Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice
Abstract
:1. Introduction
2. Results
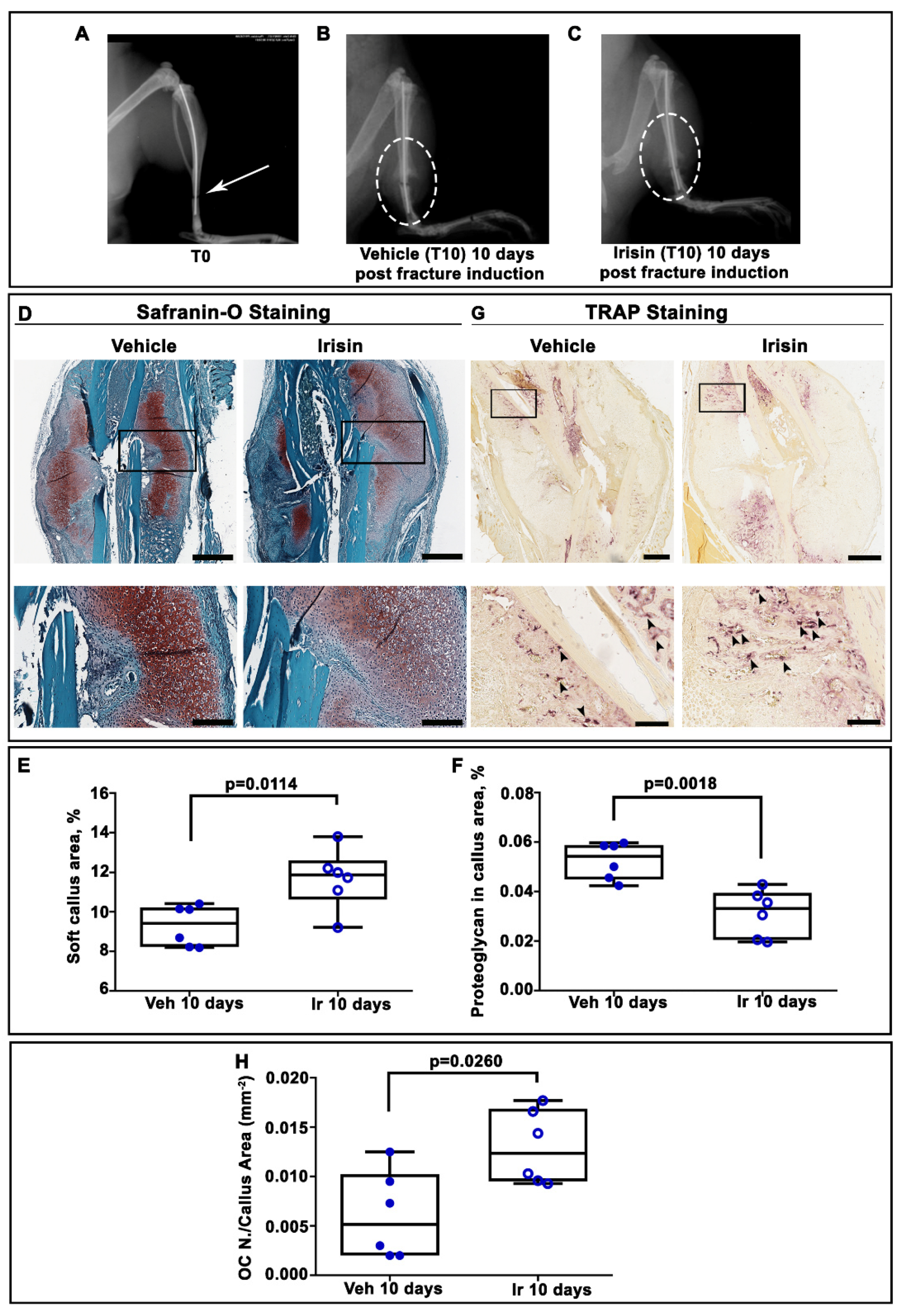
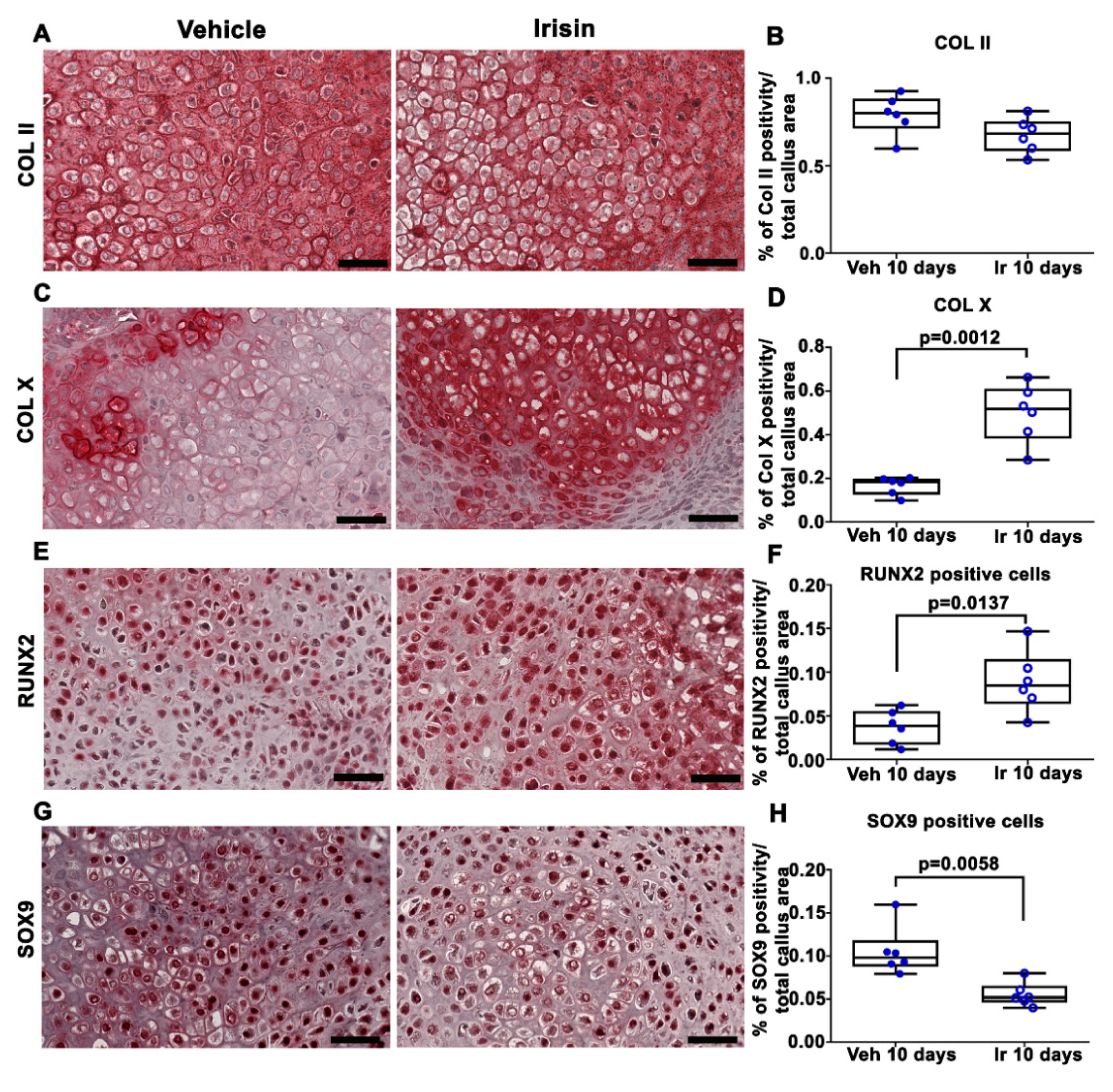
2.1. Irisin Induces Maturation of the Soft Callus at 10 Days Post-Fracture
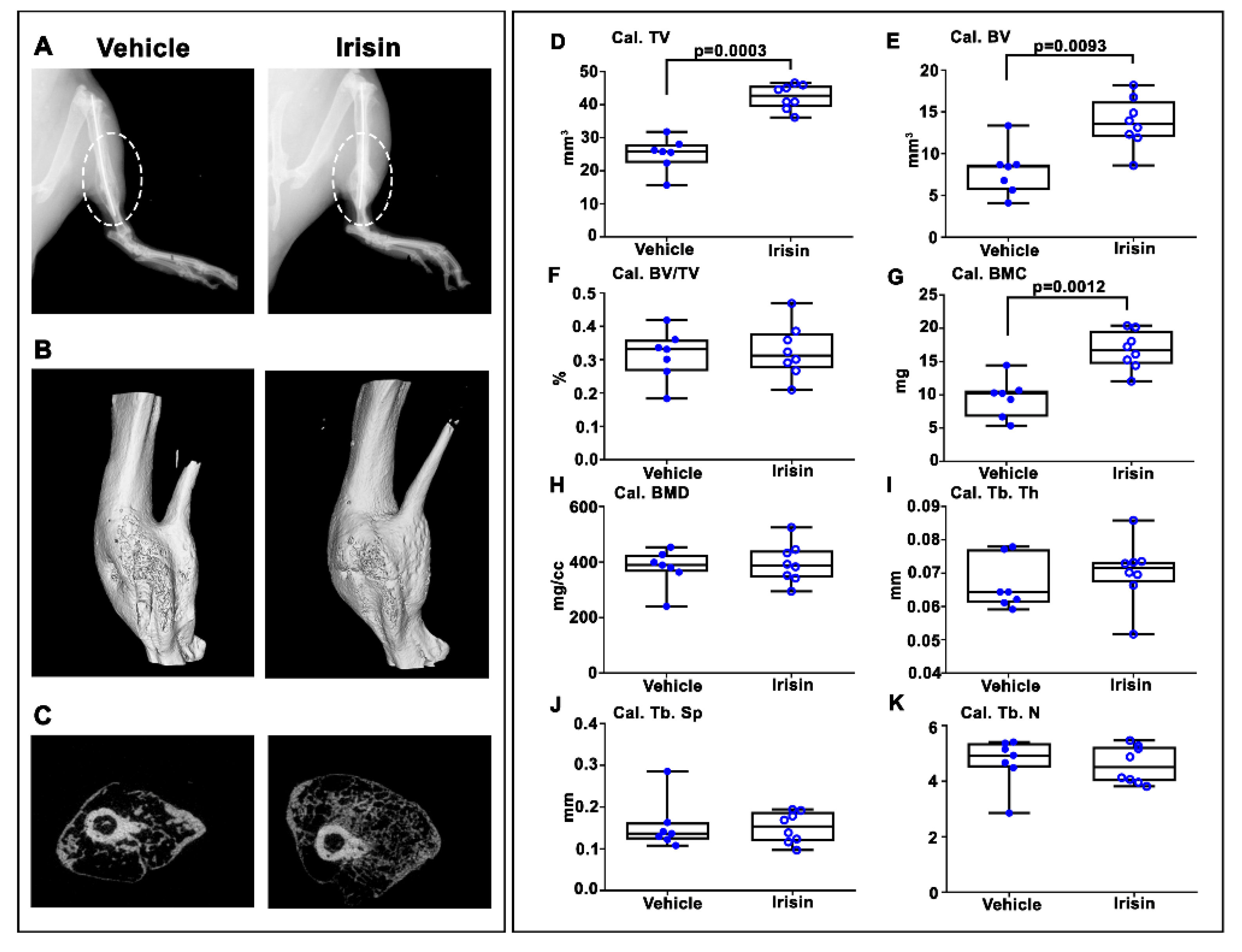
2.2. Irisin Increased Bony Callus Size at 28 Days Post-Fracture
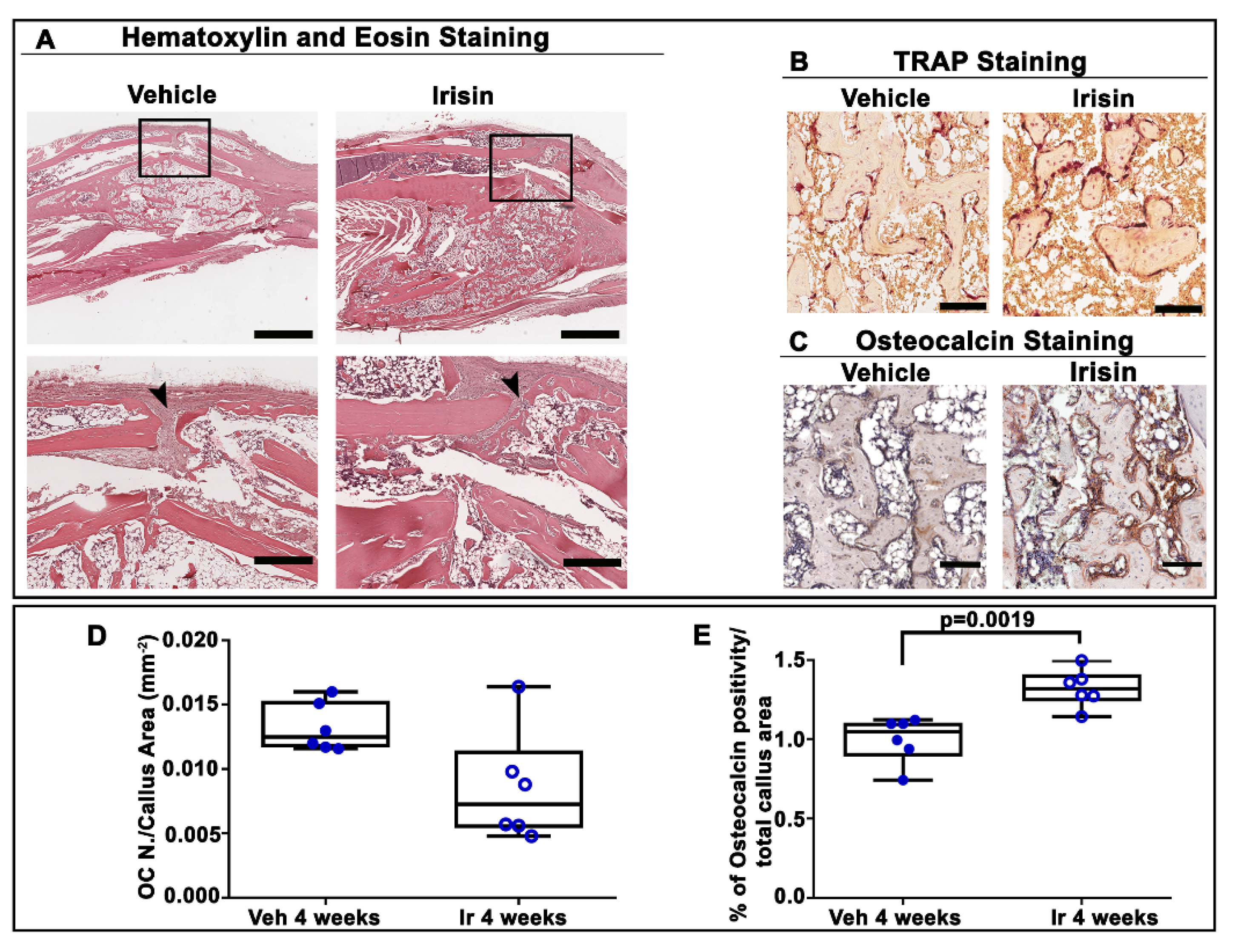
2.3. Irisin Accelerated Bony Callus Formation at 28 Days Post-Fracture
3. Discussion
4. Materials and Methods
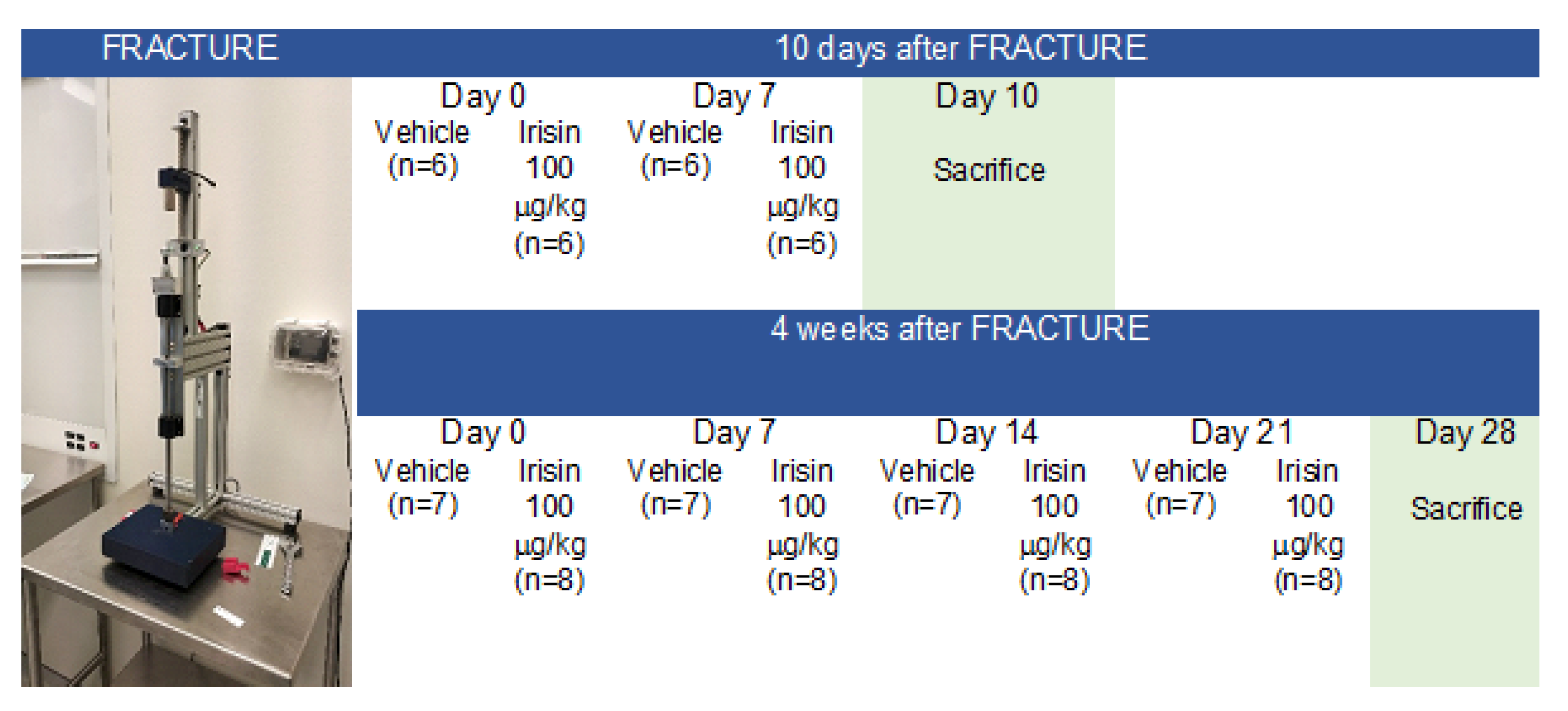
4.1. Experimental Design and Tibial Fracture Procedure
4.2. X-ray and Micro-Computed Tomography
4.3. Histological and Immunohistochemical Assays
4.4. Morphometric Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, F.; Galler, M.; Zellner, M.; Bäuml, C.; Füchtmeier, B. Total hip arthroplasty after failed osteosynthesis of proximal femoral fractures: Revision and mortality of 80 patients. J. Orthop. Surg. 2017, 25, 2309499017717869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morice, A.; Ducellier, F.; Bizot, P. Total hip arthroplasty after failed fixation of a proximal femur fracture: Analysis of 59 cases of intra- and extra-capsular fractures. Orthop. Traumatol. Surg. Res. OTSR 2018, 104, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury 2018, 49, 1461–1465. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop.Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.A.; Rivera, K.O.; Miclau, T., 3rd; Alsberg, E.; Marcucio, R.S.; Bahney, C.S. Microenvironmental Regulation of Chondrocyte Plasticity in Endochondral Repair-A New Frontier for Developmental Engineering. Front. Bioeng. Biotechnol. 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Kostenuik, P.; Mirza, F.M. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 213–223. [Google Scholar] [CrossRef]
- Liu, R.; Schindeler, A.; Little, D.G. The potential role of muscle in bone repair. J. Musculoskelet. Neuronal Interact. 2010, 10, 71–76. [Google Scholar] [PubMed]
- Colaianni, G.; Storlino, G.; Sanesi, L.; Colucci, S.; Grano, M. Myokines and Osteokines in the Pathogenesis of Muscle and Bone Diseases. Curr. Osteoporos. Rep. 2020, 18, 401–407. [Google Scholar] [CrossRef]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The effect of Irisin on bone cells in vivo and in vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell′Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [Green Version]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner.Res. Off. J. Am. Soc. Bone Mineral. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef] [Green Version]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife 2020, 9, e58172. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Q.; Zhang, L.; Ruan, J.; Zhang, X.; Chen, J.; Ma, C.; Yu, Z. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides 2018, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.C.; Drevon, C.A.; Maak, S. Irisin: Still chasing shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D′Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Sanesi, L.; Colaianni, G.; Celi, M.; Piacente, L.; D′Amato, G.; Schipani, E.; Colucci, S.; Grano, M. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 10–17. [Google Scholar] [CrossRef]
- Colaianni, G.; Faienza, M.F.; Sanesi, L.; Brunetti, G.; Pignataro, P.; Lippo, L.; Bortolotti, S.; Storlino, G.; Piacente, L.; D′Amato, G.; et al. Irisin serum levels are positively correlated with bone mineral status in a population of healthy children. Pediatric Res. 2019, 85, 484–488. [Google Scholar] [CrossRef]
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V.; et al. Irisin Correlates Positively With BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker p21 in Osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2021, 36, 305–314. [Google Scholar] [CrossRef]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cannata, F.; Cicione, C.; Papalia, R.; Denaro, V. Irisin Recovers Osteoarthritic Chondrocytes In Vitro. Cells 2020, 9, 1478. [Google Scholar] [CrossRef]
- Wang, F.S.; Kuo, C.W.; Ko, J.Y.; Chen, Y.S.; Wang, S.Y.; Ke, H.J.; Kuo, P.C.; Lee, C.H.; Wu, J.C.; Lu, W.B.; et al. Irisin Mitigates Oxidative Stress, Chondrocyte Dysfunction and Osteoarthritis Development through Regulating Mitochondrial Integrity and Autophagy. Antioxidants 2020, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Miedel, E.L.; Brisson, B.K.; Hamilton, T.; Gleason, H.; Swain, G.P.; Lopas, L.; Dopkin, D.; Perosky, J.E.; Kozloff, K.M.; Hankenson, K.D.; et al. Type III collagen modulates fracture callus bone formation and early remodeling. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.K.; Meganck, J.A.; Terkhorn, S.; Rajani, R.; Naik, A.; O′Keefe, R.J.; Goldstein, S.A.; Hankenson, K.D. Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2009, 24, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Marinaccio, C.; Ribatti, D. A simple method of image analysis to estimate CAM vascularization by APERIO ImageScope software. Int. J. Dev. Biol. 2015, 59, 217–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Komori, T. Requisite roles of Runx2 and Cbfb in skeletal development. J. Bone Miner. Metab. 2003, 21, 193–197. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [Green Version]
- van der Eerden, B.C.; Karperien, M.; Wit, J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [Green Version]
- Johansson, N.; Saarialho-Kere, U.; Airola, K.; Herva, R.; Nissinen, L.; Westermarck, J.; Vuorio, E.; Heino, J.; Kähäri, V.M. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1997, 208, 387–397. [Google Scholar] [CrossRef]
- Cawston, T.E.; Young, D.A. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2010, 339, 221–235. [Google Scholar] [CrossRef]
- Kakar, S.; Einhorn, T.A.; Vora, S.; Miara, L.J.; Hon, G.; Wigner, N.A.; Toben, D.; Jacobsen, K.A.; Al-Sebaei, M.O.; Song, M.; et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 2007, 22, 1903–1912. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Franceschi, R.T.; Luo, M.; Fan, J.; Jiang, D.; Cao, H.; Kwon, T.G.; Lai, Y.; Zhang, J.; Patrene, K.; et al. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS ONE 2009, 4, e7583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Ritter, N.M.; Farach-Carson, M.C.; Butler, W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J. Bone Miner. Res. Off. J. Am. Soc. Bone Mineral. Res. 1992, 7, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.Q.; Sicheri, F.; Howard, A.J.; Yang, D.S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 2003, 425, 977–980. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Diab, T.; Sroga, G.E.; Ural, A.; Boskey, A.L.; Gundberg, C.M.; Vashishth, D. Dilatational band formation in bone. Proc. Natl. Acad. Sci. USA 2012, 109, 19178–19183. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [Green Version]
- Posa, F.; Colaianni, G.; Di Cosola, M.; Dicarlo, M.; Gaccione, F.; Colucci, S.; Grano, M.; Mori, G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology 2021, 10, 295. [Google Scholar] [CrossRef]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. Off. J. N. Am. Spine Soc. 2014, 14, 552–559. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Metzger, C.E.; Bloomfield, S.A.; Zawieja, D.C. Inflammation-induced lymphatic architecture and bone turnover changes are ameliorated by irisin treatment in chronic inflammatory bowel disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4848–4861. [Google Scholar] [CrossRef]
- Karnes, J.M.; Daffner, S.D.; Watkins, C.M. Multiple roles of tumor necrosis factor-alpha in fracture healing. Bone 2015, 78, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Timmen, M.; Hidding, H.; Wieskötter, B.; Baum, W.; Pap, T.; Raschke, M.J.; Schett, G.; Zwerina, J.; Stange, R. Influence of antiTNF-alpha antibody treatment on fracture healing under chronic inflammation. BMC Musculoskelet. Disord. 2014, 15, 184. [Google Scholar] [CrossRef] [Green Version]
- Haffner-Luntzer, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A. Mouse Models in Bone Fracture Healing Research. Curr. Mol. Biol. Rep. 2016, 2, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colucci, S.C.; Buccoliero, C.; Sanesi, L.; Errede, M.; Colaianni, G.; Annese, T.; Khan, M.P.; Zerlotin, R.; Dicarlo, M.; Schipani, E.; et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. Int. J. Mol. Sci. 2021, 22, 10863. https://doi.org/10.3390/ijms221910863
Colucci SC, Buccoliero C, Sanesi L, Errede M, Colaianni G, Annese T, Khan MP, Zerlotin R, Dicarlo M, Schipani E, et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. International Journal of Molecular Sciences. 2021; 22(19):10863. https://doi.org/10.3390/ijms221910863
Chicago/Turabian StyleColucci, Silvia Concetta, Cinzia Buccoliero, Lorenzo Sanesi, Mariella Errede, Graziana Colaianni, Tiziana Annese, Mohd Parvez Khan, Roberta Zerlotin, Manuela Dicarlo, Ernestina Schipani, and et al. 2021. "Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice" International Journal of Molecular Sciences 22, no. 19: 10863. https://doi.org/10.3390/ijms221910863
APA StyleColucci, S. C., Buccoliero, C., Sanesi, L., Errede, M., Colaianni, G., Annese, T., Khan, M. P., Zerlotin, R., Dicarlo, M., Schipani, E., Kozloff, K. M., & Grano, M. (2021). Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. International Journal of Molecular Sciences, 22(19), 10863. https://doi.org/10.3390/ijms221910863








