Visuo-Acoustic Stimulation’s Role in Synaptic Plasticity: A Review of the Literature
Abstract
:1. Introduction
2. Neuroplasticity: Definition and Mechanisms
2.1. Biological Basis of Neuroplasticity: Microscopic Aspects
2.2. Biological Basis of Neuroplasticity: Macroscopic Aspects
3. Neuroplasticity in the Auditory System
3.1. Mechanisms of Auditory Plasticity
3.2. Biological Basis of Auditory Plasticity
4. Neuroplasticity in the Visual System
4.1. Mechanisms of Visual Neuroplasticity
4.2. Biological Basis of Visual Neuroplasticity
4.3. Visual Plasticity in Retinal Degenerative Diseases
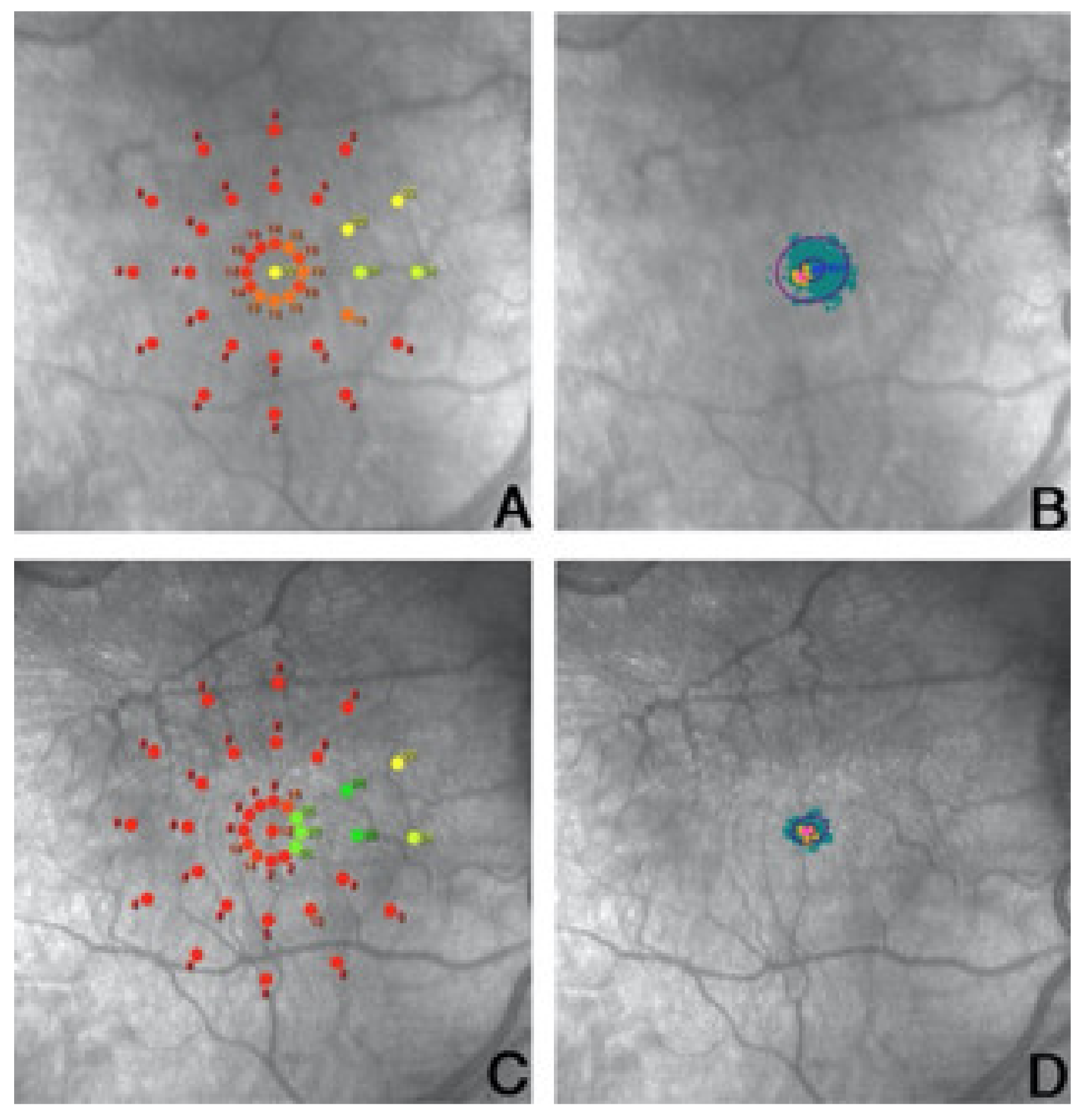
5. Microperimetric Biofeedback Training and Plasticity of the Visual Cortex
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosa, A.M.; Silva, M.F.; Ferreira, S.; Murta, J.; Castelo-Branco, M. Plasticity in the Human Visual Cortex: An Ophthalmology-Based Perspective. BioMed Res. Int. 2013, 2013, 568354. [Google Scholar] [CrossRef]
- Johnston, M.V. Plasticity in the developing brain: Implications for rehabilitation. Dev. Disabil. Res. Rev. 2009, 15, 94–101. [Google Scholar] [CrossRef]
- Bavelier, D.; Levi, D.M.; Li, R.; Dan, Y.; Hensch, T.K. Removing Brakes on Adult Brain Plasticity: From Molecular to Behavioral Interventions. J. Neurosci. 2010, 30, 14964–14971. [Google Scholar] [CrossRef]
- Dimyan, M.; Cohen, L.G. Neuroplasticity in the context of motor rehabilitation after stroke. Nat. Rev. Neurol. 2011, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Brain Plasticity and Reorganization Before, During, and After Glioma Resection. In Glioblastoma; Elsevier: Amsterdam, The Netherlands, 2016; pp. 225–236. [Google Scholar]
- Fernandez-Espejo, E.; Rodriguez-Espinosa, N. Psychostimulant Drugs and Neuroplasticity. Pharmaceuticals 2011, 4, 976–991. [Google Scholar] [CrossRef]
- Deperrois, N.; Graupner, M. Short-term depression and long-term plasticity together tune sensitive range of synaptic plasticity. PLoS Comput. Biol. 2020, 16, e1008265. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 2006, 13, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.N.; Donoghue, J.P. Plasticity and Primary Motor Cortex. Annu. Rev. Neurosci. 2000, 23, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural Substrates for the Effects of Rehabilitative Training on Motor Recovery After Ischemic Infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef] [Green Version]
- Merzenich, M.; Kaas, J.; Wall, J.; Nelson, R.; Sur, M.; Felleman, D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 1983, 8, 33–55. [Google Scholar] [CrossRef]
- Merzenich, M.; Kaas, J.; Wall, J.; Sur, M.; Nelson, R.; Felleman, D. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience 1983, 10, 639–665. [Google Scholar] [CrossRef]
- Kaas, J.H. Plasticity of Sensory and Motor Maps in Adult Mammals. Annu. Rev. Neurosci. 1991, 14, 137–167. [Google Scholar] [CrossRef]
- Holmes, G.L.; McCabe, B. Brain development and generation of brain pathologies. Int. Rev. Neurobiol. 2001, 45, 17–41. [Google Scholar] [CrossRef]
- Cruikshank, S.J.; Weinberger, N.M. Evidence for the Hebbian hypothesis in experience-dependent physiological plasticity of neocortex: A critical review. Brain Res. Rev. 1996, 22, 191–228. [Google Scholar] [CrossRef]
- Trachtenberg, J.T.; Chen, B.E.; Knott, G.W.; Feng, G.; Sanes, J.R.; Welker, E.; Svoboda, K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nat. Cell Biol. 2002, 420, 788–794. [Google Scholar] [CrossRef]
- Maletic-Savatic, M. Rapid Dendritic Morphogenesis in CA1 Hippocampal Dendrites Induced by Synaptic Activity. Science 1999, 283, 1923–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poo, M.-M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, M.; Heumann, R.; Lessmann, V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001, 20, 5887–5897. [Google Scholar] [CrossRef] [Green Version]
- Malenka, R.C.; Bear, M.F. LTP and LTD. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Huganir, R.L.; Nicoll, R.A. AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef] [Green Version]
- Feldman, D.E.; Nicoll, R.A.; Malenka, R.C. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cor-tex: LTP, LTD, and silent synapses. J. Neurobiol. 1999, 41, 92–101. [Google Scholar] [CrossRef]
- Shepherd, J.; Bear, M.F. New views of Arc, a master regulator of synaptic plasticity. Nat. Neurosci. 2011, 14, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jakkamsetti, V.; Tsai, N.-P.; Gross, C.; Molinaro, G.; Collins, K.A.; Nicoletti, F.; Wang, K.H.; Osten, P.; Bassell, G.J.; Gibson, J.R.; et al. Experience-Induced Arc/Arg3.1 Primes CA1 Pyramidal Neurons for Metabotropic Glutamate Receptor-Dependent Long-Term Synaptic Depression. Neuron 2013, 80, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, B.E.; Huber, K.M. Current Advances in Local Protein Synthesis and Synaptic Plasticity. J. Neurosci. 2006, 26, 7147–7150. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, K.M.; Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 1991, 251, 944–947. [Google Scholar] [CrossRef]
- Blitz, D.M.; Foster, K.A.; Regehr, W.G. Short-term synaptic plasticity: A comparison of two synapses. Nat. Rev. Neurosci. 2004, 5, 630–640. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens-Graham, B. New Insights into Neuron-Glia Communication. Science 2002, 298, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2007, 33, 18–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouach, N.; Glowinski, J.; Giaume, C. Activity-Dependent Neuronal Control of Gap-Junctional Communication in Astrocytes. J. Cell Biol. 2000, 149, 1513–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dityatev, A.; Schachner, M. Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 2003, 4, 456–468. [Google Scholar] [CrossRef]
- Kujala, T.; Alho, K.; Näätänen, R. Cross-modal reorganization of human cortical functions. Trends Neurosci. 2000, 23, 115–120. [Google Scholar] [CrossRef]
- Feeney, D.M.; Baron, J.C. Diaschisis. Stroke 1986, 17, 817–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossini, P.M.; Forno, G.D. Integrated technology for evaluation of brain function and neural plasticity. Phys. Med. Rehabil. Clin. 2004, 15, 263–306. [Google Scholar] [CrossRef]
- Luders, E.; Gaser, C.; Jancke, L.; Schlaug, G. A voxel-based approach to gray matter asymmetries. NeuroImage 2004, 22, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.J. Plasticity of gray matter volume: The cellular and synaptic plasticity that underlies volumetric change. Dev. Psychobiol. 2011, 53, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Kappel, V.; Moreno, A.C.D.P.; Buss, C.H. Plasticity of the auditory system: Theoretical considerations. Braz. J. Otorhinolaryngol. 2011, 77, 670–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, D.R. Plasticity in the auditory system. Hear. Res. 2018, 362, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, J.C.; King, A.J. Learning to hear: Plasticity of auditory cortical processing. Curr. Opin. Neurobiol. 2007, 17, 456–464. [Google Scholar] [CrossRef]
- Nelken, I. Stimulus-specific adaptation and deviance detection in the auditory system: Experiments and models. Biol. Cybern. 2014, 108, 655–663. [Google Scholar] [CrossRef]
- Kilgard, M.P. Cortical Map Reorganization without Cholinergic Modulation. Neuron 2005, 48, 529–530. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, J.J. Acquired hearing loss and brain plasticity. Hear. Res. 2017, 343, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive plasticity in tinnitus–triggers, mechanisms and treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Stefanescu, R.A.; Martel, D.; Shore, S.E. Tinnitus: Maladaptive auditory–somatosensory plasticity. Hear. Res. 2016, 334, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.R.; Shannon, R.V. Beyond cochlear implants: Awakening the deafened brain. Nat. Neurosci. 2009, 12, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Munro, K.J.; Lutman, M.E. The effect of speech presentation level on measurement of auditory acclimatization to amplified speech. J. Acoust. Soc. Am. 2003, 114, 484–495. [Google Scholar] [CrossRef]
- Fu, Q.-J.; Galvin, J.J. Perceptual Learning and Auditory Training in Cochlear Implant Recipients. Trends Amplif. 2007, 11, 193–205. [Google Scholar] [CrossRef]
- Pantev, C.; Dinnesen, A.; Ross, B.; Wollbrink, A.; Knief, A. Dynamics of Auditory Plasticity after Cochlear Implantation: A Longitudinal Study. Cereb. Cortex 2005, 16, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.; Yamoah, E.; Krubitzer, L. Multisensory plasticity in congenitally deaf mice: How are cortical areas functionally specified? Neuroscience 2006, 139, 1507–1524. [Google Scholar] [CrossRef] [PubMed]
- Doucet, M.E.; Bergeron, F.; Lassonde, M.; Ferron, P.; Lepore, F. Cross-modal reorganization and speech perception in cochlear implant users. Brain 2006, 129, 3376–3383. [Google Scholar] [CrossRef]
- Lee, H.-J.; Giraud, A.-L.; Kang, E.; Oh, S.-H.; Kang, H.; Kim, C.-S.; Lee, D.S. Cortical Activity at Rest Predicts Cochlear Implantation Outcome. Cereb. Cortex 2006, 17, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recanzone, G.H.; Schreiner, C.; Merzenich, M.M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J. Neurosci. 1993, 13, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Polley, D.B.; Steinberg, E.E.; Merzenich, M.M. Perceptual Learning Directs Auditory Cortical Map Reorganization through Top-Down Influences. J. Neurosci. 2006, 26, 4970–4982. [Google Scholar] [CrossRef]
- Herholz, S.C.; Zatorre, R.J. Musical Training as a Framework for Brain Plasticity: Behavior, Function, and Structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef] [Green Version]
- Carcagno, S.; Plack, C. Subcortical Plasticity Following Perceptual Learning in a Pitch Discrimination Task. J. Assoc. Res. Otolaryngol. 2010, 12, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Anomal, R.; De Villers-Sidani, E.; Merzenich, M.M.; Panizzutti, R. Manipulation of BDNF Signaling Modifies the Experience-Dependent Plasticity Induced by Pure Tone Exposure during the Critical Period in the Primary Auditory Cortex. PLoS ONE 2013, 8, e64208. [Google Scholar] [CrossRef] [Green Version]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Morishita, H.; Hensch, T.K. Critical period revisited: Impact on vision. Curr. Opin. Neurobiol. 2008, 18, 101–107. [Google Scholar] [CrossRef]
- Kotak, V.C.; Fujisawa, S.; Lee, F.A.; Karthikeyan, O.; Aoki, C.; Sanes, D.H. Hearing Loss Raises Excitability in the Auditory Cortex. J. Neurosci. 2005, 25, 3908–3918. [Google Scholar] [CrossRef] [Green Version]
- Balaram, P.; Hackett, T.; Polley, D. Synergistic Transcriptional Changes in AMPA and GABAA Receptor Genes Support Compensatory Plasticity Following Unilateral Hearing Loss. Neuroscience 2019, 407, 108–119. [Google Scholar] [CrossRef]
- Harms, L.; Parras, G.G.; Michie, P.T.; Malmierca, M.S. The Role of Glutamate Neurotransmission in Mismatch Negativity (MMN), A Measure of Auditory Synaptic Plasticity and Change-detection. Neuroscience 2021, 456, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Säljö, A.; Bao, F.; Hamberger, A.; Haglid, K.G.; Hansson, H.-A. Exposure to short-lasting impulse noise causes microglial and astroglial cell activation in the adult rat brain. Pathophysiology 2001, 8, 105–111. [Google Scholar] [CrossRef]
- Takesian, A.E.; Kotak, V.C.; Sanes, D.H. Developmental hearing loss disrupts synaptic inhibition: Implications for auditory processing. Future Neurol. 2009, 4, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Letzkus, J.; Wolff, S.B.E.; Meyer, E.; Tovote, P.; Courtin, J.; Herry, C.; Lüthi, A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nat. Cell Biol. 2011, 480, 331–335. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [Green Version]
- McKenna, T.M.; Ashe, J.H.; Weinberger, N.M. Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse 1989, 4, 30–43. [Google Scholar] [CrossRef]
- Kilgard, M.P. Cortical Map Reorganization Enabled by Nucleus Basalis Activity. Science 1998, 279, 1714–1718. [Google Scholar] [CrossRef]
- Wong, A.M. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can. J. Ophthalmol. 2012, 47, 399–409. [Google Scholar] [CrossRef]
- Daw, N.W. Critical Periods and Amblyopia. Arch. Ophthalmol. 1998, 116, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Legge, G.E.; Chung, S.T. Low Vision and Plasticity: Implications for Rehabilitation. Annu. Rev. Vis. Sci. 2016, 2, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Hubel, D.H.; Wiesel, T.N. Binocular interaction in striate cortex of kittens reared with artificial squint. J. Neurophysiol. 1965, 28, 1041–1059. [Google Scholar] [CrossRef] [Green Version]
- Wiesel, T.N.; Hubel, D.H. Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 1965, 28, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.L.; Wood, J. Amblyopia: Prevalence, natural history, functional effects and treatment. Clin. Exp. Optom. 2005, 88, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loudon, S.E.; Simonsz, H.J. The History of the Treatment of Amblyopia. Strabismus 2005, 13, 93–106. [Google Scholar] [CrossRef]
- Levi, D.M. Rethinking amblyopia 2020. Vis. Res. 2020, 176, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Ngo, C.; Nguyen, J.; Levi, D.M. Video-Game Play Induces Plasticity in the Visual System of Adults with Amblyopia. PLoS Biol. 2011, 9, e1001135. [Google Scholar] [CrossRef] [Green Version]
- Baroncelli, L.; Sale, A.; Viegi, A.; Vetencourt, J.F.M.; De Pasquale, R.; Baldini, S.; Maffei, L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp. Neurol. 2010, 226, 100–109. [Google Scholar] [CrossRef]
- Baroncelli, L.; Bonaccorsi, J.; Milanese, M.; Bonifacino, T.; Giribaldi, F.; Manno, I.; Cenni, M.C.; Berardi, N.; Bonanno, G.; Maffei, L.; et al. Enriched experience and recovery from amblyopia in adult rats: Impact of motor, social and sensory components. Neuropharmacology 2012, 62, 2388–2397. [Google Scholar] [CrossRef]
- Sale, A.; Maya-Vetencourt, J.F.; Medini, P.; Cenni, M.C.; Baroncelli, L.; De Pasquale, R.; Maffei, L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 2007, 10, 679–681. [Google Scholar] [CrossRef]
- Scholz, J.; Klein-Flugge, M.; Behrens, T.E.J.; Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009, 12, 1370–1371. [Google Scholar] [CrossRef] [Green Version]
- Karmarkar, U.R.; Dan, Y. Experience-Dependent Plasticity in Adult Visual Cortex. Neuron 2006, 52, 577–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furmanski, C.S.; Schluppeck, D.; Engel, S.A. Learning Strengthens the Response of Primary Visual Cortex to Simple Patterns. Curr. Biol. 2004, 14, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Spence, I.; Pratt, J. Playing an action video game reduces gender differences in spatial cognition. Psychol. Sci. 2007, 18, 850–855. [Google Scholar] [CrossRef]
- Green, C.S.; Li, R.; Bavelier, D. Perceptual Learning During Action Video Game Playing. Top. Cogn. Sci. 2010, 2, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Dye, M.W.G.; Green, C.S.; Bavelier, D. The development of attention skills in action video game players. Neuropsychologia 2009, 47, 1780–1789. [Google Scholar] [CrossRef] [Green Version]
- Webster, M.A. Adaptation and visual coding. J. Vis. 2011, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Cooke, S.F.; Bear, M.F. Stimulus-Selective Response Plasticity in the Visual Cortex: An Assay for the Assessment of Pathophysiology and Treatment of Cognitive Impairment Associated with Psychiatric Disorders. Biol. Psychiatry 2012, 71, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Rieke, F.; Rudd, M.E. The Challenges Natural Images Pose for Visual Adaptation. Neuron 2009, 64, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehezkel, O.; Sagi, D.; Sterkin, A.; Belkin, M.; Polat, U. Learning to adapt: Dynamics of readaptation to geometrical distortions. Vis. Res. 2010, 50, 1550–1558. [Google Scholar] [CrossRef] [Green Version]
- Mon-Williams, M.; Tresilian, J.R.; Strang, N.C.; Kochhar, P.; Wann, J.P. Improving vision: Neural compensation for optical defocus. Proc. R. Soc. B Boil. Sci. 1998, 265, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Berardi, N.; Pizzorusso, T.; Ratto, G.M.; Maffei, L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003, 26, 369–378. [Google Scholar] [CrossRef]
- Fagiolini, M.; Fritschy, J.-M.; Löw, K.; Möhler, H.; Rudolph, U.; Hensch, T.K. Specific GABAA Circuits for Visual Cortical Plasticity. Science 2004, 303, 1681–1683. [Google Scholar] [CrossRef] [Green Version]
- Fagiolini, M.; Hensch, T.K. Inhibitory threshold for critical-period activation in primary visual cortex. Nat. Cell Biol. 2000, 404, 183–186. [Google Scholar] [CrossRef]
- Harauzov, A.; Spolidoro, M.; DiCristo, G.; De Pasquale, R.; Cancedda, L.; Pizzorusso, T.; Viegi, A.; Berardi, N.; Maffei, L. Reducing Intracortical Inhibition in the Adult Visual Cortex Promotes Ocular Dominance Plasticity. J. Neurosci. 2010, 30, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Müller-Dahlhaus, F.; Paulus, W.; Ziemann, U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: Building models for the clinical use of CNS active drugs. J. Physiol. 2012, 590, 4641–4662. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Sillito, A. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience 1991, 40, 13–20. [Google Scholar] [CrossRef]
- Rasmusson, D. The role of acetylcholine in cortical synaptic plasticity. Behav. Brain Res. 2000, 115, 205–218. [Google Scholar] [CrossRef]
- Chamoun, M.; Huppe-Gourgues, F.; Legault, I.; Rosa-Neto, P.; Dumbrava, D.; Faubert, J.; Vaucher, E. Cholinergic Potentiation Improves Perceptual-Cognitive Training of Healthy Young Adults in Three Dimensional Multiple Object Tracking. Front. Hum. Neurosci. 2017, 11, 128. [Google Scholar] [CrossRef]
- Levenson, J.M.; Sweatt, J.D. Memory. Cell. Mol. Life Sci. 2006, 63, 1009–1016. [Google Scholar] [CrossRef]
- Pham, T.A.; Graham, S.J.; Suzuki, S.; Barco, A.; Kandel, E.R.; Gordon, B.; Lickey, M.E. A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn. Mem. 2004, 11, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Black, J.; Zelazny, A.M.; Greenough, W.T. Capillary and mitochondrial support of neural plasticity in adult rat visual cortex. Exp. Neurol. 1991, 111, 204–209. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.-I.; Hayashi, Y.; Sheng, M. The Importance of Dendritic Mitochondria in the Morphogenesis and Plasticity of Spines and Synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, T.A.; Klintsova, A.; Kilman, V.L.; Sirevaag, A.M.; Greenough, W.T. Induction of Multiple Synapses by Experience in the Visual Cortex of Adult Rats. Neurobiol. Learn. Mem. 1997, 68, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Lövdén, M.; Wenger, E.; Mårtensson, J.; Lindenberger, U.; Bäckman, L. Structural brain plasticity in adult learning and development. Neurosci. Biobehav. Rev. 2013, 37, 2296–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nadai, K.; Romano, M.R.; Binotto, A.; Costagliola, C.; Sato, G.; Parmeggiani, F. Clinical and Rehabilitative Management of Retinitis Pigmentosa:Up-to-Date. Curr. Genom. 2011, 12, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, Y.; Horiguchi, H.; Dumoulin, S.O.; Furuta, A.; Miyauchi, S.; Nakadomari, S.; Wandell, B.A. Task-Dependent V1 Responses in Human Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5356–5364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, V.; Ziccardi, L.; Stifano, G.; Montrone, L.; Gallinaro, G.; Falsini, B. Impact of regional retinal responses on cortical visually evoked responses: Multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin. Neurophysiol. 2010, 121, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, W.; Shi, F.; Liu, Y.; Li, J.; Qin, W.; Li, K.; Yu, C.; Jiang, T. Thick Visual Cortex in the Early Blind. J. Neurosci. 2009, 29, 2205–2211. [Google Scholar] [CrossRef] [Green Version]
- Ritter, M.; Hummer, A.; Ledolter, A.A.; Holder, G.E.; Windischberger, C.; Schmidt-Erfurth, U.M. Correspondence between retinotopic cortical mapping and conventional functional and morphological assessment of retinal disease. Br. J. Ophthalmol. 2018, 103, 208–215. [Google Scholar] [CrossRef]
- Chen, N.; Shin, K.; Millin, R.; Song, Y.; Kwon, M.; Tjan, B.S. Cortical reorganization of peripheral vision induced by simulated central vision loss. J. Neurosci. 2019, 39, 3529–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, S.-H.; Legge, G.E. Functional and cortical adaptations to central vision loss. Vis. Neurosci. 2005, 22, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, E.H.; Jacko, J.A.; Primo, S.A.; Main, K.L.; Moloney, K.P.; Kinzel, E.N.; Ginn, J. Reorganization of visual processing is related to eccentric viewing in patients with macular degeneration. Restor. Neurol. Neurosci. 2008, 26, 391–402. [Google Scholar] [PubMed]
- Wandell, B.A.; Smirnakis, S.M. Plasticity and stability of visual field maps in adult primary visual cortex. Nat. Rev. Neurosci. 2009, 10, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Cheung, S.-H.; Schuchard, R.A.; Glielmi, C.B.; Hu, X.; He, S.; Legge, G.E. Incomplete Cortical Reorganization in Macular Degeneration. Investig. Opthalmology Vis. Sci. 2010, 51, 6826–6834. [Google Scholar] [CrossRef]
- Darian-Smith, C.; Gilbert, C. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J. Neurosci. 1995, 15, 1631–1647. [Google Scholar] [CrossRef]
- Sunness, J.S.; Liu, T.; Yantis, S. Retinotopic mapping of the visual cortex using functional magnetic resonance imaging in a patient with central scotomas from atrophic macular degeneration. Ophthalmology 2004, 111, 1595–1598. [Google Scholar] [CrossRef]
- Baseler, H.; Gouws, A.; Haak, K.; Racey, C.; Crossland, M.; Tufail, A.; Rubin, G.S.; Cornelissen, F.W.; Morland, A. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat. Neurosci. 2011, 14, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Castaldi, E.; Lunghi, C.; Morrone, M.C. Neuroplasticity in adult human visual cortex. Neurosci. Biobehav. Rev. 2020, 112, 542–552. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Napolitano, G.; Fragiotta, S. Microperimetric biofeedback training fundamentals strategies and perspectives. Front. Biosci. 2018, 10, 48–64. [Google Scholar] [CrossRef] [Green Version]
- Crossland, M.D.; Culham, L.E.; Kabanarou, S.A.; Rubin, G.S. Preferred Retinal Locus Development in Patients with Macular Disease. Ophthalmology 2005, 112, 1579–1585. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Salvatore, S.; Limoli, P.G. MP-1 Biofeedback: Luminous Pattern Stimulus Versus Acoustic Biofeedback in Age Related Macular degeneration (AMD). Appl. Psychophysiol. Biofeedback 2012, 38, 11–16. [Google Scholar] [CrossRef]
- Falkenberg, H.K.; Rubin, G.S.; Bex, P.J. Acuity, crowding, reading and fixation stability. Vis. Res. 2007, 47, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda-Consolvo, T.; Otsuka, M.; Hayashi, Y.; Ishida, M.; Hayashi, A. Microperimetric Biofeedback Training Improved Visual Acuity after Successful Macular Hole Surgery. J. Ophthalmol. 2015, 2015, 572942. [Google Scholar] [CrossRef] [Green Version]
- Sborgia, G.; Niro, A.; Tritto, T.; Albano, V.; Sborgia, L.; Sborgia, A.; Donghia, R.; Giancipoli, E.; Coassin, M.; Pastore, V.; et al. Microperimetric Biofeedback Training After Successful Inverted Flap Technique for Large Macular Hole. J. Clin. Med. 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneschg, O.A.; Barboni, M.T.S.; Nagy, Z.Z.; Németh, J. Fixation stability after surgical treatment of strabismus and biofeedback fixation training in amblyopic eyes. BMC Ophthalmol. 2021, 21, 264. [Google Scholar] [CrossRef]
- Lei, H.; Schuchard, R.A. Using two preferred retinal loci for different lighting conditions in patients with central scoto-mas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1812–1818. [Google Scholar]
- Andrade, M.A.; Muro, E.M.; Morán, F. Simulation of plasticity in the adult visual cortex. Biol. Cybern. 2001, 84, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Safran, A.B.; Landis, T. Plasticity in the adult visual cortex. Curr. Opin. Ophthalmol. 1996, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.I.; Peli, E.; Knouf, N.; Kanwisher, N.G. Reorganization of Visual Processing in Macular Degeneration. J. Neurosci. 2005, 25, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Vingolo, E.M.; Salvatore, S.; Cavarretta, S. Low-Vision Rehabilitation by Means of MP-1 Biofeedback Examination in Patients with Different Macular Diseases: A Pilot Study. Appl. Psychophysiol. Biofeedback 2009, 34, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vingolo, E.M.; Cavarretta, S.; Domanico, D.; Parisi, F.; Malagola, R. Microperimetric Biofeedback in AMD Patients. Appl. Psychophysiol. Biofeedback 2007, 32, 185–189. [Google Scholar] [CrossRef]
- Salvatore, S.; Vingolo, E.M. The Mozart effect in biofeedback visual rehabilitation: A case report. Clin. Ophthalmol. 2011, 5, 1269–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratra, D.; Gopalakrishnan, S.; Dalan, D.; Ratra, V.; Damkondwar, D.; Laxmi, G. Visual rehabilitation using microperimetric acoustic biofeedback training in individuals with central scotoma. Clin. Exp. Optom. 2019, 102, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, M.; Soler, V.; Cottereau, B.; Trotter, Y. Spontaneous and training-induced cortical plasticity in MD patients: Hints from lateral masking. Sci. Rep. 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonti, E.; Budini, M.; Vingolo, E.M. Visuo-Acoustic Stimulation’s Role in Synaptic Plasticity: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 10783. https://doi.org/10.3390/ijms221910783
Tonti E, Budini M, Vingolo EM. Visuo-Acoustic Stimulation’s Role in Synaptic Plasticity: A Review of the Literature. International Journal of Molecular Sciences. 2021; 22(19):10783. https://doi.org/10.3390/ijms221910783
Chicago/Turabian StyleTonti, Emanuele, Mauro Budini, and Enzo Maria Vingolo. 2021. "Visuo-Acoustic Stimulation’s Role in Synaptic Plasticity: A Review of the Literature" International Journal of Molecular Sciences 22, no. 19: 10783. https://doi.org/10.3390/ijms221910783
APA StyleTonti, E., Budini, M., & Vingolo, E. M. (2021). Visuo-Acoustic Stimulation’s Role in Synaptic Plasticity: A Review of the Literature. International Journal of Molecular Sciences, 22(19), 10783. https://doi.org/10.3390/ijms221910783





