1. Introduction
Anthocyanins are water-soluble pigments of plants and responsible not only for the red, blue or purple colors of many flowers, but also of a spectrum of fruits and vegetables [
1]. An increasing number of studies have indicated that the consumption of anthocyanin-rich fruits and vegetables are inversely associated with inflammatory stress. Thus, a higher intake is considered to result in a lower prevalence of cancer, cardiovascular and Alzheimer’s disease [
2,
3,
4]. In particular, different types of berries such as elder-, blue-, choke- and blackberries represent rich sources of anthocyanins and are well described for their antioxidant properties [
5,
6].
These studies are mostly limited to the measurement of pro- and anti-inflammatory cytokine gene transcription and secretion [
7,
8]. However, knowledge is scarce regarding the mechanisms involved in anthocyanin activity, affecting those cytokines. The transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) represents one of the regulating parameters of cytokine secretion, being indispensable in inflammatory processes and innate immunity. It is a major player in inflammatory signal transduction promoting the expression of more than 100 target genes, most of them involved in host immune response, including pro-inflammatory cytokine interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α) or interleukin 8 (IL-8) and the anti-inflammatory interleukin 10 (IL-10) [
9,
10]. The activation of NF-κB by an inflammatory stimulus is cell-type specific. Selectivity and fine-tuning of the NF-κB-dependent transcriptional response can be achieved by selective activation and binding of different Rel/NF-κB family members to the transcription factor response element. This leads to induction of different target genes [
11]. Only a few in vitro studies, addressing the question whether polyphenols affect NF-κB activity, point out the selectivity of the transcription factor. There is evidence that polyphenols, e.g., procyanidin-B
2 and phloretin may modulate the NF-κB signaling pathway in a way that cytokine gene expression will be decreased, resulting in less cytokine secretion [
12,
13]. Moreover, an inhibiting effect of cyanidin-3
O-glucoside (Cy3glc), one of the most studied anthocyanins, on NF-κB activation has been described hinting towards the involvement of the pathway in anthocyanin-mediated immunomodulatory effects [
14].
In recent years, there has been growing interest in inflammation-related microRNAs (miRNAs) as regulating factors of cytokine expression. MiRNAs are a class of small noncoding RNAs of 18–24 nucleotides of length known to suppress the expression of protein-coding genes. The impact of miRNAs on inflammatory processes occurs post-transcriptionally and may be mediated by mRNA destabilization and/or translational repression. They are targeting a variety of different mRNAs involved in the regulation of the inflammatory response [
15,
16]. However, knowledge about their mode of action and targets is still scarce, especially in the context of inflammation. For instance, miR-146 is targeting IL-1R-associated kinase 1 (IRAK-1) and the TNF receptor associated factor 6 (TRAF-6) by decreasing the protein level. Both are important signaling proteins of the canonical NF-κB signaling pathway, which leads into the induction of pro- and anti-inflammatory cytokine gene transcription [
17,
18]. In turn, other miRNAs like miR-223 and miR-199 are controlling NF-κB activity by targeting NF-κB kinases (IKKs) [
19,
20]. Additionally, the direct regulation of cytokine mRNA may occur through miRNAs such as miR-125b, which is downregulated during inflammation to guarantee TNF-α production [
21]. MiR-125b has been described as an activator of TNF-α cytokine gene transcription during LPS stimulation. It has also been shown that miR-125b might be repressed in order to activate TNF-α gene transcription [
21]. In contrast, miR-155 and miR-146a are described as inhibitors of pro-inflammatory cytokine gene transcription, decreasing the mRNA levels of TNF-α, IL-8 and IL-6 [
17,
18]. MiR-16 has been found to be, either overexpressed or down-regulated in order to decrease or increase TNF-α stability, respectively [
22]. Some studies already indicate that different classes of polyphenols out of the class of flavanols, flavonols or stilbenes suppress cytokine gene expression via posttranscriptional regulation [
23,
24,
25]. Quercetin as well as its methoxylated metabolite/analogue isorhamnetin decrease the transcript level of miR-155 significantly at 10 µM after 6 h of incubation in RAW264.7 macrophages. In turn, miR-155 appears to target TNF-α mRNA expression [
24]. However, to the best of our knowledge, in the present study the potential impact of blackberry anthocyanins on inflammation-related miRNAs (miR-16, miR-125b, miR-155 and miR-146a) and NF-κB activity in LPS-challenged THP1 macrophages is described for the first time.
Within the scope of the European AnthoPLUS project (
www.anthoplus.com; accessed on 6 September 2021), anthocyanins of different complexity in their side chain decoration and sugar moieties were produced and assessed for their quality and properties as potential colorants and food additives. As a novel concept, test kits from different fruit juice sources were developed, so called “AnthoKits”, comprising varying fractions of purified anthocyanins [
26]. In the present study, for the first time, an AnthoKit from blackberry (
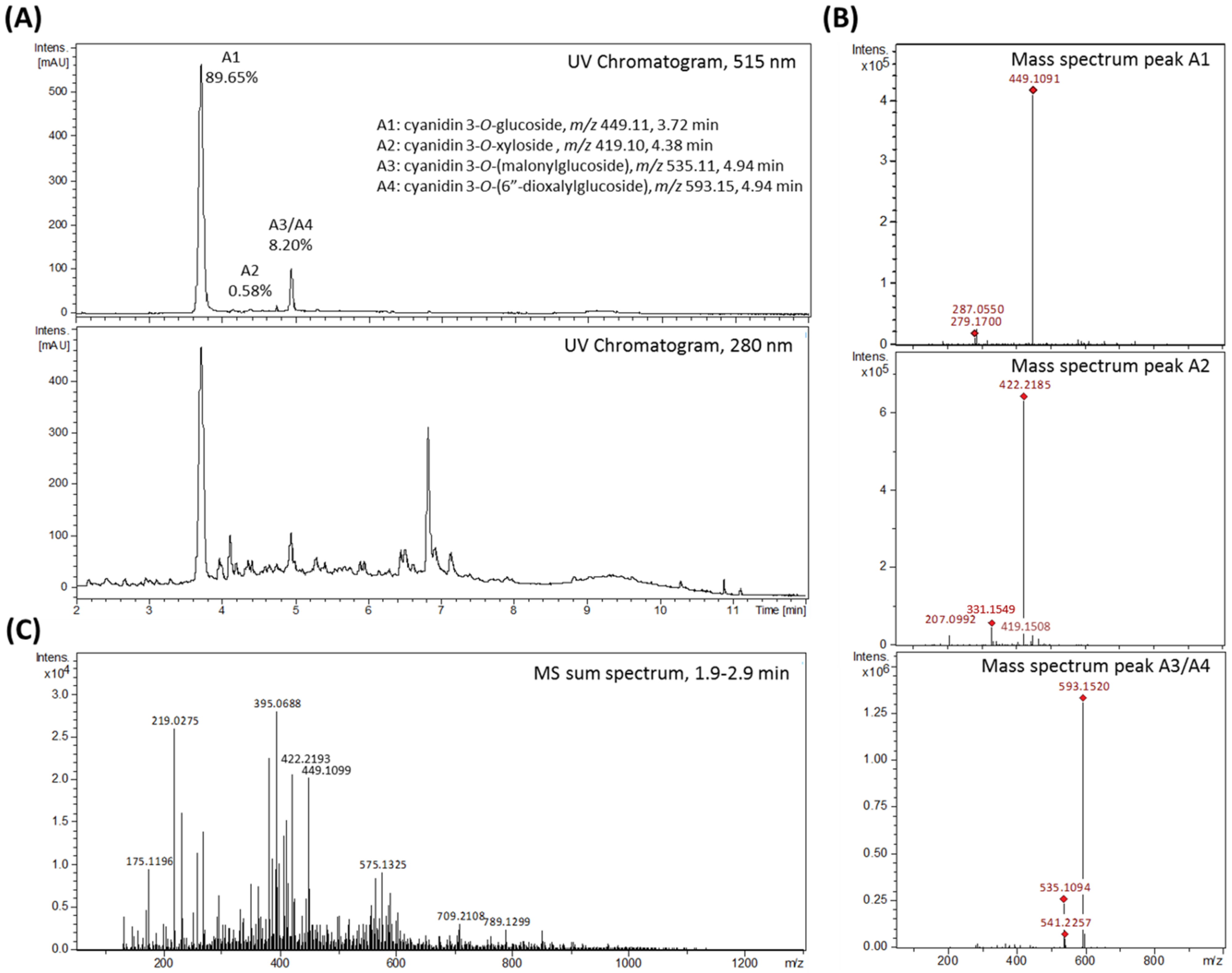
Rubus fruticosus) juice was produced and tested. The aim was to investigate the impact of blackberry constituents on LPS-induced pro-inflammatory cytokine transcription and secretion of IL-6, IL-8 and TNF-α in human THP-1 derived macrophages in plasma-relevant concentrations. The AnthoKit comprised an extract of raw blackberry juice (BB juice), four individual purified anthocyanin fractions (A1–A4,
Figure 1), a fraction of non-identified polyphenols (n.i. PP) and a mixture of the four fractions A1–A4 based on their natural composition in the blackberry juice. Well-characterized THP-1 monocytes, differentiated by treatment with phorbol-12-myristate-13-actetate (PMA) to adherent macrophages [
27], served as an in vitro cell model for intestinal macrophages, the most abundant leukocytes in the intestinal lamina propria, playing a crucial role in the intestinal immune system. One of their main functions seems to be the production of inflammatory mediators [
28]. Furthermore, this study intended to elucidate underlying mechanisms leading to inflammatory cytokine production. Hence, the ability of tested anthocyanins and mixtures to modify NF-κB activation as well as the modulation of post-transcriptionally interfering, inflammation-related miRNAs was investigated for the first time to better understand the effect of single anthocyanins in complex mixtures. In vivo, ingested anthocyanins might be already absorbed in the stomach and the small intestine passing rapidly to the blood stream. However, remaining anthocyanins which pass the upper gastrointestinal tract (GIT) represent known substrates for the gut microbiome, giving raise to degradation products such as respective aglycons, phenolic acids and phloroglucinol aldehyde [
29]. Systemic bioavailability of anthocyanins is low due to the fact that the glycosides are rapidly hydrolyzed by the gut microbiome [
30]. The stability of the liberated aglycons strongly depend on the pH of the environment. Thus, in our study not only the free aglycon cyanidin (Cy) but also the potential degradation products, phloroglucinol aldehyde (PGA) and protocatechuic acid (PCA), were included (
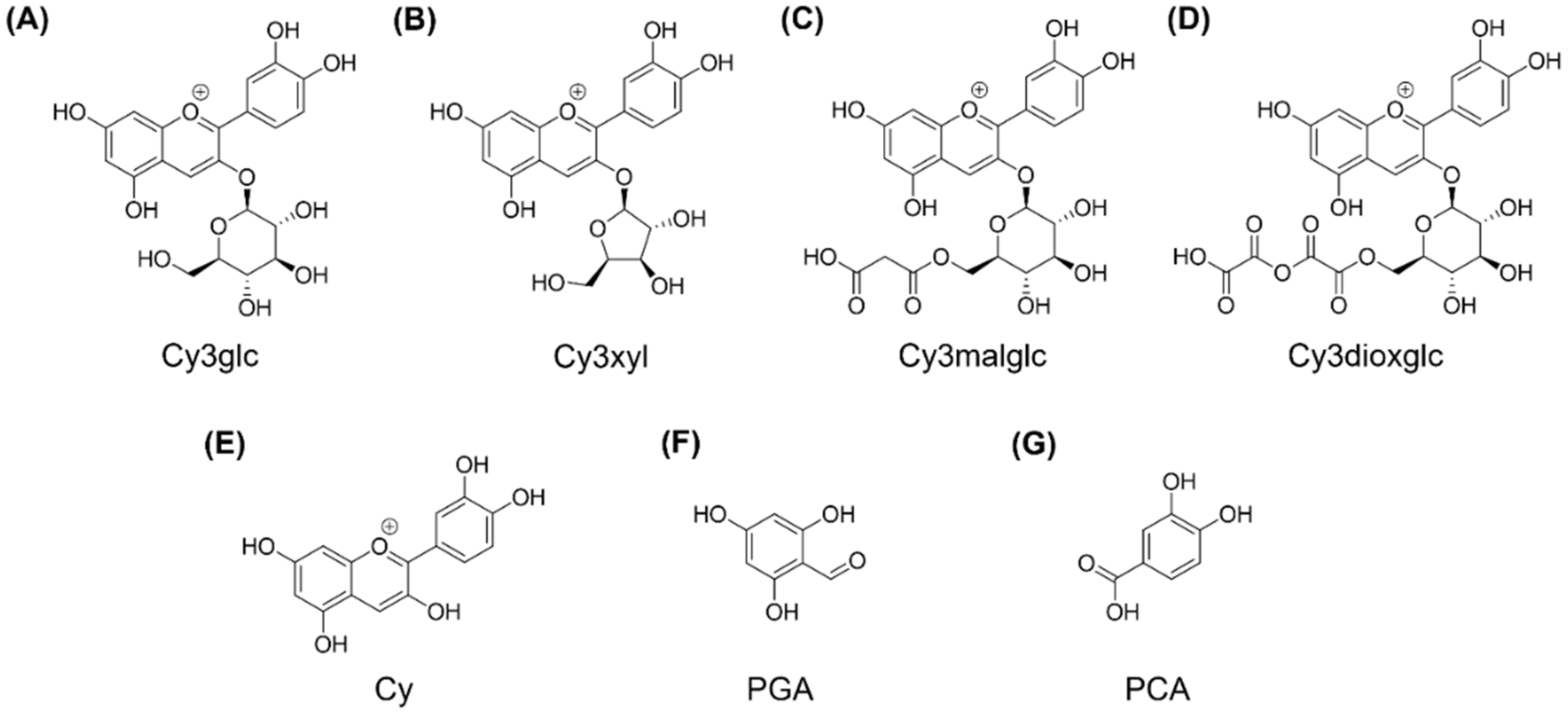
Figure 1).
3. Discussion
In the present study the modulatory effect of blackberry anthocyanins and respective degradation products on LPS-induced pro-inflammatory cytokines IL-6, TNF-α and IL-8 was investigated, to our knowledge, for the first time at physiologically relevant concentrations (0.002–5 µg/mL) in THP-1 derived macrophages. Furthermore, the impact on NF-κB activation and miRNA modulation of the single substances and complex mixtures were investigated.
Corroborated by existing literature [
34,
35], detected and purified anthocyanins in the blackberry extract were all cyanidin-based. None of the test compounds showed cytotoxic effects after 20 h of incubation in THP-1 derived macrophages (data not shown). This confirms previous findings in literature, where anthocyanins and their degradation products did not show any cytotoxic effects up to 50 µM in THP-1 cells [
8].
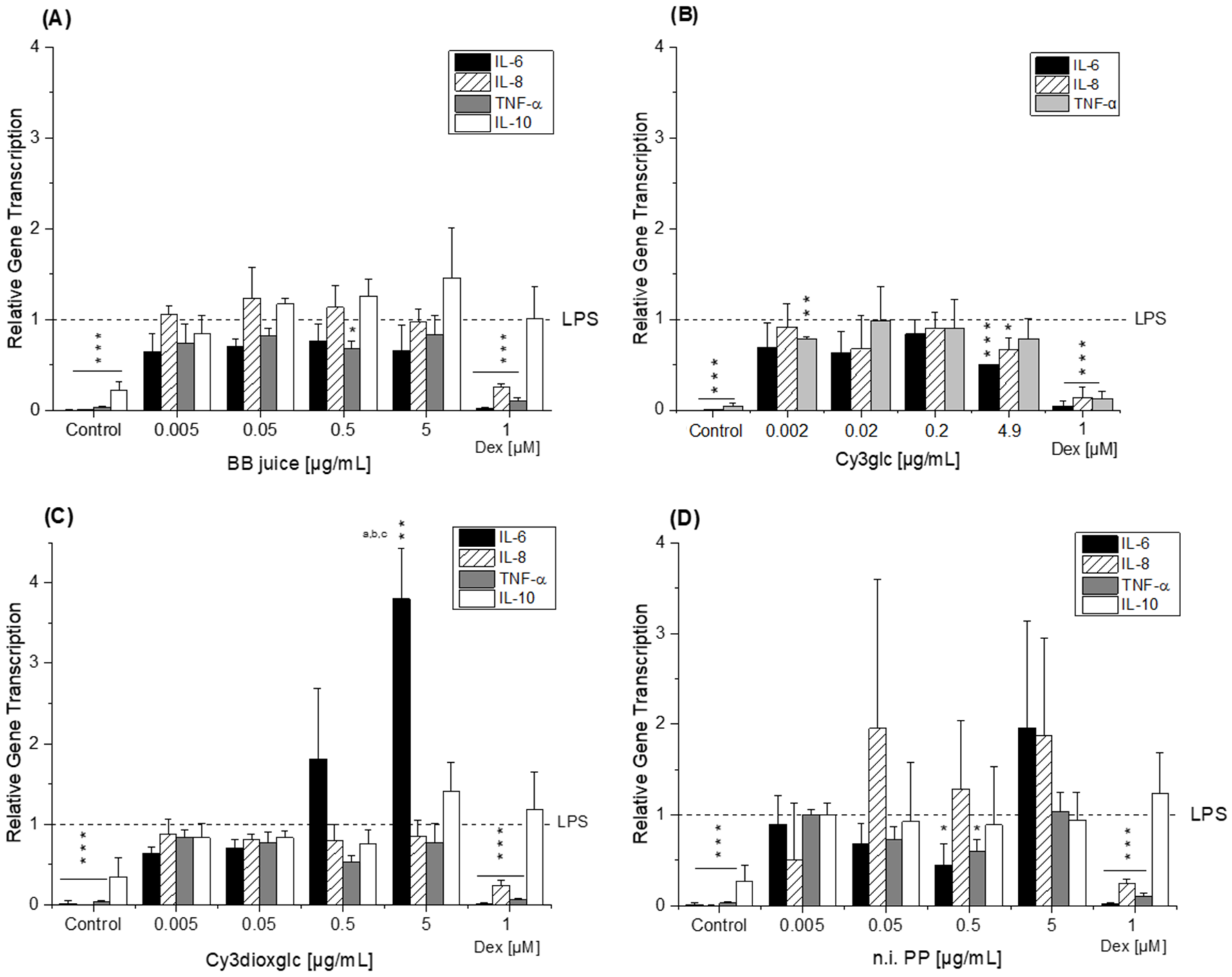
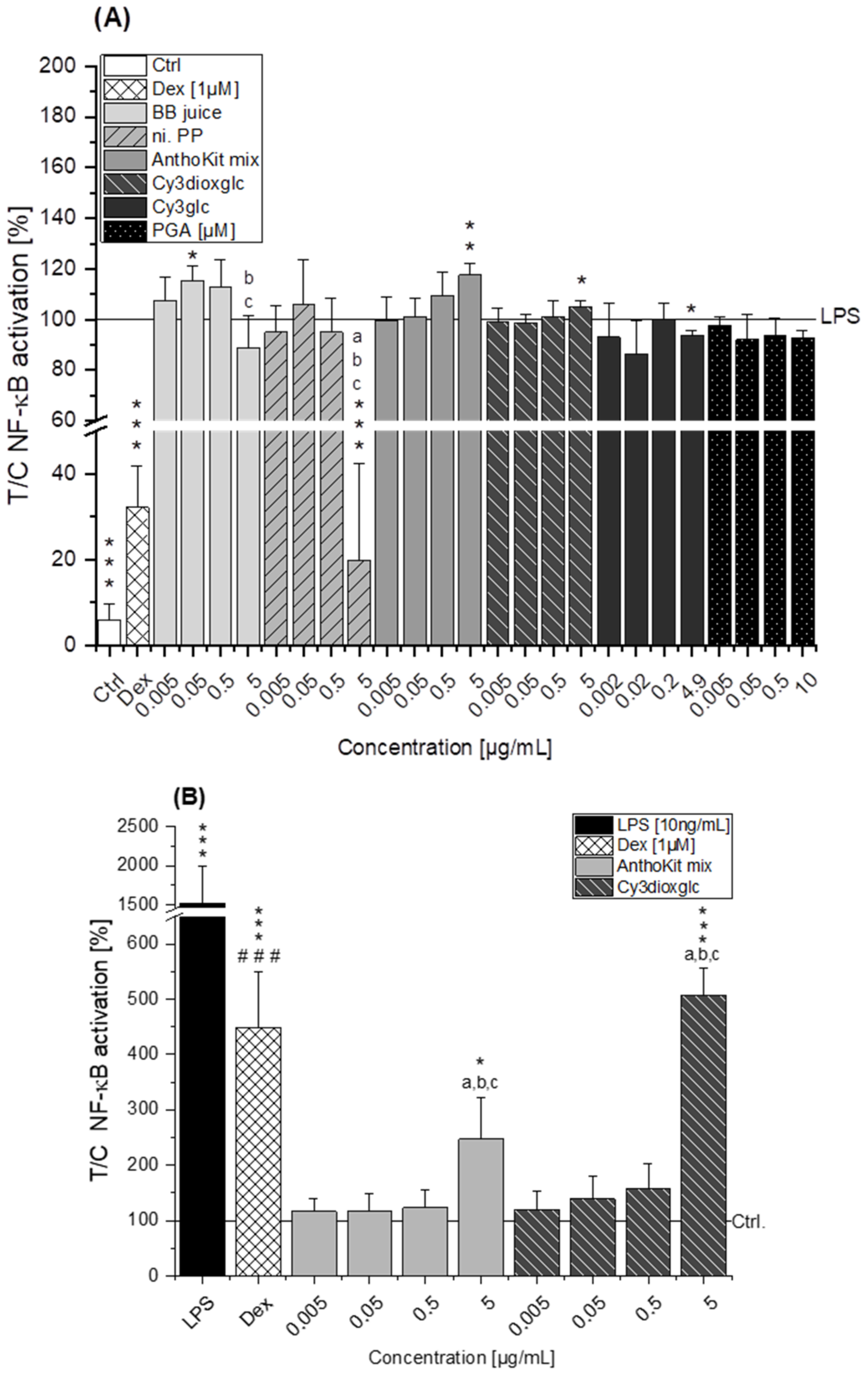
The blackberry raw juice extract, BB juice, decreased LPS-induced TNF-α transcript levels at 0.5 µg/mL and showed reducing tendencies on IL-6 transcripts (
Figure 4A). NF-κB activity was marginally affected by BB juice as it was significantly increased at 0.05 µg/mL but showed a decreasing tendency at 5 µg/mL. This reducing trend can potentially be attributed to the polyphenols present in the extract since the fraction of n.i. PP could significantly downregulate LPS-induced IL-6 and TNF-α transcripts at 0.5 µg/mL and showed a potent inhibition of LPS-induced NF-κB activity at 5 µg/mL. At this point, however, cytotoxic effects on THP1-Lucia™ NF-κB cells cannot be excluded completely as cytotoxicity was only ruled out in THP-1 derived macrophages. Paur et al. reported inhibition of LPS-induced NF-κB activity by blackberry at 100 mg/mL in U937–3κκB-LUC cells after 30 min pre-incubation and additional challenge for 6 h [
36]. Albeit the conditions and experimental set-up are different to our experiments the data support our findings, which showed moderate tendencies towards NF-κB inhibition due to the much lower concentrations applied.
Compared to the non-effective blackberry anthocyanins, Cy3xyl and C3malglc, the main anthocyanin Cy3glc significantly decreased IL-6 and IL-8 mRNA levels (
Figure 3B) accompanied with lower IL-6 protein levels (
Figure 6). Remarkably, the TNF-α transcript level was already significantly reduced at 0.002 µg/mL Cy3glc, TNF-α protein was diminished at 0.02 µg/mL. This concurs well with previous findings of Triebel et al. [
7], showing that Cy3glc has a similar pattern with respect to the effect on pro-inflammatory mediators in human colon T84 cells. However, the cells of this in vitro study were stimulated with a mixture of different cytokines instead of LPS and the applied test-concentrations of Cy3glc were obviously higher for incubation of the epithelial cell line (25–100 µM) than for the stimulated macrophages. Moreover, Cy3glc marginally yet significantly decreased LPS-induced NF-κB activation at 4.9 µg/mL (equals 10 µM), corresponding well with previous literature. Ferrari et al. showed that pre-treatment of Caco-2 cells with 20 and 40 µM Cy3glc could keep TNF-α induced NF-κB and IL-6 induction at solvent control levels [
14]. Furthermore, it could be demonstrated that Cy3glc is decreasing IκBα phosphorylation in THP-1 macrophages [
37]. Thus, Cy3glc is able to protect cells from inflammatory cytokine release. In fact, contrary to these results di Gesso et al. reported no impact of Cy3glc on NF-κB activity in THP-1 monocytes [
8]. This difference potentially reflects the discrepancy between monocytes and macrophages or is based on the shorter incubation time used. Considering that 90% of the anthocyanin fraction comprised Cy3glc, the non-protective effect of the AnthoKit mix is surprising, yet reflects potential interactions of single constituents as has been observed for the BB juice.
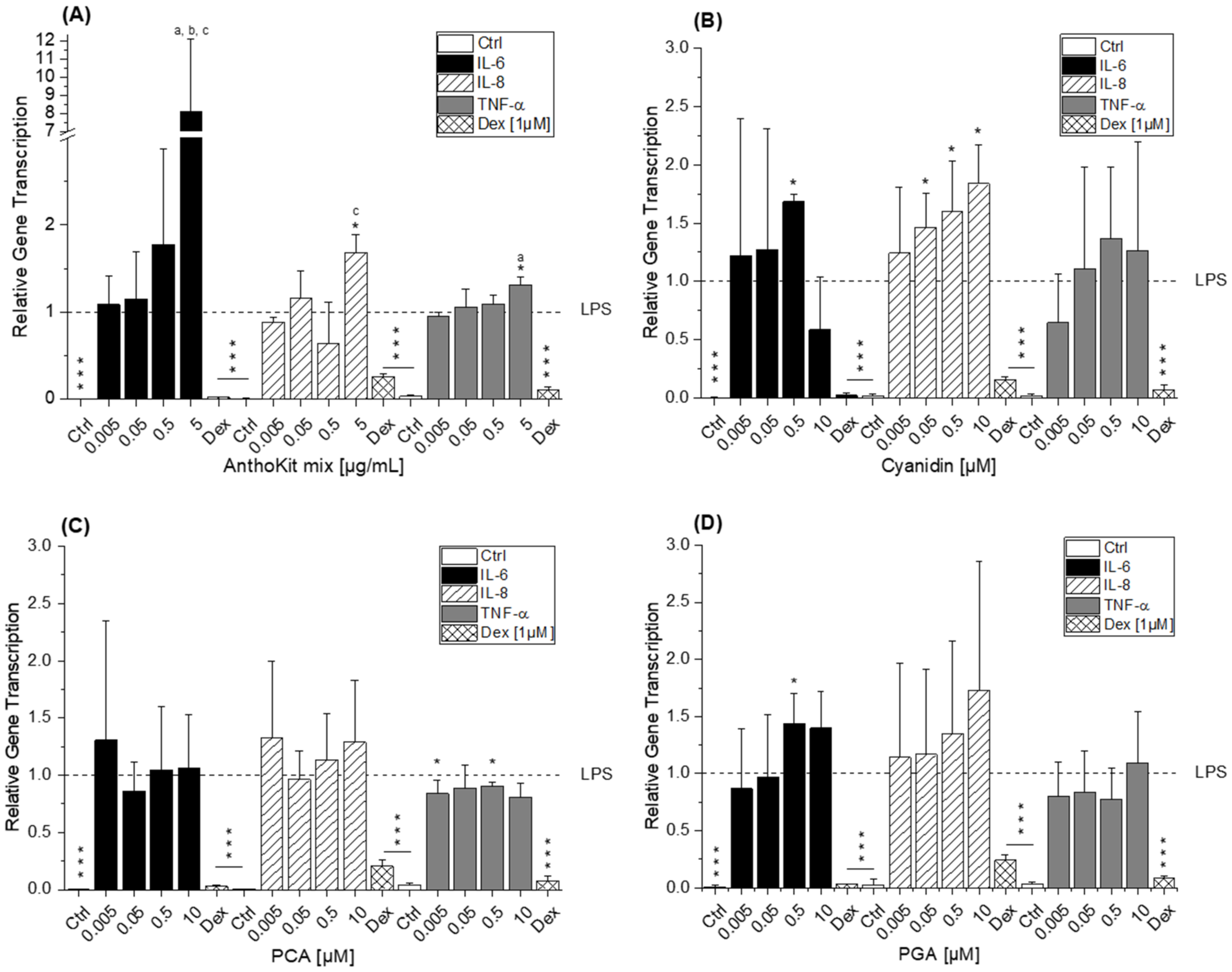
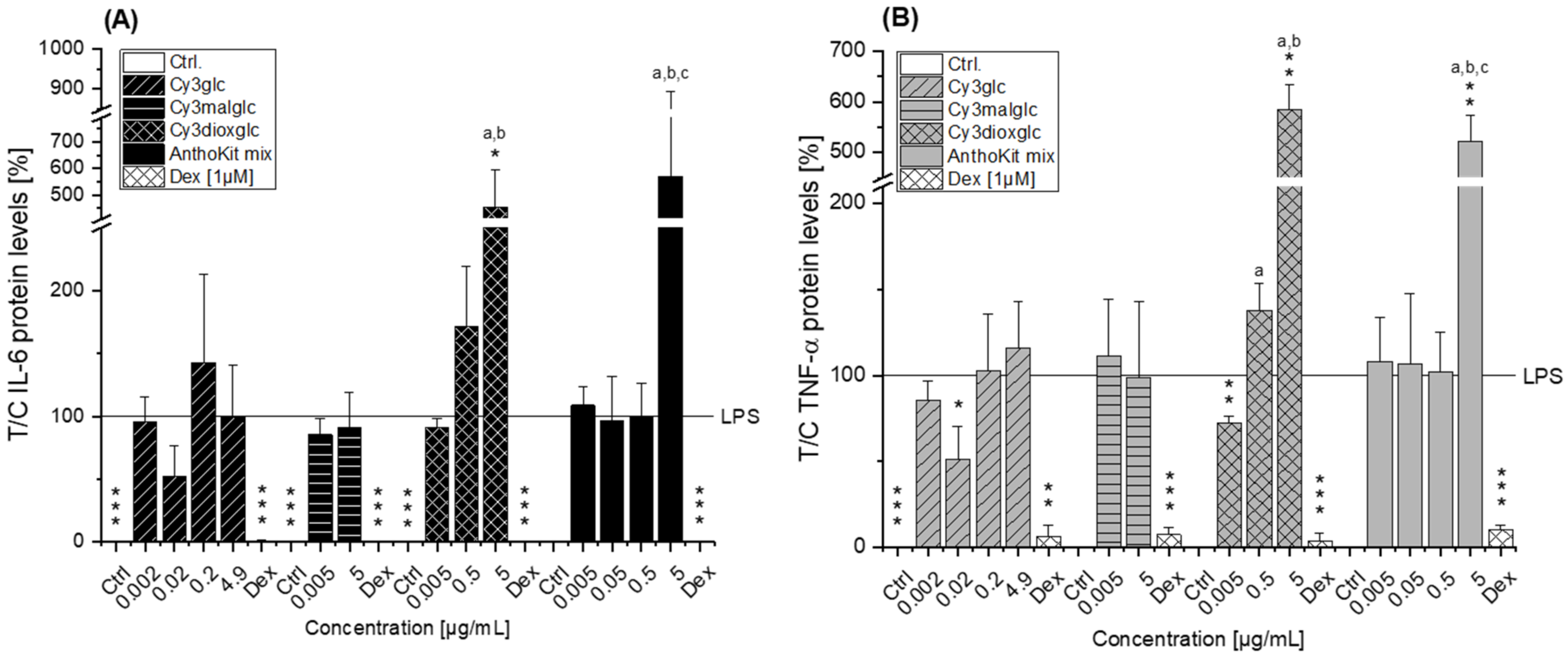
Of note, the most effective blackberry anthocyanin Cy3dioxglc additionally increased the level of IL-6 and TNF-α cytokines, suggesting pro-inflammatory properties which are supported by the minor yet significant increase of LPS-induced NF-κB activity with the highest test concentration applied. Furthermore, Cy3dioxglc alone was found to potently activate NF-κB at 5 µg/mL (
Figure 7), underpinning pro-inflammatory properties. To the best of our knowledge, the implication of Cy3dioxglc in inflammatory processes has not been described before. The AnthoKit mix, a mixture of the tested anthocyanins comprising 90% of Cy3glc and only 7% of Cy3dioxglc, still additionally elevated IL-6 and TNF-α secretion (
Figure 6), potentially intensifying the pro-inflammatory response to LPS. A similar picture was observed with NF-κB activation as 5 µg/mL AnthoKit mix could significantly increase it. This hints towards Cy3dioxglc being the potential key driver of the rather pro-inflammatory mode of action of the AnthoKit mix although only 7% of Cy3dioxglc are contained. Aboonabi et al. could show that 50 µg/mL purified anthocyanins from black currant and bilberry could reduce LPS-induced IL-6 production in primary human diabetic aortic endothelial cells [
38]. However, no Cy3dioxglc is to be expected in these extracts since black currant mainly contains anthocyanin-rutinosides and bilberry beholds a complex mixture of anthocyanins comprising predominantly delphinidin-3
O-glucoside and Cy3glc [
39,
40]. Van de Velde et al. reported significantly lower IL-6 transcript levels when incubating LPS-challenged RAW 264.7 macrophages with a crude blackberry extract and an anthocyanin- or polyphenol-enriched fraction thereof. However, the transcription of the inflammatory cytokine IL-1β could only be reduced by both of the enriched fractions whereas the crude extract showed significant induction [
41].
The aglycon Cy showed a significant pro-inflammatory tendency as well, considering the elevated IL-6 and IL-8 transcript levels after 5 h of incubation (
Figure 5B). In contrast, PCA, a potential degradation product, yielded a similar decreasing effect on TNF-α transcript levels as observed for Cy3glc. These findings differ from previous results of di Gesso et al. [
8], reporting that TNF-α mRNA was slightly increased by PCA treatment at 1 µM after 2.5 h of incubation in THP-1 monocytes. Interestingly, di Gesso and colleagues noted already after 3.5 h of incubation a slight decrease of TNF-α cytokine release with the test substance. Thus, it is quite conceivable that after 5 h of incubation the transcript level of TNF-α might have dropped even more. PGA, typically found in the colon, did not affect the inflammatory response as much as initially expected based on the significant increase of IL-6 mRNA in LPS-induced THP-1 derived macrophages (
Figure 5D). However, no impact on either cytokine secretion or NFκB activity was evident. Earlier findings by Kropat et al. have shown, that PGA activated the Nrf2 transcription factor in a Chinese hamster ovary (CHO) cell-based reporter gene assay. It also induced the transcription of anti-oxidative Nrf2 dependent gene haeme oxygenase 1 (HO-1) in HT29 colon carcinoma cells [
42]. As a logical consequence, pro-oxidative properties, which are often linked to inflammatory properties, were attributed to the final degradation product PGA. However, the apparent lack of consistency in the case of PGA may be the reflection of an overlay with mechanisms other than NF-κB-mediated fine tuning. For instance, inflammation-related miRNAs affecting cytokine secretion levels post-transcriptionally.
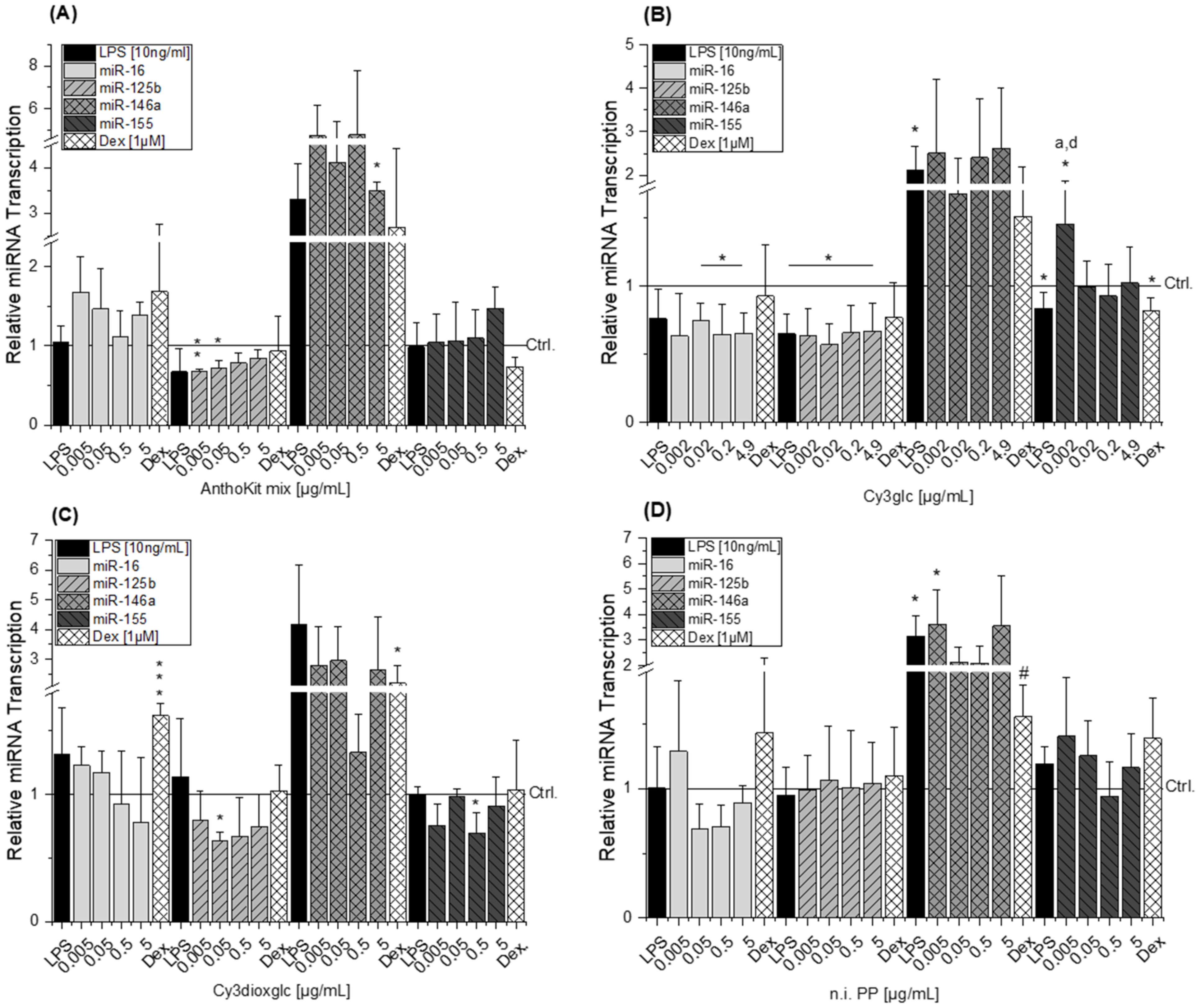
MicroRNAs have been reported as molecular targets of polyphenols [
43,
44,
45], underlining their capacity to interfere in biological processes. All miRNAs tested are targeting IL-6, IL-8 and TNF-α mRNA. Either by direct mRNA-binding in presence of RNA-binding proteins or by targeting other signaling molecules involved in signal transduction such as myeloid differentiation primary response 88 (MyD88), inhibitor of nuclear factor kappa B kinase subunit epsilon (IKKε) or TRAF-6 [
15,
46,
47,
48]. With respect to the response of THP-1 derived macrophages to LPS, the results on miRNAs predominantly correspond to the literature. Transcript levels of miR-146a in non-stimulated THP-1 cells were significantly lower than in LPS-stimulated cells, indicating an up-regulation of miR-146a levels in response to LPS. These findings are in line with previously reported results by Taganov et al. who identified miR-146a as one of several endotoxin-responsive genes in THP-1 cells [
18]. However, Dex was not able to convincingly suppress miR-146a with statistical significance in all experimental setups as limited amounts of test compounds did not allow sufficient biological replicates. Furthermore, miR-146a has previously been reported to negatively regulate the translation of IL-6 [
49]. This corresponds well with the observed reducing tendency of 0.5 µg/mL Cy3dioxglc which occurs concomitantly with the induction of IL-6 protein levels. However, for the highest concentration of Cy3dioxglc and the AnthoKit mix the effect on miR-146a could not be observed despite the potent induction of IL-6 suggesting that other posttranscriptional regulatory mechanisms are involved in stabilizing IL-6 translation. Tili et al. reported decreased levels of miR-125b in LPS-stimulated murine RAW 264.7 macrophages in comparison to non-stimulated cells. Contrary to that,, they observed an up-regulation of miR-155 [
21], which was not the case for the THP-1 derived macrophages in the present study (
Figure 7). However, there is still considerable ambiguity with regard to the role of miR-155. A number of studies have demonstrated that both inhibition and overexpression of miR-155 is negatively regulating the expression of cytokines, e.g., IL-1β or IL-8 [
50,
51]. On the other hand, miR-155 seems to be essential for the expression of TNF-α and IL-6. Indeed, some scientific reports have demonstrated that miR-155 is increased in response to inflammatory processes at an early stage in order to enhance TNF-α production [
25,
52]. Nevertheless, Nahid et al. found that miR-155 transcript levels are rarely affected during LPS stimulation in THP-1 derived macrophages, whereas TNF-α transcript levels and cytokine release were potently induced [
17]. Taken together, the findings by former investigations are contradictory.
Boesch-Saadatmandi et al. investigated the impact of the flavonols quercetin and isorhamnetin on LPS-induced TNF-α transcription as well as cytokine release in RAW 264.7 macrophages after 2 and 24 h of incubation, respectively [
24]. The results show that quercetin and isorhamnetin decrease TNF-α mRNA and cytokine level, respectively. Additionally, they studied whether miR-155 transcript levels are altered after 6 h of incubation considering the potential implication of the miRNA in regulating TNF-α expression. The obtained data indicate that both flavonols antagonize LPS-induced miR-155 transcript levels. The authors suggest that the decrease of miRNA transcript level may relate to the inhibition of NF-κB activation and thus, result in the reduction of pro-inflammatory TNF-α. However, our results do not support the observations regarding the impact of bioactive food constituents on miR-155 transcription, although, Cy3glc reduces pro-inflammatory cytokine expression (
Figure 3B and
Figure 5) and NF-κB activation (
Figure 6A). This is not exceptional considering incubation time and concentration of an inflammatory stimulus as crucial factors in influencing the expected response. On the one hand, the observed increase of miR-155 after 20 h of incubation with Cy3glc might thus be considered as a response to the continuous repression of pro-inflammatory cytokine mRNA. On the other hand, other targets of miR-155 include several upstream signaling molecules of the NF-κB pathway, such as MyD88, IKKε or Tak1-binding protein 2 (TAB2) [
15,
21]. As a result, the observed induction of miR-155 after Cy3glc incubation potentially blocks NF-κB pathway activation leading to suppressed cytokine expression. The results demand further evaluation for fully understanding how the anthocyanin Cy3glc affects miRNA levels. The impact of Cy3dioxglc is even more complex and puzzling as it reduces miR-125b and miR-155 at a concentration of 0.05 or 0.5 µg/mL, respectively, and hence, is signaling protective effects against LPS. However, this contradicts the further activation of LPS-induced NF-κB activation and IL-6 transcription and secretion observed with Cy3dioxglc.
Taken together, plasma-relevant concentrations of blackberry anthocyanins display different and partly controversial modulatory effects regarding the inflammatory response of THP-1 derived macrophages to LPS. As the blackberry extract, comprising all tested anthocyanins, had no major impact on the cellular stress response, it nicely shows that these effects are well balanced in a mixture. The study also demonstrates that the bioactivity of anthocyanins is not always associated with anti-inflammatory properties. Depending on the composition and the applied concentration also pro-inflammatory effects might occur. It might further be speculated whether these, at least in the present study, limited pro-inflammatory effects are contributing to some hormesis-like effects. However if so, the pro-inflammatory nature of respective bioactive constituents should be taken into account when fortified preparations such as food supplements for regular human consumption are provided.
4. Materials and Methods
4.1. Chemicals and Plant Material
Cyanidin-3O-glucoside chloride (Cy3glc) and cyanidin chloride (Cy) were purchased from Extrasynthése (Lyon, France). Protocatechuic acid (PCA) and phloroglucinol aldehyde (PGA) were obtained from Carl Roth (Karlsruhe, Germany) and Sigma Aldrich (Munich, Germany), respectively. All used compounds had a purity ≥ 95% (HPLC). Stock solutions of the test compounds were prepared at concentrations of 0.005, 0.05, 0.5 and 10 mM in ethanol (acidified, 80% EtOH). Phorbol 12-myristate 13-actetate (PMA; TLC purity ≥ 99%, PMA), lipopolysaccharides from Escherichia coli (LPS) and dexamethasone (Dex) were purchased from Sigma Aldrich and were dissolved in DMSO, cell culture medium and EtOH (80%, v/v), respectively. Freshly squeezed blackberry juice without any further processing was obtained from Bayernwald Früchteverwertung KG (Hengersberg, Germany). The juice was frozen and stored at −80 °C until further use.
4.2. Anthocyanin Quantification by UV/Vis Spectral Photometric Detection
Total anthocyanin content was quantified by means of the pH differential method described by Giusti and Wrolstad [
53]. In brief, the absorbance of pH = 1.0 (25 mM KCl) and pH = 4.5 (0.4 M Na-acetate) buffer-diluted samples was measured at 515 nm and 700 nm with a SPECTRAMAX
TM Pro UV-visible spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Measurements were done in duplicates. The differential absorbance was calculated using the following equation: A = (A515–A700) pH 1.0—(A515–A700) pH 4.5. The total anthocyanin content was then calculated based on reference curves for cyanidin-3
O-glucoside in the range from 0.001 mg/mL to 0.05 mg/mL.
4.3. LC-MS Analysis
Prior to injection samples were diluted with solvent A (water + 0.5% formic acid, FA) in a ratio of 1:5 and incubated one hour at 4 °C to allow formation of potential precipitates. After centrifugation the clear supernatant was transferred to a sample vial and 3 µL of this mixture were injected per sample.
LC-UV/MS profiling was carried out using a combination of an ultra-performance liquid chromatography (UPLC) instrument (H-class, Waters, Milford, MA, USA) with photodiode array (PDA) detection coupled to an ultra-high-resolution time of flight mass spectrometer (UHR-TOF–MS, maXis Impact, Bruker Daltonics, Bremen, Germany) for MS detection. LC separation was based on a method described previously [
54]. For MS detection, the outlet of the PDA detector was coupled to the electrospray ionization (ESI) source (positive ion mode at 260 °C dry temperature, 3 bar nebulizer gas pressure, 4000 V capillary voltage and a dry gas flow of 11 L/min) of the UHR-TOF-MS instrument using a splitter directing 83% of the eluate into the source. The instrument was operated in MS full scan mode at 3 Hz acquisition speed using the following settings: mass range
m/z 50–1300, hexapole radio frequency (RF) voltage 800 V peak-to-peak (Vpp), collision energy 8 eV, funnel 1 RF of 300 Vpp, a funnel 2 RF of 600 Vpp, prepulse storage time 5 µs, transfer time 50 µs, collision cell RF of 500 Vpp. Instrument calibration was done with 10 mM sodium formate solution (12.5 mL H
2O, 12.5 mL isopropanol, 50 µL concentrated FA, and 250 µL 1M NaOH).
For LC-MS/MS fragmentation analyses of the blackberry raw juice the instrument was operated in MS full scan mode as indicated above and with auto-MS/MS scan mode activated. MS fragmentation was realized by collision-induced dissociation (CID) with argon at 1.5 mTorr in the quadrupole collision cell of the instrument using the following settings: molecular mass 300 Da (width 5 Da) with 27, 22, and 17 eV for charge state 1, 2, and 3 respectively; molecular mass 500 Da (width 6 Da) with 32, 27, and 22 eV for charge state 1, 2, and 3 respectively; molecular mass 1000 Da (width 8 Da) with 45, 40, and 35 eV for charge state 1, 2, and 3 respectively; molecular mass 1500 Da (width 10 Da) with 45, 40, and 35 eV for charge state 1, 2, and 3 respectively. Detection of precursor ions was set to 3 times at an absolute threshold of 5000 counts. The smart exclusion option was activated with exclusion after 15 acquired spectra and a release time of 0.5 min. MS-fragmentation analysis of the final fractions (anthocyanin A1, A2, A3, A4 and polyphenols) were conducted by directly injecting the previously with acetonitrile and 0.1% FA to a final anthocyanin concentration of about 0.1 µg/µL diluted samples into the ionization source of the UHR-TOF-MS instrument. Mass spectra were acquired and main molecular ions selected for MS-fragmentation analysis, at a flow rate of 100 µL/h, an accordingly adjusted collision energy specific for each compound, a width of 3 and a spectra rate of 1–3 Hz depending on the compound.
LC-UV/MS data were analyzed by means of Bruker Compass DataAnalysis version 4.1 software. The annotation of detected compounds is based on comparison of the measured retention times and molecular ion masses with available reference standards, as well as with literature data, and fragmentation pattern as obtained from LC-MS/MS experiments.
4.4. Anthocyanin Isolation and Preparation of Blackberry AnthoKit
Based on 400 mL blackberry juice with an anthocyanin content of 0.25 µg/µL a juice raw extract was prepared, containing 100 mg of total anthocyanins for subsequent purifications. Therefore, the juice was first filtered through four layers of miracloth paper. Degreasing was realized by two-phase-partitioning, thoroughly mixing the extract with two volumes (v/v) of n-heptane and allowing phase separation overnight. The lower phase (degreased extract) has been carefully collected after phase separation. Rotary evaporation in vacuo was conducted to remove the remaining organic solvent. The extract was than adjusted to an end concentration of 2% methanol (MeOH) and 0.5% FA. A stepwise filtration of the so prepared defatted extract through nylon net filters was carried out down to 0.2 µm pore size (filtration in vacuum). This sample was named raw juice and was analyzed by LC-UV/MS and LC-MS/MS as stated above.
To obtain a preliminary separation of the various anthocyanins present in this mixture and to remove low molecular weight contaminations (e.g., salt and sugar), the raw juice was loaded on a solid phase extraction column (RediSep RF Gold® C18Aq column, 15.5 g, 20–40 micron, 10–100 mg capacity, 13.5 mL column volume (CV), Teledyne Isco, Lincoln, NE, USA) equilibrated with 3 CV 2% methanol, 0.5% FA. Compound elution was realized by step gradients at 2%, 10%, 25%, 40% and 98% of MeOH with 0.5% FA using 7.4 CV each, at a flow rate of about 20 mL/min utilizing a Chromabond® vacuum chamber (Machery-Nagel, Düren, Germany). Resulting fractions were analyzed by LC-UV/MS. Fractions containing anthocyanins were then used for further anthocyanin isolation, which were the three crude fractions from elution with 10, 25, and 40% MeOH, 0.5% FA. Other fractions containing polyphenols, but no anthocyanins, were combined and named polyphenols fraction. The volume and the content of organic solvent of these fractions were reduced under vacuum by means of rotary evaporation.
For final anthocyanin separation and purification, the isolated crude extracts were filtered through a cellulose acetate filter with 0.45 µm pore size, in vacuo. Subsequently the extracts were loaded on a preparative HPLC column (Gemini® 5 µm NX-C18 110 A, LC Column 250 × 30 mm, AXIA™ Packed, Phenomenex, Aschaffenburg, Germany) equipped with an appropriate pre-column (SecurityGuard PREP Cartridge Gemini-NX C18 15 × 30 mm) and equilibrated with solvent A (water + 2% of 5% ammonium formiate in FA). Compound elution was realized by a linear gradient from 2–30% acetonitrile (solvent B) within 170 min at a flow rate of 10 mL/min utilizing a Varian ProStar chromatography instrument (Agilent Technologies, Santa Clara, CA, USA) with UV monitoring at 280 nm and 515 nm. Resulting fractions, showing anthocyanin specific absorption at 515 nm, were analyzed by LC-UV/MS and properly combined. Finally, four anthocyanin fractions enriched in the main blackberry anthocyanins Cy3glc (further named anthocyanin A1), cyanidin-3O-xyloside (Cy3xyl; anthocyanin A2), cyanidin-3O-(malonylglucoside) (Cy3malglc; anthocyanin A3) and cyanidin-3O-(6″-dioxalylglucoside) (Cy3dioxglc; anthocyanin A4), were obtained. Again, other fractions containing polyphenols, but no anthocyanins, were combined and added to the polyphenol fraction. The volume and the content of organic solvent of the final pure isolate fractions were reduced under vacuum by means of a rotation evaporator.
A final purification and enrichment step was performed to remove remaining low molecular weight contaminations (e.g., salt and sugar). Each fraction (anthocyanin A1, A2, A3, A4 and polyphenols) was adjusted to a final concentration of 2% methanol, 0.5% FA. In addition, 10 mL of the original blackberry juice were mixed with 10 mL of 2% methanol, 0.5% FA and then cleared by filtration through a cellulose acetate filter with 0.45 µm pore size, in vacuum. Each fraction (A1, A2, A3, A4, polyphenols and the cleared blackberry raw juice) was loaded on a separate solid phase extraction cartridge (Chromabond® C18, 70 mL, 10 g, Macherey-Nagel), which was conditioned with 3 CV of 2% methanol, 0.5% FA. The flow through was observed to be clear and the anthocyanins bound to the C18 column matrix. Remaining sugars and salts were washed off with two column volumes of 2% methanol, 0.5% FA. Elution of the bound anthocyanins and polyphenols was performed with 98% methanol, 0.5% FA.
For preparation of the AnthoKit mix we combined the individual purified anthocyanin fractions based on their natural composition in the blackberry juice; namely 90% (
w/
w) of anthocyanin A1, 1% of anthocyanin A2, 2% of anthocyanin A3 and 7% of anthocyanin A4, as illustrated in
Supplementary Figure S2. All resulting fractions (anthocyanin A1, A2, A3, A4 and ni. polyphenols) and the AnthoKit mix were dried under liquid nitrogen. The obtained dry powder was covered with argon and stored at −20 °C until further use.
4.5. Determination of Glucose, Fructose and Sucrose
Determination of soluble sugars in each fraction (anthocyanin A1–A4, polyphenols and the cleared blackberry raw juice) was performed as described previously through sequential enzymatic degradation of glucose, fructose and sucrose; using one unit each of glucose-6-phosphate dehydrogenase (Hoffmann-La Roche, Basel, Switzerland) for baseline generation, and then hexokinase (Sigma-Aldrich, Munich, Germany), phosphogluco-isomerase (Sigma-Aldrich) and invertase (β-fructofuranosidase, Sigma-Aldrich), respectively [
26,
55]. Quantities were calculated based on reference curves for authentic standards in the range from 0.1 mM to 1.0 mM.
4.6. Cell Culture
THP-1 monocytes were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). THP-1 cells were cultured in RPMI 1640 cell culture medium containing 2 g/L glucose (Thermo Fisher Scientific, Vienna, Austria) and supplemented with 10% heat inactivated fetal calf serum and 45 units/mL (1%) penicillin/streptomycin (Thermo Fisher Scientific) at 37 °C and 5% CO2 in a humidified atmosphere. The THP1-LuciaTM NF-κB cells used for NF-κB activity measurements were purchased from Invivogen (Toulouse, France). THP1-Lucia NF-κB cells were maintained in RPMI 1640 cell culture medium containing 4.5 g/L glucose and 2.383 g/L HEPES (Thermo Fisher Scientific) supplemented with 10% (v/v) heat inactivated FCS, 1% penicillin/streptomycin and 100 µg/mL of normocin (Invivogen, Toulouse, France) at 37 °C and 5% CO2 in a humidified atmosphere. To maintain the stability of the reporter gene cell clone, cells were grown under selection pressure with 100 µg/mL of zeocin (Invivogen, Toulouse, France) every second cell passage. Cell density was kept between 2∙105 and 1∙106 cells/mL and the number of cell passage did not exceed 20 passages.
4.7. THP-1 Cell Differentiation
THP-1 and THP1-Lucia™ NF-κB monocytes were seeded into six-well plates at a cell density of 1.2∙10
5 cells/mL and were subsequently differentiated into macrophages during 72 h with PMA (10 ng/mL) as described by Park et al. [
56] and Kollarova et al. [
27]. The cell culture medium for differentiation was removed and THP-1 macrophages were subsequently cultivated for 24 h in PMA-free culture medium prior the incubation with the test compounds.
4.8. Cell Viability Assay
Cell viability after 2 h of pre-incubation with blackberry AnthoKit components (0.005–5 µg/mL), Cy3glc, Cy, PCA and PGA (0.005–10 µM) and subsequent additional stimulation with LPS from
E. coli (10 ng/mL, 18 h) was tested by performing the alamar blue (Thermo Fisher Scientific) cell viability assay [
32]. The non-fluorescent assay reagent resazurin is absorbed by living cells. In the cytosol it is reduced to resorufin which is highly fluorescent. For assay performance, the cell culture medium was removed from monocytes after the incubation with test compounds, cells were washed with PBS and incubated with resazurin in FCS-free cell culture medium at a final concentration of 10% (
v/
v). After 1 h of incubation at 37 °C and 5% CO
2 an aliquot of the cell culture medium was transferred into a 96-well plate and the fluorescence of the reduced resazurin was measured with the Gen5 Microplate Reader (BioTek Instruments, Vienna, Austria) using a fluorescence excitation wavelength of 530 nm. The read out of the fluorescence emission was conducted at 560 nm.
4.9. Quantitative Real-Time PCR
Quantitative real-time PCR (qRT-PCR) was used for transcription analysis of IL-6, IL-8, TNF-α and IL-10 cytokines as well as miR-16, miR-125b, miR-155 and miR-146a. THP-1 derived macrophages were pre-incubated for 2 h with respective anthocyanins at concentrations between 0.0024–5 µg/mL or in the case of the degradation products Cy, PCA and PGA at concentrations of 0.005, 0.05, 0.5 and 10 µM with a final solvent concentration of 0.1% (
v/
v). Subsequently, cells were co-incubated with LPS (10 ng/mL) for 3 h for cytokine gene transcription analysis, or for 18 h for miRNA transcription measurements. The duration for LPS stimulation was chosen according to the monitoring of Chanput et al. showing that LPS-induced IL-6, IL-8 and TNF-α transcript levels reached their optimum between 3 and 6 h [
33]. Dex (1 µM) and 0.1% of EtOH (80%) served as positive and solvent control, respectively. After cell lysis with Qiazol (Qiagen, Hilden, Germany) total RNA was extracted using the miRNeasy Kit (RNA size ≥ 18 nucleotides, Qiagen) according to the instructions of the manufacturer’s protocol. RNA purity and quantity was determined with the NanoDrop 2000 (Thermo Fisher Scientific). Following the instructions of the manufacturer’s protocol, 1 µg of total RNA was reverse transcribed into complementary DNA (cDNA) using the miScript II RT Kit (Qiagen). Gene specific cDNA was exponentially amplified by performing RT-PCR using the StepOne™Plus System (Applied Biosystems, Thermo Fisher Scientific). qRT-PCR was conducted in a 96-well plate using the miScript SYBR Green PCR Kit (Qiagen) and the following gene- or miRNA-specific primer assays (Qiagen). QuantiTect Primer Assays: Hs_GAPDH_1_SG; Hs_ACTB_1_SG; Hs_TNF_1_SG; Hs_IL6_1_SG; Hs_IL10_1_SG; RT
2 qPCR Primer Assay: human IL8; miScript Primer Assays: Hs_RNU6-2_11 (detected transcript: NR_002752); Hs_SNORD68_11 (detected transcript: NR_002450); Hs_miR-16_2 (sequence: 5′UAGCAGCACGUAAAUAUUGGCG); Hs_miR-125b_1 (sequence: 5′UCCCUGAGACCCUAACUUGUGA); Hs_miR-146a_1 (sequence: 5′UGAGAACUGAAUUCCAUGGGUU); Hs_miR-155_2 (sequence: 5′UUAAUGCUAAUCGUGAUAGGGGU). The amplification protocol consisted of an initial activation step of the HotStarTaq polymerase for 15 min at 95 °C, 40 cycles of denaturation for 15 s at 94 °C, annealing for 30 s at 55 °C and extension for 30 s at 70 °C, followed by melting curve analysis. Relative gene transcript and miRNA transcript levels were calculated by applying the 2
−ΔΔCt method as described by Schmittgen and Livak [
57,
58]. Ct-values of the mRNA and miRNA target genes were normalized to the average of the Ct-values of the control genes ACTB and GAPDH or RNU6 and SNORD68, respectively. Subsequently, normalized data were compared to the calibrator (respective control sample).
4.10. Multiplex Immunoassay
In order to study the impact of selected polyphenols on TNF-α, IL-6 and IL-10 cytokine release a multiplex immunoassay was performed. For this, differentiated THP-1 macrophages were pre-incubated for 2 h with respective anthocyanins and their degradation products Cy, PCA and PGA (0.0024–5 µg/mL and 0.005, 0.05, 0.5 and 10 µM, respectively) and subsequently stimulated with LPS (10 ng/mL) for 18 h. The time of incubation with LPS was selected according to the results described by Chanput et al. [
33], where THP-1 macrophages reached a plateau for TNF-α and IL-6 secretion after 18 h of incubation. Afterwards, supernatants were removed and centrifuged at 10.000×
g (4 °C) to separate secreted cytokines from cell debris. Samples were stored at −80 °C until further analysis. To determine the cytokine concentration of TNF-α, IL-6 and IL-10, the supernatants were prepared with the ProcartaPlex Human Basic Kit and ProcartaPlex Human TNF-α, IL-6 and IL-10 Simplex (Affymetrix, Vienna, Austria) according to the instructions of the manufacturer’s protocol. Briefly, a mixture of magnetic beads was dispensed in a 96-well plate, carrying antibodies against the respective target cytokines, and 50 µL of each cytokine sample was added per well and incubated for 2 h. Once the cytokines attached to the corresponding antibody, a second cytokine dependent antibody was added and incubated for 30 min. The secondary antibody allowed afterwards the attachment of the reporter streptavidin-phycoerythrin conjugate (SA-PE), that permits the quantification of secreted cytokines with the Luminex 200 System (BioRad, Vienna, Austria), a bead-based flow cytometer, measuring the fluorescence of the bound SA-PE.
4.11. NF-κB Reporter Gene assay
The question, whether the NF-κB signaling pathway is affected by blackberry anthocyanins or the degradation product PGA was addressed by applying a NF-κB reporter gene assay. After differentiation into macrophages, NF-κB-inducible Luc reporter cells (THP1-Lucia™ NF-κB cells) were pre-incubated for 2 h with Cy3glc or PGA (0.005–10 µM) and subsequently stimulated with LPS (10 ng/mL) for 18 h, as recommended in the manufacturer’s protocol to induce luciferase gene expression by activation of NF-κB. The quantification of secreted Lucia luciferase in cell culture media is based on the catalytic oxidation of the coelenterazine-based luminescence assay reagent (QUANTI-Luc™) which leads to the emission of visible blue light (λ = 465–493 nm). For this, an aliquot of luciferase containing supernatant was transferred (10 µL) into a 96-well plate and fully automated 50 µL of QUANTI-Luc™ (Invivogen, Toulouse, France) were injected into each well with a dispenser. The resulting luminescence was measured with the Gen5 Microplate Reader (BioTek Instruments).
4.12. Statistical Analysis
Presented data are the means + SD of at least three independent biological replicates. The one sample Student’s t-test was used to compare significant differences between LPS-stimulated cells and non-stimulated cells, cells treated with the compound of interest or Dex treated cells (positive control), respectively. To verify significant differences between the test concentrations of the test-substance the analysis was performed by using one-way ANOVA followed by Holm-Bonferroni post-hoc test. The obtained results were considered as statistically different with p ≤ 0.05.