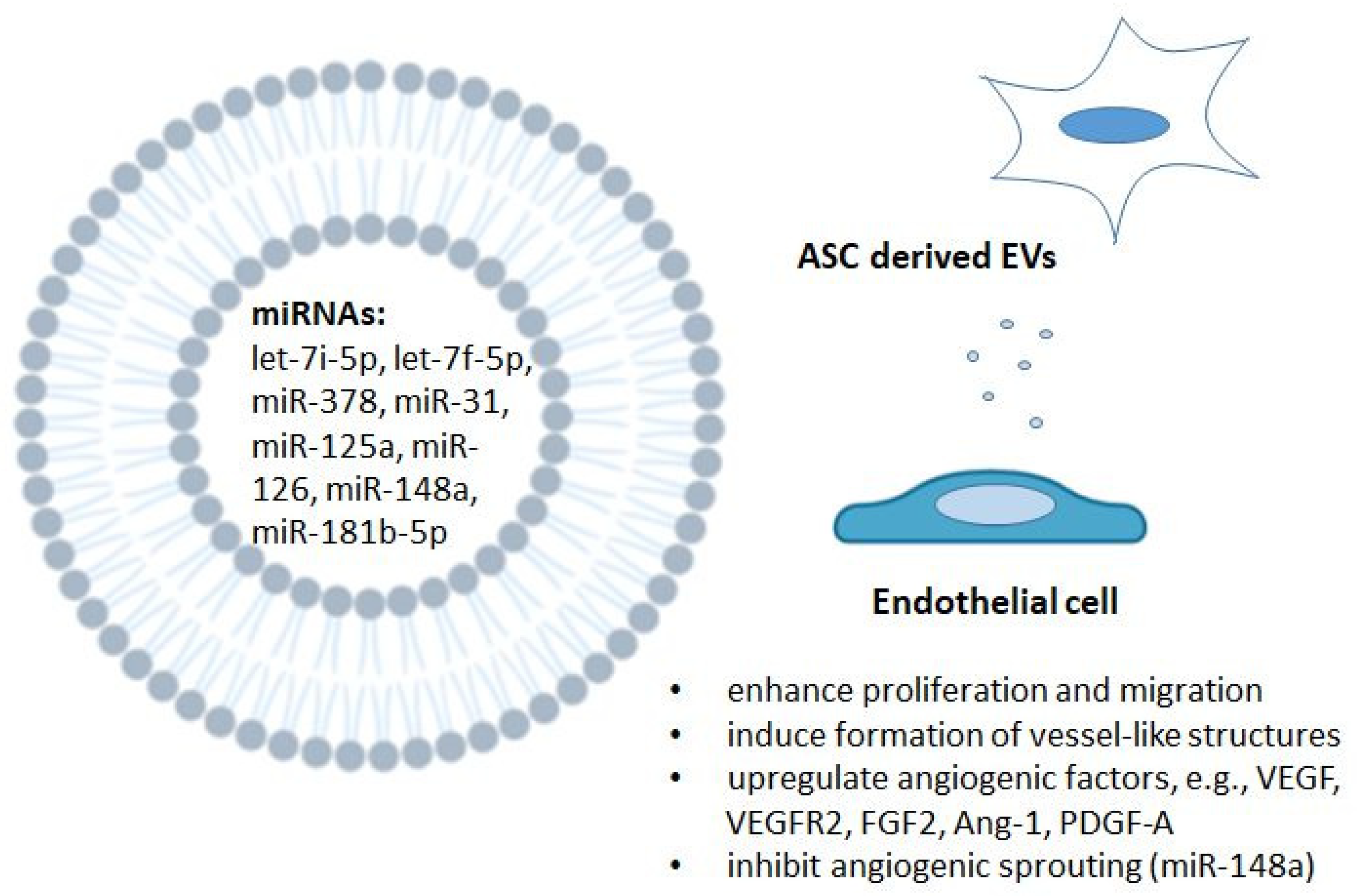
5.5. The Role of Selected ASC-Derived MicroRNAs in Endothelial Cells and Angiogenesis
5.6. Hypoxic Conditions Augment the Pro-Angiogenic Properties of ASC-Derived EVs
5.7. Negative Impact of Obesity and Metabolic Syndrome on the Angiogenic Potential of ASC-EVs
6. Physical Forces Influencing the Potential of ASCs and Their EVs for Angiogenesis
7. Implications of ASCs and Their EVs towards Clinical Application
7.1. A prevascularized Transplant for Wound Treatment
7.2. ASC-Derived EVs in Fat Grafting
7.3. ASC EVs Facilitate Angiogenesis in a Diabetic Environment and during Wound Healing
7.4. ASC-Derived EVs and Cardioprotection
7.5. Clinical Efficacy of ASCs and ASC-Derived EVs in Treatment of Ischemic Diseases
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| α-SMA | α-smooth muscle actin |
| ac-LDL | Acetylated low-density lipoprotein |
| AGO-1 | Argonaute 1 |
| AKT | Protein kinase B |
| AMI | Acute myocardial infarction |
| Ang-1 | Angiopoietin 1 |
| ANGPTL4 | Angiopoietin-like 4 protein |
| ASC | Adipose-derived stem/stromal cell |
| AT-EC | Adipose tissue-derived endothelial cell |
| BEC | Blood vascular endothelial cell |
| BMEC | Brain microvascular endothelial cell |
| BM-MSC | Bone marrow-derived mesenchymal stem/stromal cell |
| CAM | Chorioallantoic membrane |
| CBD-EC | Cord blood-derived endothelial cell |
| CCL | Chemokine (C-C motif) ligand |
| CD | Cluster of differentiation |
| CM | Conditioned medium |
| CXCL | C-X-C motif chemokine |
| CXCR | C-X-C chemokine receptor |
| d-hASC | Diabetic human adipose-derived stem/stromal cell |
| DLL4 | Delta-like 4 |
| EC | Endothelial cell |
| ECFC | Endothelial colony-forming cell |
| EGF | Epidermal growth factor |
| EGM | Endothelial cell growth medium |
| E-MSC | Endometrium-derived mesenchymal stem/stromal cell |
| eNOS | Endothelial cell nitric oxide synthase |
| EPC | Endothelial progenitor cell |
| ERK | Extracellular signal-regulated kinase |
| ETS1 | V-ets avian erythroblastosis virus E26 oncogene homolog 1 |
| EV | Extracellular vesicle |
| FGF | Fibroblast growth factor |
| FIH-1 | Factor inhibiting hypoxia inducible factor 1 |
| FOXO1 | Forkhead box protein O1 |
| HAMEC | Human adipose-derived microvascular endothelial cell |
| hASC | Human adipose-derived stem/stromal cell |
| HDMEC | Human dermal microvascular endothelial cell |
| h-exo | Hypoxic exosome |
| HGF | Hepatocyte growth factor |
| HIF-1α | Hypoxia-inducible factor-1-alpha |
| HMEC | Human microvascular endothelial cell |
| Hsp | Heat shock protein |
| HUVEC | Human umbilical vein endothelial cell |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| LEC | Lymphatic endothelial cell |
| LIPUS | Low-intensity pulsed ultrasound stimulation |
| LMEC | Lung microvascular endothelial cell |
| MCP | Monocyte chemoattractant protein |
| MetS | Metabolic syndrome |
| miR, miRNA | Micro-RNA |
| MMP | Matrix metalloproteinase |
| n-EV | Healthy donor extracellular vesicle |
| n-exo | Normoxic exosome |
| NG2 | Neural/glial antigen 2 |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor-E2-related factor 2 |
| OEC | Outgrowth endothelial cell |
| PCL | Polycaprolactone |
| PDGF | Platelet-derived growth factor |
| PDGFR | Platelet-derived growth factor receptor |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| Pl-EC | Placenta-derived endothelial cell |
| PlGF | Platelet-derived growth factor |
| SCF | Stem cell factor |
| SDF | Stromal cell-derived factor |
| SIRT1 | Sirtuin 1 |
| TF | Transcription factor |
| TGF-β | Transforming growth factor beta |
| Tie | Tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains |
| TNF | Tumor necrosis factor |
| TRPM7 | Transient receptor potential melastatin 7 |
| UC-MSC | Umbilical cord-derived mesenchymal stem/stromal cell |
| VE-cadherin | Vascular endothelial cadherin |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| vWf | von Willebrand factor |
References
- Tousoulis, D.; Charakida, M.; Stefanadis, C. Endothelial function and inflammation in coronary artery disease. Postgrad. Med. J. 2008, 84, 368–371. [Google Scholar] [CrossRef]
- Chao, C.Y.L.; Cheing, G.L.Y. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes/Metab. Res. Rev. 2009, 25, 604–614. [Google Scholar] [CrossRef]
- Woywodt, A.; Gerdes, S.; Ahl, B.; Erdbruegger, U.; Haubitz, M.; Weissenborn, K. Circulating Endothelial Cells and Stroke: Influence of Stroke Subtypes and Changes During the Course of Disease. J. Stroke Cerebrovasc. Dis. 2012, 21, 452–458. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.-H.; Tsai, F.-C.; Deng, W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Johnson, T.; Liu, D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Verseijden, F.; Sluijs, S.J.P.-V.; Pavljasevic, P.; Hofer, S.O.; Van Osch, G.J.; Farrell, E. Adult Human Bone Marrow– and Adipose Tissue–Derived Stromal Cells Support the Formation of Prevascular-like Structures from Endothelial Cells In Vitro. Tissue Eng. Part A 2010, 16, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Merfeld-Clauss, S.; Lupov, I.P.; Lu, H.; March, K.L.; Traktuev, D.O. Adipose Stromal Cell Contact with Endothelial Cells Results in Loss of Complementary Vasculogenic Activity Mediated by Induction of Activin A. Stem Cells 2015, 33, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Guven, S.; Zimoch, J.; Zapiórkowska, N.A.; Biedermann, T.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Martin, I.; Scherberich, A.; Reichmann, E.; et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr. Surg. Int. 2016, 32, 17–27. [Google Scholar] [CrossRef]
- Periasamy, R.; Elshaer, S.L.; Gangaraju, R. CD140b (PDGFRβ) Signaling in Adipose-Derived Stem Cells Mediates Angiogenic Behavior of Retinal Endothelial Cells. Regen. Eng. Transl. Med. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Sun, X.; Altalhi, W.; Nunes, S.S. Vascularization strategies of engineered tissues and their application in cardiac regeneration. Adv. Drug Deliv. Rev. 2016, 96, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Aghi, M.; Chiocca, E.A. Contribution of Bone Marrow-Derived Cells to Blood Vessels in Ischemic Tissues and Tumors. Mol. Ther. 2005, 12, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Kirton, J.P.; Xu, Q. Endothelial precursors in vascular repair. Microvasc. Res. 2010, 79, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.; Deanfield, J. Functions of the healthy endothelium. Coron. Artery Dis. 2001, 12, 485–491. [Google Scholar] [CrossRef]
- Chong, M.; Ng, W.K.; Chan, J.K.Y. Concise Review: Endothelial Progenitor Cells in Regenerative Medicine: Applications and Challenges. Stem Cells Transl. Med. 2016, 5, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Hager, G.; Holnthoner, W.; Wolbank, S.; Husa, A.-M.; Godthardt, K.; Redl, H.; Gabriel, C. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy 2013, 15, 1426–1435. [Google Scholar] [CrossRef]
- Strassburg, S.; Nienhueser, H.; Stark, G.B.; Finkenzeller, G.; Torio-Padron, N. Human Adipose-Derived Stem Cells Enhance the Angiogenic Potential of Endothelial Progenitor Cells, But Not of Human Umbilical Vein Endothelial Cells. Tissue Eng. Part A 2013, 19, 166–174. [Google Scholar] [CrossRef]
- Freiman, A.; Shandalov, Y.; Rozenfeld, D.; Shor, E.; Segal, S.; Ben-David, D.; Meretzki, S.; Egozi, D.; Levenberg, S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell. Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tiss. Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Safford, K.M.; Hicok, K.C.; Safford, S.D.; Halvorsen, Y.-D.C.; Wilkison, W.O.; Gimble, J.M.; Rice, H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2002, 294, 371–379. [Google Scholar] [CrossRef]
- Brzoska, M.; Geiger, H.; Gauer, S.; Baer, P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem. Biophys. Res. Commun. 2005, 330, 142–150. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef]
- Hsiao, S.T.F.; Asgari, A.; Lokmic, Z.T.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative Analysis of Paracrine Factor Expression in Human Adult Mesenchymal Stem Cells Derived from Bone Marrow, Adipose, and Dermal Tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells. Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Miranville, A.; Heeschen, C.; Sengenès, C.; Curat, C.; Busse, R.; Bouloumié, A. Improvement of Postnatal Neovascularization by Human Adipose Tissue-Derived Stem Cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Lindner, V.; Majack, R.A.; Reidy, M.A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J. Clin. Investig. 1990, 85, 2004–2008. [Google Scholar] [CrossRef]
- Beitz, J.G.; Kim, I.S.; Calabresi, P.; Frackelton, A.R. Human microvascular endothelial cells express receptors for platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 1991, 88, 2021–2025. [Google Scholar] [CrossRef]
- Risau, W.; Drexler, H.; Mironov, V.; Smits, A.; Siegbahn, A.; Funa, K.; Heldin, C.-H. Platelet-Derived Growth Factor is Angiogenic in Vivo. Growth Factors 1992, 7, 261–266. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Rissanen, T.T.; Vajanto, I.; Hartikainen, J. Vascular Endothelial Growth Factors: Biology and Current Status of Clinical Applications in Cardiovascular Medicine. J. Am. Coll. Cardiol. 2007, 49, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Karsan, A.; Yee, E.; Poirier, G.G.; Zhou, P.; Craig, R.; Harlan, J.M. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. Am. J. Pathol. 1997, 151, 1775–1784. [Google Scholar] [PubMed]
- Spyridopoulos, I.; Brogi, E.; Kearney, M.; Sullivan, A.B.; Cetrulo, C.; Isner, J.M.; Losordo, D.W. Vascular Endothelial Growth Factor Inhibits Endothelial Cell Apoptosis Induced by Tumor Necrosis Factor-α: Balance Between Growth and Death Signals. J. Mol. Cell. Cardiol. 1997, 29, 1321–1330. [Google Scholar] [CrossRef]
- Madri, J.A.; Pratt, B.M.; Tucker, A.M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 1998, 106, 1375–1384. [Google Scholar] [CrossRef]
- Goumans, M.-J.; Lebrin, F.; Valdimarsdottir, G. Controlling the Angiogenic SwitchA Balance between Two Distinct TGF-b Receptor Signaling Pathways. Trends Cardiovasc. Med. 2003, 13, 301–307. [Google Scholar] [CrossRef]
- Bussolino, F.; DI Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef]
- Park, I.S.; Kim, S.H.; Jung, Y.; Rhie, J.-W.; Kim, S.-H. Endothelial differentiation and vasculogenesis induced by three-dimensional adipose-derived stem cells. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2013, 296, 168–177. [Google Scholar] [CrossRef]
- Almalki, S.G.; Valle, Y.L.; Agrawal, D.K. MMP-2 and MMP-14 Silencing Inhibits VEGFR2 Cleavage and Induces the Differentiation of Porcine Adipose-Derived Mesenchymal Stem Cells to Endothelial Cells. Stem Cells Transl. Med. 2017, 6, 1385–1398. [Google Scholar] [CrossRef]
- Ning, H.; Liu, G.; Lin, G.; Yang, R.; Lue, T.F.; Lin, C.-S. Fibroblast Growth Factor 2 Promotes Endothelial Differentiation of Adipose Tissue-Derived Stem Cells. J. Sex. Med. 2009, 6, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells. Stem Cell Res. Ther. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Harris, W.M.; Plastini, M.; Kappy, N.; Ortiz, T.; Chang, S.; Brown, S.; Carpenter, J.P.; Zhang, P. Endothelial Differentiated Adipose-Derived Stem Cells Improvement of Survival and Neovascularization in Fat Transplantation. Aesthetic Surg. J. 2019, 39, 220–232. [Google Scholar] [CrossRef]
- Fischer, L.J.; McIlhenny, S.; Tulenko, T.; Golesorkhi, N.; Zhang, P.; Larson, R.; Lombardi, J.; Shapiro, I.; DiMuzio, P.J. Endothelial Differentiation of Adipose-Derived Stem Cells: Effects of Endothelial Cell Growth Supplement and Shear Force. J. Surg. Res. 2009, 152, 157–166. [Google Scholar] [CrossRef]
- Colazzo, F.; Alrashed, F.; Saratchandra, P.; Carubelli, I.; Chester, A.H.; Yacoub, M.H.; Taylor, P.M.; Somers, P. Shear stress and VEGF enhance endothelial differentiation of human adipose-derived stem cells. Growth Factors 2014, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Villalobos, M.A.; Choron, R.L.; Chang, S.; Brown, S.A.; Carpenter, J.P.; Tulenko, T.N.; Zhang, P. Fibroblast growth factor and vascular endothelial growth factor play a critical role in endotheliogenesis from human adipose-derived stem cells. J. Vasc. Surg. 2017, 65, 1483–1492. [Google Scholar] [CrossRef]
- Bekhite, M.; Finkensieper, A.; Rebhan, J.; Huse, S.; Schultze-Mosgau, S.; Figulla, H.-R.; Sauer, H.; Wartenberg, M. Hypoxia, Leptin, and Vascular Endothelial Growth Factor Stimulate Vascular Endothelial Cell Differentiation of Human Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2014, 23, 333–351. [Google Scholar] [CrossRef]
- Fromer, M.W.; Chang, S.; Hagaman, A.L.; Koko, K.; Nolan, R.S.; Zhang, P.; Brown, S.A.; Carpenter, J.P.; Caputo, F.J. The endothelial cell secretome as a novel treatment to prime adipose-derived stem cells for improved wound healing in diabetes. J. Vasc. Surg. 2018, 68, 234–244. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998, 8, 588–594. [Google Scholar] [CrossRef]
- Arderiu, G.; Peña, E.; Aledo, R.; Juan-Babot, O.; Crespo, J.; Vilahur, G.; Oñate, B.; Moscatiello, F.; Badimon, L. MicroRNA-145 Regulates the Differentiation of Adipose Stem Cells Toward Microvascular Endothelial Cells and Promotes Angiogenesis. Circ. Res. 2019, 125, 74–89. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.; Li, H.; Li, A.; Yuan, X.; Wang, Q.; Xiu, R. Enhanced matrix metalloproteinases-2 activates aortic endothelial hypermeability, apoptosis and vascular rarefaction in spontaneously hypertensive rat. Clin. Hemorheol. Microcirc. 2014, 57, 325–338. [Google Scholar] [CrossRef]
- Kiran, M.S.; Viji, R.I.; Kumar, S.V.; Prabhakaran, A.A.; Sudhakaran, P.R. Changes in expression of VE-cadherin and MMPs in endothelial cells: Implications for angiogenesis. Vasc. Cell 2011, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Li, S.; Zhang, Y.; Lu, L.; Cui, L.; Guo, F.F. Hypoxia promotes differentiation of adipose-derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Djonov, V.; Volarevic, V. Molecular mechanisms underlying therapeutic potential of pericytes. J. Biomed. Sci. 2018, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef]
- Lee, S.; Zeiger, A.; Maloney, J.M.; Kotecki, M.; Van Vliet, K.J.; Herman, I.M. Pericyte actomyosin-mediated contraction at the cell–material interface can modulate the microvascular niche. J. Phys. Condens. Matter. 2010, 22, 194115. [Google Scholar] [CrossRef] [PubMed]
- Mendel, T.A.; Clabough, E.B.D.; Kao, D.S.; Demidova-Rice, T.N.; Durham, J.T.; Zotter, B.C.; Seaman, S.A.; Cronk, S.M.; Rakoczy, E.P.; Katz, A.J.; et al. Pericytes Derived from Adipose-Derived Stem Cells Protect against Retinal Vasculopathy. PLoS ONE 2013, 8, e65691. [Google Scholar] [CrossRef]
- Rohringer, S.; Hofbauer, P.; Schneider, K.H.; Husa, A.-M.; Feichtinger, G.; Peterbauer-Scherb, A.; Redl, H.; Holnthoner, W. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis 2014, 17, 921–933. [Google Scholar] [CrossRef]
- Pill, K.; Melke, J.; Mühleder, S.; Pultar, M.; Rohringer, S.; Priglinger, E.; Redl, H.R.; Hofmann, S.; Holnthoner, W. Microvascular Networks From Endothelial Cells and Mesenchymal Stromal Cells From Adipose Tissue and Bone Marrow: A Comparison. Front. Bioeng. Biotechnol. 2018, 6, 156. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Q.; Shao, X.; Zhang, T.; Xue, C.; Shi, S.; Zhao, D.; Lin, Y. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 2017, 50, e12390. [Google Scholar] [CrossRef]
- Holnthoner, W.; Hohenegger, K.; Husa, A.-M.; Muehleder, S.; Meinl, A.; Peterbauer-Scherb, A.; Redl, H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J. Tissue Eng. Regen. Med. 2015, 9, 127–136. [Google Scholar] [CrossRef]
- Mazzocchi, A.R.; Man, A.J.; Desormeaux, J.-P.S.; Gaborski, T.R. Porous Membranes Promote Endothelial Differentiation of Adipose-Derived Stem Cells and Perivascular Interactions. Cell. Mol. Bioeng. 2014, 7, 369–378. [Google Scholar] [CrossRef]
- Kang, M.-L.; Kim, H.-S.; You, J.; Choi, Y.S.; Kwon, B.-J.; Park, C.H.; Baek, W.; Kim, M.S.; Lee, Y.J.; Im, G.-I.; et al. Hydrogel cross-linking–programmed release of nitric oxide regulates source-dependent angiogenic behaviors of human mesenchymal stem cell. Sci. Adv. 2020, 6, eaay5413. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, F.; Both, S.K.; Prins, H.-J.; Helder, M.N.; Pan, J.; Cui, F.-Z.; Jansen, J.A.; van den Beucken, J.J. In vitro and in vivo angiogenic capacity of BM-MSCs/HUVECs and AT-MSCs/HUVECs cocultures. Biofabrication 2014, 6, 015005. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, L.; Schaupper, M.; Mühleder, S.; Schimek, K.; Hasenberg, T.; Marx, U.; Priglinger, E.; Redl, H.; Holnthoner, W. Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices. Front. Bioeng. Biotechnol. 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Nassiri, S.M.; Rahbarghazi, R.; Siavashi, V.; Araghi, A. Endothelial juxtaposition of distinct adult stem cells activates angiogenesis signaling molecules in endothelial cells. Cell Tissue Res. 2015, 362, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neoh, K.G.; Kang, E.-T. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med. 2018, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Petrillo, S.; Zicola, E.; Roato, I.; Ferracini, R.; Tolosano, E.; Altruda, F.; Carossa, S.; Mussano, F.; Munaron, L. The Crosstalk Between Osteodifferentiating Stem Cells and Endothelial Cells Promotes Angiogenesis and Bone Formation. Front. Physiol. 2019, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.; Spitz, S.; Rothbauer, M.; Jordan, C.; Purtscher, M.; Zirath, H.; Schuller, P.; Eilenberger, C.; Ali, S.F.; Mühleder, S.; et al. Engineering of three-dimensional pre-vascular networks within fibrin hydrogel constructs by microfluidic control over reciprocal cell signaling. Biomicrofluidics 2018, 12, 042216. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.E.B.; Beckenkamp, L.R.; Sobral, L.; Fantacini, D.M.C.; Melo, F.U.F.; Borges, J.S.; Leopoldino, A.; Kashima, S.; Covas, D.T. Pre-culture in endothelial growth medium enhances the angiogenic properties of adipose-derived stem/stromal cells. Angiogenesis 2018, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Xu, Z.; Ikegami, Y.; Yamane, S.; Tsurashima, M.; Ijima, H. Co-culture of mesenchymal stem cells and human umbilical vein endothelial cells on heparinized polycaprolactone/gelatin co-spun nanofibers for improved endothelium remodeling. Int. J. Biol. Macromol. 2020, 151, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhu, S.; Fanai, M.L.; Wang, J.; Cai, J.; Feng, J. 3D co-culture model of endothelial colony-forming cells (ECFCs) reverses late passage adipose-derived stem cell senescence for wound healing. Stem Cell Res. Ther. 2020, 11, 355. [Google Scholar] [CrossRef]
- Bachmann, S.; Jennewein, M.; Bubel, M.; Guthörl, S.; Pohlemann, T.; Oberringer, M. Interacting adipose-derived stem cells and microvascular endothelial cells provide a beneficial milieu for soft tissue healing. Mol. Biol. Rep. 2020, 47, 111–122. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Moreno-Luna, R.; Zhou, B.; Pu, W.T.; Melero-Martin, J.M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 2012, 15, 443–455. [Google Scholar] [CrossRef]
- Nakanishi, C.; Nagaya, N.; Ohnishi, S.; Yamahara, K.; Takabatake, S.; Konno, T.; Hayashi, K.; Kawashiri, M.-A.; Tsubokawa, T.; Yamagishi, M. Gene and Protein Expression Analysis of Mesenchymal Stem Cells Derived From Rat Adipose Tissue and Bone Marrow. Circ. J. 2011, 75, 2260–2268. [Google Scholar] [CrossRef]
- Gallo, R.L.; Dorschner, R.A.; Takashima, S.; Klagsbrun, M.; Eriksson, E.; Bernfield, M. Endothelial cell surface alkaline phosphatase activity is induced by IL-6 released during wound repair. J. Investig. Dermatol. 1997, 109, 597–603. [Google Scholar] [CrossRef][Green Version]
- Lin, Z.-Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Massague, J. The Transforming Growth Factor-beta Family. Annu. Rev. Cell Biol. 1990, 6, 597–641. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-W.; Chen, W.-L.; Huang, S.-M.; Chan, J.Y.-H. Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J. 2019, 33, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Prater, D.N.; Merfeld-Clauss, S.; Sanjeevaiah, A.R.; Saadatzadeh, M.R.; Murphy, M.; Johnstone, B.H.; Ingram, D.A.; March, K.L. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res. 2009, 104, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, S.; Nienhueser, H.; Stark, G.B.; Finkenzeller, G.; Torio-Padron, N. Co-culture of adipose-derived stem cells and endothelial cells in fibrin induces angiogenesis and vasculogenesis in a chorioallantoic membrane model. J. Tissue Eng. Regen. Med. 2016, 10, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, F.; Mei, H.; Wang, S.; Cheng, L. Human adipose mesenchymal stem cells show more efficient angiogenesis promotion on endothelial colony-forming cells than umbilical cord and endometrium. Stem Cells Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Fernández-Francos, S.; Eiro, N.; Costa, L.; Escudero-Cernuda, S.; Fernández-Sánchez, M.; Vizoso, F. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric Cancer Originating from Bone Marrow-Derived Cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Røsland, G.V.; Svendsen, A.; Torsvik, A.; Sobala, E.; Mc Cormack, E.; Immervoll, H.; Mysliwietz, J.; Tonn, J.-C.; Goldbrunner, R.; Lonning, P.E.; et al. Long-term Cultures of Bone Marrow–Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation. Cancer Res. 2009, 69, 5331–5339. [Google Scholar] [CrossRef]
- Haarer, J.; Johnson, C.L.; Soeder, Y.; Dahlke, M.H. Caveats of mesenchymal stem cell therapy in solid organ transplantation. Transpl. Int. 2015, 28, 1–9. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- MacIsaac, Z.M.; Shang, H.; Agrawal, H.; Yang, N.; Parker, A.; Katz, A.J. Long-term in-vivo tumorigenic assessment of human culture-expanded adipose stromal/stem cells. Exp. Cell Res. 2012, 318, 416–423. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in Plasma Exosome is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2018, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing Into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0174303. [Google Scholar] [CrossRef]
- Eirin, A.; Riester, S.M.; Zhu, X.-Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Q.; Xu, Q.; Liu, L.; Jiang, B. MiR-148a inhibits angiogenesis by targeting ERBB3. J. Biomed. Res. 2011, 25, 170–177. [Google Scholar] [CrossRef]
- Kim, H.; Ko, Y.; Park, H.; Zhang, H.; Jeong, Y.; Kim, Y.; Noh, M.; Park, S.; Kim, Y.-M.; Kwon, Y.-G. MicroRNA-148a/b-3p regulates angiogenesis by targeting neuropilin-1 in endothelial cells. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Lee, D.Y.; Deng, Z.; Wang, C.-H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for Endothelial MicroRNA Expression and Angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Wang, Y.; Long, K.; Wang, X.; Jin, L.; Tang, Q.; Zhu, L.; Tang, G.; Li, X.; et al. MiR-532-5p alleviates hypoxia-induced cardiomyocyte apoptosis by targeting PDCD4. Gene 2018, 675, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Huang, L.-F.; Zhao, L.; Zeng, Z.; Wang, X.; Cao, D.; Yang, L.; Ye, Z.; Chen, X.; Liu, B.; et al. Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells. Genes Dis. 2020, 7, 225–234. [Google Scholar] [CrossRef]
- Xing, Y.; Hou, J.; Guo, T.; Zheng, S.; Zhou, C.; Huang, H.; Chen, Y.; Sun, K.; Zhong, T.; Wang, J.; et al. microRNA-378 promotes mesenchymal stem cell survival and vascularization under hypoxic-ischemic conditions in vitro. Stem Cell Res. Ther. 2014, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Dhar, G.; Majumder, M.; Haque, I.; Mehta, S.; Van Veldhuizen, P.J.; Banerjee, S.K.; Banerjee, S. Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1. Mol. Cancer 2010, 9, 209. [Google Scholar] [CrossRef]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: Implications for neovascularization. Expert Opin. Biol. Ther. 2010, 10, 57–71. [Google Scholar] [CrossRef]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Zhou, M.; Li, Y.; Wang, J.; Yuan, Q.; Xiao, P.; Sun, J.; Wang, Z. Extracellular Vesicles from Human Adipose-Derived Stem Cells for the Improvement of Angiogenesis and Fat-Grafting Application. Plast. Reconstr. Surg. 2019, 144, 869–880. [Google Scholar] [CrossRef]
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Hu, X.; Wang, Z.; Wu, S.; Yi, Y. Extracellular vesicles derived from human adipose-derived stem cells promote the exogenous angiogenesis of fat grafts via the let-7/AGO1/VEGF signalling pathway. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, B.; Cai, J.; Wei, Y.; Jiang, Z.; Desjardins, H.E.; Adams, A.E.; Li, S.; Kao, H.-K.; Guo, L. Exosomes Are Comparable to Source Adipose Stem Cells in Fat Graft Retention with Up-Regulating Early Inflammation and Angiogenesis. Plast. Reconstr. Surg. 2019, 144, 816e–827e. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.; Yamashita, T.; Tu, T.C.; Kato, T.; Ohneda, K.; Sato, F.; Ohneda, O. Microvesicles enhance the mobility of human diabetic adipose tissue-derived mesenchymal stem cells in vitro and improve wound healing in vivo. Biochem. Biophys. Res. Commun. 2016, 473, 1111–1118. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified ADSCs promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1 mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Z.; Qi, Y.; Zhang, W.; Zhang, C.; Jiang, M.; Deng, S.; Wang, H. Exosomes from SIRT1-Overexpressing ADSCs Restore Cardiac Function by Improving Angiogenic Function of EPCs. Mol. Ther. Nucleic Acids 2020, 21, 737–750. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, L.; Zhang, B.; Li, B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J. Cell. Biochem. 2020, 121, 2089–2102. [Google Scholar] [CrossRef] [PubMed]
- Togliatto, G.; Dentelli, P.; Gili, M.; Gallo, S.; Deregibus, C.; Biglieri, E.; Iavello, A.; Santini, E.; Rossi, C.; Solini, A.; et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: Impact on clinical applications. Int. J. Obes. 2016, 40, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Y.; Zhang, Y.; Liu, J.; Xu, Z. Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen–Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J. Mol. Neurosci. 2018, 65, 74–83. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Han, Y.-D.; Bai, Y.; Yan, X.-L.; Ren, J.; Zeng, Q.; Li, X.-D.; Pei, X.-T.; Han, Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef]
- Han, Y.; Ren, J.; Bai, Y.; Pei, X.; Han, Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019, 109, 59–68. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Wang, H.-W.; Huang, T.-S.; Lo, H.-H.; Huang, P.-H.; Lin, C.-C.; Chang, S.-J.; Liao, K.-H.; Tsai, C.-H.; Chan, C.-H.; Tsai, C.-F.; et al. Deficiency of the MicroRNA-31–MicroRNA-720 Pathway in the Plasma and Endothelial Progenitor Cells from Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 857–869. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Greco, S.; De Simone, M.; Colussi, C.; Zaccagnini, G.; Fasanaro, P.; Pescatori, M.; Cardani, R.; Perbellini, R.; Isaia, E.; Sale, P.; et al. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009, 23, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, T.-C.; Jan, Y.-H.; Lin, F.-M.; Wang, W.-C.; Xiao, H.; Wang, Y.-T.; Sun, W.; Cui, X.; Li, Y.-S.; et al. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J. Clin. Investig. 2013, 123, 1057–1067. [Google Scholar] [CrossRef]
- Meng, Y.; Eirin, A.; Zhu, X.-Y.; Tang, H.; Chanana, P.; Lerman, A.; Van Wijnen, A.J.; Lerman, L.O. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytom. Part A 2018, 93, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Ferguson, C.M.; Zhu, X.-Y.; Saadiq, I.M.; Tang, H.; Lerman, A.; Lerman, L.O. Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem Cell Res. 2020, 47, 101877. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.L.; Huang, H.H.; Chen, T.; Ju, K.C.; Kuo, S.M. Angiogenesis-promoting effect of LIPUS on hADSCs and HUVECs cultured on collagen/hyaluronan scaffolds. Mater. Sci. Eng. C 2019, 102, 22–33. [Google Scholar] [CrossRef]
- Landry, P.S.; Sadasivan, K.K.; Marino, A.A.; Albright, J.A. Electromagnetic Fields Can Affect Osteogenesis by Increasing the Rate of Differentiation. Clin. Orthop. Relat. Res. 1997, 338, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Bodamyali, T.; Bhatt, B.; Hughes, F.; Winrow, V.; Kanczler, J.; Simon, B.; Abbott, J.; Blake, D.; Stevens, C. Pulsed Electromagnetic Fields Simultaneously Induce Osteogenesis and Upregulate Transcription of Bone Morphogenetic Proteins 2 and 4 in Rat Osteoblastsin Vitro. Biochem. Biophys. Res. Commun. 1998, 250, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004, 18, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Grassi, C.; D’Ascenzo, M.; Torsello, A.; Martinotti, G.; Wolf, F.; Cittadini, A.; Azzena, G.B. Effects of 50Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium 2004, 35, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Śmieszek, A.; Lewandowski, D.; Toker, N.Y. The influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissue. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Hato, T.; Tabata, M.; Oike, Y. The Role of Angiopoietin-Like Proteins in Angiogenesis and Metabolism. Trends Cardiovasc. Med. 2008, 18, 6–14. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, A.; Tao, C.; Li, X.; Jin, P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2013, 441, 675–680. [Google Scholar] [CrossRef]
- Kimura, K.; Nagano, M.; Salazar, G.; Yamashita, T.; Tsuboi, I.; Mishima, H.; Matsushita, S.; Sato, F.; Yamagata, K.; Ohneda, O. The Role of CCL5 in the Ability of Adipose Tissue-Derived Mesenchymal Stem Cells to Support Repair of Ischemic Regions. Stem Cells Dev. 2014, 23, 488–501. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.J.; Danesh, F.R. MicroRNA-29c Is a Signature MicroRNA under High Glucose Conditions That Targets Sprouty Homolog 1, and Its in Vivo Knockdown Prevents Progression of Diabetic Nephropathy. J. Biol. Chem. 2011, 286, 11837–11848. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Zeng, J.; Chen, J.; Wang, Y.; Chen, J.; Li, Y.; Barati, M.T.; Wintergerst, K.A.; Pan, K.; et al. Elevating CXCR7 Improves Angiogenic Function of EPCs via Akt/GSK-3β/Fyn-Mediated Nrf2 Activation in Diabetic Limb Ischemia. Circ. Res. 2017, 120, e7–e23. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Qu, B.; Li, Y.-M.; Yang, L.-B.; Fan, K.-X.; Zheng, H.; Huang, H.-D.; Gu, J.-W.; Kuang, Y.-Q.; Ma, Y. NFAT5 protects astrocytes against oxygen–glucose–serum deprivation/restoration damage via the SIRT1/Nrf2 pathway. J. Mol. Neurosci. 2017, 61, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, B.; Cheng, B.; Liu, Y.; Zhang, B.; Wang, X.; Lin, X.; Yang, B.; Gong, G. Crocin Alleviates Myocardial Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress via Regulation of miR-34a/Sirt1/Nrf2 Pathway. Shock 2019, 51, 123–130. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Database. Available online: www.clinicaltrials.gov (accessed on 1 October 2021).
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Cui, M.; Gao, X.; Sun, D.; Qin, X.; Narsinh, K.; Li, C.; Jia, H.; Li, C.; et al. Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res. Cardiol. 2013, 108, 333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Cheng, K.; Zhang, R.; Narsinh, K.; Li, S.; Li, X.; Qin, X.; Zhang, R.; Li, C.; et al. Activation of Liver X Receptor Improves Viability of Adipose-Derived Mesenchymal Stem Cells to Attenuate Myocardial Ischemia Injury Through TLR4/NF-κB and Keap-1/Nrf-2 Signaling Pathways. Antioxid. Redox Signal. 2014, 21, 2543–2557. [Google Scholar] [CrossRef]
- Shevchenko, E.K.; Makarevich, P.I.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Sysoeva, V.Y.; Tkachuk, V.A.; Parfyonova, Y.V. Transplantation of modified human adipose derived stromal cells expressing VEGF165 results in more efficient angiogenic response in ischemic skeletal muscle. J. Transl. Med. 2013, 11, 138. [Google Scholar] [CrossRef]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, X.; Shi, J.; Gao, S.; Zhu, Y.; Gu, T.; Shi, E. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J. Thorac. Cardiovasc. Surg. 2019, 157, 508–517. [Google Scholar] [CrossRef]
- Hood, J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
| Factor | Source of ASC | Authors | Details |
|---|---|---|---|
| Semisolid methylcellulose medium | Human | [34] | Expression of CD31 and vWf and formation of vessel-like structures in vitro. In vivo neovascularization. Dedifferentiation of mature adipocytes into ECs. |
| Endothelial cell growth medium containing VEGF and IGF | Human | [35] | Expression of CD31 and vWf in vitro. Increased capillary density and blood flow in ischemic hindlimb in vivo. |
| Endothelial cell growth medium containing VEGF and FGF-2, Matrigel coating | Human | [4] | Ac-LDL uptake in vitro. Expression of CD31, VE-cadherin and eNOS in vitro and in vivo. Improved blood perfusion in vivo. Blockade of PI3K inhibits differentiation. |
| Endothelial cell growth medium containing FGF-2 | Rat | [51] | Ac-LDL uptake; expression of CD31, vWf and eNOS; and formation of tube-like structures on Matrigel in vitro. Blockade of FGF-2 inhibits differentiation. |
| Endothelial cell growth medium containing VEGF | Pig | [52] | Endothelial cell morphology and increase in ERK phosphorylation in vitro. ERK inhibition decreases the expression of CD31 and VE-cadherin. Blockade of VEGFR2 inhibits ERK. |
| Endothelial cell growth medium containing VEGF, FGF-2, EGF and IGF-1 | Human | [53] | Change in morphology; induced expression of CD31, vWf and eNOS; and formation of cord-like structures on Matrigel in vitro. Improved fat graft retention and neovascularization in vivo. |
| Endothelial cell growth supplement, shear force | Human | [54] | Ac-LDL uptake and expression of CD31 in vitro. No expression of eNOS or vWf. |
| Shear stress + VEGF | Human | [55] | Expression of CD31, VE-cadherin, vWf, eNOS and VEGFR1 and -2 in vitro. |
| Added FGF-2 or FGF-2 + VEGF | Human | [56] | Expression of CD31, vWf, eNOS and VE-cadherin and formation of capillary-like structures on Matrigel in vitro. Blockade of FGF receptor inhibits differentiation. |
| Hypoxia in combination with leptin and VEGF | Human | [57] | Expression of CD31, VE-cadherin, vWf, VEGR2 and eNOS and increased sprout formation on Matrigel in vitro. Blockade of AKT inhibits differentiation. |
| HUVEC priming | Human | [58] | Change in morphology and increased expression of CD31, vWf and eNOS. Formation of capillary-like tube networks on Matrigel. |
| 3D cell culturing | Human | [49] | Formation of 3D cell mass induced hypoxia and expression of VEGF, IL-8 and CD31 among other angiogenic factors. Formation of vascular structures in vivo when implanted in mice. |
| Silencing of MMP-2 and MMP-14 | Pig | [50] | Increased expression of CD31 and VE-cadherin, formation of capillary tubes and ac-LDL uptake in vitro. Decreased cleavage of VEGFR2. |
| EC Type | Source of ASC | Authors | Details and Effect on Vessel Formation |
|---|---|---|---|
| HUVEC | Human | [8] | Culturing of fibrin-embedded spheroids induced organization into prevascular-like structures expressing CD34 and α-SMA. |
| OEC | Human | [72] | Induced formation of CD31-positive branching vessel structures in a fibrin matrix. Expression of MMP-14 in the invading sprouts. Elevated VEGF secretion. |
| HUVEC | Human | [75] | Improved capillary network formation and expression of CD31, vWf, VEGF and MMPs in HA/gelatin gel. Enhanced vascularization in a 3D-printed composite scaffold. |
| AT-EC | Human | [10] | Vascular network with continuous endothelial lumen formation. |
| HUVEC | Human | [76] | Induced formation of vessel-like structures on Thermanox (2D) and in collagen gel (3D). |
| BEC + LEC | Human | [77] | In a triculture in fibrin gel, LEC and BEC form separate networks, which are dependent on ASC contact. Lymphatic network is dependent on VEGF-C. |
| HUVEC, rat LMEC | Rat | [78] | Improved tubulogenesis in Matrigel. Upregulation of VEGF, Ang-2, VEGFR2 and Tie-2 in HUVECs. |
| EPC, HUVEC | Human | [21] | Increased VEGF secretion and formation of capillary-like structures with longer sprouts in ASC/EPC co-culture but not in ASC/HUVEC co-culture. Blockade of VEGFR2 inhibits capillary-like structure formation. |
| HUVEC | Human | [79] | Enhanced calcium deposition and secretion of BMP-2 and VEGF, which were further increased by electrical stimulation. |
| CBD-EC | Human | [9] | The co-culture induces activin A expression in ASCs and secretes lower levels of angiogenic factors compared with ASC culture. |
| HMEC | Human | [80] | Improved capillary network by osteodifferentiating ASCs. ECs enhance the production of VEGF, PDGF-B and FGF-2 in osteodifferentiating ASCs. |
| HUVEC, OEC | Human | [69] | Proximity of ASCs required for mature network formation in fibrin gel. ASCs induce and stabilize EC networks by developing pericyte characteristics and by protein secretion. |
| HUVEC | Human | [70] | Induced network formation and deposition of basal lamina components in a co-culture in fibrin. ASCs differentiate toward a pericyte phenotype. |
| HUVEC | Human | [73] | ASCs show pericyte-like behavior and differentiation into ECs in a co-culture over a porous membrane. |
| HUVEC | Human | [74] | ASCs exhibit EC-like phenotype in a co-culture in nitric-oxide-releasing gel. Increased sprouting in the beginning of cultures. |
| HAMEC, HUVEC | Human | [22] | HAMEC/ASC co-culture induces the most organized and complex vascular network expressing CD31 and α-SMA in a 3D scaffold. |
| Mouse BMEC | Mouse | [71] | IGF-1 enhances the formation of vessel-like structures and upregulates the expression of angiogenic factors via PI3K/AKT pathway in collagen gel. |
| HUVEC | Human | [81] | Indirect flow enhances EC sprouting but fails to form vascular networks in fibrin gel, while direct flow inhibits prevascular network formation. |
| HUVEC | Human | [82] | Pre-culture of ASCs in EGM-2 improves the formation of tube-like structures in a co-culture. |
| HUVEC | Rat | [83] | Enhanced CD31 expression on co-spun nanofiber substrate. |
| ECFC | Human | [84] | Co-culture in a hyaluronic acid gel reverses late-passage ASC senescence and shows increased amount of CD31-positive cells. |
| HDMEC | Human | [85] | Myofibroblast differentiation of ASCs attenuated in co-culture. Hypoxia increases expression of IL-6. Increased expression of VEGF compared with EC culture. |
| Context | Source of ASC | Authors | Effect of ASC EVs |
|---|---|---|---|
| Angiogenesis | Human | [127] | Stimulate in vitro and in vivo angiogenesis. PDGF enhances EV secretion in ASCs. |
| Angiogenesis | Human | [129] | Promote angiogenesis in vitro and in vivo via miRNA-31. |
| Angiogenesis | Human | [12] | Promote angiogenesis in vitro and in vivo via miRNA-125a. |
| Angiogenesis, fat grafting | Human | [128] | Promote angiogenesis and fat grafting in vivo. |
| Angiogenesis, fat grafting | Human | [130] | Improve survival of fat graft by angiogenesis promotion via let-7/argonaute 1/VEGF pathway. |
| Angiogenesis, fat grafting | Mouse | [131] | ASC-EVs comparable to ASCs in aiding fat graft survival via angiogenesis promotion and fat graft volume retention. |
| Wound healing | Human | [13] | Promote in vivo wound healing via activation of the AKT and ERK pathways. Promote angiogenesis. |
| Wound healing, diabetic environment | Human | [132] | Healthy EVs can upregulate the expression of genes important to wound healing. Enhance the mobility of diabetic ASCs to the wound site in vitro and in vivo. |
| Wound healing, diabetic environment | Human | [133] | Promote wound healing in a diabetic foot ulcer model in vivo. Enhanced effect with nuclear factor-E2-related factor 2 (Nrf2) |
| Wound healing, diabetic environment | Mouse | [134] | Exosome containing wound-healing gel promotes wound healing and angiogenesis in diabetic environment in vivo. |
| Wound healing, diabetic environment | Human | [135] | mmu_circ_0000250-modified ASC-EVs promote wound healing in vivo. |
| Myocardial infarction | Rat | [23] | miRNA-126 overexpression prevents myocardial damage and promotes angiogenesis in vivo. |
| Myocardial infarction | Mouse | [136] | SIRT-overexpressing ASC-EVs promote survival and myocardial function by promoting angiogenesis via Nrf2 in vivo. |
| Myocardial infarction | Human | [137] | Inhibit cardiomyocyte apoptosis, reduce infarction area and increase microvascular density in vivo. |
| Obesity | Human | [138] | Obesity decreases pro-angiogenic effect of EVs via impairment of miR-126 content. |
| Hypoxia, angiogenesis | Rat | [139] | Promote angiogenesis via miRNA-181b in oxygen–glucose deprivation in vitro. |
| Hypoxia, angiogenesis | Human | [140] | Hypoxia treatment of ASCs promotes EV-induced angiogenesis via protein kinase A (PKA) signaling pathway. |
| Hypoxia, angiogenesis, fat grafting | Human | [141] | Promote survival of fat graft by promoting angiogenesis and reducing inflammation. Hypoxia pretreatment of ASCs can enhance the effects. |
| Hypoxia, angiogenesis, fat grafting | Human | [142] | Hypoxia treatment of ASCs promotes EV-induced angiogenesis and fat grafting in vivo. |
| Hypoxia, angiogenesis | Human | [143] | EVs from hypoxia-conditioned ASCs are a more potent angiogenesis inducer than EVs without preconditioning. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautiainen, S.; Laaksonen, T.; Koivuniemi, R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 10890. https://doi.org/10.3390/ijms221910890
Rautiainen S, Laaksonen T, Koivuniemi R. Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(19):10890. https://doi.org/10.3390/ijms221910890
Chicago/Turabian StyleRautiainen, Swarna, Timo Laaksonen, and Raili Koivuniemi. 2021. "Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells" International Journal of Molecular Sciences 22, no. 19: 10890. https://doi.org/10.3390/ijms221910890
APA StyleRautiainen, S., Laaksonen, T., & Koivuniemi, R. (2021). Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells. International Journal of Molecular Sciences, 22(19), 10890. https://doi.org/10.3390/ijms221910890






