Distinct Mechanisms Account for In Vitro Activation and Sensitization of TRPV1 by the Porphyrin Hemin
Abstract
:1. Introduction
2. Results
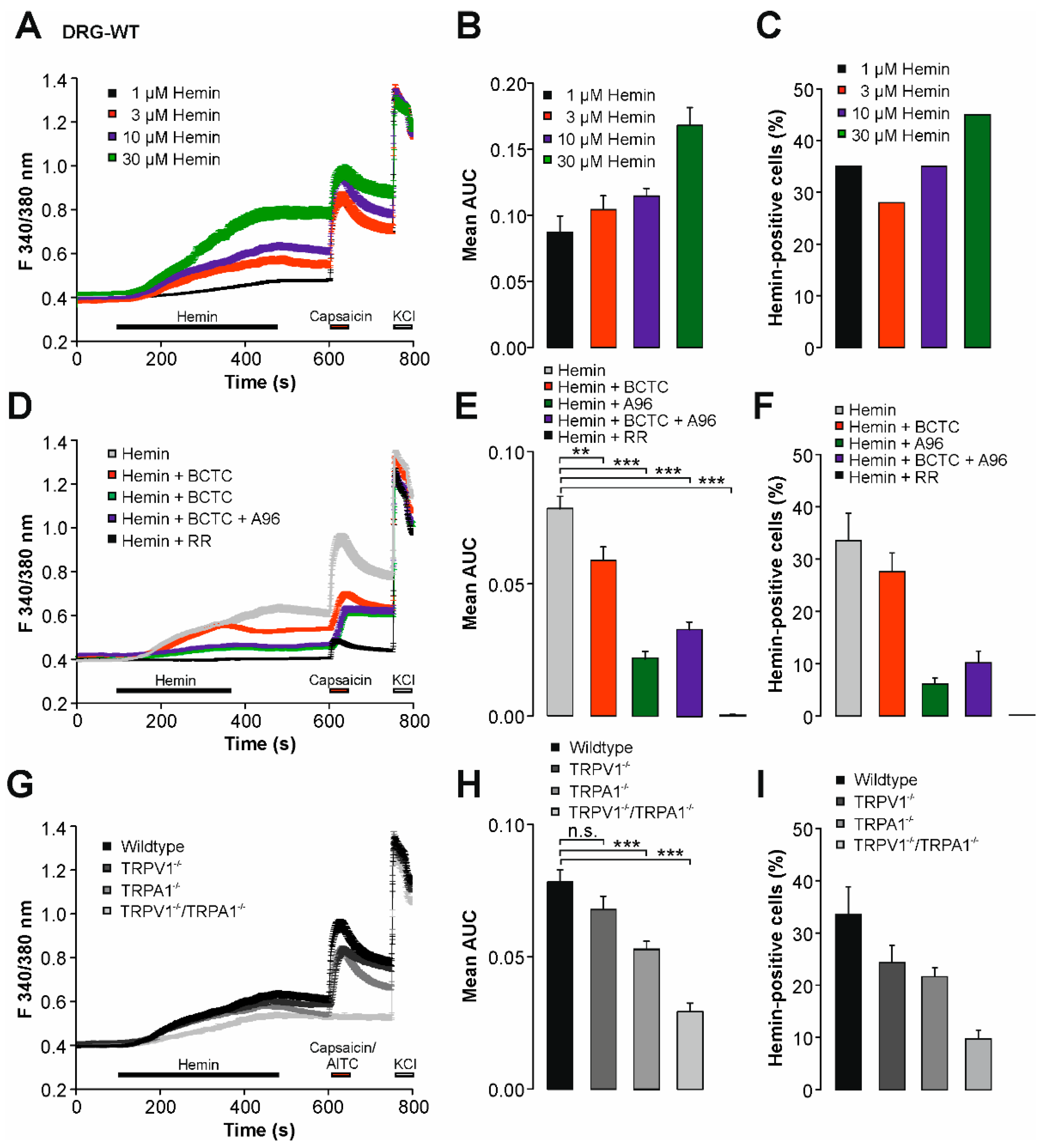
2.1. Hemin Induces a Calcium Influx in Mouse DRG Neurons
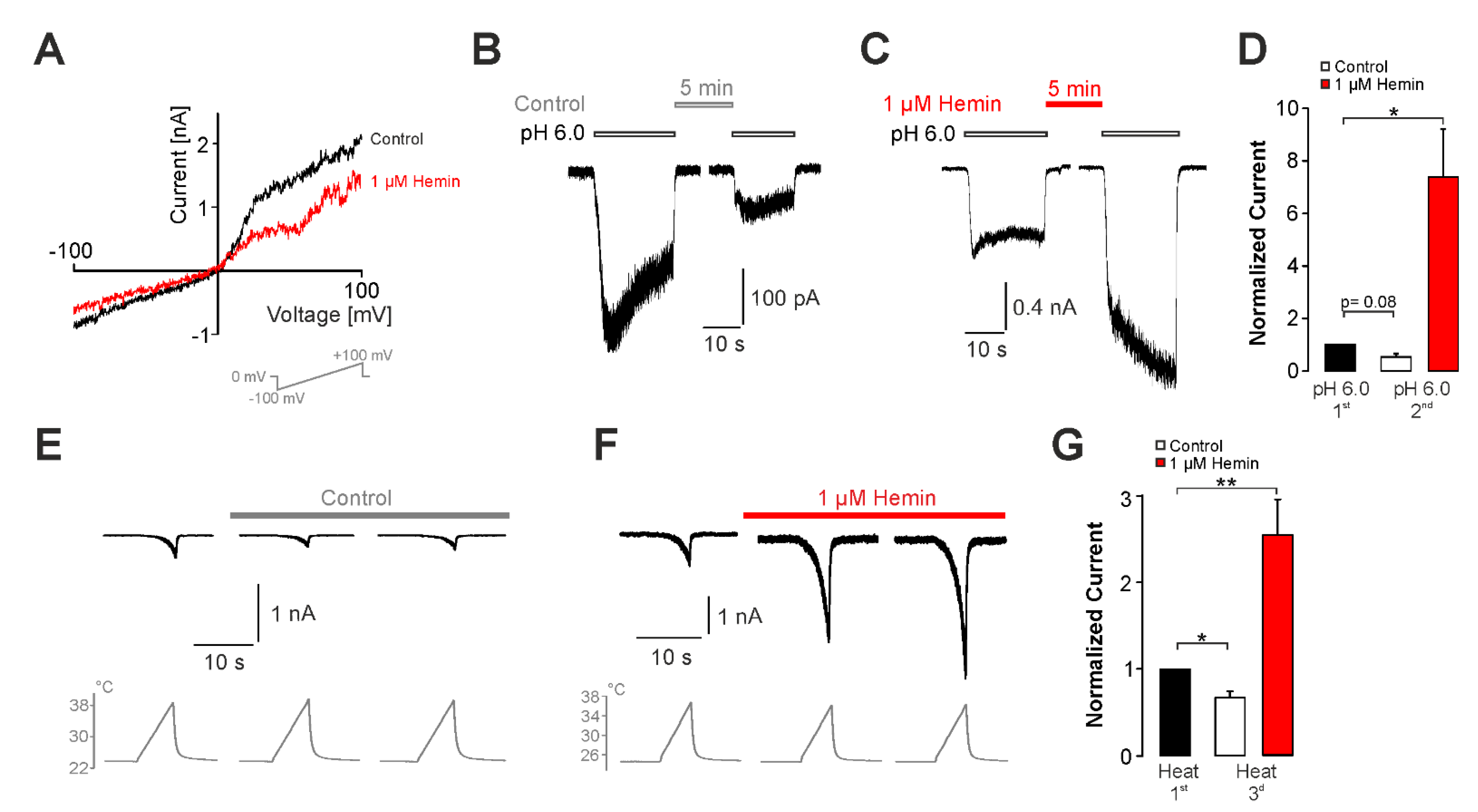
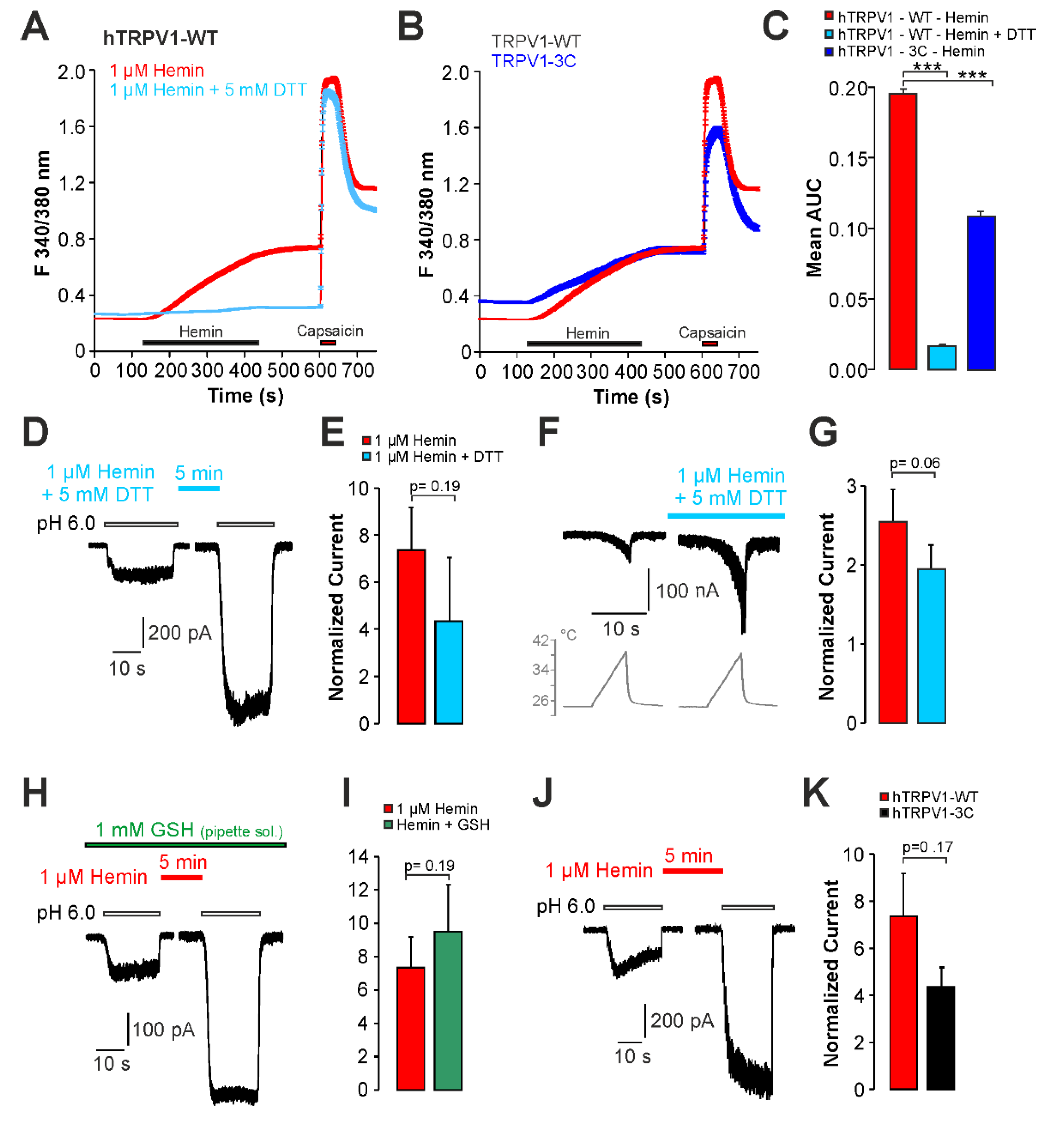
2.2. Hemin Induces an Activation and Sensitization of hTRPV1 Expressed in HEK293t Cells
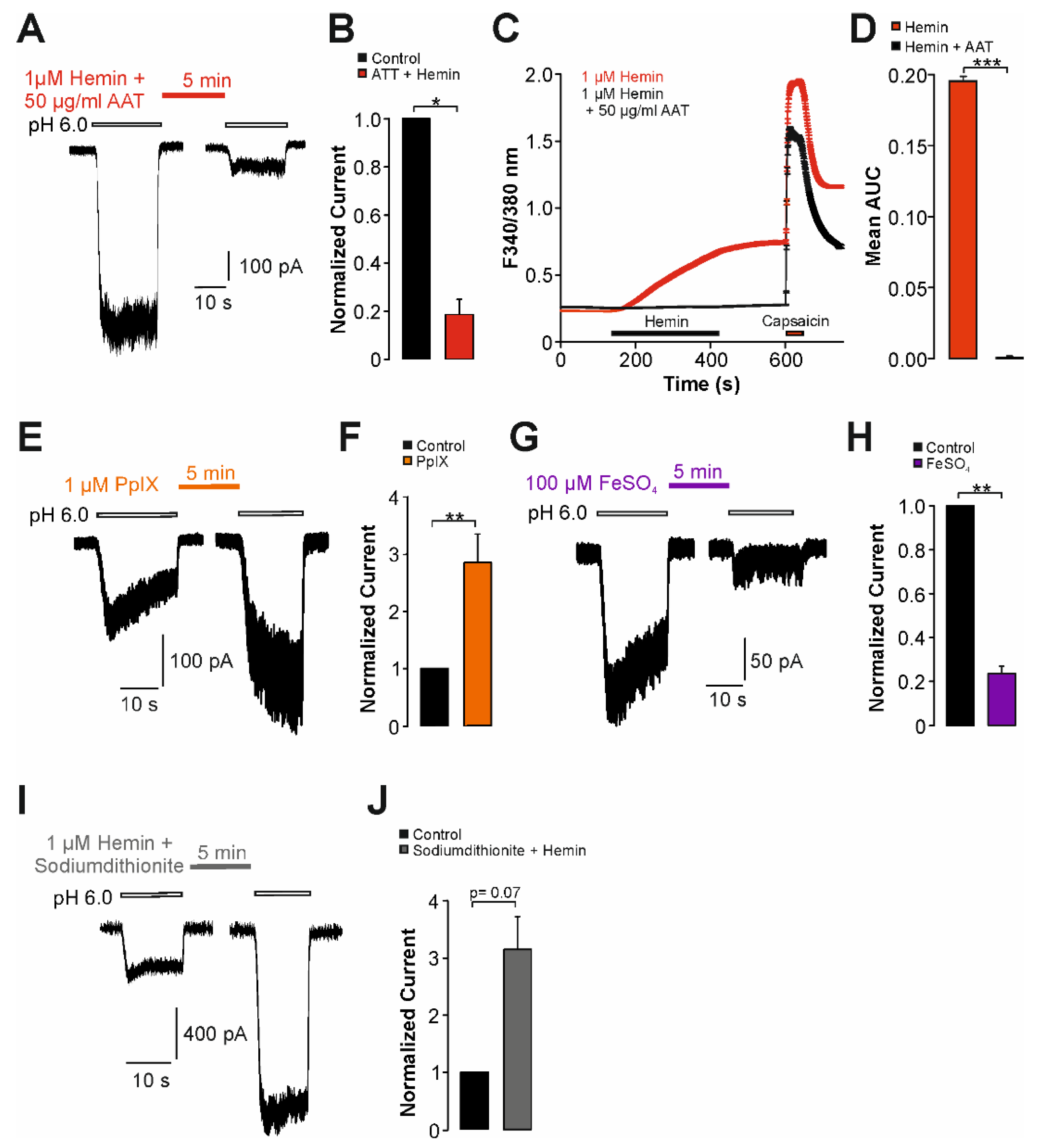
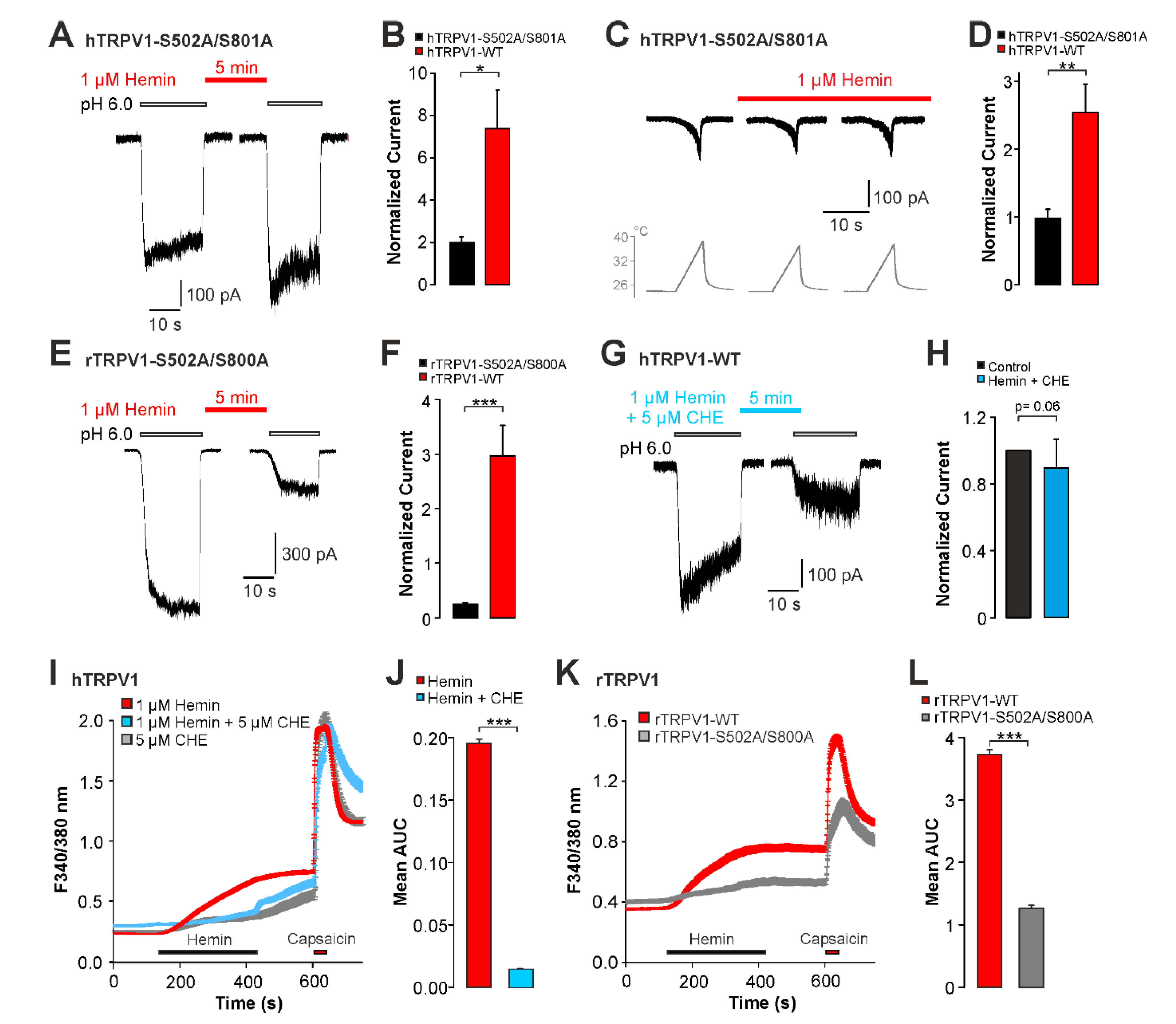
2.3. Sensitization of TRPV1 by Hemin Is Mediated by PKC
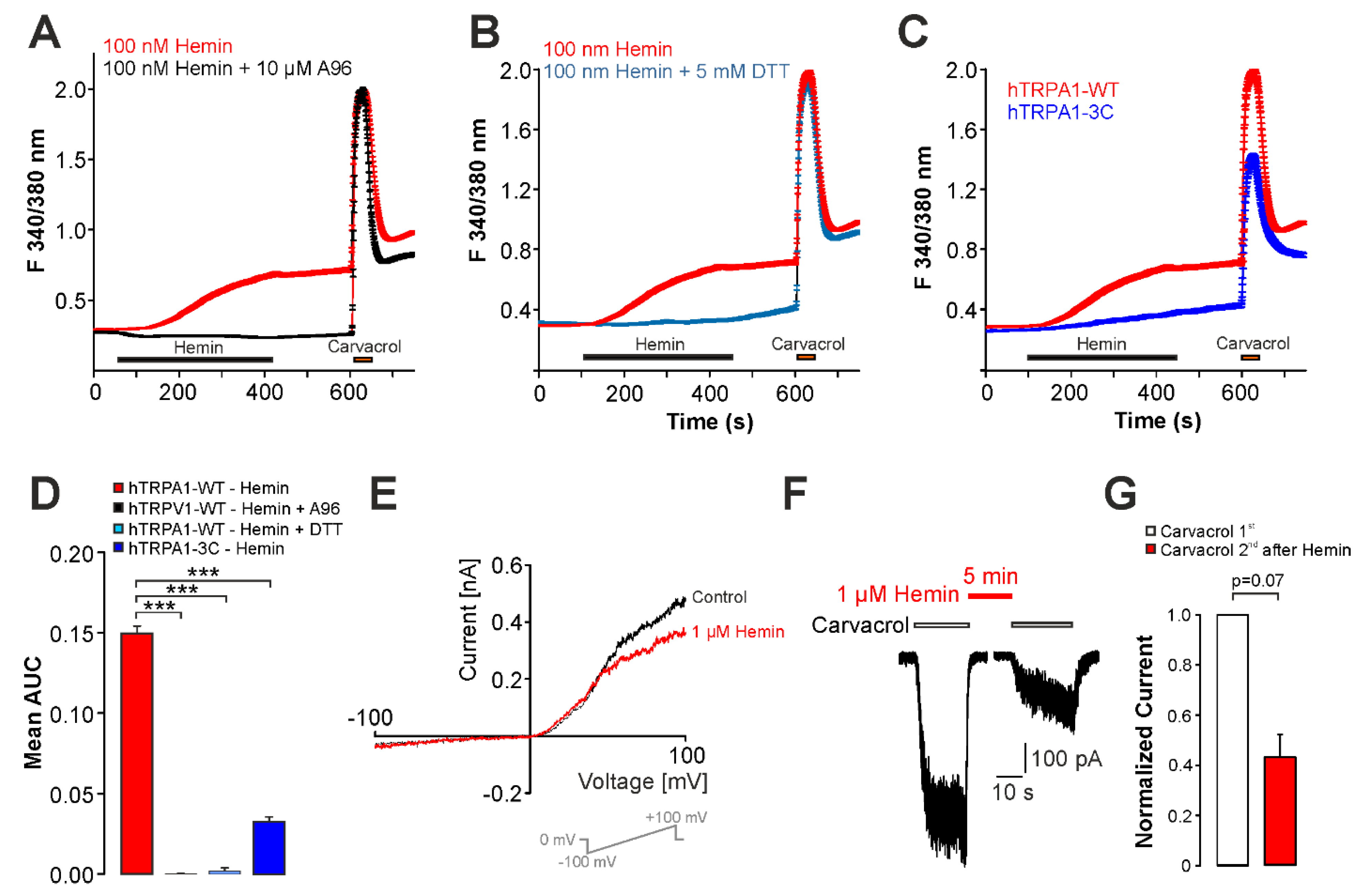
2.4. UVA-Light Triggers Direct Activation of TRPV1 and TRPA1 by Hemin
3. Discussion
4. Materials and Methods
4.1. Cell Culture and cDNA
4.2. Chemicals
4.3. Whole-Cell Patch Clamp
4.4. Calcium Imaging
4.5. Dorsal Root Ganglion Neuron Culture
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bevan, S.; Quallo, T.; Andersson, D.A. Trpv1. Handb. Exp. Pharmacol. 2014, 222, 207–245. [Google Scholar]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Gulinello, M.; Frenette, P.S. Nociceptors protect sickle cell disease mice from vaso-occlusive episodes and chronic organ damage. J. Exp. Med. 2021, 218, 218. [Google Scholar] [CrossRef] [PubMed]
- Baral, P.; Umans, B.D.; Li, L.; Wallrapp, A.; Bist, M.; Kirschbaum, T.; Wei, Y.; Zhou, Y.; Kuchroo, V.K.; Burkett, P.R.; et al. Nociceptor sensory neurons suppress neutrophil and gammadelta t cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 2018, 24, 417–426. [Google Scholar] [CrossRef]
- Cohen, J.A.; Edwards, T.N.; Liu, A.W.; Hirai, T.; Jones, M.R.; Wu, J.; Li, Y.; Zhang, S.; Ho, J.; Davis, B.M.; et al. Cutaneous trpv1(+) neurons trigger protective innate type 17 anticipatory immunity. Cell 2019, 178, 919–932.e914. [Google Scholar] [CrossRef]
- Agarwal, N.; Taberner, F.J.; Rangel Rojas, D.; Moroni, M.; Omberbasic, D.; Njoo, C.; Andrieux, A.; Gupta, P.; Bali, K.K.; Herpel, E.; et al. Sumoylation of enzymes and ion channels in sensory neurons protects against metabolic dysfunction, neuropathy, and sensory loss in diabetes. Neuron 2020, 107, 1141–1159.e1147. [Google Scholar] [CrossRef]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-innervating nociceptor neurons regulate peyer’s patch microfold cells and sfb levels to mediate salmonella host defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef]
- Huang, W.; Rubinstein, J.; Prieto, A.R.; Thang, L.V.; Wang, D.H. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension 2009, 53, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of neural transient receptor potential channels in peripheral inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef] [PubMed]
- Hillery, C.A.; Kerstein, P.C.; Vilceanu, D.; Barabas, M.E.; Retherford, D.; Brandow, A.M.; Wandersee, N.J.; Stucky, C.L. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011, 118, 3376–3383. [Google Scholar] [CrossRef] [Green Version]
- Sadler, K.E.; Stucky, C.L. Neuronal transient receptor potential (trp) channels and noxious sensory detection in sickle cell disease. Neurosci. Lett. 2019, 694, 184–191. [Google Scholar] [CrossRef]
- Vincent, L.; Vang, D.; Nguyen, J.; Gupta, M.; Luk, K.; Ericson, M.E.; Simone, D.A.; Gupta, K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013, 122, 1853–1862. [Google Scholar] [CrossRef] [Green Version]
- Sadler, K.E.; Zappia, K.J.; O’Hara, C.L.; Langer, S.N.; Weyer, A.D.; Hillery, C.A.; Stucky, C.L. Chemokine (c-c motif) receptor 2 mediates mechanical and cold hypersensitivity in sickle cell disease mice. Pain 2018, 159, 1652–1663. [Google Scholar] [CrossRef]
- Immenschuh, S.; Vijayan, V.; Janciauskiene, S.; Gueler, F. Heme as a target for therapeutic interventions. Front. Pharmacol. 2017, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Telen, M.J.; Malik, P.; Vercellotti, G.M. Therapeutic strategies for sickle cell disease: Towards a multi-agent approach. Nat. Rev. Drug Discov. 2019, 18, 139–158. [Google Scholar] [CrossRef]
- Mohan, S.; Glushakov, A.V.; Decurnou, A.; Narumiya, S.; Dore, S. Contribution of pge2 ep1 receptor in hemin-induced neurotoxicity. Front. Mol. Neurosci. 2013, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Coburger, I.; Yang, K.; Bernert, A.; Wiesel, E.; Sahoo, N.; Swain, S.M.; Hoshi, T.; Schonherr, R.; Heinemann, S.H. Impact of intracellular hemin on n-type inactivation of voltage-gated k(+) channels. Pflugers Arch. 2020, 472, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xu, R.; Heinemann, S.H.; Hoshi, T. The rck1 high-affinity ca2+ sensor confers carbon monoxide sensitivity to slo1 bk channels. Proc. Natl. Acad. Sci. USA 2008, 105, 4039–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem can bind to and inhibit mammalian calcium-dependent slo1 bk channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Graca-Souza, A.V.; Arruda, M.A.; de Freitas, M.S.; Barja-Fidalgo, C.; Oliveira, P.L. Neutrophil activation by heme: Implications for inflammatory processes. Blood 2002, 99, 4160–4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.; Qu, L.; Wang, S.; Kim, M.; Bennett, D.; Ro, J.; Caterina, M.J.; Chung, M.K. Phosphorylation of trpv1 s801 contributes to modality-specific hyperalgesia in mice. J. Neurosci. 2019, 39, 9954–9966. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase c. Nature 2000, 408, 985–990. [Google Scholar] [CrossRef]
- Chuang, H.H.; Lin, S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc. Natl. Acad. Sci. USA 2009, 106, 20097–20102. [Google Scholar] [CrossRef] [Green Version]
- Janciauskiene, S.; Tumpara, S.; Wiese, M.; Wrenger, S.; Vijayan, V.; Gueler, F.; Chen, R.; Madyaningrana, K.; Mahadeva, R.; Welte, T.; et al. Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J. Leukoc. Biol. 2017, 102, 1127–1141. [Google Scholar] [CrossRef]
- Babes, A.; Ciotu, C.I.; Hoffmann, T.; Kichko, T.I.; Selescu, T.; Neacsu, C.; Sauer, S.K.; Reeh, P.W.; Fischer, M.J.M. Photosensitization of trpa1 and trpv1 by 7-dehydrocholesterol: Implications for the smith-lemli-opitz syndrome. Pain 2017, 158, 2475–2486. [Google Scholar] [CrossRef]
- Pantke, S.; Fricke, T.C.; Eberhardt, M.J.; Herzog, C.; Leffler, A. Gating of the capsaicin receptor trpv1 by uva-light and oxidants are mediated by distinct mechanisms. Cell Calcium 2021, 96, 102391. [Google Scholar] [CrossRef]
- Chiabrando, D.; Fiorito, V.; Petrillo, S.; Bertino, F.; Tolosano, E. Heme: A neglected player in nociception? Neurosci. Biobehav. Rev. 2021, 124, 124–136. [Google Scholar] [CrossRef]
- Chiabrando, D.; Castori, M.; di Rocco, M.; Ungelenk, M.; Giesselmann, S.; Di Capua, M.; Madeo, A.; Grammatico, P.; Bartsch, S.; Hubner, C.A.; et al. Mutations in the heme exporter flvcr1 cause sensory neurodegeneration with loss of pain perception. PLoS Genet. 2016, 12, e1006461. [Google Scholar] [CrossRef]
- Bertino, F.; Firestone, K.; Bellacchio, E.; Jackson, K.E.; Asamoah, A.; Hersh, J.; Fiorito, V.; Destefanis, F.; Gonser, R.; Tucker, M.E.; et al. Heme and sensory neuropathy: Insights from novel mutations in the heme exporter feline leukemia virus subgroup c receptor 1. Pain 2019, 160, 2766–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.N.; Guo, L.H.; Li, Q.J.; Liu, L. The role of ho/co in the spinal nociception transmission and hyperalgesia of rats induced by formalin. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2013, 29, 58–62. [Google Scholar] [PubMed]
- Li, X.; Clark, J.D. Heme oxygenase type 2 plays a role in formalin-induced nociception. Pain 2000, 86, 75–80. [Google Scholar] [CrossRef]
- Babes, A.; Kichko, T.I.; Selescu, T.; Manolache, A.; Neacsu, C.; Gebhardt, L.; Reeh, P.W. Psoralens activate and photosensitize transient receptor potential channels ankyrin type 1 (trpa1) and vanilloid type 1 (trpv1). Eur. J. Pain 2021, 25, 122–135. [Google Scholar] [CrossRef]
- Babes, A.; Sauer, S.K.; Moparthi, L.; Kichko, T.I.; Neacsu, C.; Namer, B.; Filipovic, M.; Zygmunt, P.M.; Reeh, P.W.; Fischer, M.J. Photosensitization in porphyrias and photodynamic therapy involves trpa1 and trpv1. J. Neurosci. 2016, 36, 5264–5278. [Google Scholar] [CrossRef] [Green Version]
- Fricke, T.C.; Echtermeyer, F.; Zielke, J.; de la Roche, J.; Filipovic, M.R.; Claverol, S.; Herzog, C.; Tominaga, M.; Pumroy, R.A.; Moiseenkova-Bell, V.Y.; et al. Oxidation of methionine residues activates the high-threshold heat-sensitive ion channel trpv2. Proc. Natl. Acad. Sci. USA 2019, 116, 24359–24365. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmaers, N.E.; Wiegand, S.B.; Herzog, C.; Echtermeyer, F.G.; Eberhardt, M.J.; Leffler, A. Distinct Mechanisms Account for In Vitro Activation and Sensitization of TRPV1 by the Porphyrin Hemin. Int. J. Mol. Sci. 2021, 22, 10856. https://doi.org/10.3390/ijms221910856
Palmaers NE, Wiegand SB, Herzog C, Echtermeyer FG, Eberhardt MJ, Leffler A. Distinct Mechanisms Account for In Vitro Activation and Sensitization of TRPV1 by the Porphyrin Hemin. International Journal of Molecular Sciences. 2021; 22(19):10856. https://doi.org/10.3390/ijms221910856
Chicago/Turabian StylePalmaers, Natalie E., Steffen B. Wiegand, Christine Herzog, Frank G. Echtermeyer, Mirjam J. Eberhardt, and Andreas Leffler. 2021. "Distinct Mechanisms Account for In Vitro Activation and Sensitization of TRPV1 by the Porphyrin Hemin" International Journal of Molecular Sciences 22, no. 19: 10856. https://doi.org/10.3390/ijms221910856
APA StylePalmaers, N. E., Wiegand, S. B., Herzog, C., Echtermeyer, F. G., Eberhardt, M. J., & Leffler, A. (2021). Distinct Mechanisms Account for In Vitro Activation and Sensitization of TRPV1 by the Porphyrin Hemin. International Journal of Molecular Sciences, 22(19), 10856. https://doi.org/10.3390/ijms221910856





