Incomptine A Induces Apoptosis, ROS Production and a Differential Protein Expression on Non-Hodgkin’s Lymphoma Cells †
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Incomptine A (IA) Isolation
4.2. Chemicals
4.3. Cell Cultures and Reagents
4.3.1. Cytotoxic Activity
4.3.2. Annexin V/DAPI Apoptosis Assay
4.3.3. Reactive Oxygen Species Assay
4.3.4. Cell Lysis and iTRAQ-Based Proteomic Analysis
4.3.5. Nano LC-MS/MS Analyses
4.3.6. Protein Identification
4.3.7. Differential Protein Analysis
4.3.8. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calzada, F.; Ramirez-Santos, J.; Valdes, M.; Garcia-Hernandez, N.; Pina-Jiménez, E.; Ordoñez-Razo, R.M. Evaluation of acute oral toxicity, brine shrimp lethality, and antilymphoma activity of geranylgeraniol and Annona macroprophyllata Leaf extracts. Rev. Bras. Farmacogn. 2020, 30, 301–304. [Google Scholar] [CrossRef]
- Howard, S.C.; McCormick, J.; Pui, C.H.; Buddington, R.K.; Harvey, R.D. Preventing and managing toxicities of high-dose methotrexate. Oncologist 2016, 21, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Calzada, F.; Yepez-Mulia, L.; Tapia-Contreras, A.; Alfredo, O. Antiprotozoal and antibacterial properties of Decachaeta incompta. Rev. Latinoamer. Quim. 2009, 37, 97–103. [Google Scholar]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Baer-Dubowska, W.; Gnojkowski, J.; Chmiel, J. Inhibicja enzymów przemiany glikolitycznej przez laktony seskwiterpe nowe w limfocytach stymulowanych fitohemaglutynina [Inhibition of glycolytic enzymes by sesquiterpene lactones in phytohemagglutinin-stimulated lymphocytes]. Folia Med. Cracov. 1980, 22, 393–402. [Google Scholar] [PubMed]
- Ritterson, L.C.; Tolan, D.R. Targeting of several glycolytic enzymes using RNA interference reveals aldolase affects cancer cell proliferation through a non-glycolytic mechanism. J. Biol. Chem. 2012, 287, 42554–42563. [Google Scholar]
- Velazquez-Dominguez, J.; Marchat, L.A.; Lopez-Camarillo, C.; Mendoza-Hernandez, G.; Sanchez Espindola, E.; Calzada, F.; Ortega-Hernandez, A.; Sanchez-Monroy, V.; Ramirez-Moreno, E. Effect of the sesquiterpene lactone incomptine A in the energy metabolism of Entamoeba histolytica. Exp. Parasitol. 2013, 135, 503–510. [Google Scholar]
- Guerrero, C.; Taboada, J.; Diaz, J.B.; Oliva, A.; Ortega, A. Incomptinas A y B dos heliangolidas aisladas de Decachaeta incompta. Estudio preliminar de las actividades biologicas de incomptina B. Rev. Latinoamer. Quim. 1994, 23, 142–147. [Google Scholar]
- Calzada, F.; Valdes, M.; Barbosa, E.; Velazquez, C.; Bautista, E. Evaluation of antipropulsive activity of Decachaeta in compta (DC) King and Robinson and its sesquiterpene lactones on induced hyperperistalsis in rats. Phcog. Mag. 2020, 16, S272–S275. [Google Scholar] [CrossRef]
- Calzada, F.; Solares-Pascasio, J.I.; Valdes, M.; Garcia-Hernandez, N.; Velazquez, C.; Ordoñez-Razo, R.M.; Barbosa, E. Antilymphoma potential of the ethanol extract and rutin obtained of the leaves from Schinus molle Linn. Phcog. Res. 2018, 10, 119–123. [Google Scholar] [CrossRef]
- Abdolmohamma, M.H.; Fouladdel, S.; Shafiee, A.; Amin, G.; Ghaffari, S.; Azizi, E. Anticancer effects and cell cycle analysis on human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to doxorubicin. J. Pharm. Sci. 2008, 16, 112–118. [Google Scholar]
- Perumal, A.; AlSalhi, M.S.; Kanakarajan, S.; DEvanesan, S.; Selvaraj, R.; Tamizhazhagan, V. Phytochemical evaluation and anticancer activity of rambutan (Nephelium lappaceum) fruit endocarp extracts against human hepatocellular carcinoma (HepG-2) cells. Saudi J. Biol. Sci. 2021, 28, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Douros, J. Current Current status of the NCI plant and animal product program. J. Nat. Prod. 1982, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponeko, V.; Hay, N.; Gartel, A. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; JMDucore Storms, D.H. Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells. Cancer Lett. 2007, 254, 119–127. [Google Scholar] [CrossRef]
- Gautam, P.; Nair, S.C.; Gupta, M.K.; Rakesh, S.; Polisetty, R.V.; Uppin, M.S.; Sundaram, C.; Puligopu, A.K.; Ankathi, P.; Purohit, A.K.; et al. Proteins with Altered Levels in Plasma from Glioblastoma Patients as Revealed by iTRAQ-Based Quantitative Proteomic Analysis. PLoS ONE 2012, 7, e46153. [Google Scholar]
- Li, S.; Su, X.; Jin, Q.; Li, G.; Sun, Y.; Abdullah, M.; Cai, Y.; Lin, Y. iTRAQ-Based identification of proteins related to lignin synthesis in the pear pollinated with pollen from different varieties. Molecules 2018, 23, 548. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, S.; Zhang, X.; Li, W.; Luo, S.; Sun, Z.; Nie, R. iTRAQ-based quantitative proteomic analysis of processed Euphorbia lathyrism L. for reducing the intestinal toxicity. Proteome Sci. 2018, 16, 8. [Google Scholar] [CrossRef]
- Tran, P.N.; Tate, C.J.; Ridgway, M.; Saliba, K.J.; Kirk, K. Human dihydrofolate reductase influences the sensitivity of the malaria parasite Plasmodium falciparum to ketotifen-A cautionary tale in screening transgenic parasites. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 179–183. [Google Scholar] [CrossRef][Green Version]
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de novo synthesis increases breast cancer stemness and metastasis via cGMP-PKG-MAPK signaling pathway. PLoS Biol. 2020, 18, e3000872. [Google Scholar] [CrossRef]
- Spurlock, C.F., III; Tossberg, J.T.; Fuchs, H.A.; Olsen, N.J.; Aune, T.M. Methotrexate increases expression of cell cycle checkpoint genes via JNK activation. Arthritis Rheum. 2012, 64, 1780–1789. [Google Scholar] [CrossRef]
- Herman, S.; Zurgil, N.; Deutsch, M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm. Res. 2005, 54, 273–280. [Google Scholar] [CrossRef]
- Canellos, G.P.; Skarin, A.T.; Rosenthal, D.S.; Moloney, W.C.; Frei, E. III. Methotrexate as a single agent and in combination chemotherapy for the treatment of non-Hodgkin’s lymphoma of unfavorable histology. Cancer Treat. Rep. 1981, 65, 125–129. [Google Scholar]
- Kuwana, T.; King, L.E.; Cosentino, K.; Suess, J.; Garcia-Saez, A.J.; Gilmore, A.P.; Newmeyer, D.D. Mitochondrial residence of the apoptosis inducer BAX is more important than BAX oligomerization in promoting membrane permeabilization. J. Biol. Chem. 2020, 295, 1623–1636. [Google Scholar] [CrossRef]
- Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione transferases: Potential targets to overcome chemoresistance in solid tumors. Int. J. Mol. Sci. 2018, 19, 3785. [Google Scholar] [CrossRef]
- Lang, J.Y.; Ma, K.; Guo, J.X.; Sun, H. Oxidative stress induces B lymphocyte DNA damage and apoptosis by upregulating p66shc. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1051–1060. [Google Scholar] [PubMed]
- Bridge, G.; Rashid, S.; Martin, S.A. DNA mismatch repair and oxidative DNA damage: Implications for cancer biology and treatment. Cancers 2014, 6, 1597–1614. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Bella, S.D.; Marco, P.D.; Emanuele, S.; Fiore, R.D.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed]
- Kordes, U.; Krappmann, D.; Heissmeyer, V.; Ludwing, W.D.; Scheidereit, C. Transcription factor NF-κB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000, 14, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-κB signaling: Balancing life and death—A new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Xu, L.; Xu, M.; Tong, X. Effects of aerobic glycolysis on pathogenesis and drug resistance of non-Hodgkin lymphoma. Zhejiang Da Xue Xue Bao Yi Xue Ban 2019, 48, 219–223. [Google Scholar] [PubMed]
- Tang, Y.; Yang, X.; Feng, K.; Hu, C.; Li, S. High expression of aldolase A is associated with tumor progression and poor prognosis in hepatocellular carcinoma. J. Gastrointest. Oncol. 2021, 12, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Gizak, A.; Wisniewski, J.; Heron, P.; Mamczur, P.; Sygusch, J.; Rakus, D. Targeting a moonlighting function of aldolase induces apoptosis in cancer cells. Cell Death Dis. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Wallberg, F.; Tenev, T.; Meier, P. Analysis of Apoptosis and Necroptosis by Fluorescence-Activated Cell Sorting. Cold Spring Harb. Protoc. 2016, 2016, 087387. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P.A.; Guzman, M.L.; Mayani, H.; Chavez-Gonzalez, A. Parthenolide and DMAPT induce cell death in primitive CML cells through reactive oxygen species. J. Cell. Mol. Med. 2018, 22, 4899–4912. [Google Scholar] [CrossRef]

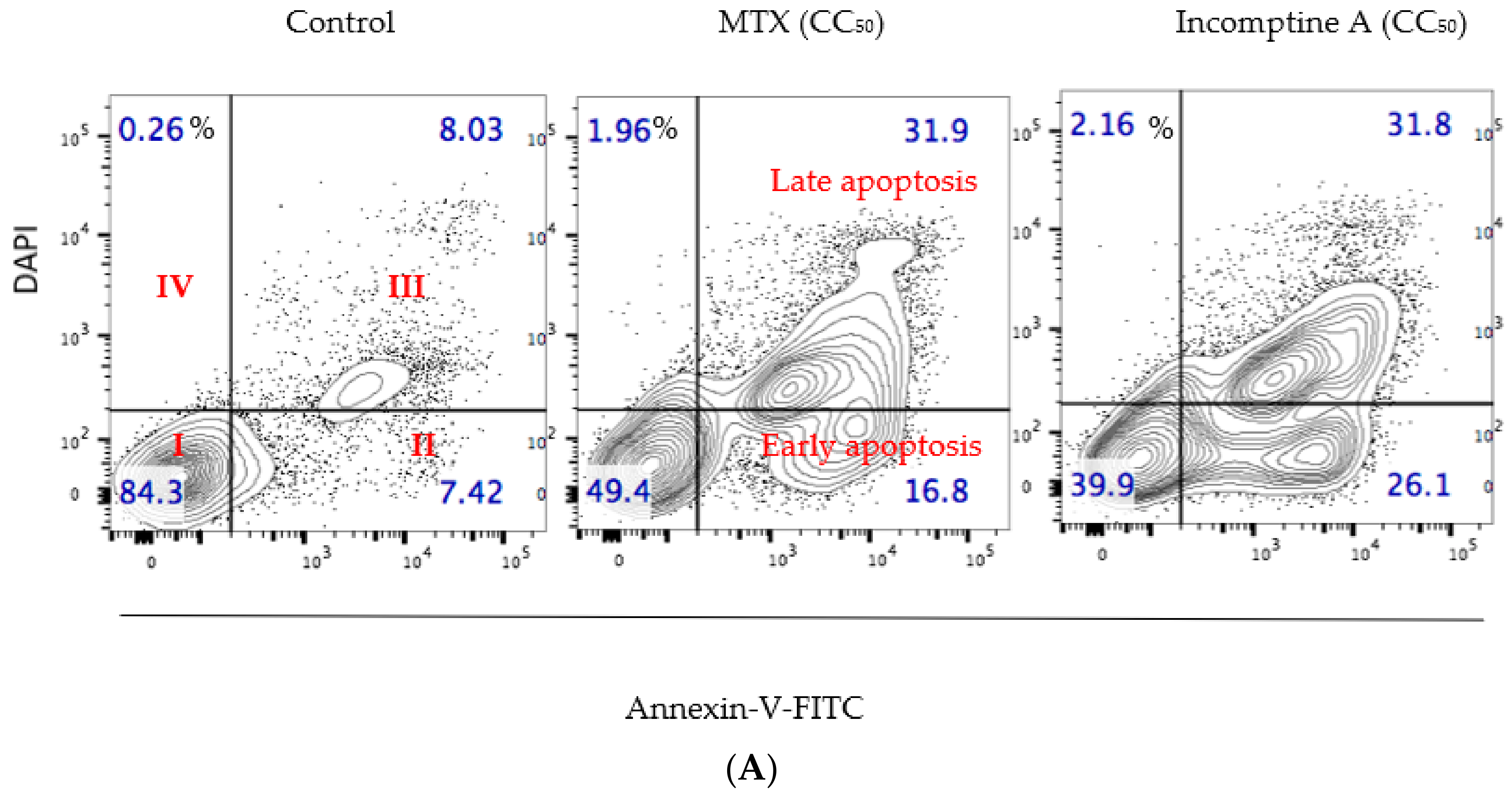
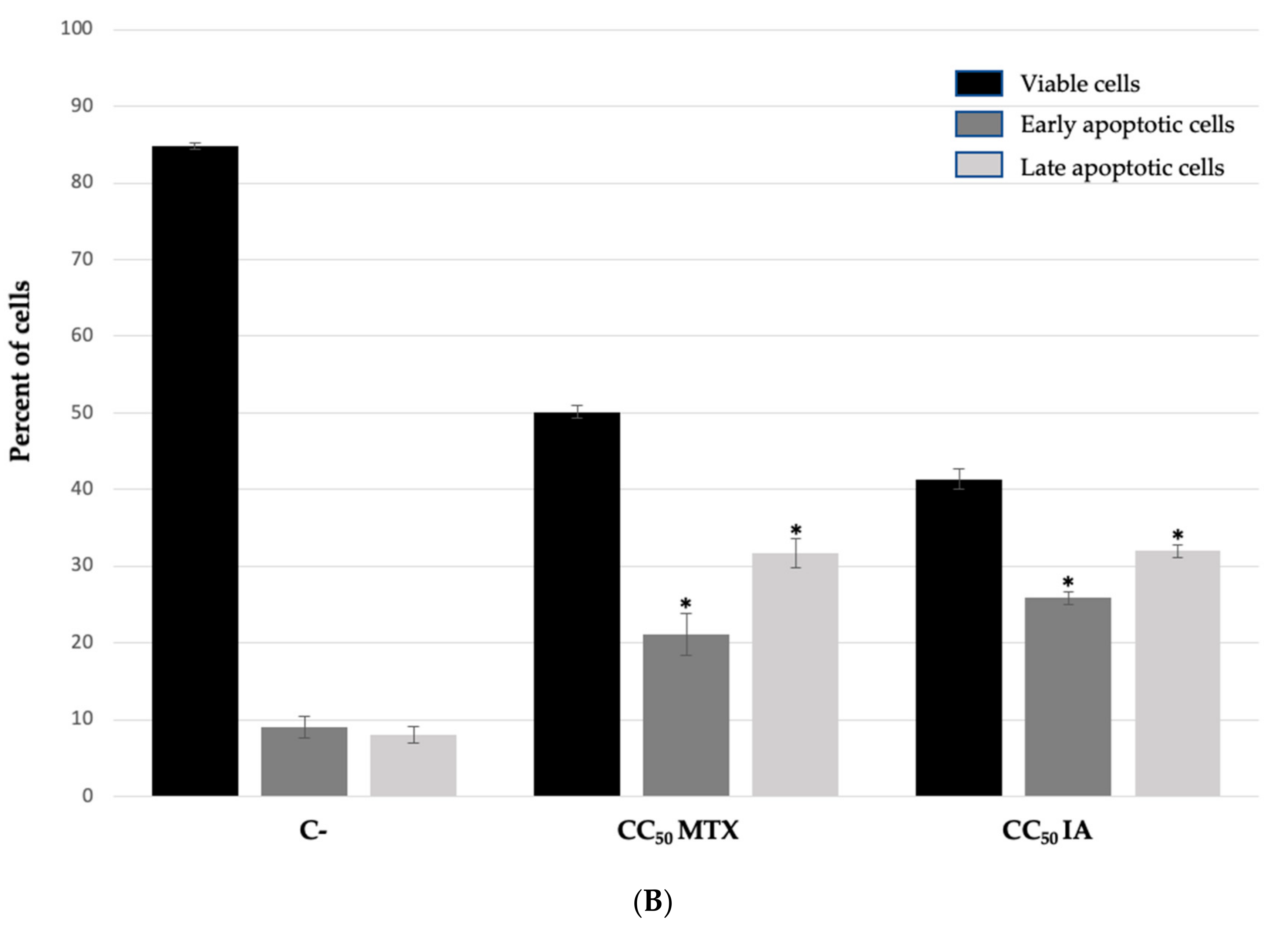
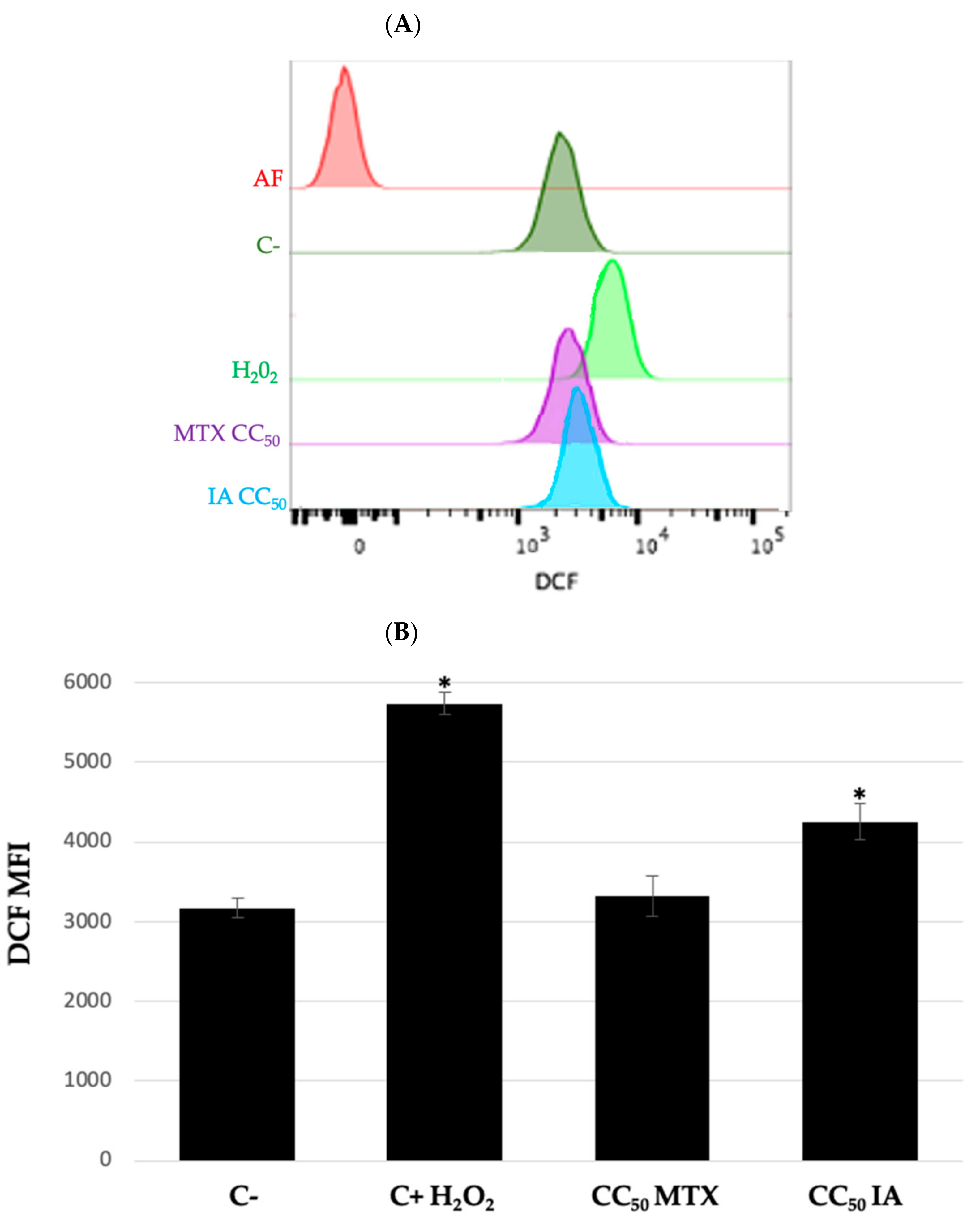
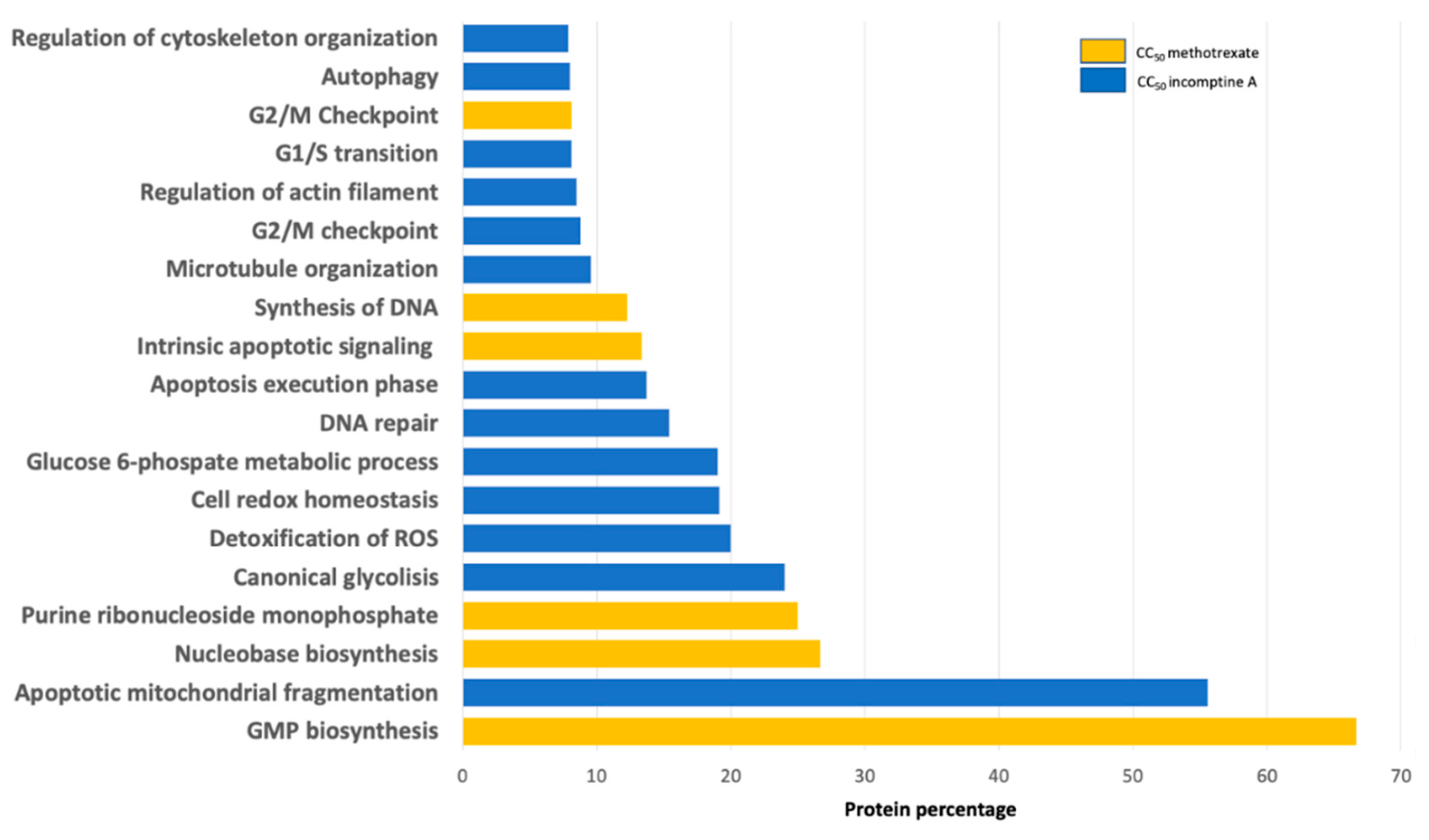

| NHL Cell Line | Incomptine A (IA) CC50 (mM) a | Methotrexate CC50 (mM) a |
|---|---|---|
| U-937 | 0.12 ± 0.02 | 0.5 ± 0.004 |
| Farage | 2.3 ± 0.55 | 1.5 ± 0.01 |
| SU-DHL-2 | 3.2 ± 0.04 | 1.3 ± 0.01 |
| REC-1 | 3.5 ± 0.01 | 8.1 ± 0.05 |
| Protein ID | Protein Name | Gene Name | MTX (FC) | IA (FC) |
|---|---|---|---|---|
| Q07812 | Apoptosis regulator BAX | BAX | 1.12 | 1.75 |
| Q53EL6 | Programmed cell death protein 4 | PDCD4 | 1.98 | 2.80 |
| Q92597 | NDRG1 protein | NDRG1 | 1.53 | 1.64 |
| Q16548 | Bcl-2-related protein A1 | BCL2A1 | 0.77 | 0.53 |
| Q9NYF8 | Bcl-2-associated transcription factor 1 | BCLAF1 | 0.56 | 0.26 |
| P00374 | Dihydrofolate reductase | DHFR | 1.42 | 1.77 |
| P11586 | Tetrahydrofolate synthase | MTHFD1 | 1.29 | 2.1 |
| P60891 | Ribose phosphate pyrophosphokinase 1 | PRPS1 | 1.65 | 2.24 |
| P10620 | Microsomal glutathione S-transferase 1 | MGST1 | 0.76 | 0.59 |
| Q9Y2Q3 | Glutathione S-transferase kappa 1 | GSTK1 | 0.65 | 0.49 |
| Q06830 | Peroxiredoxin-1 | PRDX1 | 0.93 | 2.74 |
| P32119 | Peroxiredoxin-2 | PRDX2 | 1.21 | 1.95 |
| P30041 | Peroxiredoxin-6 | PRDX6 | 1.27 | 2.29 |
| O14920 | Inhibitor of nuclear factor kappa-B kinase subunit beta | IKBKB | 1.23 | 1.73 |
| P19838 | Nuclear factor NF-kappa-B p105 subunit | NFKB1 | 1.11 | 1.68 |
| Q04206 | Nuclear factor NF-kappa-B p65 subunit | RELA | 1.79 | 1.62 |
| Q04864 | c-Rel | REL | 1.45 | 1.9 |
| P42224 | Signal transducer and activator of transcription 1-alpha/beta | STAT1 | 1.50 | 1.97 |
| P30281 | G1/S-specific cyclin-D3 | CCND3 | 1.21 | 0.57 |
| P43246 | DNA mismatch repair protein Msh2 | MSH2 | 1.39 | 1.95 |
| O95352 | Autophagy protein 7 | ATG7 | 1.40 | 2.73 |
| Q9NT62 | Autophagy protein 3 | ATG3 | 1.31 | 2.21 |
| P11279 | Lysosomal associated membrane protein 1 | LAMP1 | 0.91 | 1.60 |
| P13473 | Lysosomal associated membrane protein 2 | LAMP2 | 1.47 | 1.86 |
| P00338 | L-lactate dehydrogenase A chain | LDHA | 0.97 | 0.54 |
| P07195 | L-lactate dehydrogenase B chain | LDHB | 0.78 | 0.51 |
| P04075 | Fructose-bisphosphate aldolase A | ALDOA | 1.06 | 0.34 |
| P18669 | Phosphoglycerate mutase 1 | PGAM1 | 1.25 | 0.62 |
| O43707 | Alpha actinin 4 | ACTN4 | 1.27 | 0.51 |
| Q6IRU2 | Tropomyosin alpha-4 chain | TPM4 | 0.96 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pina-Jiménez, E.; Calzada, F.; Bautista, E.; Ordoñez-Razo, R.M.; Velázquez, C.; Barbosa, E.; García-Hernández, N. Incomptine A Induces Apoptosis, ROS Production and a Differential Protein Expression on Non-Hodgkin’s Lymphoma Cells. Int. J. Mol. Sci. 2021, 22, 10516. https://doi.org/10.3390/ijms221910516
Pina-Jiménez E, Calzada F, Bautista E, Ordoñez-Razo RM, Velázquez C, Barbosa E, García-Hernández N. Incomptine A Induces Apoptosis, ROS Production and a Differential Protein Expression on Non-Hodgkin’s Lymphoma Cells. International Journal of Molecular Sciences. 2021; 22(19):10516. https://doi.org/10.3390/ijms221910516
Chicago/Turabian StylePina-Jiménez, Emmanuel, Fernando Calzada, Elihú Bautista, Rosa María Ordoñez-Razo, Claudia Velázquez, Elizabeth Barbosa, and Normand García-Hernández. 2021. "Incomptine A Induces Apoptosis, ROS Production and a Differential Protein Expression on Non-Hodgkin’s Lymphoma Cells" International Journal of Molecular Sciences 22, no. 19: 10516. https://doi.org/10.3390/ijms221910516
APA StylePina-Jiménez, E., Calzada, F., Bautista, E., Ordoñez-Razo, R. M., Velázquez, C., Barbosa, E., & García-Hernández, N. (2021). Incomptine A Induces Apoptosis, ROS Production and a Differential Protein Expression on Non-Hodgkin’s Lymphoma Cells. International Journal of Molecular Sciences, 22(19), 10516. https://doi.org/10.3390/ijms221910516










